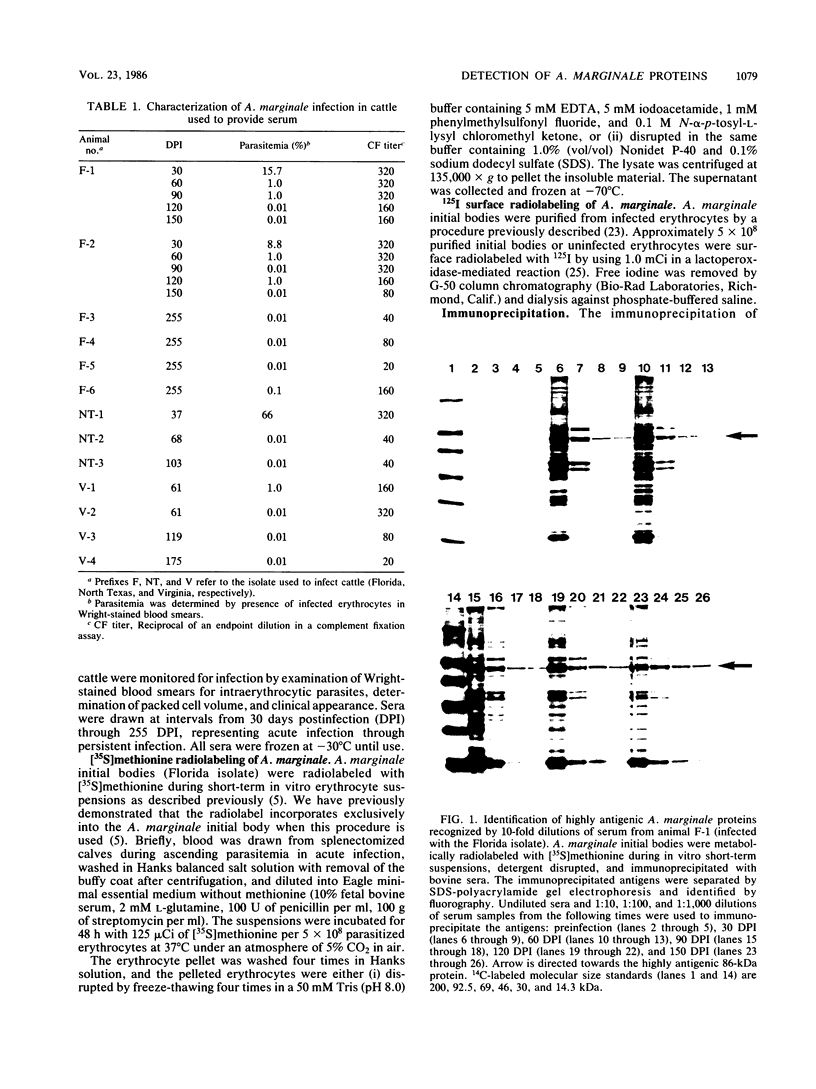
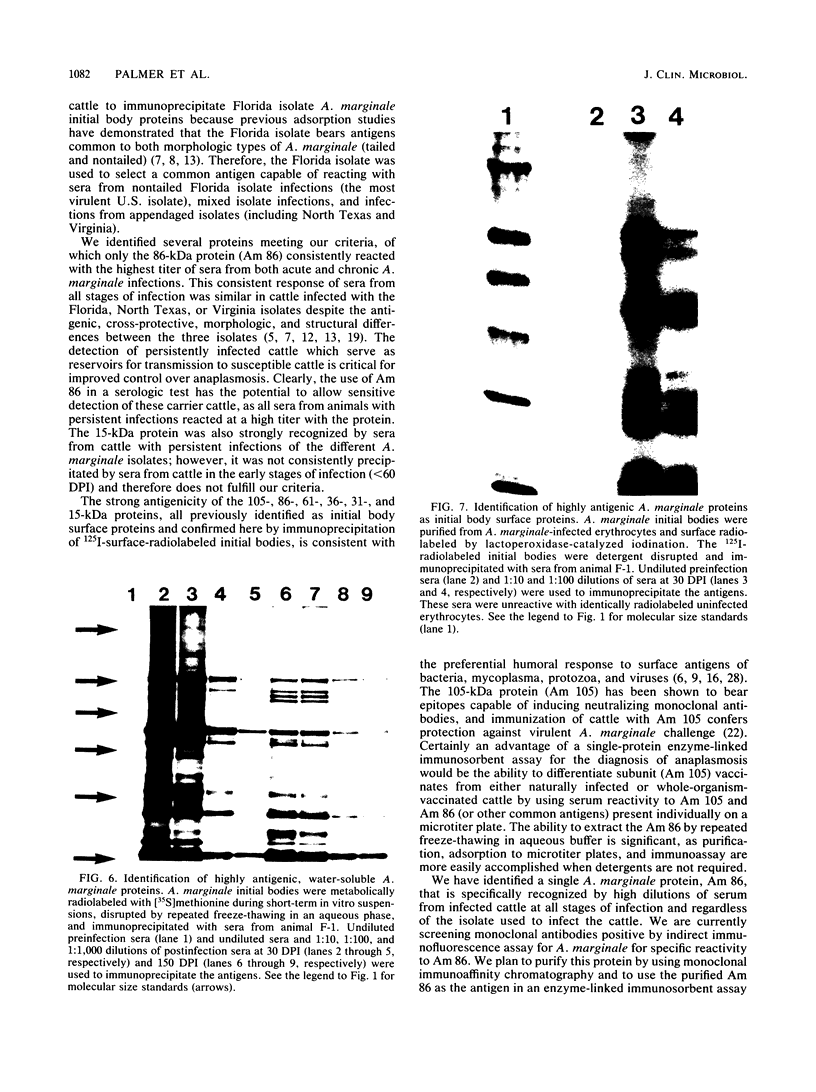
Abstract
The establishment and maintenance of anaplasmosis-free cattle herds is impaired due to the lack of a rapid, sensitive, and specific serologic test to detect persistently infected cattle which serve as carriers for the organism. To develop an improved diagnostic test for anaplasmosis we screened Anaplasma marginale initial body proteins to identify a protein common to antigenically different isolates that is recognized by the host immune system at all stages of infection. Seronegative cattle were infected with either the Florida, Virginia, or North Texas isolate of A. marginale and monitored for infection by daily examination of Wright-stained blood smears for parasitized erythrocytes. Sera from cattle at different stages of infection, from acute through persistent, were used to immunoprecipitate A. marginale proteins that were metabolically radiolabeled with [35S]methionine or surface radiolabeled with 125I. Multiple A. marginale proteins were recognized by using sera either undiluted or at 1:10; however, only four or five proteins were sufficiently antigenic to elicit antibody reactive with a 1:1,000 serum dilution. A single protein with an apparent molecular mass of 86 kilodaltons was consistently recognized at all stages of infection regardless of the isolate used to infect the cattle. This protein was demonstrated to be on the surface of the A. marginale initial body and to be water soluble. We propose use of this 86-kilodalton protein to develop an improved serologic test for diagnosis of bovine anaplasmosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amerault T. E., Rose J. E., Martin W. E., Roby T. O. Effects of phenol on card-agglutination and complement-fixation tests for bovine anaplasmosis. Am J Vet Res. 1980 Mar;41(3):435–438. [PubMed] [Google Scholar]
- Barbet A. F., Anderson L. W., Palmer G. H., McGuire T. C. Comparison of proteins synthesized by two different isolates of Anaplasma marginale. Infect Immun. 1983 Jun;40(3):1068–1074. doi: 10.1128/iai.40.3.1068-1074.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans J. A., Cohen S. Immunology of malaria. Annu Rev Microbiol. 1983;37:25–49. doi: 10.1146/annurev.mi.37.100183.000325. [DOI] [PubMed] [Google Scholar]
- Goff W. L., Winward L. D. Detection of geographic isolates of Anaplasma marginale, using polyclonal bovine antisera and microfluorometry. Am J Vet Res. 1985 Nov;46(11):2399–2400. [PubMed] [Google Scholar]
- Gonzalez E. F., Long R. F., Todorovic R. A. Comparisons of the complement-fixation, indirect fluorescent antibody, and card agglutination tests for the diagnosis of bovine anaplasmosis. Am J Vet Res. 1978 Sep;39(9):1538–1541. [PubMed] [Google Scholar]
- Johnson G. C., Barbet A. F., Klevjer-Anderson P., McGuire T. C. Preferential immune response to virion surface glycoproteins by caprine arthritis-encephalitis virus-infected goats. Infect Immun. 1983 Aug;41(2):657–665. doi: 10.1128/iai.41.2.657-665.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREIER J. P., RISTIC M. Anaplasmosis. X. Morphologic characteristics of the parasites present in the blood of calves infected with the Oregon strain of Anaplasma marginale. Am J Vet Res. 1963 Jul;24:676–687. [PubMed] [Google Scholar]
- KREIER J. P., RISTIC M. Anaplasmosis. XI. Immunoserologic characteristics of the parasites present in the blood of calves infected with the Oregon strain of Anaplasma marginale. Am J Vet Res. 1963 Jul;24:688–696. [PubMed] [Google Scholar]
- Kocan K. M., Venable J. H., Brock W. E. Ultrastructure of anaplasmal inclusions (Pawhuska isolate) and their appendages in intact and hemolyzed erythrocytes and in complement-fixation antigen. Am J Vet Res. 1978 Jul;39(7):1123–1130. [PubMed] [Google Scholar]
- Kocan K. M., Venable J. H., Hsu K. C., Brock W. E. Ultrastructural localization of anaplasmal antigens (Pawhuska isolate) with ferritin-conjugated antibody. Am J Vet Res. 1978 Jul;39(7):1131–1135. [PubMed] [Google Scholar]
- Kuttler K. L., Winward L. D. Serologic comparisons of 4 Anaplasma isolates as measured by the complement-fixation test. Vet Microbiol. 1984 Apr;9(2):181–186. doi: 10.1016/0378-1135(84)90033-6. [DOI] [PubMed] [Google Scholar]
- Leith D. K., Trevino L. B., Tully J. G., Senterfit L. B., Baseman J. B. Host discrimination of Mycoplasma pneumoniae proteinaceous immunogens. J Exp Med. 1983 Feb 1;157(2):502–514. doi: 10.1084/jem.157.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther D. G., Cox H. U., Nelson W. O. Comparisons of serotests with calf inoculations for detection of carriers in anaplasmosis- vaccinated cattle. Am J Vet Res. 1980 Dec;41(12):2085–2086. [PubMed] [Google Scholar]
- Löhr K. F., Ross J. P., Meyer H. Studies on homologous and heterologous antibody responses to infections with Anaplasma marginale and A. centrale using the indirect fluorescent antibody and capillary tube agglutination tests. Z Tropenmed Parasitol. 1973 Mar;24(1):86–95. [PubMed] [Google Scholar]
- McGuire T. C., Palmer G. H., Goff W. L., Johnson M. I., Davis W. C. Common and isolate-restricted antigens of Anaplasma marginale detected with monoclonal antibodies. Infect Immun. 1984 Sep;45(3):697–700. doi: 10.1128/iai.45.3.697-700.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer G. H., Barbet A. F., Davis W. C., McGuire T. C. Immunization with an isolate-common surface protein protects cattle against anaplasmosis. Science. 1986 Mar 14;231(4743):1299–1302. doi: 10.1126/science.3945825. [DOI] [PubMed] [Google Scholar]
- Palmer G. H., McGuire T. C. Immune serum against Anaplasma marginale initial bodies neutralizes infectivity for cattle. J Immunol. 1984 Aug;133(2):1010–1015. [PubMed] [Google Scholar]
- Rovis L., Barbet A. F., Williams R. O. Characterisation of the surface coat of Trypanosoma congolense. Nature. 1978 Feb 16;271(5646):654–656. doi: 10.1038/271654a0. [DOI] [PubMed] [Google Scholar]
- Shapiro S. Z., August T. The use of immunoprecipitation to study the synthesis and cleavage processing of viral proteins. J Immunol Methods. 1976;13(2):153–159. doi: 10.1016/0022-1759(76)90153-8. [DOI] [PubMed] [Google Scholar]
- Swift B. L., Thomas G. M. Bovine anaplasmosis: elimination of the carrier state with injectable long-acting oxytetracycline. J Am Vet Med Assoc. 1983 Jul 1;183(1):63–65. [PubMed] [Google Scholar]
- Tabatabai L. B., Deyoe B. L. Characterization of salt-extractable protein antigens from Brucella abortus by crossed immunoelectrophoresis and isoelectricfocusing. Vet Microbiol. 1984 Oct;9(6):549–560. doi: 10.1016/0378-1135(84)90017-8. [DOI] [PubMed] [Google Scholar]