Abstract
Brain development is characterized by maturational processes that span the period from childhood through adolescence to adulthood, but little is known whether and how developmental processes differ during these phases. We analyzed the development of functional networks by measuring neural synchrony in EEG recordings during a Gestalt perception task in 68 participants ranging in age from 6 to 21 years. Until early adolescence, developmental improvements in cognitive performance were accompanied by increases in neural synchrony. This developmental phase was followed by an unexpected decrease in neural synchrony that occurred during late adolescence and was associated with reduced performance. After this period of destabilization, we observed a reorganization of synchronization patterns that was accompanied by pronounced increases in gamma-band power and in theta and beta phase synchrony. These findings provide evidence for the relationship between neural synchrony and late brain development that has important implications for the understanding of adolescence as a critical period of brain maturation.
Keywords: oscillations, synchrony, adolescence, electroencephalography, Gestalt perception
Developmental psychology and brain research has focused mainly on the early pre- and postnatal periods as critical windows for the organization and functional adjustment of neural circuitry. These studies revealed the important role of early experience in shaping cortical networks and emphasized the decline of neuronal plasticity with age. However, more recent evidence suggests that brain development and its susceptibility to epigenetic influences extends far beyond the early postnatal stages and in humans comes to an end only around age 20.
Emerging evidence from anatomy and physiology suggests that later developmental periods, such as adolescence, may have a crucial impact on the organization of cortical circuitry. Anatomically, the volume and organization of white matter increases continuously (1–3), whereas the volume of cortical gray matter increases only until the onset of adolescence and then decreases again (4–6). Physiologically, there is evidence that dopamine–NMDA receptor interactions in prefrontal cortex (7) mature only after adolescence, and there are data suggesting late maturation of GABAergic neurotransmission (8).
These findings indicate important changes in anatomical and physiological parameters during late developmental periods, but the functional implications of these changes are poorly understood. The putative relevance of these changes is highlighted by the fact that the onset of brain disorders, such as schizophrenia, that cause lasting emotional and cognitive dysfunctions often occurs during the transition from adolescence to adulthood (9). Thus, not only the early but also the late maturational processes are likely to be critical, especially for the development of higher cognitive functions.
We investigated the development of functional networks by examining age-dependent changes in task-related neural oscillations and synchrony in EEG recordings in children, adolescents, and adults. The participants (n = 68) were between the ages of 6 and 21 years. We focused on neural synchrony for 2 reasons. First, evidence indicates that the development of cortical networks depends on neuronal activity, whereby the temporal correlations of activity play an important role in determining the occurrence of circuit modifications (10). Second, precise synchronization of oscillations and neuronal discharges supports temporal coordination of distributed brain processes and is an important criterion for the functional maturity of networks (11).
To examine the relationship between neural synchrony and the development of functional networks, EEG data were acquired during the perception of Mooney faces (Fig. 1 A and B) (12) and were analyzed for spectral power as well as for phase synchronization of induced oscillations. Mooney faces were chosen as stimuli because their perception is associated with increased synchronization of oscillatory activity in the beta and gamma band and with the coherent activation of extended functional networks (13, 14). Therefore, we expected to find developmental changes in the topology of interareal synchronization and perhaps also in the frequency of the bands in which this synchronization occurs.
Fig. 1.
The top row shows an upright Mooney face (A) and an inverted/scrambled version of the same image (B). The middle row shows behavioral data for detection rates (%) in the face condition (C) and the no-face condition (D). The bottom row shows behavioral data for reaction times (ms) in the face condition (E) and the no-face condition (F).
Our data show that the developmental increase in rate of detection and the reduction in reaction time were accompanied by increases in neural synchrony in the theta, beta, and gamma bands. Quite unexpectedly, however, this development was not linear. During the late adolescent period, there was a phase during which functional networks underwent reorganization, reflected by significant reductions in phase synchrony and induced gamma-band power. This reorganization phase was followed by a marked increase in neural synchrony, highlighting the important role of late developmental processes for the maturation of functional networks.
Results
Behavioral Results.
We analyzed the percentage of correct and incorrect responses as well as reaction times (RTs) for the face and no-face conditions (Fig. 1 C–F). In the face condition, older participants detected significantly more face stimuli than younger participants [F(4, 63) = 13.61, P < 0.0001; Posthoc least significant difference (LSD) tests: adult > late childhood, adult > early childhood, late adolescence > early childhood, early adolescence > early childhood, late childhood > early childhood]. No significant differences among groups were found in the detection rates in the no-face condition [F(4, 63) = 0.46, P = 0.7].
ANOVAs for RTs revealed that older participants responded significantly faster than younger ones, both in the face condition [F(4, 63) = 8.43, P < 0.0001; Posthoc LSD tests: adult > late childhood, adult > early childhood, late adolescence > early childhood, early adolescence > late childhood, early adolescence > early childhood, late childhood > early childhood] and in the no-face condition [F(4, 63) = 4.15, P < 0.005; Posthoc LSD tests: adult > early childhood, early adolescence > late adolescence, early adolescence > late childhood, early adolescence > early childhood]. Specifically, in both conditions, a pronounced decrease in RTs was observed until early adolescence that was then followed by a transient increase of 100–150 ms in the late adolescent groups relative to early adolescent and adult participants.
Time Frequency Analysis.
For the analysis of age-dependent changes in EEG signals, we focused on the face condition because it is associated with prominent induced oscillatory activity (13, 14). The analysis of responses to the inverted condition is reported in SI Text. Across analyses, we concentrated on a post-stimulus interval (100–400 ms) that had been identified previously as related to cognitive processes underlying the construction of coherent object representations (15).
Across groups, task-induced oscillatory activity was found predominantly in the theta and gamma frequency range between 100 and 300 ms. Consistent with previous research (13, 14), we found that adults, when exposed to stimuli in the face condition, showed enhanced gamma-band activity between 160 and 280 ms after stimulus onset (Fig. 2A). This induced activity was maximal over parietal electrodes and was significantly higher in the face condition than in the no-face condition (electrodes Pz, P2, P4, P04) [γ1-band (50–75 Hz) time interval: 160–280 ms, t (13) = 2.62 P < 0.05]. No differences between conditions were found, however, for beta-band activity (electrodes Pz, P2, P4, P04) [β-band (13–30 Hz) time interval: 160–280 ms, t (13) = 0.8, P = 0.54].
Fig. 2.
Spectral power in the beta and gamma bands in the face condition. All values are expressed in standard deviations in reference to the baseline. (Left Column) Spectral power (13–75 Hz) across all electrodes. (A–E) Adult (A); late adolescence (B); early adolescence (C); late childhood (D); early childhood (E). (Middle Column) Topography for 30–75 Hz frequency band between 100 and 300 ms. (F) Adults and (G–J) difference maps (adults − younger) for younger age groups relative to adult participants. (Right Column) (K) Group comparison across all electrodes of spectral power in the 30–75 Hz range between 100 and 300 ms. (L) Group comparison across parietal electrodes of spectral power in the 30–75 Hz range between 100 and 300 ms (electrodes CPz, P1, P2, Pz, PO3, PO4).
Task-related changes also were observed in the theta and alpha bands. Theta-band activity increased 100 ms after stimulus onset, reached a maximum at ≈200 ms (Fig. S1), and was particularly prominent over frontal regions, consistent with the role of theta oscillations in the maintenance of short-term memories (16). The power of theta oscillations over frontal electrodes was significantly higher in the face condition [θ-band (4–7 Hz) time interval: 100–300 ms: t (13) = 2.35, P = 0.05]. Similarly, alpha activity averaged across all electrodes was significantly higher in response to stimuli in the face condition than in the no-face condition [α-band (8–12 Hz) time interval: 100–300 ms, t (13) = 2.43, P < 0.05].
Developmental Changes in Spectral Power.
The analyses of age-dependent changes in EEG power revealed a significant increase in gamma-band activity over all electrodes with age (Fig. 2 A–K) [γ-band (30–75 Hz) time interval: 100–300 ms, F(4, 63) = 3.58 P < 0.01, Posthoc LSD: adult > late adolescence, adult > late childhood, adult > early childhood] that was found both for high and low gamma-band activity [γ1-band (30–50 Hz) time interval: 100–300 ms, F(4, 63) = 3.43, P < 0.01, Posthoc LSD: adult > late adolescence, adult > late childhood, adult > early childhood; γ2-band (50–75 Hz) time interval: 100–300 ms, F(4, 63) = 2.78, P < 0.05, Posthoc LSD: adult > late adolescence, adult > early childhood]. Differences among groups were most pronounced over parietal regions (Fig. 2L) (electrodes CPz, P1, P2, Pz, PO3, PO4) [γ-band (30–75 Hz) time interval: 100–300 ms, F(4, 63) = 6.06, P < 0.0001, Posthoc LSD: adult > all groups, early adolescence > early childhood]. Moreover, the differences between late adolescent and adult participants were particularly pronounced because there was a transient reduction of gamma-band activity during adolescence (Fig. 2K). A similar pattern was observed for the power of beta-band oscillations, which also showed a transient reduction during the late adolescent period (all electrodes) [β-band (13–30 Hz) time interval: 100–300 ms, F(4, 63) = 2.75, P < 0.05, Posthoc LSD: adult > late adolescence, late childhood > late adolescence, early childhood > late adolescence].
A further significant age-dependent increase was found for induced theta activity averaged across all electrodes (Fig. S1) [θ-band (4–7 Hz) time interval: 100–300 ms, F(4, 63) = 3.95, P < 0.01, Posthoc LSD: adult > early childhood, late adolescence > early childhood, early adolescence > early childhood]. These changes remained significant when considering only the signals from frontal electrodes (AF3, AFz, AF4, F1, Fz, AF4) (Fig. S1L) [θ-band (4–7 Hz) time interval: 100–300 ms, F(4, 63) = 4.07, P < 0.005, Posthoc LSD: adult > late childhood, adult > early childhood, late adolescence > early childhood, early adolescence > early childhood]. In contrast, no differences among groups were found for activity in the alpha band [α-band (8–12 Hz) time interval: 100–300 ms, F(4, 63) = 0.30, P = 0.88.]
Phase Synchronization.
We examined phase synchrony as an index of the precision of synchronization of oscillatory activity across electrodes. Phase synchrony is a direct measure of synchronization that is not confounded by the amplitude of the signal and therefore is a reliable index of synchronization (17). Moreover, phase synchrony is a measure for correlations between neuronal groups at least 2 cm apart; thus it reflects mainly long-distance coordination, whereas spectral power reflects local synchronization of neuronal populations with a spatial extension in the range of 1 cm (11).
Similar to the induced spectral power, phase synchrony showed a task-dependent increase. These changes were most prominent in the theta and beta bands. In the adult group, beta phase synchrony averaged across all electrode pairs increased 100 ms after stimulus onset (Fig. 3A), and this increase lasted for about 200 ms. As in previous studies (11, 12), phase synchrony was stronger in the face condition than in the no-face condition, but these differences did not reach statistical significance [β-band (13–30 Hz) time interval: 120–220 ms, t (13) = 1.83, P = 0.11]. Possible reasons for this difference are that performance was close to the ceiling level (detection rate 92%) because presentation times were longer (400 ms) than in previous experiments and that there were more stimuli in the face condition than in the no-face condition (142 vs. 96). Both factors weakened the statistical power of the tests.
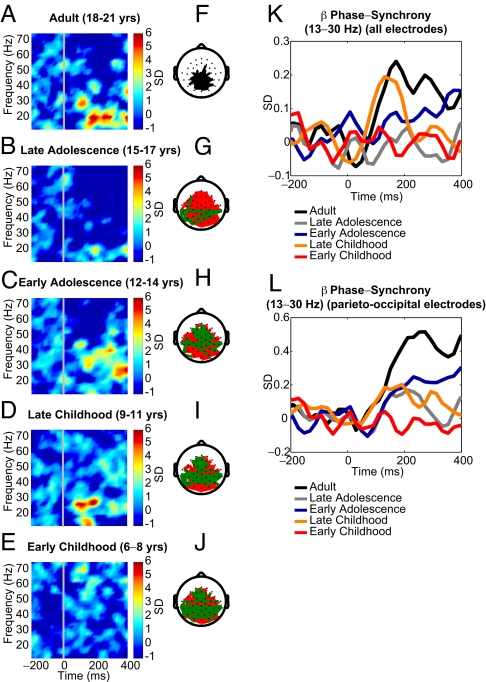
Fig. 3.
Phase synchrony in the beta and gamma bands in the face condition. All values are expressed in standard deviations in reference to the baseline. (Left Column) Phase synchrony (13–75 Hz) across all electrodes. (A–E) Adult (A); late adolescence (B); early adolescence (C); late childhood (D); early childhood (E). (Middle Column) Topography for 13–30 Hz frequency band between 100 and 300 ms. (F) Adult group. Synchrony between electrodes is indicated by black lines, which are drawn only if the synchrony value is beyond a 1-tailed probability of P < 0.0000000002. (G–J) Difference maps for younger age groups relative to adult participants. Black lines indicate a significant increase (P < 0.0003) in synchrony in adults compared with the younger group. Green lines indicate a significant increase (P < 0.0003) in synchrony for the younger group relative to adults. (K) Group comparison for all electrodes of phase synchrony in the 13–30 Hz frequency range between 100 and 300 ms. (L) Group comparison for all parieto-occipital electrodes in the 13–30 Hz frequency range between 100 and 300 ms.
Phase synchrony also increased in the theta band 100 ms after stimulus onset, remained elevated for 200 ms, and was strongest in the 4–7 Hz frequency range. Topographically, phase synchronization was pronounced between bilateral frontal and fronto-parietal electrodes (Fig. 4), suggesting top-down modulation of sensory regions through frontal cortex (18, 19). However, theta phase synchrony did not differ between stimulus conditions [θ-band (4–7 Hz) time interval: 100–300 ms t (13) = .31, P = 0.75].
Fig. 4.
Phase synchrony in the theta and alpha bands in the face condition. All values are expressed in standard deviations in reference to the baseline. (Left Column) Phase synchrony (4–12 Hz) across all electrodes. (A–E) Adult (A); late adolescence (B); early adolescence (C); late childhood (D); early childhood (E). (Middle Column) Topography for 4–7 Hz frequency band between 100 and 300 ms. (F) Adult group. Synchrony between electrodes is indicated by black lines, which are drawn if the synchrony value is beyond a 1-tailed probability of P < 0.0000000001. (G–J) Difference maps for younger age groups relative to adult participants. Black lines indicate a significant increase (P < 0.0000000001) in synchrony in adults compared with the younger group. Green lines indicate a significant increase (P < 0.0000000001) in synchrony for the younger group relative to adults. (K) Group comparison of phase synchrony in the 4–7 Hz frequency range between 100 and 300 ms for all electrodes.
In contrast to activity in the beta and theta frequency ranges, modulation of phase synchrony in the alpha and gamma bands was relatively small and showed no significant differences between conditions (all electrodes) [α-band (8–12 Hz) time interval: 100–300 ms, t (13) = 0.43, P = 0.68; γ-band (30–75 Hz) time interval: 100–300 ms, t (13) = −0.21, P = 0.84].
Developmental Changes in Phase Synchronization.
During development, significant increases in phase synchrony were observed for oscillatory activity in the theta, beta, and gamma frequency ranges. In contrast, no significant differences were found for alpha phase synchrony [α-band (8–12 Hz) time interval: 100–300 ms, F(4, 63) = 0.23, P = 0.92.]
The increase in phase synchrony averaged across all electrode pairs was statistically significant in the beta band [β-band (13–30 Hz) time interval: 100–300 ms, F(4, 63) = 2.61, P < 0.05] (Fig. 3 A–K). The developmental pattern was similar to that observed for induced gamma-band power. During late adolescence, phase synchrony in this frequency band underwent a transitory reduction before it increased again to reach adult levels (Posthoc LSD test: adult > late adolescence, adult > early childhood, late childhood > late adolescence). Although a similar pattern was present for phase synchrony in the lower gamma band, differences among groups did not reach statistically significant levels [γ1-band (30–50 Hz) time interval: 100–300 ms, F(4, 63) = 1.49, P = 0.22].
Phase synchrony in the theta band averaged across all electrode pairs increased continuously during childhood and early adolescence and showed a further increase between late adolescence and adulthood (Fig. 4 A–K). Overall, the increase with age was statistically significant [θ-band (4–7 Hz) time interval: 100–300 ms, F(4, 63) = 2.81, P < 0.05]. Posthoc LSD tests confirmed that the increases in theta phase synchrony in the adults and in the late adolescent and early childhood groups were significant.
Topographical Analysis of Phase Synchronization.
First, we analyzed the topography of phase synchronization in the 13–30 Hz frequency range between 100 and 300 ms for all groups. In the adult group, beta phase synchrony patterns were focused almost exclusively among and between occipital and parietal electrodes (Fig. 3F). In the younger age groups, long-range synchronization was found predominantly between frontal and temporal areas as well as between the 2 hemispheres (Fig. 3 G–J). Phase synchrony among and between parietal and occipital electrodes generally was absent.
To examine developmental changes over visual areas more precisely, we selected parieto-occipital electrodes (CP1, CP2, CP5, CP3, CP4, CP6, P9, P7, P3, P1, Pz, P2, P4, P8, P10, PO3, PO4, O1, Oz, O2) (Fig. 3L and Fig. S2) for which the age-dependent differences in beta phase synchrony were particularly pronounced. We found a strong increase of phase synchrony with age over parieto-occipital electrodes in the beta frequency range [β-band (13–30 Hz) time interval: 100–300 ms, F(4, 63) = 5.32, P < 0.0001, Posthoc LSD: adult > all groups, early adolescence > early childhood, late childhood > early childhood]. In addition to activity in the beta band, we observed an increase in phase synchrony in the lower gamma band with age [γ1-band (30–50 Hz) time interval: 100–300 ms, F(4, 63) = 2.91, P < 0.05, Posthoc LSD: adult > late childhood, adult > early childhood, early adolescence > early childhood].
The topology of phase-synchrony patterns also changed in the theta band. In the adult group, theta phase synchrony was observed between widely distributed electrode pairs (Fig. 4F). Difference maps between adult participants and younger age groups revealed an increase with age in synchrony patterns between frontal and occipito-parietal electrodes and between the 2 hemispheres (Fig. 4 F–J).
Correlations Between Neural Synchrony, Age, and Behavior.
We calculated 2-tailed Pearson correlations as well as hierarchical regression analyses to examine the relationship between spectral power, phase synchrony, age, and behavior (Table S1 and Fig. S3). There was a significant negative correlation between age and RTs and a positive correlation between age and detection rate. Age correlated positively with induced theta-band power, theta phase synchrony, induced gamma-band power, and beta/gamma phase synchrony.
Significant correlations also were found for neural synchrony and behavioral performance across all age groups. Faster RTs correlated significantly with increased phase synchrony over parieto-occipital electrodes in the beta/gamma frequency range as well as with amplitude increases in the gamma and theta bands. Moreover, higher detection rates of face stimuli were significantly correlated with increased spectral power in the gamma and theta bands and with beta/gamma phase synchrony over parieto-occipital electrodes.
We used hierarchical regression analysis to examine the relative contributions of age and task performance on neural synchrony (Table S2). Results showed that age was the only significant predictor for increased neural synchrony during development.
Discussion
The results of this study highlight the relevance of late developmental processes for the maturation of cortical networks and may have important implications for the understanding of both normal and pathological brain development. In adult participants, perception of Mooney faces was associated with stimulus-induced increases in both local and long-range synchronization in the theta, beta, and gamma frequency ranges. Consistent with the role of induced gamma-band activity in the construction of coherent object representations (15), we found an increase in gamma-band power between 200 and 300 ms during the successful grouping of stimulus elements into a coherent percept in the face condition. In addition there was an increase in beta phase synchronization, reflecting long-range coordination of oscillatory activity, and an increase in both the power and phase of induced theta oscillations. It has been proposed that enhanced theta activity over frontal cortex and long-range synchronization in the theta frequency range are associated with the maintenance of task-specific information in short-term memory and with top-down control (18).
The developmental changes were characterized by a selective increase in the amplitude of theta- and gamma-band oscillations and an enhancement of long-range synchronization in theta, beta, and gamma frequency ranges. These changes were accompanied by improvements in RTs and detection rates. However, these changes were not continuous, because there was a transient decrease in beta phase synchrony. This phase was followed by a further increase in the power of oscillations in the theta and gamma bands and a recovery in phase synchronization at beta and gamma frequencies.
Interestingly, this recovery was associated with a reorganization of the synchronization patterns from a widespread to a more focal distribution. Beta phase synchronization became most pronounced among and between parietal and occipital electrodes. In addition, there was a selective increase in theta phase synchronization among frontal brain regions as well as among anterior and posterior brain regions. The emergence of long-range theta phase synchronization may be related to the strong increase of induced gamma oscillations during this last development stage, because fluctuations in gamma power are phase-locked to theta oscillations (20). These results suggest a reorganization of functional networks during late adolescence that seems to be associated with a transitory destabilization of functions and perhaps with a heightened vulnerability of the developmental process.
Additional analyses showed that the present data are not the result of (i) differences among groups in the number of trials, because adjusting the number of trials did not change the current results (see SI Text), (ii) changes in baseline activity across developmental stages, because groups did not show significant differences in baseline activity except for theta power that could account for the development of task-related neural synchrony (Fig. S4, and SI Text), or (iii) saccadic artifacts. After re-analyzing their data with an average reference montage, Yuval-Greenberg et al. demonstrated that gamma band oscillations resulting from saccadic artifacts have a frontal distribution (see fig. 1 of ref. 21). We followed this analysis approach and clearly show that the present data do not show a maximum over frontal electrodes (Fig. S5).
Finally, the developmental effects are not specific to upright Mooney faces, because similar differences also were observed for responses in the no-face condition (see SI Text). Thus, the data suggest a general change in cortical processing during adolescence that directly reflects continued maturation in physiological and anatomical parameters.
Relationship to Previous Research.
The present results are compatible with and extend the developmental data on developmental changes in fMRI activity patterns in a variety of cognitive tasks (22–25). These studies revealed a developmental pattern in which brain areas critical for task performance become increasingly activated (26). Activation of frontal and parietal regions was found to be more prominent and focused in adult participants than in children and adolescents during tasks involving working memory, attention, and face processing.
Recently, we have demonstrated that the amplitude of the BOLD signal is closely and positively correlated with the entrainment of neurons into synchronized gamma band oscillations (27). Thus, the fMRI data are fully compatible with the conclusions drawn from the present results, i.e., that the ability of cortical networks to engage in precisely synchronized high-frequency oscillations increases during development and is a hallmark of maturity.
The changes seen in local and long-range synchronization during development are consistent with changes observed both in anatomy and neurotransmitter systems. The development of cortico-cortical connections is a possible correlate of the changes observed in neural synchrony, because synchronization in the high-frequency (gamma and beta) ranges is mediated mainly by cortico-cortical connections (28, 29). Ashtari and colleagues (1) recently showed that white matter maturation continues after adolescence in several brain regions.
In addition, the generation of synchronized, oscillatory activity is related to several neurotransmitter systems. GABAergic neurons play a pivotal role in the primary generation of high-frequency oscillatory activity and their local synchronization, whereas glutamatergic connections seem to control their strength, duration, and long-range synchronization (30, 31). Recent evidence points to important changes in these neurotransmitter-systems during adolescence (7, 8).
Late Adolescence as a Critical Period of Brain Development.
We propose that the pronounced changes in neural synchrony seen during the transition from late adolescence to early adulthood reflect a critical developmental period that is associated with a rearrangement of functional networks and with an increase of the temporal precision and spatial focusing of neuronal interactions. The expected increases in oscillation frequency and synchrony during childhood and early adolescence were followed by an unexpected but significant reduction of phase synchronization in the beta frequency range during the late adolescent period, suggesting that cortical networks undergo a transient destabilization before the emergence of mature cortical networks.
This reorganization of cortical activity during late adolescence in our study is compatible with findings from functional and structural imaging (1, 32) that suggest a non-monotonic trajectory for the development of cortical networks during this period. In addition, the presence of behavioral and psychological disturbances during adolescence is consistent with this view (33). Subsequent recovery of synchrony and the newly emerging networks are likely to underlie the stabilization of cognitive representations, increased cognitive control through enhanced top-down modulation by frontal brain regions, and an increase in cognitive resources through a shift toward more focused processing modes.
We believe that there is a causal relation between the maturation of network properties capable of supporting precise synchronization of high-frequency oscillations and the emergence of cognitive abilities for the following reasons. Schizophrenia is associated with impairments of exactly these abilities (34), and these impairments as well as the first psychotic symptoms typically manifest themselves during the period of adolescence during which the spatiotemporal patterns of neuronal synchrony attain the precision characteristic of the mature brain. There now is consistent evidence that neural synchrony is disturbed in patients with schizophrenia. Both local and long-range synchronization is reduced, and this reduction is particularly marked in the beta and gamma frequency ranges (13, 35, 36).
Conclusions
In summary, our findings of a prolonged development of task-related oscillatory activity and synchrony demonstrate that functional networks undergo important maturational processes following early childhood that have not been reported previously. Specifically, the changing patterns of synchronous, oscillatory activity during adolescence seem to reflect a major reorganization of cortical networks that may have profound implications for the understanding of both normal development and developmental disorders, such as schizophrenia, that typically emerge during this period.
Materials and Methods
Participants.
Sixty-eight participants in the age range of 6–21 years were recruited from the local community. Written informed consent was obtained from all parents after the study procedures were described. Participants were divided into 5 age groups: 6–8 years (early childhood, n = 13, mean age: 7.2 years, 10 males), 9–11 years (late childhood, n = 14, mean age: 10.1, 9 males), 12–14 years (early adolescence, n = 13, mean age: 13.4, 10 males), 15–17 years (late adolescence, n = 15, mean age: 16 years, 6 males), and 18–21 years (adult, n = 14, mean age: 20.7, 6 males). The 5 groups did not differ in sex distribution (χ2(4) = 6.89, P = 0.14) or in handedness (χ2(4) = 7.09, P = 0.53).
Behavioral Assessment.
Participants were screened for a history of psychiatric and neurological disorders and current drug abuse. Ophthalmological assessment included monocular and binocular visual acuity. Participants whose native language was German were examined by means of 2 subtests of the Hamburg Wechsler Intelligenztest für Kinder (HAWIK-III) und Erwachsene (HAWIE-R) (37). Participants (n = 6) whose native language was not German were examined by means of the Culture Fair Test (CFT) − 20 (38). Scores of the vocabulary subtest of the HAWIE were transformed into standardized, age-normed scores. The groups did not differ in verbal IQ [F(4, 63) = 1.87, P = 0.13].
Stimuli and Task.
Participants were presented Mooney faces, a visual closure task consisting of degraded pictures (Fig. 1 A and B) in which all shades of gray are removed, leaving the shadows rendered in black and the highlights in white. For the experimental condition, 36 Mooney faces were selected in their original orientation and once after vertical mirroring (face condition). From these 36 Mooney faces, 24 images were presented upside-down. In addition,12 of the inverted Mooney faces were scrambled by selecting various stimulus features to decrease the likelihood of perceiving a face in the inverted stimuli. Both inverted and inverted/scrambled Mooney faces were combined in the no-face condition. All stimuli subtended a visual angle of ≈7 × 10°.
Stimuli were presented on a 19-inch computer screen. A fixation cross was presented in the center of the screen between trials. After a training block of 8 trials (4 stimuli not included in the experimental block), participants received 4 blocks of experimental trials with a total number of 142 stimuli in the upright and 96 stimuli in the inverted condition. The stimuli were presented in a controlled, random order, ensuring that a given Mooney face appeared only once in each block. Each stimulus was presented once in each combination of orientation and facing direction in the first 2 blocks. The next 2 blocks were an exact repetition of the first 2 blocks. Stimuli were presented for 400 ms with an interstimulus interval of 3500–4500 ms.
Participants were instructed to report the perception of a face as quickly as possible regardless of orientation by pressing 1 of 2 buttons with their index fingers. The buttons were mounted on a response pad. The assignment of the response button to the corresponding condition was randomized across participants. Thus, half the participants responded to a face stimulus with their right index finger, and the other half responded with their left index finger.
Electrophysiological Recording and Analysis.
EEG activity was recorded from 62 scalp sites using the BrainAmp amplifier (Brain Products) and Braincap equidistant electrode cap (Falk Minow Services). All channels were referenced during recording to an electrode (FCz) with a forehead ground and impedance of < 5 kΩ. An additional electrode was placed on the infraorbital ridge of the right eye to record the vertical electrooculogram (EOG). The EEG and EOG were digitized with a sampling rate of 500 Hz. The initial bandpass recording filter was set at 0.01–100 Hz.
For the analysis, EEG data were referenced to electrodes TP9 and TP10. This reference was chosen because it was unlikely to be involved in widespread synchronous activity. Principal component analysis (PCA) (39) was used to identify eye-blink artifacts by their distinct topography and to remove their contribution from the subject's data. As an extra measure against eye artifacts, activity recorded on the foremost line of electrodes (AF5, AF6, AF7, AF8, F7, F5, F6, F7, F8) was omitted from further analysis.
The digitized signals were analyzed by means of a windowed Fourier transform (window length: 192 ms, step 20 ms, window overlap 90% for the 13–75 Hz frequency range, and window length: 400 ms, step 20 ms, 95% overlap for the 4–12 Hz frequency range). Signal windows were zero padded to 512 points to obtain an interpolated frequency resolution of approximately 1 Hz per frequency bin. For every time window and frequency bin, amplitude and phase values were computed as reported previously (13, 17). These amplitude and phase values were evaluated in the 4–75 Hz frequency range.
Time frequency charts of both phase synchrony and spectral power were normalized to a baseline before the stimulus onset. The normalization involves subtracting the baseline average and dividing by the baseline standard deviation on a frequency-by-frequency basis. For activity in the theta (4–7 Hz) and alpha (8–12 Hz) frequency ranges, we used a time window from −600 to −200 ms. Data in the beta (13–30 Hz) and gamma (30–75 Hz) frequency ranges were analyzed with time window of −700 to −100 ms. The baselines for high and low frequencies were adapted to the different length of the analyzing window to exclude the influence of any post-stimulus activity in the normalization.
Spurious volume conduction can mimic bona fide neural synchronization if the synchronization occurs with zero or π phase lag (40). [However, see Vicente, et al. (41) for a different perspective.] This situation can occur when a single powerful dipole activates consistently at the same time throughout the trials. In such an eventuality, the near-by electrodes show zero phase locking, and the distant ones show π phase locking. To counter this possibility, the windows exhibiting zero phase locking and π phase locking were eliminated from the analysis. In particular, vectors representing phase differences of 0 ± 1° were multiplied by zero, thus effectively taking them out of the subsequent computations of phase locking.
Statistical Analysis.
Only trials in which participants responded correctly were considered for analysis. Behavioral and EEG data were analyzed with 2-tailed t-tests. The alpha-level was set at .05 for all tests. Posthoc comparisons were carried out with Fishers LSD test.
For differences among groups, we first analyzed the EEG signal from all electrodes by testing the activity averaged across all electrodes within specific frequency bands. If significant differences emerged among groups, we then chose a region of interest (ROI) to further investigate these differences. ROIs were chosen on the basis of maximum activity and prior experimental findings.
Supplementary Material
Acknowledgments.
This work was supported by the Max Planck Society and by Grant 01GWS055 from the Bundesministerium für Bildung und Forschung (P.J. U. and W.S.). E.R. was supported by the Hertie-Stiftung through the Frankfurt Institute of Advanced Studies, by Grant 1070846 from the Fondo National des Desarrollo Cientifico y Tecnológico, and by a joint collaboration grant from La Comisión Nacional de Investigación Científica and Deutscher Akademischer Austausch Dienst.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900390106/DCSupplemental.
References
- 1.Ashtari M, et al. White matter development during late adolescence in healthy males: A cross-sectional diffusion tensor imaging study. NeuroImage. 2007;35:501–510. doi: 10.1016/j.neuroimage.2006.10.047. [DOI] [PubMed] [Google Scholar]
- 2.Paus T, et al. Structural maturation of neural pathways in children and adolescents: In vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- 3.Snook L, Paulson LA, Roy D, Phillips L, Beaulieu C. Diffusion tensor imaging of neurodevelopment in children and young adults. NeuroImage. 2005;26:1164–1173. doi: 10.1016/j.neuroimage.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 4.Huttenlocher PR. Synaptic density in human frontal cortex—developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 5.Sowell ER, et al. Mapping cortical change across the human life span. Nature Neuroscience. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- 6.Gogtay N, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tseng KY, O'Donnell P. Post-pubertal emergence of prefrontal cortical up states induced by D1-NMDA co-activation. Cereb Cortex. 2005;15:49–1557. doi: 10.1093/cercor/bhh107. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto T, et al. Protracted developmental trajectories of GABA(A) receptor alpha1 and alpha2 subunit expression in primate prefrontal cortex. Biol Psychiatry. doi: 10.1016/j.biopsych.2009.01.004. in press. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Häfner H, Maurer K, Loffler W, Riecher-Rossler A. The influence of age and sex on the onset and early course of schizophrenia. Br J Psychiatry. 1993;162:80–86. doi: 10.1192/bjp.162.1.80. [DOI] [PubMed] [Google Scholar]
- 10.Singer W. Development and plasticity of cortical processing architectures. Science. 1995;270:758–764. doi: 10.1126/science.270.5237.758. [DOI] [PubMed] [Google Scholar]
- 11.Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: Phase synchronization and large-scale integration. Nature Reviews Neuroscience. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- 12.Mooney CM, Ferguson GA . A new closure test. Canadian Journal of Psychology. 1951;5:129–133. doi: 10.1037/h0083540. [DOI] [PubMed] [Google Scholar]
- 13.Uhlhaas PJ, et al. Dysfunctional long-range coordination of neural activity during Gestalt perception in schizophrenia. J Neurosci. 2006;26:8168–8175. doi: 10.1523/JNEUROSCI.2002-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez E, et al. Perception's shadow: Long-distance synchronization of human brain activity. Nature. 1999;397:430–433. doi: 10.1038/17120. [DOI] [PubMed] [Google Scholar]
- 15.Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends in Cognitive Science. 1999;3:151–162. doi: 10.1016/s1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- 16.Jensen O, Tesche C. Frontal theta activity in humans increases with memory load in a working memory task. Eur J Neurosci. 2002;15:1395–1399. doi: 10.1046/j.1460-9568.2002.01975.x. [DOI] [PubMed] [Google Scholar]
- 17.Lachaux JP, Rodriguez E, Martinerie J, Varela FJ. Measuring phase synchrony in brain signals. Hum Brain Mapp. 1999;8:194–208. doi: 10.1002/(SICI)1097-0193(1999)8:4<194::AID-HBM4>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Stein A, Chiang C, König P. Top-down processing mediated by interareal synchronization. Proc Natl Acad Sci USA. 2000;97:14748–14753. doi: 10.1073/pnas.97.26.14748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizuhara H, Yamaguchi Y. Human cortical circuits for central executive function emerge by theta phase synchronization. NeuroImage. 2007;36:232–244. doi: 10.1016/j.neuroimage.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 20.Canolty RT, et al. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006;313:1626–1628. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuval-Greenberg S, Tomer O, Keren AS, Nelken I, Deouell LY. Transient induced gamma-band response in EEG as a manifestation of miniature saccades. Neuron. 2008;58:429–441. doi: 10.1016/j.neuron.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 22.Brown TT, et al. Developmental changes in human cerebral functional organization for word generation. Cereb Cortex. 2005;15:275–290. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- 23.Crone EA, Wendelken C, Donohue S, van Leijenhorst L, Bunge SA. Neurocognitive development of the ability to manipulate information in working memory. Proc Natl Acad Sci USA. 2006;103:9315–9320. doi: 10.1073/pnas.0510088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golarai G, et al. Differential development of high-level visual cortex correlates with category-specific recognition memory. Nature Neuroscience. 2007;10:512–522. doi: 10.1038/nn1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adleman NE, et al. A developmental fMRI study of the Stroop color-word task. NeuroImage. 2002;16:61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- 26.Durston S, Casey BJ. A shift from diffuse to focal cortical activity with development. Developmental Science. 2006;9:1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- 27.Niessing J, et al. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science. 2005;309:948–951. doi: 10.1126/science.1110948. [DOI] [PubMed] [Google Scholar]
- 28.Engel AK, König P, Kreiter AK, Singer W. Interhemispheric synchronization of oscillatory neuronal responses in cat visual cortex. Science. 1991;252:1177–1179. doi: 10.1126/science.252.5009.1177. [DOI] [PubMed] [Google Scholar]
- 29.Löwel S, Singer W. Selection of intrinsic horizontal connections in the visual cortex by correlated neuronal activity. Science. 1992;255:209–212. doi: 10.1126/science.1372754. [DOI] [PubMed] [Google Scholar]
- 30.Wang XJ, Buzsaki G. Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model. J Neurosci. 1996;16:6402–6413. doi: 10.1523/JNEUROSCI.16-20-06402.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Traub RD, Bibbig A, LeBeau FE, Buhl EH, Whittington MA. Cellular mechanisms of neuronal population oscillations in the hippocampus in vitro. Annu Rev Neurosci. 2004;27:247–278. doi: 10.1146/annurev.neuro.27.070203.144303. [DOI] [PubMed] [Google Scholar]
- 32.Hare TA, et al. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo-task. Biol Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 34.Phillips WA, Silverstein SM. Convergence of biological and psychological perspectives on cognitive coordination in schizophrenia. Behav Brain Sci. 2003;26:65–82. doi: 10.1017/s0140525x03000025. [DOI] [PubMed] [Google Scholar]
- 35.Spencer KM, et al. Abnormal neural synchrony in schizophrenia. J Neurosci. 2003;19:7407–7411. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uhlhaas PJ, Haenschel C, Nikolic D, Singer W. The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr Bull. 2008;34:927–943. doi: 10.1093/schbul/sbn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tewes U. Hamburg-Wechsler-Intelligence Scale for Adults-Revision. Bern: HAWIE-R (translated from German) (Huber; 1991. [Google Scholar]
- 38.Weiss RH. Basic Intelligence Test. Braunschweig: CFT 20) (translated from German). (Westermann; 1987. [Google Scholar]
- 39.Ille N, Berg P, Scherg M. Artifact correction of the ongoing EEG using spatial filters based on artifact and brain signal topographies. Journal of Clinical Neurophysiology. 2002;19:113–124. doi: 10.1097/00004691-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Nolte G, et al. Identifying true brain interaction from EEG data using the imaginary part of coherency. Clinical Neurophysiology. 2004;115:2292–2307. doi: 10.1016/j.clinph.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 41.Vicente R, Gollo LL, Mirasso CR, Fischer I, Pipa G. Dynamical relaying can yield zero time lag neuronal synchrony despite long conduction delays. Proc Natl Acad Sci USA. 2008;105:17157–17162. doi: 10.1073/pnas.0809353105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.