Abstract
Tyrosine phosphorylation plays a critical role in regulating cellular function and is a central feature in signaling cascades involved in oncogenesis. The regulation of tyrosine phosphorylation is coordinately controlled by kinases and phosphatases (PTPs). Whereas activation of tyrosine kinases has been shown to play vital roles in tumor development, the role of PTPs is much less well defined. Here, we show that the receptor protein tyrosine phosphatase delta (PTPRD) is frequently inactivated in glioblastoma multiforme (GBM), a deadly primary neoplasm of the brain. PTPRD is a target of deletion in GBM, often via focal intragenic loss. In GBM tumors that do not possess deletions in PTPRD, the gene is frequently subject to cancer-specific epigenetic silencing via promoter CpG island hypermethylation (37%). Sequencing of the PTPRD gene in GBM and other primary human tumors revealed that the gene is mutated in 6% of GBMs, 13% of head and neck squamous cell carcinomas, and in 9% of lung cancers. These mutations were deleterious. In total, PTPRD inactivation occurs in >50% of GBM tumors, and loss of expression predicts for poor prognosis in glioma patients. Wild-type PTPRD inhibits the growth of GBM and other tumor cells, an effect not observed with PTPRD alleles harboring cancer-specific mutations. Human astrocytes lacking PTPRD exhibited increased growth. PTPRD was found to dephosphorylate the oncoprotein STAT3. These results implicate PTPRD as a tumor suppressor on chromosome 9p that is involved in the development of GBMs and multiple human cancers.
Keywords: glioblastoma multiforme, methylation, mutation
Loss of tumor suppressor function leads to the initiation and progression of cancer (1, 2). Inactivation of tumor suppressor genes can result from both genetic mechanisms such as mutation and deletion or epigenetic mechanisms such as DNA hypermethylation (3, 4). Identification of these genes has provided insight into the biological processes underlying oncogenesis, but the key tumor suppressors in many cancers, such as glioblastoma multiforme (GBM), remain poorly defined.
We previously identified the receptor protein tyrosine phosphatase delta (PTPRD) as a gene that predicts for poor prognosis in breast and colon cancer (4). PTPRD is a member of the highly conserved family of receptor protein tyrosine phosphatases (PTPs), several members of which have been implicated in tumorigenesis (5). The gene encodes a transmembrane protein with a cytoplasmic tyrosine phosphatase domain. The PTPRD gene is located within an area of the genome, chromosome 9p, found to be frequently lost in neuroblastoma, gliomas, lung cancer, and other malignancies (6–9). Some deletions of PTPRD have been noted in several of these studies. However, its close proximity to CDKN2A on chromosome 9p has complicated interpretations. In addition, in independent work during the course of our investigations, PTPRD mutations have been detected in a lung cancer genome study, although no functional validation of the alterations is noted (10–12). Together with these data, our study shows that PTPRD is a broadly altered tumor suppressor gene in human malignancies (13).
Using a multifaceted genomic analysis approach, we have determined that PTPRD is a frequent target of inactivation via both genetic and epigenetic mechanisms in GBM and other human cancers. Our concordance data on methylation and copy number analysis show that independent and nonoverlapping mechanisms (genetic and epigenetic) inactivate this gene and are strong genomic evidence indicating that PTPRD loss is not simply driven by CDKN2A loss. In addition, we also show that loss of PTPRD expression associates with gliomas of poor prognosis, loss of PTPRD results in altered growth of astrocytes, PTPRD dephosphorylates STAT3 and regulates the STAT3 pathway, and mutations in PTPRD abrogate the ability to regulate STAT3. Taken together, our data provide insight into the molecular underpinnings of this tumor suppressor gene.
Results and Discussion
PTPRD Is Deleted in GBM.
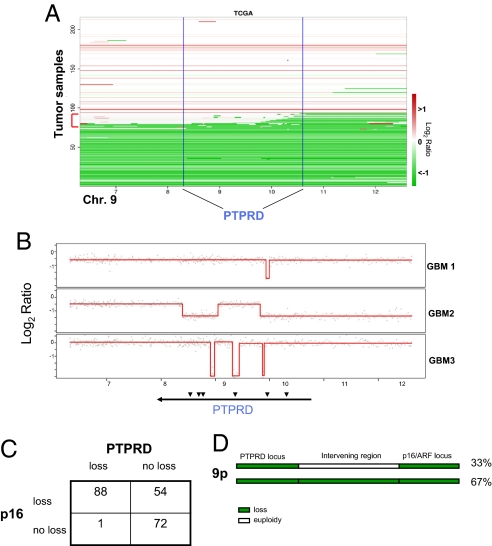
Canonical tumor suppressor genes such as p16INK4A, PTEN, etc., are often subject to homozygous deletion (14). To determine whether PTPRD is deleted in GBM, we examined array comparative genomic hybridization (aCGH) results and determined copy number alterations (CNAs) at the PTPRD locus (9p23–24) in 215 GBM tumors. This dataset was obtained as part of The Cancer Genome Atlas (TCGA) initiative (15). Of note, although the aCGH data for the 9p locus is available, PTPRD has not been analyzed by the TCGA sequencing and methylation analysis efforts. As expected, we observed that 9p loss is a frequent event in GBM, with ≈40% of tumors exhibiting loss (log2 ratio less than −0.25) or homozygous deletion (log2 ratio less than −1) in the region (Fig. 1A) (16). To separate PTPRD loss from CDKN2A (p16INK4A) loss, we examined this region in detail. Loss of regions of various sizes on 9p, encompassing both the PTPRD and CDKN2A genes, was found in a significant proportion of tumors. Both loss and homozygous deletion of the PTPRD gene but not surrounding genes was observed. Importantly, in some tumors, intragenic homozygous deletions were found in the PTPRD gene that removed PTPRD exons, but not surrounding genes, thus defining a minimal common region of deletion at the PTPRD locus (Fig. 1 A and B). These changes were significantly above the background rate expected (Q-value = 3.65E−8).
Fig. 1.
Deletion of PTPRD in GBM. (A) aCGH profile analysis of 215 GBM tumors from TCGA (4/14/2008 data freeze). Segmentation data for the area surrounding PTPRD on chromosome 9p is shown. Tumors are sorted by amount of loss at the PTPRD locus for convenient viewing. Chromosomal gain or loss is represented by a color gradient (red, gain; green, loss). x axis represents genomic location along chromosome 9p (Mbps). The blue bars represent the boundaries of the PTPRD gene. Red bracket marks tumors that possess intragenic deletions of PTPRD. (B) PTPRD is subject to focal, intragenic deletions. Shown are aCGH profiles of selected tumors with intragenic deletions of PTPRD. Probes are plotted along chromosome 9p according to log2 ratio. The red line represents averaged log2 ratio trend. The black arrows show PTPRD exons encompassed by intragenic deletions. (C) Frequency of loss of PTPRD and p16INK4A. Loss is defined as a log2 ratio less than −0.25 to −2.00. Coordinate loss of both genes is indicated. (D) Diagram depicting the nature of loss events in the genomic region encompassing PTPRD and p16INK4A. Depicted is a summary of data generated from analysis of aCGH data. Shown is the region of chromosome 9p in which PTPRD (9p23–24) and p16INK4A (9p21) are located, as well as the intervening DNA. Black bars do not represent exact borders but serve only to summarize the data. Green represents loss of copy number and white represents euploidy. Thirty-three percent occur as a result of two distinct loss events separated by intervening DNA which is euploid (no CNA).
Examination of regions of allelic loss has been crucial for the identification of cancer genes. Loss of the PTPRD and p16INK4A loci on chromosome 9p are very frequent events, and examination of the patterns of loss as a function of genomic location is instructive. Particularly interesting is the relationship between PTPRD loss (9p23–24) and loss of the nearby p16INK4A gene (9p21). Fig. 1C summarizes the frequency of loss events for PTPRD, p16INK4A, or both genes. Of tumors where PTPRD and p16INK4A undergo loss, 29 show distinct loss in each region via separate events (i.e., copy number decreases at PTPRD and p16INK4A with euploidy in the DNA lying between these genes). In another 59 tumors, a single large event eliminates both genes simultaneously (Fig. 1D). This finding indicates that, in the region of 9p we are examining, there are at least 2 separate loci driving genetic loss, one at PTPRD and another at p16. Together, the unbiased identification of PTPRD as a target of microdeletions, as well as frequent larger deletions strongly suggests that PTPRD is targeted for inactivation during GBM pathogenesis. Moreover, PTPRD is a tumor suppressor that is located near p16INK4A, and there may exist selective pressure for codeletion of both genes. This finding is consistent with the widely held hypothesis that there exists more than one tumor suppressor gene in the region of 9p21–24 (other than p16INK4A) (17, 18).
Epigenetic Silencing of PTPRD.
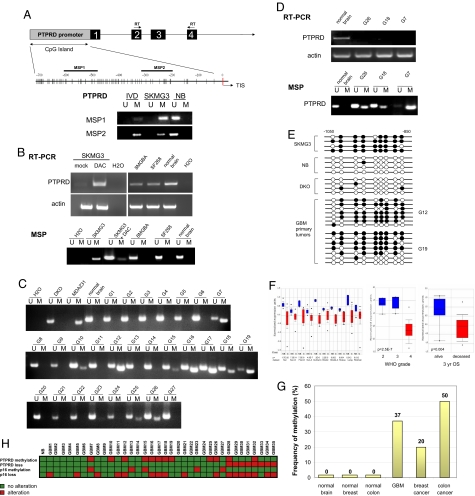
Inactivation of tumor suppressor genes can result from both genetic mechanisms, such as deletion, or epigenetic mechanisms, such as DNA hypermethylation (3, 19). Epigenetic silencing via promoter CpG island hypermethylation has been shown to be a predominant mechanism by which tumor suppressors are inactivated in cancers (20). In some malignancies, such as chronic lymphocytic leukemia, hypermethylation can be a very common mode of tumor suppressor inactivation (21). In glioma, the 2 major mechanisms of p16INK4A inactivation are deletion and hypermethylation (22); in some tumors, methylation occurs and in others, deletion occurs. Given that tumor suppressors that play key roles are frequently targeted by multiple modes of inactivation (4, 19), we wondered whether PTPRD was silenced by hypermethylation in GBM tumors that did not possess deletions in the gene. PTPRD possesses a canonical promoter CpG island (Fig. 2A). We designed methylation-specific PCR (MSP) assays to assess the methylation status of the PTPRD gene in GBM cell lines and primary tumors. We found that PTPRD was unmethylated and expressed in normal brain tissue (NB) (Fig. 2 A and B). A methylated PTPRD promoter is strictly associated with loss of PTPRD expression. PTPRD was methylated and silenced in SKMG3, and the gene was unmethylated and expressed in 2 other GBM lines, 8MGBA and SF268. Treatment of SKMG3 with the DNA methyltransferase (DNMT) inhibitor 5-aza-deoxycytidine (DAC) resulted in demethylation of the PTPRD promoter and restored expression of the gene (Fig. 2B).
Fig. 2.
PTPRD is subject to frequent epigenetic silencing in GBM. (A) Shown is promoter structure of the PTPRD gene (Top). Numbered boxes denote exons and TIS denotes the transcriptional start site. Two independent MSP assays were developed to detect hypermethylated PTPRD and both produce identical results (Bottom). IVD, in vitro methylated genomic DNA; SKMG3, GBM cell line; NB, normal brain. U denotes the presence of unmethylated alleles and M denotes the presence of methylated alleles. The locations assayed by RT-PCR and MSP are noted. (B) Epigenetic silencing of PTPRD expression caused by hypermethylation. Results are shown for RT-PCR (Top) and MSP (Bottom). PTPRD is silenced and hypermethylated in the GBM cell line SKMG3. After treatment with the DNMT inhibitor DAC, PTPRD becomes demethylated and expression is restored. (C) Frequent hypermethylation of PTPRD in primary GBM tumors. Double knockout (DKO), control for unmethylated PTPRD alleles derived from a cell line in which DNMT1 and 3b were knocked out (44). MDA-MB 213 was previously found to undergo silencing of PTPRD and is used as a positive control for methylated alleles (4). (D) Loss of PTPRD expression in primary GBM tumors with hypermethylated PTPRD. Representative samples are shown. (E) Bisulfite sequencing of the PTPRD promoter. Black circles represent methylated CpG dinucleotides. White circles represent unmethylated CpG dinucleotides. (F) Decreased expression of PTPRD in malignant glioma is associated with poor clinical prognosis. Left shows representative data across multiple independently published microarray datasets. Datasets used are labeled. P values for significance are shown. Each pair of plots denotes normalized expression for normal brain (NB, blue) versus glioma (G, red). Shaded boxes = 25th–75th percentile. Whiskers = 10th–90th percentile, and asterisks represent range. Bars = median. Middle and Right show box plots demonstrating decreased PTPRD expression in gliomas with increasing WHO grade and those with poorer survival. All datasets were analyzed as previously described (23) and are listed in Table S1. The second and third graphs, Shia dataset (Table S1). (G) PTPRD is methylated in several primary human cancers, but not in corresponding normal tissues. Methylation was detected by MSP and confirmed with bisulfite sequencing. The data from colon and breast cancer described in our previous study are shown here to enable comparison with methylation frequencies in GBM (4). (H) Concordance analysis of genomic and epigenomic inactivation of PTPRD and p16INK4A. Map shows the presence (red) or absence (green) of both loss and epigenetic inactivation in p16INK4A and PTPRD, in the same tumor set. Analysis of copy number was performed with genomic qPCR and methylation was performed with MSP.
Next, we analyzed the methylation status of PTPRD in primary GBM tumors. Genomic quantitative PCR was used to identify a set of tumors that did not possess deletions in PTPRD. PTPRD was methylated in 37% (10/27) of tumors (Fig. 2C). As was the case with the cell lines, hypermethylation of the PTPRD promoter was strictly associated with loss of gene expression (Fig. 2D). Bisulfite sequencing confirmed the results obtained with MSP (Fig. 2E). Based on these data, it is clear that inactivation of PTPRD can occur not only by deletion, but also by epigenetic silencing, and that this is a very frequent event in GBM.
If PTPRD is both deleted and epigenetically silenced in malignant gliomas, transcriptome analyses should demonstrate lower PTPRD mRNA levels in gliomas compared with normal brain tissue. To address this question, we analyzed an extensive expression microarray database (Oncomine), using large numbers of expression profiles from published microarray datasets (23) (Tables S1 and S2). The microarray metaanalysis algorithms and statistical analysis used were as previously described (24). PTPRD expression levels were found to be significantly lower in nearly every available dataset comparing normal brain with glioma (Fig. 2F). Moreover, PTPRD expression was significantly lower in malignant gliomas of high World Health Organization (WHO) grade as compared with gliomas of lower WHO grade. Patients with WHO grade IV tumors (GBM) have poorer survival than patients with low grade astrocytomas (WHO I and II) or anaplastic astrocytomas (WHO III), and as expected, tumors from patients with poorer survival have decreased PTPRD levels (Fig. 2F). Previously, we detected cancer-specific hypermethylation of PTPRD in colon and breast cancer (4). Together with these data, it appears that epigenetic silencing of PTPRD is a cancer-specific event common to multiple malignancies (Fig. 2G). The data from colon and breast cancer described in our previous study are shown here to enable comparison with methylation frequencies in GBM (4).
Concordance Analysis of PTPRD Alterations.
We then determined the concordance of loss versus methylation of PTPRD and p16INK4A (Fig. 2H). The genomic and epigenetic inactivation events in PTPRD are for the most part mutually exclusive (P < 0.05). Importantly, in some tumors, p16INK4A is genetically lost but PTPRD is methylated instead, which is consistent with the hypothesis that PTPRD inactivation is not simply driven by p16 loss because these 2 genes undergo different mechanisms of inactivation in the same tumor sample. Furthermore, concordance analysis of PTPRD and p16INK4A status shows that although 2 tumors show methylation at both genes, PTPRD methylation does not usually occur concomitantly with p16INK4A methylation because p16INK4A is mostly inactivated by deletion. These data, in addition to the copy number data above, show that PTPRD is a separate tumor suppressor locus independent of CDKN2A.
PTPRD Is Mutated in GBM and Other Human Malignancies.
To determine whether PTPRD mutations are present in GBM and other human tumors, we sequenced all exons of the gene in 222 human cancers (48 GBM, 22 lung, 24 squamous cell carcinoma of the head and neck, 32 prostate, 60 colon, and 36 thyroid). Exons were amplified by PCR from cancer genomic DNA samples and directly sequenced (see Materials and Methods). Whenever a presumptive mutation was identified, we verified that the change did not correspond to a known single-nucleotide polymorphism and attempted to determine whether it was somatically acquired (i.e., tumor specific) by examining the sequence of the gene in genomic DNA from normal tissue of the same patient. Using this strategy, we identified somatic mutations in 3 different types of human malignancies. PTPRD mutations were found in 3 of 48 (6%) GBMs, 2 of 22 (9%) lung cancers, and 3 of 24 (13%) squamous cell carcinomas of the head and neck (Tables 1 and S3). No mutations were found in prostate cancers, colon carcinomas, or thyroid cancers.
Table 1.
Somatic Mutations of PTPRD in Human Cancers
| Cancer type | Genomic position | Normal genotype | Tumor genotype | Amino acid change | SIFT prediction | Domain |
|---|---|---|---|---|---|---|
| Glioblastoma | 8476143 | GTC | ATC | V892I | deleterious | InterPro IPR003961 |
| Fibronectin, type III | ||||||
| Glioblastoma | 8366672 | CAA | TAA | Q1481X | deleterious, | InterPro IPR000242 |
| stop codon | Protein-tyrosine phosphatase | |||||
| Glioblastoma | 8474270 | CGT | TGT | R1088C | deleterious | InterPro IPR003961 |
| Fibronectin, type III | ||||||
| Lung | 8461063 | CCT | ACT | P1146T | deleterious | |
| Lung | 8489811 | GTA | ATA | V720I | tolerated | InterPro IPR003961 |
| Fibronectin, type III | ||||||
| Squamous, head and neck | 8439782 | CCT | ACT | P1311T | deleterious | |
| Squamous, head and neck | 8511492 | CCA | CTA | P249L | deleterious | InterPro IPR003598 |
| Immunoglobulin C2 | ||||||
| Squamous, head and neck | 8511315 | CTG | CCG | L308P | deleterious | InterPro IPR003598 |
| Immunoglobulin C2 |
All somatic mutations identified were nonsynonymous. Nearly all mutations occurred in known functional domains of the predicted protein product. The mutation Q1481X is predicted to result in a truncated protein product lacking a functional C-terminal phosphatase domain. Other alterations identified were missense mutations and are predicted to be deleterious (Table 1). Importantly, these missense mutations are located in conserved areas of the PTPR gene family and correspond to locations of mutations found in PTPRT in colon cancer (25). These include mutations in the fibronectin and phosphatase domains of PTPRT (Q987K, N1128I, etc.).
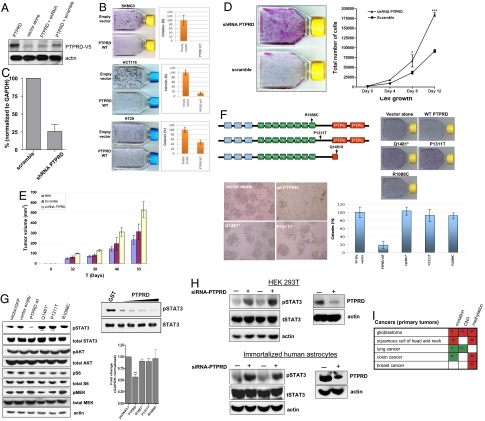
Little is known about the functional role of PTPRD. Other related PTPs have been shown to regulate cell growth and/or apoptosis. We have found that PTPRD is expressed in normal brain tissue, human astrocytes (Figs. 2D and 3C), and other human tissues, including colon and breast (4). To determine whether ectopic expression of PTPRD can inhibit cancer cell growth, we transfected wild-type PTPRD into human GBM and colon cancer cells. Transfection resulted in production of PTPRD protein (Fig. 3A and Fig. S1). Expression of PTPRD in human cancer cell lines potently inhibited cell growth, as seen by the substantial decrease in the number of neomycin-resistant colonies compared with empty vector (Fig. 3B and Fig. S2). Transfection of wild-type but not mutant PTPRD into 293 cells resulted in reduced growth of transfected cells (Fig. S3). Next, we assessed the effects of loss of PTPRD expression in immortalized primary human astrocytes (IHA) (26). We knocked down PTPRD expression in astrocytes by expressing an shRNA targeting the gene (Fig. 3 A and C). Knockdown of PTPRD resulted in increased cell growth compared with a control expressing a scrambled shRNA (Fig. 3D). This result was due to a significant increase in the rate of growth of the cells lacking PTPRD expression (Fig. 3D). Knockdown with 2 other shRNAs targeting PTPRD but not scrambled sequence produced the same results (Fig. S4). The tumor-forming ability of IHAs in which PTPRD was knocked down was measured by using a mouse xenograft model. Depletion of PTPRD resulted in significantly enhanced tumor growth (Fig. 3E). Importantly, expression of PTPRD with cancer-specific mutations (Q1481X, R1088C, and P1311T) resulted in a decreased ability to inhibit cell growth as compared with wild-type PTPRD (Fig. 3F), demonstrating that these mutations have clear functional consequences.
Fig. 3.
Tumor suppressive properties of PTPRD. (A) Immunoblot showing HEK 293T cells transfected with wild-type PTPRD cDNA, vector alone (pcDNA 3.1-V5), PTPRD + shRNA targeting PTPRD, and PTPRD + scrambled shRNA. Antibody against the V5 epitope (Invitrogen) was used to detect the PTPRD-V5 fusion protein. Actin was used as a loading control. (B) Expression of PTPRD suppresses growth of human cancer cells. PTPRD was transfected into the cell lines indicated, and the cells were cultured for 2 weeks in media containing G418. (C) shRNA knockdown of PTPRD expression in primary human astrocytes. Expression was measured using quantitative PCR. Results normalized to GAPDH. Assay was performed in triplicate. (D) Knockdown of PTPRD in IHA results in increased growth rate. Left shows cultures of either astrocytes with PTPRD knocked down or control cells (scramble) plated at equal cell numbers and cultured for 10 days. Right shows a growth curve comparing rate of proliferation of astrocytes with PTPRD knocked down versus control. Five thousand cells were plated in both cases. All experiments were performed in triplicate. All graphs report mean ± SD. *, P < 0.05; ***, P < 0.001. (E) PTPRD depletion results in increased tumor growth. Immortalized human astrocytes (IHA) expressing shRNA that depleted PTPRD resulted in faster tumor growth than a scrambled control in a mouse xenograft model. The astrocytes used were immortalized by transfection with E6, E7, hTERT, and ras (25). Error bars show ± SD. P < 0.05 for all comparisons of PTPRD shRNA versus astrocytes alone and scramble shRNA. (F) PTPRD mutations found in human cancers abrogate growth suppressive properties. Top shows the mutations that were tested. Shown are bright field pictures and colony formation assays of HCT116 cells transfected with empty vector, wild-type PTPRD, and the 3 mutant PTPRD alleles indicated. PTPc, phosphatase domain; FN3, fibronectin type III domain; IGC2, Ig C2 domain. (G) PTPRD dephosphorylates and regulates STAT3. Left shows the levels of phospho and total proteins indicated after wild-type and mutant PTPRD transfection into HEK 293T cells. Top Right shows blot demonstrating that PTPRD directly dephosphorylates STAT3 in vitro. GST-PTPRD fusion protein (1–20 μg) was incubated with immunoprecipitated STAT3-Flag and analyzed by Western blot. Bottom Right bar graph shows that SOCS3 mRNA levels decrease after wild-type PTPRD transfection. Values represent normalized expression measured using qPCR. **, P = 0.0019). (H) Knockdown of PTPRD increases the phosphorylation of STAT3. siRNA was used to knockdown PTPRD in the indicated cells. PTPRD was detected by Western blot using an anti-PTPRD antibody. Total STAT3 and phospho-STAT3 were detected as above. (I) Summary of PTPRD alterations in primary human cancers. The presence of a type of alteration as detected in the current study is denoted by red. Number indicates observed frequency of event. Green indicates evidence of the event in the literature (4, 11, 12, 44, 45).
PTPRD Dephosphorylates STAT3, an Activity Abrogated by Cancer-Specific Mutations.
How does the PTPRD phosphatase suppress tumor cell growth? To answer this question, we performed an analysis of candidate pathways and identified the oncoprotein STAT3 as a potential substrate of PTPRD. Transfection of wild-type PTPRD resulted in the specific dephosphorylation of STAT3 at tyrosine 705, a residue that must be phosphorylated for STAT3 to be active (27) (Fig. 3G). Dephosphorylation was not seen with any other phospho-proteins we examined other than STAT3 in our analysis of candidate pathways (Fig. 3G and Fig. S5). Furthermore, and most importantly, STAT3 dephosphorylation was not seen after expression of PTPRD harboring cancer-specific mutations that we identified. Levels of the STAT3 transcriptional target suppressor of cytokine signaling 3 (SOCS3) also decreased after transfection of wild-type but not mutant PTPRD (Fig. 3G). These mutations abrogate the ability of the gene to block both tumor cell growth and STAT3 phosphorylation, establishing a mechanistic link for the loss of function mutations.
We have demonstrated that PTPRD regulates STAT3 phosphorylation, but have not yet shown that the dephosphorylation was directly mediated by PTPRD rather than a downstream phosphatase. To address this point, we made a GST-PTPRD fusion protein and incubated it in vitro with immunoprecipitated STAT3. Incubation with GST-PTPRD but not GST alone resulted in a dose-dependent decrease in phospho-STAT3, indicating that STAT3 is a direct substrate of PTPRD (Fig. 3G). Examination of the protein sequence of PTPRD revealed that the cytoplasmic domain possesses 3 consensus STAT3 binding motifs (PYXXQ) (28). Moreover, knockdown of PTPRD using siRNA in both HEK 293T cells and human astrocytes results in an increase in STAT3 phosphorylation (Fig. 3H).
The combination of genetic, epigenetic, and cellular data demonstrates that PTPRD is a tumor suppressor gene in malignant glioma and other human cancers. Our results, along with previously identified alterations in PTPRD, strongly suggest that this gene is a broadly inactivated tumor suppressor in human malignancies and may play an important role in tumor development (8–13). Our results show that PTPRD dysfunction occurs in a number of tumor types (Fig. 3I) (4). Importantly, there exists strong evidence that there is more than one tumor suppressor gene on 9p near 9p21 (9p21 includes p16INK4A) in multiple human cancers (18, 29, 30). Our data strongly indicates that PTPRD is one of these tumor suppressor genes and is likely to be fundamentally important for oncogenesis. Also, it should be noted that PTPRT also can regulate STAT3, and it will be interesting to analyze the relative roles of these 2 genes in human cancer (31). STAT3 is a well documented oncoprotein (32–34). In GBM, aberrant activation of STAT3 is very common (35). Inhibition of STAT3 activity has been shown to slow or arrest growth in GBM (36–39).
The function of PTPRD is consistent with the function of other PTPs implicated in tumor suppression and with their general role of antagonizing growth-stimulating signaling pathways (40). Our results indicate that PTPRD may act as a tumor suppressor by regulating cell growth. Moreover, our finding that PTPRD expression is preferentially lost in GBM versus gliomas of lower WHO grade suggests that loss of this gene plays an important role in the progression rather than the initiation of malignant gliomas.
PTPs such as PTPRD regulate signaling pathways that may be amenable to therapeutic targeting in tumor cells. Although pharmacologic reactivation of PTPs is difficult, identification of corresponding kinases that phosphorylate common substrates could provide therapeutic targets, such as in the case of PTEN and AKT. This approach may have broad therapeutic implications as PTPRD alterations exist in >50% of GBMs, as well as in other human malignancies.
Materials and Methods
See SI Text for full methods.
Tumor Samples and aCGH Analysis.
Tumors from the Memorial Sloan–Kettering Cancer Center were obtained after patient consent and with institutional review board approval. In our analysis, processing and analysis of data were performed as described (41).
Methylation and Gene Expression Analysis.
Selection of primers used for MSP and determinants for CpG island localization and designation was accomplished by using MSPPrimer (42). MSP was performed as previously described (43). Bisulfite sequencing and RT-PCR was performed as previously described (4).
Supplementary Material
Acknowledgments.
We thank Thomas Landers, Igor Dolgalev, Sabrena Thomas, and Benjamin Golas for their exceptional technical expertise and Stephen B. Baylin, Eric Holland, Neal Rosen, Ross Levine, and members of the Sawyers laboratory for helpful discussions and comments. This work was supported in part by The Cancer Genome Atlas project (C.B., M.L., C.S., and J.M.); The Brain Tumors Funders' Collaborative (I.K.M., T.C.C., P.S.M., and L.L.); The Flight Attendants Medical Research Institute (T.A.C.); The American Society for Clinical Oncology; The Society of Memorial Sloan–Kettering Foundation; and The Louis Gerstner Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900571106/DCSupplemental.
References
- 1.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 2.Futreal PA, et al. A census of human cancer genes. Nat Rev Cancer. 2004;4:177–183. doi: 10.1038/nrc1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ponder BA. Cancer genetics. Nature. 2001;411:336–341. doi: 10.1038/35077207. [DOI] [PubMed] [Google Scholar]
- 4.Chan TA, et al. Convergence of mutation and epigenetic alterations identifies common genes in cancer that predict for poor prognosis. PLoS Med. 2008;5:823–838. doi: 10.1371/journal.pmed.0050114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ostman A, Hellberg C, Bohmer FD. Protein-tyrosine phosphatases and cancer. Nat Rev Cancer. 2006;6:307–320. doi: 10.1038/nrc1837. [DOI] [PubMed] [Google Scholar]
- 6.Stallings RL, et al. High-resolution analysis of chromosomal breakpoints and genomic instability identifies PTPRD as a candidate tumor suppressor gene in neuroblastoma. Cancer Res. 2006;66:3673–3680. doi: 10.1158/0008-5472.CAN-05-4154. [DOI] [PubMed] [Google Scholar]
- 7.Sato M, et al. Identification of chromosome arm 9p as the most frequent target of homozygous deletions in lung cancer. Genes Chromosomes Cancer. 2005;44:405–414. doi: 10.1002/gcc.20253. [DOI] [PubMed] [Google Scholar]
- 8.Purdie KJ, et al. Allelic imbalances and microdeletions affecting the PTPRD gene in cutaneous squamous cell carcinomas detected using single nucleotide polymorphism microarray analysis. Genes Chromosomes Cancer. 2007;46:661–669. doi: 10.1002/gcc.20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Purdie KJ, et al. Single nucleotide polymorphism array analysis defines a specific genetic fingerprint for well-differentiated cutaneous SCCs. J Invest Dermatol. 2009 doi: 10.1038/jid.2008.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao X, et al. Homozygous deletions and chromosome amplifications in human lung carcinomas revealed by single nucleotide polymorphism array analysis. Cancer Res. 2005;65:5561–5570. doi: 10.1158/0008-5472.CAN-04-4603. [DOI] [PubMed] [Google Scholar]
- 11.Weir BA, et al. Characterizing the cancer genome in lung adenocarcinoma. Nature. 2007;450:893–898. doi: 10.1038/nature06358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding L, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–1075. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solomon DA, et al. Mutational inactivation of PTPRD in glioblastoma multiforme and malignant melanoma. Cancer Res. 2008;68:10300–10306. doi: 10.1158/0008-5472.CAN-08-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sherr CJ. Principles of tumor suppression. Cell. 2004;116:235–246. doi: 10.1016/s0092-8674(03)01075-4. [DOI] [PubMed] [Google Scholar]
- 15.McLendon R, et al. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bello MJ, et al. Molecular and cytogenetic analysis of chromosome 9 deletions in 75 malignant gliomas. Genes, Chromosomes Cancer. 1994;9:33–41. doi: 10.1002/gcc.2870090107. [DOI] [PubMed] [Google Scholar]
- 17.Cook AL, et al. CDKN2A is not the principal target of deletions on the short arm of chromosome 9 in neuroendocrine (Merkel cell) carcinoma of the skin. Int J Cancer. 2001;93:361–367. doi: 10.1002/ijc.1352. [DOI] [PubMed] [Google Scholar]
- 18.Pollock PM, Welch J, Hayward NK. Evidence for three tumor suppressor loci on chromosome 9p involved in melanoma development. Cancer Res. 2001;61:1154–1161. [PubMed] [Google Scholar]
- 19.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nature Reviews. 2002;3:415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 20.Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349:2042–2054. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- 21.Raval A, et al. Downregulation of death-associated protein kinase 1 (DAPK1) in chronic lymphocytic leukemia. Cell. 2007;129:879–890. doi: 10.1016/j.cell.2007.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furnari FB, et al. Malignant astrocytic glioma: Genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 23.Rhodes DR, et al. ONCOMINE: A cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6:1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rhodes DR, et al. Large-scale meta-analysis of cancer microarray data identifies common transcriptional profiles of neoplastic transformation and progression. Proc Natl Acad Sci USA. 2004;101:9309–9314. doi: 10.1073/pnas.0401994101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z, et al. Mutational analysis of the tyrosine phosphatome in colorectal cancers. Science. 2004;304:1164–1166. doi: 10.1126/science.1096096. [DOI] [PubMed] [Google Scholar]
- 26.Sonoda Y, et al. Formation of intracranial tumors by genetically modified human astrocytes defines four pathways critical in the development of human anaplastic astrocytoma. Cancer Res. 2001;61:4956–4960. [PubMed] [Google Scholar]
- 27.Inoue M, Minami M, Matsumoto M, Kishimoto T, Akira S. The amino acid residues immediately carboxyl-terminal to the tyrosine phosphorylation site contribute to interleukin 6-specific activation of signal transducer and activator of transcription 3. J Biol Chem. 1997;272:9550–9555. doi: 10.1074/jbc.272.14.9550. [DOI] [PubMed] [Google Scholar]
- 28.Shao H, et al. Structural requirements for signal transducer and activator of transcription 3 binding to phosphotyrosine ligands containing the YXXQ motif. The J Biol Chem. 2004;279:18967–18973. doi: 10.1074/jbc.M314037200. [DOI] [PubMed] [Google Scholar]
- 29.Porterfield BW, Olopade OI, Rowley JD, Diaz MO. Analysis of tumor suppressor gene on human chromosome 9 in mouse x human somatic cell hybrids. Somat Cell Mol Genet. 1994;20:391–400. doi: 10.1007/BF02257456. [DOI] [PubMed] [Google Scholar]
- 30.Illei PB, Rusch VW, Zakowski MF, Ladanyi M. Homozygous deletion of CDKN2A and codeletion of the methylthioadenosine phosphorylase gene in the majority of pleural mesotheliomas. Clin Cancer Res. 2003;9:2108–2113. [PubMed] [Google Scholar]
- 31.Zhang X, et al. Identification of STAT3 as a substrate of receptor protein tyrosine phosphatase T. Proc Natl Acad Sci USA. 2007;104:4060–4064. doi: 10.1073/pnas.0611665104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bromberg JF, et al. Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 33.Gao SP, et al. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest. 2007;117:3846–3856. doi: 10.1172/JCI31871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhong Z, Wen Z, Darnell JE., Jr Stat3: A STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 35.Rahaman SO, et al. Inhibition of constitutively active Stat3 suppresses proliferation and induces apoptosis in glioblastoma multiforme cells. Oncogene. 2002;21:8404–8413. doi: 10.1038/sj.onc.1206047. [DOI] [PubMed] [Google Scholar]
- 36.Konnikova L, Kotecki M, Kruger MM, Cochran BH. Knockdown of STAT3 expression by RNAi induces apoptosis in astrocytoma cells. BMC Cancer. 2003;3:23–29. doi: 10.1186/1471-2407-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fuh B, et al. LLL-3 inhibits STAT3 activity, suppresses glioblastoma cell growth and prolongs survival in a mouse glioblastoma model. British Journal of Cancer. 2009;100:106–112. doi: 10.1038/sj.bjc.6604793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li GH, et al. STAT3 silencing with lentivirus inhibits growth and induces apoptosis and differentiation of U251 cells. Journal of Neuro-oncology. 2009;91:165–174. doi: 10.1007/s11060-008-9696-0. [DOI] [PubMed] [Google Scholar]
- 39.Su Y, et al. JSI-124 inhibits glioblastoma multiforme cell proliferation through G(2)/M cell cycle arrest and apoptosis augment. Cancer Biology & Therapy. 2008;7:1243–1249. doi: 10.4161/cbt.7.8.6263. [DOI] [PubMed] [Google Scholar]
- 40.Mohi MG, et al. Prognostic, therapeutic, and mechanistic implications of a mouse model of leukemia evoked by Shp2 (PTPN11) mutations. Cancer Cell. 2005;7:179–191. doi: 10.1016/j.ccr.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 41.Wiedemeyer R, et al. Feedback circuit among INK4 tumor suppressors constrains human glioblastoma development. Cancer Cell. 2008;13:355–364. doi: 10.1016/j.ccr.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brandes JC, Carraway H, Herman JG. Optimal primer design using the novel primer design program: MSPprimer provides accurate methylation analysis of the ATM promoter. Oncogene. 2007;26:6229–6237. doi: 10.1038/sj.onc.1210433. [DOI] [PubMed] [Google Scholar]
- 43.Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR: A novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA. 1996;93:9821–9826. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rhee I, et al. DNMT1 and DNMT3b cooperate to silence genes in human cancer cells. Nature. 2002;416:552–556. doi: 10.1038/416552a. [DOI] [PubMed] [Google Scholar]
- 45.Sjoblom T, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.