Abstract
Myocardial infarction results in loss of cardiomyocytes, scar formation, ventricular remodelling, and eventually heart failure. In recent years, cell therapy has emerged as a potential new strategy for patients with ischaemic heart disease. This includes embryonic and bone marrow derived stem cells. Recent clinical studies showed ostensibly conflicting results of intracoronary infusion of autologous bone marrow derived stem cells in patients with acute or chronic myocardial infarction. Anyway, these results have stimulated additional clinical and pre-clinical studies to further enhance the beneficial effects of stem cell therapy. Recently, the existence of cardiac stem cells that reside in the heart itself was demonstrated. Their discovery has sparked intense hope for myocardial regeneration with cells that are obtained from the heart itself and are thereby inherently programmed to reconstitute cardiac tissue. These cells can be detected by several surface markers (e.g. c-kit, Sca-1, MDR1, Isl-1). Both in vitro and in vivo differentiation into cardiomyocytes, endothelial cells and vascular smooth muscle cells has been demonstrated, and animal studies showed promising results on improvement of left ventricular function. This review will discuss current views regarding the feasibility of cardiac repair, and focus on the potential role of the resident cardiac stem and progenitor cells. (Neth Heart J 2009;17:199-207.)
Keywords: ischaemic heart disease, stem cell therapy, regeneration, cardiac stem cell
Myocardial infarction results in loss of cardiomyocytes, scar formation, ventricular remodelling, and eventually heart failure. Pharmacological, catheterbased, and surgical interventions have led to improved survival of patients with coronary artery disease, although these techniques fail to regenerate dead myocardium. Consequently, the reduced mortality is accompanied by increased morbidity due to ischaemic heart failure. In recent years, cell therapy has emerged as a potential new strategy.1 The ultimate goals of cell therapy are myocardial regeneration and neovascularisation, leading to clinical improvement without severe adverse effects. Important features of stem cells include self renewal, clonogenicity, and ability to differentiate into cardiomyocytes, endothelial cells and vascular smooth muscle cells. Recent studies in animal models of myocardial infarction have demonstrated that various subsets of adult primitive cells can regenerate functional cardiomyocytes with improvement in cardiac structure and function. Small clinical trials using adult bone marrow derived stem cell therapy in patients with myocardial infarction and ischaemic cardiomyopathy have recapitulated these beneficial effects in humans, with infarct size reduction and improvement of cardiac function. Recently, the existence of cardiac stem cells that reside in the heart itself was demonstrated.2 These cells are capable of differentiation into both myocytes and vascular cells, leading to functional improvement in animal models of heart failure. Their discovery has sparked intense hope for myocardial regeneration with cells that are obtained from the heart itself and are thereby inherently programmed to reconstitute cardiac tissue. This review will discuss current views regarding the feasibility of cardiac repair, and focus on the potential role of the resident cardiac stem and progenitor cells.
Stem cells for cardiac repair
Several phenotypically distinct cell populations have been utilised for cardiac regeneration, and the relative merits of one cell over another remain to be determined.1 In general, totipotent (e.g., embryonic), pluripotent (e.g., bone marrow mesenchymal stem cells) and multipotent (e.g., tissue specific) stem cells can be distinguished.3-5 The last-mentioned have less ability to differentiate than embryonic stem cells. Embryonic stem cells have been shown to be able to differentiate into cardiomyocytes and all cell lines necessary for formation of new blood vessels.6-8 However, aside from ethical issues, no clinical studies using embryonic stem cells for myocardial regeneration have been initiated because of the possibility of teratoma formation.4,9 On the other hand, it has been shown that several pluripotent or somatic stem cells are able to differentiate into cardiomyocytes and/or lead to neovascularisation, e.g., haematopoetic (CD34+, CD45+),10,11 mesenchymal (CD34−, CD45−)12-14 and endothelial progenitor (CD133+) stem cells.15,16
Experimental evidence for cardiac repair using stem cells
The first experimental evidence for cardiac repair was provided by a unique observation by Quaini and colleagues. Analysis of post-transplant organs in recipients of sex-mismatched heart transplantations indicated the existence of a circulating pool of stem cells that is capable of differentiation into myocytes, coronary arterioles, and capillaries.17 Cases in which a male patient received a heart from a female donor showed that primitive cells translocated from the recipient to the graft. The Y chromosome was used to detect migrated undifferentiated cells expressing stem-cell antigens (Sca-1, c-kit, MDR1), and to discriminate between primitive cells derived from the recipient and those derived from the donor. Y chromosome positive myocytes made up 7 to 10% of those in the donor hearts and were highly proliferative. These results showed a high level of cardiac chimerism caused by the migration of primitive cells from the recipient to the grafted heart.
Next, Orlic and colleagues showed that transplantation of bone marrow derived stem cells can lead to a regenerative response in a mouse model of myocardial infarction.11 They used lineage-negative (Lin) bone marrow cells from transgenic mice expressing enhanced green fluorescent protein; cells were separated by fluorescence-activated cell sorting on the basis of c-kit expression. Nine days after transplantation, these injected cells in the border zone of infarcted myocardium led to newly formed myocardium that occupied a large area of the infarcted portion of the ventricle. The developing tissue comprised both proliferating myocytes and vascular structures. The authors concluded that locally delivered bone marrow cells can generate myocardium de novo, ameliorating the outcome of coronary artery disease. Other groups were not able to replicate these findings. Murry et al. used a different strategy by studying a transgenic mouse line with a reporter gene for the cardiac specific α-myosin heavy chain (MHC-nLAC).18 Bone marrow derived haematopoietic stem cells (lin−, c-kit+) from these transgenic mice were transplanted into infarcted myocardium in non-transgenic, histocompatible recipient mice. Although donor cells were detected in the hearts, none of these cells had differentiated into cardiomyocytes. They concluded that this failure of transplanted stem cells to contribute to formation of new cardiomyocytes may call into question the mechanistic underpinnings of clinical trials. Surprisingly, the idea that clinical trials should be withheld on the basis of a negative mouse study was editorialised.19 Fortunately, however, cardiologists did not heed this exhortation and several trials of stem cells have been performed in patients with ischaemic heart disease.
Clinical trials with bone marrow derived stem cells
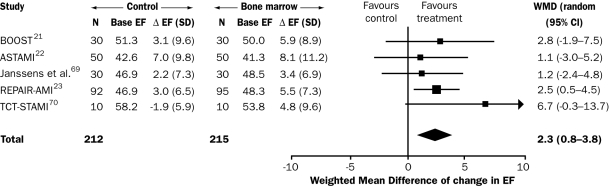
Autologous cells should be used preferably for clinical purposes to obviate tissue rejection. At present, bone marrow is the most frequent source of cells used for clinical studies in cardiac repair. The first clinical trial (in ten patients) was reported in 2002.20 In 2006, three large randomised clinical trials were published in the New England Journal of Medicine: the ASTAMI, REPAIR-AMI and TOPCARE-CHD trials.21-23 These studies showed ostensibly conflicting results of intracoronary infusion of autologous bone marrow derived stem cells in patients with acute or chronic myocardial infarction, but the discrepancy is only apparent because the ASTAMI study did not use optimal techniques to preserve the function of bone marrow cells. A metaanalysis of randomised and cohort studies showed a significant increase in left ventricular ejection fraction at four months of 2.3 to 3.7% (figure 1).24,25 Although it has been suggested that this improvement is not clinically important,26 many researchers in the field consider these results encouraging, particularly since these were only the first attempts. The results have stimulated additional clinical and preclinical studies to further enhance the beneficial effects of stem cell therapy. The current debate on differences between clinical studies and their results is focused on several parameters, such as endpoint measurement (echocardiography versus MRI), timing of cell delivery, and viability and/or number of cells to be transplanted.25,27-30 Most of all, the type of cell will determine the ultimate success of stem cell therapy for ischaemic heart disease. In this context, the recent discovery of cardiac stem cells that reside in the heart is both important and very promising.
Figure 1.
Estimation of the effect of intracoronary injection of non-mobilised bone marrow cells on LV ejection fraction after acute MI. Metaanalysis including current randomised controlled trials. Test for heterogeneity P=0.68 and test for overall effect P=0.002. Follow-up was four months in the REPAIR-AMI trial23 and in the study by Janssens et al.31 six months in the ASTAMI trial22 and in the TCT-STAMI,32 and 18 months in the BOOST.21 Base=baseline, EF=ejection fraction, D=change from baseline to follow-up, WMD=weighted mean difference. (Adapted with permission from Hirsch et al.)25
Resident cardiac stem cells
The heart has been classically considered a post-mitotic organ. However, it has been suggested that a responsive stem cell pool resides in the adult myocardium, and may influence adaptation of the post-natal heart.33,34 Recent studies have shown evidence for actual regeneration by differentiation of stem cells into cardiomyocytes.35-37 Although myocardial infarcts do not regenerate spontaneously, the endogenous repair mechanisms that are present in the heart can be manipulated. Recently, adult cardiac stem cells (CSCs) were discovered. These cells reside in the heart itself and are inherently programmed to reconstitute cardiac tissue. Indeed, resident CSCs have the ability to proliferate and differentiate into cardiomyocytes, smooth muscle cells, and endothelial cells.38 It is suggested that CSCs account for physiological turnover in absence of injury. Thus, they have the potential to regenerate the infarcted heart. For stem cell therapy, these cells need to be harvested, cultured and delivered to the infarcted heart. CSCs represent a logical source to exploit in cardiac regeneration therapy because, unlike other adult stem cells, they are intrinsically programmed to generate cardiac tissue and to increase cardiac tissue viability.
Characterisation of cardiac stem cells using different surface markers
CSCs are undifferentiated cells, some of which express transcription factors for early myocyte lineage (e.g. Nkx2.5, GATA-4, MEF2). CSCs can be detected by several surface markers (e.g. c-kit, Sca-1, MDR1, Isl-1). None of these markers are specific for CSCs; they are found in haematopoietic stem cells, but also in other cell types. Whether these cells are true cardiac stem cells remains to be ascertained. An issue of biological and clinical relevance is whether newly formed myocytes are derived from CSCs that accumulated in the heart during early development, or are the progeny of bone marrow stem cells that home to the myocardium later in life. In favour of the first possibility is the fact that c-kit+ cells migrate during foetal growth and form colonies in several organs, including the heart. However, the earlier-mentioned study by Quaini et al. demonstrates that the heart contains ‘new heart cells’ derived from the recipient of a donor heart.17 Chemotaxis of haematopoietic stem cells is modulated by stem cell factor, which promotes their migration to specific sites, suggesting that stem cells may have been stored in the heart as remnants from the cardiac primordia. Moreover, CSCs differ from other types of stem cells as they are typically Lin−, CD34− and CD45−. Endothelial progenitor cells, in addition to expressing c-kit, are also positive for CD34. CD45 expression has been considered a marker of haematopoietic specification for even the most primitive stem cells.39 Because most c-kit+ cells of bone marrow origin are also positive for CD45 and other blood lineage markers, it is possible that CSCs either represent a different subpopulation or they have resided in the myocardium long enough to have lost the epitopes of the blood cell lineages.
Several groups have isolated and expanded undifferentiated cells from adult heart tissue, based on different stem cell and progenitor antigens and other characteristics: c-kit+, sca-1+, side population cells, cardiospheres, and Islet1+ cardiac progenitor cells.2,35,40-46 These cells will be discussed hereafter; table 1 represents their most important properties.
c-kit+ cardiac stem cells
The tyrosine kinase receptor c-kit is the receptor for stem cell factor and is important for proliferation, migration, differentiation, and secretion. Beltrami et al. isolated cardiac stem cells from rat hearts (age 20–25 months), using c-kit antibody.2 The isolated population contained approximately 90% c-kit+ cells. In general these cells are negative for myocyte, endothelial and smooth muscle cells and fibroblast cytoplasmatic proteins. They did express markers for cardiac or myocyte lineage (e.g. GATA-4, GATA-5, Nkx2.5 and MEF2), whereas skeletal muscle, blood cell lineage and neural markers were not detected (see also table 1). In differentiation medium, these c-kit+ cells expressed signs of biochemical differentiation into either myocytes, smooth muscle cells or endothelial cells. Although they observed biochemical differentiation, these changes did not result in a mature phenotype. In vitro myocytes did not contain organised structures, lacked the presence of sarcomers, and were not able to contract spontaneously. However, when c-kit+ lin− cells were injected in the myocardium after infarction more animals survived in the experimental group. The injected cells multiplied and differentiated over time giving rise to a regenerated myocardium. The regenerated myocardium consisted of small myocytes, capillaries, and arterioles resembling a neonatal myocardium. The expression of many cardiac genes and proteins could be detected in the newly formed myocytes (table 1).
Table 1.
Overview of specific properties (e.g. stem cell markers, cardiac transcription factors) of different cardiac stem cells: c-kit+, sca-1+, side population cells, cardiospheres, and Islet1+ cardiac progenitor cells.
| CSC type | Ref | Tissue | Stem cell markers | Cardiac specific TF (at isolation) | Cardiac myocyte gene* (in vitro diff.) | In vivo myocytic diff. | In vivo functional studies | |
|---|---|---|---|---|---|---|---|---|
| Positive | Negative | |||||||
|
c-kit |
2,47,49 | Rat Pig Human |
CD34 CD45 Lineage |
GATA4 GATA5 MEF2C NKX2.5 |
α-sarcomeric actin, cardiac myosin, desmin, connexin 43 | Yes (also into SMC, EC, fibroblast) | Yes | |
| Sca-1 | 35,37,41,46 | Mouse Human |
Sca-1 CD31 |
c-kit (+/−) CD34 CD45 Lineage |
GATA4 GATA5 MEF2C NKX2.5 |
Troponin I, α-sarcomeric actin, cardiac myosin, MHC (α and β), desmin, connexin 43 | Yes | Yes |
| Side population | 33,43,44 | Mouse | Abcg2 Sca-1 |
CD31 c-kit (low) CD34 (low) CD45(low) |
GATA4 MEF2C NKX2.5 |
Troponin, α-cardiac actinin | ND | ND |
| Cardiospheres | 40,51 | Mouse Human |
c-kit, Sca-1 MDR |
CD34 Lineage |
GATA4 | Troponin, α-MHC | ND | Yes |
| Islet-1 | 45 | Neonatal mouse | Ils-1 | Sca-1 c-kit CD31 |
NKX2.5 GATA4 |
Troponin T, α-cardiac actinin | ND | ND |
* Cardiac myocytic markers were negative at isolation. CSC=cardiac stem cell, TF= transcription factors, ND=not determined, diff=differentiation, MHC=myosin heavy chain.
Recently, Bearzi and colleagues established conditions for the isolation and expansion of c-kit-positive human CSCs from small samples of myocardium.47 Human CSCs differentiated predominantly into cardiomyocytes and, to a lesser extent, into smooth muscle cells and endothelial cells. Potentially, these cells can be isolated and expanded in vitro for subsequent autologous regeneration of non vital myocardium in patients affected by heart failure of ischaemic and nonischaemic origin, which is important for entering the clinical arena.
Stem cell antigen-1 positive (Sca-1+) cardiac stem cells
While Beltrami et al. made their selection based on c-kit, other groups based the isolation of progenitor cells on another stem cell marker, stem cell antigen (Sca-1).34 It is involved in cell signalling and cell adhesion. Schneider's group isolated cardiac cells by using magnetic anti-biotin microbeads recognising Sca1+-biotin labelled cells.41 They could be grown and subsequently differentiated, in the presence of 5-azacytidine (5-aza) – a cytosine analogue, into cells expressing cardiac genes and protein which were not expressed initially. Sca-1+ heart derived cells are small interstitial cells and CD31+, located adjacent to the basal lamina. They do not express blood lineage markers, c-kit, Flt-1, Flk-1, vascular endothelial cadherin, von Willebrand factor and the haematopoietic stem cells marker, CD45 and CD34 (table 1). Telomerase activity is observed in Sca-1+ cells, suggesting self renewal capacity of these cells. Initially no cardiac proteins are expressed but some cardiac transcription factors could be detected. Upon stimulation with 5-aza, cardiac genes and structural proteins were expressed. In vivo studies were performed in an ischaemia /reperfusion model in mice. Given intravenously, Sca-1+ cells homed to injured myocardium. By using a Cre/Lox donor/recipient pair, differentiation was shown to an equal extent with and without fusion to host cells.
Matsuura et al. also use Sca-1+ cells.35 They showed that murine cardiac Sca-1+ cells are able to differentiate into spontaneously beating cardiomyocytes upon oxytocin stimulation. Only a small number of cardiac cells (0.3%) were Sca-1+, some of which expressed CD45, CD34 and c-kit (40%, 10% and 10% respectively). Two weeks after oxytocin stimulation, the majority of Sca-1+ cells were fast-replicating small round cells with a high nucleus/cytoplasm ratio. Sca-1+/CD45− differentiated into cardiomyocytes, and could also be differentiated into other lineages (when stimulated in a different environment) supporting the multipotent potency of Sca-1+ cells. The results indicated that Sca-1+ cells in the adult murine heart have potential as stem cells and may contribute to the regeneration of injured hearts.
Recently Goumans and Doevendans' group reported the isolation of a human progenitor cell based on Sca-1.37,46 Although Sca-1 has not been described in humans, a mouse Sca-1 antibody is able to bind a homogenous population in the foetal and adult human heart. This population, defined as cardiomyocyte progenitor cells (CMPC), could easily be expanded. So far there are no data of in vivo studies using CMPCs, but in vitro results are promising since they were able to differentiate into mature cardiomyocytes, and beat spontaneously upon 5-aza stimulation. Less than 15% of the cultured Sca-1+ cells differentiated into myocytes, which expressed organised sarcomers upon differentiation with absorbic acid. This effect was strongly enhanced by adding TGF-β. Connexin 40 and 43 were expressed on the cell membrane in a typical gapjunctional pattern, indicating coupling and communication between differentiated CMPC, which is essential for functional cardiomyocytes.
Side population cells
Another progenitor cell population is called sidepopulation (SP) cells. SP cells are found in several adult tissues and are able to differentiate into organ specific cells from the tissue in which they originate. Side populations are isolated based on the expression of the ATP binding cassette transporter, Abcg2, by effluxing Hoechst 33342.48 Abcg2 is expressed in embryonic cells indicating expression during development. Besides in embryonic cells, Abcg2 could also be detected in the developing and adult heart, which would suggest that there is still a potential for development or (re)generation in the adult heart.33 Cardiac SP cells express several stem cell markers in different quantities (Sca1+, c-kit+) and they are CD31−, CD34+ and CD45− (table 1). After co-culturing with cardiac cells in cardiomyocyte specific medium, cardiac SP are able to express α-actin indicating cardiomyocyte differentiation. They also express several transcriptional regulators involved in cell-cycling (e.g. Notch and TGF-β), suggesting a self-renewal capacity as seen in ES. It has been suggested that these SP cells function as a progenitor cell population for the development, maintenance and repair of the heart.43 In addition, Pfister et al. showed that CD31−/Sca-1+ SP cells represent a distinct cardiac progenitor cell population that is capable of cardiomyogenic differentiation into mature cardiomyocytes.44
Cardiospheres
In 2004, another cell population was isolated from cardiac tissue which has the properties of cardiac stem cells.40 These self-adherent clusters of undifferentiated cells were termed ‘cardiospheres’ and grow as selfadherent clusters from subcultures of postnatal atrial or ventricular human biopsy specimens and from murine hearts. They are clonogenic, express stem and endothelial progenitor cell antigens/markers, and appear to have the properties of adult cardiac stem cells (table 1). Both in vitro and in vivo they differentiate into the major specialised cell types of the heart: myocytes (i.e., cells demonstrating contractile activity and/or showing cardiomyocyte markers) and vascular cells (i.e., cells with endothelial or smooth muscle markers).
Isl-1+ cells
In the hearts of newborn rodents and humans, CSCs are found that do not express c-kit and sca-1.45 They are identified by the expression of the transcription factor islet-1. They also express factors that are known to be involved in the early stage of cardiogenesis (e.g. Nkx2.5, GATA4) (table 1). When co-cultured with neonatal myocytes, these cells were able to differentiate and adopt the cardiomyocyte phenotype, including electrical and contractile properties. The clinical application of Isl-1+ cells seems limited, as they are extremely scarce, and have so far only be detected in neonatal tissues.
In vivo experiments with CSCs
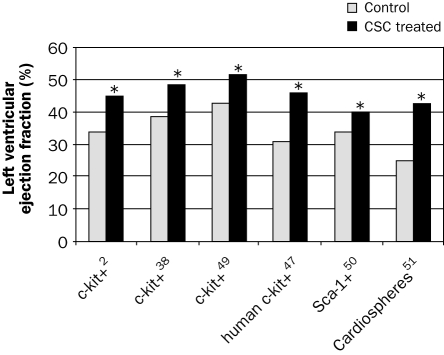
Cardiac stem cells are expected to be introduced from bench to bedside, as in vitro differentiation and proliferation has been shown. Several studies have been performed to document left ventricular improvement in animal models of myocardial infarction; the results on left ventricular ejection fraction are summarised in figure 2. Beltrami injected 1×105 c-kit+ cells or placebo (PBS) in two border zone regions of a five-hour-old myocardial infarction in rats (n=22).2 These cells or their progeny reconstituted myocardium formed of new vessels and myocytes with the characteristics of young cells, encompassing up to 70% of the ventricle, as shown by BrdU labelling. Moreover, left ventricular function at 20 days, as measured by echocardiography, improved significantly as compared with the placebo group (34±3 vs. 45±10%). Cell implantation also reduced infarct size and LV dilation, increased wall thickness and ejection fraction, and significantly improved end-diastolic pressure, developed pressure and dP/dt.
Figure 2 .
Overview of animal studies evaluating the effect on left ventricular ejection fraction at two to five weeks after CSCs therapy for myocardial infarction. X-axis: tested cells from adult heart tissue, based on different stem cell and progenitor antigens and other characteristics. All these in vivo studies were done in rodents, except the study by Bolli et al.,49 which was performed in pigs. For other details see text. * p<0.05.
In a study by Dawn et al., rats (n=80) were subjected to a 90-minute coronary occlusion and four hours of reperfusion.38 In total, 1×106 c-kit+ cells were infused intracoronarily. At five weeks left ventricular function had significantly improved as compared with the control group (38.9±2 vs. 48.4±2%). They also showed that CSCs were able to induce regeneration, and decrease myocardial infarct size by 29%. Bolli's group also showed a marked improvement in a large animal (porcine) model of myocardial infarction.49 Thirty days after intracoronary infusion of CSCs during cardiac catheterisation, the left ventricular ejection fraction improved significantly (42.9±2.3 vs. 51.7±2.0). These studies indicate the clinical potential for the use of CSCs.
Bearzi et al. used human CSC in immunodeficient mice (n=25) and immunosuppressed rats (n=19).47 After myocardial infarction, these human cells were able to generate a chimeric heart, which contained human myocardium composed of myocytes, coronary resistance arterioles and capillaries. It was structurally and functionally integrated in the rodent heart, and contributed to the cardiac performance, as shown by echocardiography and haemodynamic measurements. For instance, left ventricular ejection fraction at two weeks was 46 compared with 31% in the control groups without human CSC treatment.
Wang et al. investigated the role of Sca-1+/CD31− cardiac progenitor cells in a postinfarction model in mice.50 Three weeks after transplantation, attenuated functional decline and adverse structural remodelling was seen in the infarcted mouse heart, as evidenced by increased left ventricular ejection fraction, decreased end-diastolic and systolic dimension, a significant increase of myocardial neovascularisation, and modest cardiomyocyte regeneration. Attenuation of left ventricular remodelling was accompanied by remarkably improved myocardial bioenergetic characteristics. Importantly, cell transplantation with Sca-1− cells did not attenuate left ventricular remodelling.
Smith et al. transplanted human derived cardiospheres into the borderzone of myocardial infarction in immunodeficient mice.51 These cells were derived from biopsy specimens in humans. After 20 days, the percentage of viable myocardium within the infarct zone was greater in the cardiosphere CSC-treated group than in the control group; likewise, left ventricular ejection fraction was significantly higher in the cardiosphere CSC-treated group (42.8±3.3% vs. 25.0±2.0% for control group).
In summary, thus far all animal studies published with CSCs have shown a significant improvement in cardiac performance at two to five weeks. The effects of CSC transplantation in these animal studies on left ventricular ejection fraction after myocardial infarction are summarised in figure 2. Additional trials in animal models should elucidate the long-term effects and safety of these cells in the setting of ischaemic heart failure, prior to designing clinical trials.
Future directions
Several questions regarding cell therapy remain that are of interest for ongoing and future research projects. First, the mechanism for clinical effects (i.e. improvement of left ventricular function) remains unknown.52-59 Do these cells have a true regenerative capacity (i.e. new myocyte and blood vessel formation), or are paracrine effects responsible? The Dutch multicentre HEBE trial will potentially address this issue. The trial has randomised 200 patients with acute myocardial infarction in three arms, in addition to standard therapy: infusion of bone marrow derived mononuclear cells, peripheral mononuclear cells and no additional therapy.25,60 Primary endpoint is left ventricular function at four months, assessed by MRI. Enrolment was completed in April 2008, and the final results are expected at the end of 2008.
Second, what is the optimal strategy for cell delivery? The ultimate goal is delivery of enough cells to the injured myocardium that will actually engraft and survive to maximise restoration of function. To this end, several approaches are being used:1,61,62 (1) percutaneous intracoronary infusion during short balloon occlusion of the artery that supplies the area of interest, (2) direct injection into the ventricular wall during open chest surgery, or (3) percutaneous intramyocardial injection using an endomyocardial injection catheter guided by an electromechanically mapping system.63-66 Tracking of the transplanted stem cells (using scintigraphy, PET and/or MRI) will allow the investigator to monitor how many cells will remain in the heart.67 A head-to-head comparison, using optimal stem cell tracking modalities, is currently ongoing. Safety issues in cardiac cell transplantation include: abnormal cellular differentiation, arrhythmias, restenosis, accelerated atherosclerosis, calcifications, microinfarctions and coronary obstruction.4
Third, what is the difference between the various CSCs (c-kit+, sca-1+, SP cells, cardiospheres, and Islet-1+) that have been isolated so far? Maybe all or some of these cells originate from a common primitive cell that resides in the heart, but are at different stages of development.
Lastly, how can we optimise differentiation and survival of the transplanted cells at the region of interest? Interactive signalling is important for mobilisation, homing, survival, proliferation and differentiation of stem cells. In general, the receptor tyrosine kinase signalling pathway is important for survival of cells by inhibition of apoptosis and is also involved in orchestrating the cross talk between reservoir (stem cell niche) and target area (infarcted myocardium). Several growth factors act as ligands in a part of this pathway that signals via mitogen-activated protein kinases, phosphatidyl inositol 3 kinase, and Akt. Preclinical studies have shown that interference with this axis leads to improved homing and survival of stem cells.68-72 In this view, molecular techniques (e.g. growth factor therapy, transduction with viral vectors, optimising culture medium) to modify the stem cell for better homing, differentiation and survival are of specific interest.
Concluding remarks
It is now apparent that the heart contains relatively primitive cells that have the potential to differentiate into cardiomyocytes, endothelial cells and smooth muscle cells. The main advantage of these cardiac stem cells over other types of cells lies in their heightened predisposition to adopt the cardiac muscle fate. Several subtypes of CSCs have been described, and clinical improvement in animal models of myocardial infarction has been shown, both for autologous and humanderived CSCs. Cardiac stem cell therapy could change the fundamental approach to the treatment of heart disease, as it has produced improved left ventricular function in animal models of myocardial infarction. Translational research projects are ongoing to elucidate the mechanism of success. Isolation, expansion and delivery of these cells should be optimised before heading for clinical trials in patients. Data so far are in favour of this exciting field of research with CSCs, which can potentially improve the outlook for millions of patients with ischaemic heart disease.
Acknowledgement
S.A.J. Chamuleau was supported by a Fellowship Grant (2006) of the Interuniversity Cardiology Institute of the Netherlands (ICIN) to follow a postdoc fellowship at the Institute of Molecular Cardiology at the University of Louisville, KY, USA.
References
- 1.Dimmeler S, Zeiher AM, Schneider MD. Unchain my heart: the scientific foundations of cardiac repair. J Clin Invest 2005;115: 572–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 2003;114:763–76. [DOI] [PubMed] [Google Scholar]
- 3.Fijnvandraat AC, Moorman AF. [Stem cells: biology and possible application to myocardial infarct]. Ned Tijdschr Geneeskd 2004; 148:1186–91. [PubMed] [Google Scholar]
- 4.Oettgen P. Cardiac stem cell therapy; Need for optimization of efficacy and safety monitoring. Circulation 2006;114:353–8. [DOI] [PubMed] [Google Scholar]
- 5.de Muinck E, Thompson C, Simons M. Progress and prospects: Cell based regenerative therapy for cardiovascular disease. Gene Ther 2006;13:659–71. [DOI] [PubMed] [Google Scholar]
- 6.Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, et al. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest 2001;108:407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caspi O, Huber I, Kehat I, Habib M, Arbel G, Gepstein A, et al. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J Am Coll Cardiol 2007;50:1884–93. [DOI] [PubMed] [Google Scholar]
- 8.Gepstein L. Derivation and potential applications of human embryonic stem cells. Circ Res 2002;91:866–76. [DOI] [PubMed] [Google Scholar]
- 9.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science 1998;282:1145–7. [DOI] [PubMed] [Google Scholar]
- 10.Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest 2001;107:1395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature 2001;410:701–5. [DOI] [PubMed] [Google Scholar]
- 12.Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest 1999;103:697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 2002;105:93–8. [DOI] [PubMed] [Google Scholar]
- 14.Atsma DE, Fibbe WE, Rabelink TJ. Opportunities and challenges for mesenchymal stem cell-mediated heart repair. Curr Opin Lipidol 2007;18:645–9. [DOI] [PubMed] [Google Scholar]
- 15.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med 2001;7:430–6. [DOI] [PubMed] [Google Scholar]
- 16.Szmitko PE, Fedak PW, Weisel RD, Stewart DJ, Kutryk MJ, Verma S. Endothelial progenitor cells: new hope for a broken heart. Circulation 2003;107:3093–100. [DOI] [PubMed] [Google Scholar]
- 17.Quaini F, Urbanek K, Beltrami AP, Finato N, Beltrami CA, Nadal-Ginard B, et al. Chimerism of the transplanted heart. N Engl J Med 2002;346:5–15. [DOI] [PubMed] [Google Scholar]
- 18.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature 2004;428:664–8. [DOI] [PubMed] [Google Scholar]
- 19.Chien KR. Stem cells: lost in translation. Nature 2004;428:607–8. [DOI] [PubMed] [Google Scholar]
- 20.Strauer BE, Brehm M, Zeus T, Kostering M, Hernandez A, Sorg RV, et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation 2002;106:1913–8. [DOI] [PubMed] [Google Scholar]
- 21.Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med 2006;355:1222–32. [DOI] [PubMed] [Google Scholar]
- 22.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med 2006;355:1199–209. [DOI] [PubMed] [Google Scholar]
- 23.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med 2006;355:1210–21. [DOI] [PubMed] [Google Scholar]
- 24.Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med 2007;167:989–97. [DOI] [PubMed] [Google Scholar]
- 25.Hirsch A, Nijveldt R, van der Vleuten PA, Tio RA, van der Giessen WJ, Marques KM, et al. Intracoronary infusion of autologous mononuclear bone marrow cells in patients with acute myocardial infarction treated with primary PCI: Pilot study of the multicenter HEBE trial. Catheter Cardiovasc Interv 2008;71:273–81. [DOI] [PubMed] [Google Scholar]
- 26.Rosenzweig A. Cardiac Cell Therapy – Mixed Results from Mixed Cells. N Engl J Med 2006;355:1274–7. [DOI] [PubMed] [Google Scholar]
- 27.Arnesen H, Lunde K, Aakhus S, Forfang K. Cell therapy in myocardial infarction. Lancet 2007;369:2142–3. [DOI] [PubMed] [Google Scholar]
- 28.Boyle AJ, Schulman SP, Hare JM. Stem cell therapy for cardiac repair. Circulation 2006;114:339–52. [DOI] [PubMed] [Google Scholar]
- 29.Fuster V, Sanz J, Viles-Gonzalez JF, Rajagopalan S. The utility of magnetic resonance imaging in cardiac tissue regeneration trials. Nat Clin Pract Cardiovasc Med 2006;(3 Suppl 1):S2–7. [DOI] [PubMed] [Google Scholar]
- 30.Bartunek J, Wijns W, Heyndrickx GR, Vanderheyden M. Timing of intracoronary bone-marrow-derived stem cell transplantation after ST-elevation myocardial infarction. Nat Clin Pract Cardiovasc Med 2006;3(Suppl 1):S52–6. [DOI] [PubMed] [Google Scholar]
- 31.Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: doubleblind, randomised controlled trial. Lancet 2006;367:113–21. [DOI] [PubMed] [Google Scholar]
- 32.Ge J, Li Y, Qian J, Shi J, Wang Q, Niu Y, et al. Efficacy of emergent transcatheter transplantation of stem cells for treatment of acute myocardial infarction (TCT-STAMI). Heart 2006;92:1764–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hierlihy AM, Seale P, Lobe CG, Rudnicki MA, Megeney LA. The post-natal heart contains a myocardial stem cell population. FEBS Lett 2002;530:239–43. [DOI] [PubMed] [Google Scholar]
- 34.Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, et al. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med 2001;344:1750–7. [DOI] [PubMed] [Google Scholar]
- 35.Matsuura K, Nagai T, Nishigaki N, Oyama T, Nishi J, Wada H, et al. Adult Cardiac Sca-1-positive Cells Differentiate into Beating Cardiomyocytes. J Biol Chem 2004;279:11384–91. [DOI] [PubMed] [Google Scholar]
- 36.Anversa P, Nadal-Ginard B. Myocyte renewal and ventricular remodelling. Nature 2002;415:240–3. [DOI] [PubMed] [Google Scholar]
- 37.Goumans MJ, de Boer TP, Smits AM, Laake LW, van Vliet P, Metz CHG, et al. TGF-beta1 induces efficient differentiation of human cardiomyocyte progenitor cells into functional cardiomyocytes in vitro. Stem Cell Res 2008;1:138–49. [DOI] [PubMed] [Google Scholar]
- 38.Dawn B, Stein AB, Urbanek K, Rota M, Whang B, Rastaldo R, et al. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proc Natl Acad Sci U S A 2005;102:3766–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nadin BM, Goodell MA, Hirschi KK. Phenotype and hematopoietic potential of side population cells throughout embryonic development. Blood 2003;102:2436–43. [DOI] [PubMed] [Google Scholar]
- 40.Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res 2004; 95:911–21. [DOI] [PubMed] [Google Scholar]
- 41.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A 2003;100:12313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Vliet P, Sluijter JP, Doevendans PA, Goumans MJ. Isolation and expansion of resident cardiac progenitor cells. Expert Rev Cardiovasc Ther 2007;5:33–43. [DOI] [PubMed] [Google Scholar]
- 43.Martin CM, Meeson AP, Robertson SM, Hawke TJ, Richardson JA, Bates S, et al. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev Biol 2004;265:262–75. [DOI] [PubMed] [Google Scholar]
- 44.Pfister O, Mouquet F, Jain M, Summer R, Helmes M, Fine A, et al. CD31− but Not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res 2005;97: 52–61. [DOI] [PubMed] [Google Scholar]
- 45.Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature 2005;433:647–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Vliet P, Roccio M, Smits AM, van Oorschot AA, Metz CH, van Veen TA, et al. Progenitor cells isolated from the human heart: a potential cell source for regenerative therapy. Neth Heart J 2008;16:163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, et al. Human cardiac stem cells. Proc Natl Acad Sci U S A 2007;104:14068–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med 2001;7: 1028–34. [DOI] [PubMed] [Google Scholar]
- 49.Bolli R, Jneid H, Tang XL, Dawn B, Rimoldi O, Mosna F, et al. Intracoronary administration of cardiac stem cells improves cardiac function in pigs with old infarction. Circulation 2006;114(suppl II):II-239 (abstract). [Google Scholar]
- 50.Wang X, Hu Q, Nakamura Y, Lee J, Zhang G, From AH, et al. The role of the sca-1+/CD31− cardiac progenitor cell population in postinfarction left ventricular remodeling. Stem Cells 2006;24: 1779–88. [DOI] [PubMed] [Google Scholar]
- 51.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation 2007;115:896–908. [DOI] [PubMed] [Google Scholar]
- 52.Fazel S, Cimini M, Chen L, Li S, Angoulvant D, Fedak P, et al. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest 2006;116:1865–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anversa P, Leri A, Kajstura J. Cardiac Regeneration. J Am Coll Cardiol 2006;47:1769–76. [DOI] [PubMed] [Google Scholar]
- 54.Bolli R. Foreword – focused issue on cardiac repair by stem cells. Basic Res Cardiol 2005;100:469–70. [DOI] [PubMed] [Google Scholar]
- 55.Chien KR. Lost and found: cardiac stem cell therapy revisited. J Clin Invest 2006;116:1838–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J 2006;20:661–9. [DOI] [PubMed] [Google Scholar]
- 57.Timmers L, Kiang Lim S, Arslan F, Armstrong JS, Hoefer IE, Doevendans PA, et al. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Research 2008;1:129–37. [DOI] [PubMed] [Google Scholar]
- 58.Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Amano K, Iba O, et al. Improvement of collateral perfusion and regional function by implantation of peripheral blood mononuclear cells into ischemic hibernating myocardium. Arterioscler Thromb Vasc Biol 2002;22:1804–10. [DOI] [PubMed] [Google Scholar]
- 59.Yoshioka T, Ageyama N, Shibata H, Yasu T, Misawa Y, Takeuchi K, et al. Repair of infarcted myocardium mediated by transplanted bone marrow-derived CD34+ stem cells in a nonhuman primate model. Stem Cells 2005;23:355–64. [DOI] [PubMed] [Google Scholar]
- 60.Hirsch A, Nijveldt R, van der Vleuten PA, Biemond BJ, Doevendans PA, van Rossum AC, et al. Intracoronary infusion of autologous mononuclear bone marrow cells or peripheral mononuclear blood cells after primary percutaneous coronary intervention: Rationale and design of the HEBE trial – A prospective, multicenter, randomized trial. Am Heart J 2006;152:434–41. [DOI] [PubMed] [Google Scholar]
- 61.Freyman T, Polin G, Osman H, Crary J, Lu M, Cheng L, et al. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J 2006;27:1114–22. [DOI] [PubMed] [Google Scholar]
- 62.Sherman W, Martens TP, Viles-Gonzalez JF, Siminiak T. Catheterbased delivery of cells to the heart. Nat Clin Pract Cardiovasc Med 2006;(3 Suppl 1):S57–64. [DOI] [PubMed] [Google Scholar]
- 63.Ripa RS, Wang Y, Jorgensen E, Johnsen HE, Hesse B, Kastrup J. Intramyocardial injection of vascular endothelial growth factor-A165 plasmid followed by granulocyte-colony stimulating factor to induce angiogenesis in patients with severe chronic ischaemic heart disease. Eur Heart J 2006;27:1785–92. [DOI] [PubMed] [Google Scholar]
- 64.Smits PC, van Langenhove G, Schaar M, Reijs A, Bakker WH, van der Giessen WJ, et al. Efficacy of percutaneous intramyocardial injections using a nonfluoroscopic 3-D mapping based catheter system. Cardiovasc Drugs Ther 2002;16:527–33. [DOI] [PubMed] [Google Scholar]
- 65.Vale PR, Losordo DW, Milliken CE, McDonald MC, Gravelin LM, Curry CM, et al. Randomized, single-blind, placebo-controlled pilot study of catheter-based myocardial gene transfer for therapeutic angiogenesis using left ventricular electromechanical mapping in patients with chronic myocardial ischemia. Circulation 2001;103:2138–43. [DOI] [PubMed] [Google Scholar]
- 66.Beeres SL, Bax JJ, Dibbets-Schneider P, Stokkel MP, Fibbe WE, van der Wall EE, et al. Intramyocardial injection of autologous bone marrow mononuclear cells in patients with chronic myocardial infarction and severe left ventricular dysfunction. Am J Cardiol 2007;100:1094–8. [DOI] [PubMed] [Google Scholar]
- 67.San Roman JA, Fernandez-Aviles F. The role of noninvasive imaging techniques in the assessment of stem cell therapy after acute myocardial infarction. Nat Clin Pract Cardiovasc Med 2006;3(Suppl 1):S38–41. [DOI] [PubMed] [Google Scholar]
- 68.Li Q, Li B, Wang X, Leri A, Jana KP, Liu Y, et al. Overexpression of Insulin-like Growth Factor-1 in Mice Protects from Myocyte Death after Infarction, Attenuating Ventricular Dilation, Wall Stress, and Cardiac Hypertrophy. J Clin Invest 1997;100:1991–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Urbanek K, Rota M, Cascapera S, Bearzi C, Nascimbene A, De Angelis A, et al. Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circ Res 2005;97:663–73. [DOI] [PubMed] [Google Scholar]
- 70.Linke A, Muller P, Nurzynska D, Casarsa C, Torella D, Nascimbene A, et al. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci U S A 2005;102:8966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Davis ME, Hsieh PCH, Takahashi T, Song Q, Zhang S, Kamm RD, et al. Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc Natl Acad Sci U S A 2006;103: 8155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med 2003;9: 1195–201. [DOI] [PubMed] [Google Scholar]