Abstract
Development of natural killer (NK) cells is thought to depend on interactions between NK progenitors and the bone marrow (BM) microenvironment; however, little is known about the molecular signals involved. Here we show that lymphotoxin (LT) provides an important signal for the development of both NK cells and NK/T cells. LTα−/− mice show marked reduction in splenic and BM NK and NK/T cell numbers and dramatically impaired NK and NK/T cell function. Mice deficient in either tumor necrosis factor receptor (TNFR)-I or TNFR-II have normal numbers of NK and NK/T cells, implying that neither of the TNFRs nor soluble LTα3 is required for development of these cell types. Reciprocal BM transfers between LTα−/− and wild-type mice suggest that close interactions between membrane LT-expressing NK cell precursors and LT-responsive radioresistant stromal cells are necessary for NK cell development. When LT-deficient BM cells are incubated with IL-15, NK cells are formed. In addition, LT-deficient BM cells produce IL-15 after activation. Thus, membrane LT appears to deliver a signal for NK cell development that is either independent of IL-15 or upstream in the IL-15 pathway. These results reveal a novel function for membrane LT in NK and NK/T cell development. They also support a cellular and molecular mechanism by which NK cell precursors themselves deliver essential signals, through the membrane ligand, that induce the microenvironment to promote further NK cell and NK/T cell development.
In their soluble forms, lymphotoxin α (LTα) and tumor necrosis factor α (TNFα) are structurally related homotrimers (LTα3 and TNFα3) that show similar biological activities by binding to either of the two defined tumor necrosis factor receptors (TNFR), TNF receptor I (TNFR-I) and TNF receptor II (TNFR-II). These receptor–ligand interactions lead to activation of a wide variety of inflammatory responses (1–5). LTα can also associate with membrane LTβ to form the membrane-bound LTα1β2 heterotrimer. This membrane ligand has no measurable affinity for TNFR-I or TNFR-II but rather shows high affinity for the LTβ receptor (LTβR) (1, 4, 5). Whereas LTα and LTβ are expressed in activated T, B, and NK cells, the LTβR is expressed exclusively in nonlymphoid tissues. Thus, the biologic activities of membrane LT may depend on interactions between ligand-bearing BM-derived cells with LTβR-expressing stromal cells.
LTα−/−, LTβ−/−, and LTβR−/− mice all manifest profoundly defective lymph node and Peyer’s patch development and altered splenic microarchitecture (6–11), demonstrating a key role of membrane LT–LTβR interactions in secondary lymphoid tissue organogenesis. Administration of a soluble LTβR–Ig Fcγ fusion protein (LTβR–Ig) or blocking anti-LTβ mAb to pregnant mice showed that the essential membrane LT signals for peripheral lymphoid organogenesis were delivered during the second half of gestation (12, 13). Because membrane LT-dependent lymph node development is retained in scid mice and in RAG-1−/− or RAG-2−/− mice, it is clear that membrane LT-expressing, non-T, non-B cells are essential for secondary lymphoid organ development. The NK lineage is the only recognized non-T, non-B cell bone marrow (BM)-derived lineage reported to express LT (1, 2). We, therefore, considered that there might be disturbed development or function of NK cells in LTα−/− mice. Here, we report that lack of LT but not TNF leads to an impairment of both NK cell and NK/T cell development. IL-15, known to be induced through interferon regulatory factor I (IRF-1) (14, 15), is an essential cytokine for NK (16) and NK/T (17) cell development. Our observation that IL-15 can bypass the developmental block of LTα−/− BM cells in vitro suggests that membrane LT on NK lineage cells and LTβR on stromal cells may regulate the development of NK and NK/T cells by using a mechanism that may be independent or upstream of the IL-15/IRF-1 pathway. This study reveals not only an important function for membrane LT, but also provides an example of a membrane ligand regulating the development of NK cells through an interaction with LTβR expressed on cells within the BM microenvironment.
MATERIALS AND METHODS
Mice, Antibodies, and Soluble Receptor.
LTα−/− mice were initially prepared on a mixed 129/Sv × C57BL/6 background (6) and maintained by interbreeding under specific pathogen-free conditions. LTα−/− mice and their LTα+/+ littermates were therefore available on similar genetic backgrounds. Where indicated, the targeted allele was backcrossed six times to C57BL/6 mice (N6 heterozygotes). The N6 mice were then intercrossed to produce LTα−/− mice on the C57BL/6 background. TNFR-I−/− and TNFR-II−/− mice (C57BL/6 background) were provided by Jacques J. Peschon (Immunex) (18). TNFα−/− mice were provided by Michael Marino and Lloyd Old (Ludwig Institute for Cancer Research, New York). Animal care and use were in accordance with institutional guidelines. Murine LTβR-human IgG1 Fc and human lymphocyte function-associated antigen (LFA)-3–human IgG1 Fc fusion proteins and anti-LTβ mAb were generously provided by J. Browning (Biogen) (4, 12). For some experiments, LTβR–Ig fusion protein was prepared in our own laboratory. Anti-CD3ɛ, anti-NK1.1 (PK136), anti-pan NK (DX-5), and anti-B220 antibodies conjugated with FITC or phycoerythrin were all obtained from PharMingen. Splenocytes and BM cells were stained and analyzed by two-color flow cytometry on a FACScan fluorescence-activated cell sorter (Becton Dickinson). Rabbit polyclonal anti-asialo-GM-1 (anti-ASGM1) was obtained from Wako Chemicals (Richmond, VA).
NK Cytotoxicity Assay.
Fresh splenocytes from C57BL/6 and C57BL/6-LTα−/− mice, recovered by using a Lympholyte-M gradient (Cedarlane Laboratories), were used in a standard 51Cr-release assay against YAC-1 cells for natural killing ability in vitro by using several effector/target ratios (20). Spontaneous 51Cr release was <15% of maximum release.
NK-Dependent BM Rejection Assay.
After lethal γ-irradiation (9.5 Gy from a 137Cs source) on day 0, mixed (129/Sv × C57BL/6) LTα−/− mice or their wild-type (wt) littermates received 3 × 106 BM cells from C57BL/6-B2 m−/− (The Jackson Laboratory) via tail vein injection. On day 5, recipient mice were injected intravenously with 3 μCi (1 Ci = 37 GBq) of [125I]deoxyuridine and 1 × 10−11 mol of FUdR. On day 6, the spleens were removed and rinsed with PBS, and the radioactivity was counted with a γ-counter. Incorporation of radioactivity into the spleens was used as an index of hematopoietic precursor cell proliferation (21, 22). Where indicated, wt mice were treated with 50 μl of anti-NK cell polyclonal antibody, ASGM-1, i.v. 2 days before BM transfer to remove endogenous NK cell activity.
Analysis of NK/T Cell Function.
IL-4 production by NK/T cells was determined as described (23). Groups of three mice were treated with 2 μg of anti-CD3 mAb (2C11) by i.v. tail vein injection. Ninety minutes later, the spleens were removed and a single cell suspension was produced and cultured in RPMI 1640 plus 10% FBS at 1 × 107 cells per ml for 2 h. Culture supernatants were harvested, and IL-4 levels were measured by using a mouse IL-4 ELISA kit (Endogen, Cambridge, MA).
Reciprocal Transfer of BM and Spleen Cells.
BM (2 × 106) and spleen (50 × 106) cells were transferred to γ-irradiated (10.5 Gy from 137Cs source for BM transfers, 7 Gy for spleen transfers) recipients were prepared as described (11). Four to eight weeks after BM transfer, spleens and BM were collected, and the numbers of cells in various lymphocyte subsets were determined by using flow cytometry. Where indicated, BM cells from 129/SvJ and C57BL/6J or LTα−/− mice were mixed in a 1:1 ratio and 2 × 106 mixed cells were transferred into lethally irradiated 129/SvJ recipient mice. Four to six weeks later, recipient splenocytes were collected for analysis. When isolated splenocytes were transferred, recipient spleens were collected for analysis 10 days after irradiation and transfer. The numbers of NK1.1+ cells were determined by using flow cytometry as described above.
In Vitro Generation of NK cells.
BM cells (50 × 106) were cultured for 7 days in RPMI 1640 containing 10% FBS and 100 ng/ml human recombinant IL-15 (gift from Thomas Waldmann, National Cancer Institute). Cells recovered from cultures were analyzed by using flow cytometry for expression of NK1.1 and CD3 and for cytotoxic activity with YAC-1 cells as target.
Analysis of IL-15 Gene Expression.
Total RNA was prepared from freshly isolated BM cells or BM cells cultured in vitro with lipopolysaccharide (30 μg/ml) and IFN-γ (100 units/ml) for 6 h. RNase protection assay of murine IL-15 mRNA was carried out by using the RiboQuant kit (PharMingen) according to the manufacturer’s instructions.
RESULTS AND DISCUSSION
Impaired NK and NK/T Cell Development and Function in LTα−/− Mice.
By using flow cytometry, we found that the numbers of NK cells in the spleens of LTα−/− mice (NK1.1+, CD3−) were greatly and consistently reduced compared with wt mice (6.8 fold) (Fig. 1A). A variable reduction of NK cells (25–75% in five individual experiments) was also observed in the BM of LTα−/− mice. This result suggested that there was a primary failure of NK cell development in the BM, although there might also have been reduced release of NK cells from the marrow to the periphery.
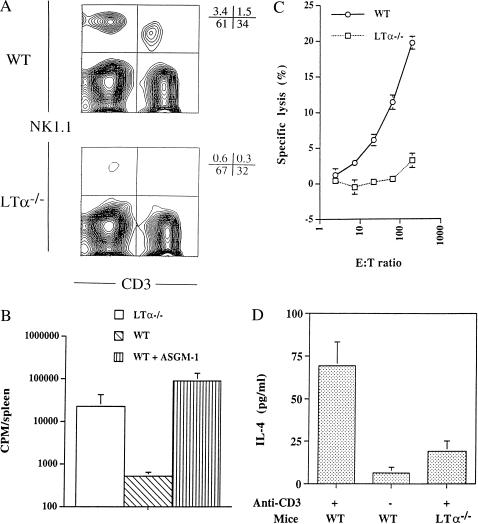
Figure 1.
NK cell development and function are severely impaired in C57BL/6 LTα−/− mice. (A) Lower number of NK cells in LTα−/− mice. Splenocytes from 8- to 12-week-old wt and LTα−/− mice were collected and stained for the NK cell marker PK136 (for NK1.1) and the T cell marker CD3. Data from one of five representative experiments are shown. The total numbers of nucleated cells recovered from the spleens and BM of wt and LTα−/− mice were similar. (B) LTα−/− mice failed to reject MHC class I-deficient BM cells in vivo. Irradiated wt and LTα−/− mice (four in each group) were infused with BM cells from β2-microglobulin-deficient donors. BM rejection was measured as described in Materials and Methods. Error bars represent SD of splenic uptake of 125I[UdR] in individual mice. This experiment was repeated once with similar results. (C) Impaired NK cytotoxicity in LTα−/− mice. Fresh splenocytes from C57BL/6 and C57BL/6-LTα−/− mice were used in a standard 51Cr-release assay for natural killing ability in vitro. Error bars represent SD for the six replicates performed for each data point. A similar defect was found with in vivo poly[I]⋅poly[C]-stimulated splenocytes (K.I., data not shown). (D) Lack of NK/T activity in LTα−/− mice. NK/T activity was assessed as production of IL-4 by mice injected with 2 μg of monoclonal anti-CD3 antibody (2C11). For experiments in B and D, the LTα−/− mutation was on a mixed 129/Sv × C57BL/6 background, and wt mice were LTα−/− littermate controls with similar genetic backgrounds.
The developmental relationship of CD3− NK cells to NK/T cells has not yet been well defined. A close relationship between the two lineages is suggested by their concomitant deficiency in IRF-1-deficient mice and by their shared expression of genes encoded in the NK gene complex that are not generally expressed by other cells (14, 24, 25). Of interest, the numbers of NK/T cells were, like the numbers of NK cells, also reduced in LTα−/− compared with wt mice (Fig. 1A).
We next studied whether the reduced number of NK cells in LTα−/− mice significantly affected NK activity. LTα−/− mice failed to reject BM transferred from β2-microglobulin-deficient donors. Rejection of MHC class I-deficient BM in vivo, which is normally NK cell-dependent, was dramatically impaired in LTα−/− mice (Fig. 1B). Freshly isolated splenocytes from LTα−/− mice also failed to mediate cytotoxicity of YAC-1 target cells in vitro (Fig. 1C). Similar data were obtained from poly[I:C]-stimulated splenocytes. Thus, the reduced number of NK cells in the LTα−/− strain is coupled with significant impairment of NK cell activity measured in vivo and in vitro. In addition, we observed a dramatic decrease in NK/T cell function in LTα−/− mice as assessed by IL-4 production after i.v. administration of anti-CD3 mAb (Fig. 1D). The shared requirement for LT to support the development of both CD3− NK cells and CD3+ NK/T cells adds support to a developmental relationship between these two lineages.
Essential Interaction Between Membrane LT and LTβR for NK Cell Development.
LTα−/− mice lack both soluble LTα3 that signals through TNFR-I or TNFR-II and membrane LT that binds LTβR (1, 2). To determine which of these ligand–receptor interactions is responsible for defective NK cell development, we analyzed TNFα−/−, TNFR-I−/− and TNFR-II−/− mice. In all three of these gene-targeted mouse strains, the numbers of NK and NK/T cells were similar to those in wt mice (Table 1), indicating that LTα3/TNF receptor or TNF/TNF receptor signaling is not essential for NK cell development. Rather, membrane LT/LTβR-mediated signaling is required. To investigate this directly, we used LTβR–Ig fusion protein to block membrane LT activity in developing wt mice; the splenic NK cell number was markedly reduced (Fig. 2). Although LTβR–Ig can also bind human LIGHT, another recently identified membrane-associated TNF family member (26), administration of anti-LTβ mAb (which does not interact with LIGHT) also resulted in a reduction in NK cell numbers comparable to treatment with LTβR–Ig (data not shown). Thus, the data indicate that a signal from membrane LT-expressing cells (LTα1β2+) to responsive (LTβR+) cells is required for normal NK cell development.
Table 1.
The development and maintenance of NK cells by membrane LT but not by soluble LTα3 or TNFα3
| Mice | n | NK1.1+CD3− | NK1.1+CD3+ | NK1.1−CD3+ |
|---|---|---|---|---|
| wt | 5 | 3.4 ± 0.5 | 1.2 ± 0.3 | 31 ± 6 |
| LTα−/− | 5 | 0.5 ± 0.2* | 0.4 ± 0.1* | 34 ± 3 |
| TNFR-I−/− | 4 | 2.9 ± 0.4 | 1.1 ± 0.2 | 26 ± 3 |
| TNFR-II−/− | 2 | 2.6 | 1.2 | 35 |
| TNFα−/− | 3 | 3.3 ± 0.9 | 1.1 ± 0.3 | 31 ± 5 |
Values reported represent cells with the indicated phenotypes expressed as means (percentages) ± SD or mean of two experiments.
, P < 0.01 for wt vs. LTα−/− mice.
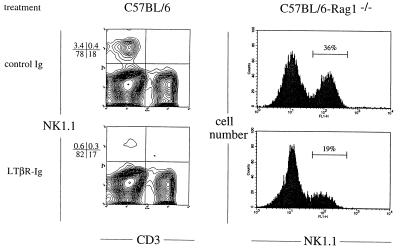
Figure 2.
NK cell development is blocked by treatment of mice with LTβR–Ig. Pregnant mice at gestational day 16 were treated with LTβR–Ig (25 μg) i.p., and each offspring was treated again on day 7 after birth. Splenocytes collected at day 28 after birth were stained for expression of NK1.1. (Lower) Splenocytes from mice treated with LTβR–Ig. (Upper) Control C57BL/6J mice treated with 25 μg of human LFA-1–Ig fusion protein and C57BL/6-RAG-1−/− mice treated with 25 μg of mouse total IgG. (Left) Data from C57BL/6J splenocytes. (Right) Data for C57BL/6-RAG-1−/− splenocytes. Similar data were obtained in four additional experiments with a range of NK cell reduction from 40–80%.
It is well recognized that RAG-1−/− and scid mice retain functional NK cells (27). To investigate whether this robust NK cell development that occurs independent of T and B cells is also dependent on membrane LT, we treated developing RAG-1−/− mice with LTβR–Ig. A 40–80% reduction of splenic NK cells was readily detected at 5 weeks of age (Fig. 2). Because the NK lineage itself is one of the major membrane LT-expressing lineages other than B and T lymphocytes, these experiments suggest that NK cells may depend on the expression of membrane LT on the NK lineage itself.
LT-Dependent BM Microenvironment for NK Cell Development.
We have previously shown that certain elements of the LTα-dependent lymphoid tissue microenvironment are plastic whereas others are developmentally fixed (11). To test whether introduction of LTα-expressing cells into an LTα-deficient microenvironment can restore NK cell development, we transferred BM cells from wt C57BL/6 mice into lethally irradiated C57BL/6 LTα−/− recipients. Four to eight weeks after reconstitution, splenic NK cells were not restored even though the numbers of splenic B and T cells had been restored. These data suggest that an LTα-dependent microenvironment is essential for NK cell development and that absence of this microenvironment is developmentally fixed in adult LTα−/− recipients (Table 2).
Table 2.
LT-dependent interactions between NK precursors and BM stromal cells
| Donor | Recipient | n | NK1.1+ DX-5+ | NK1.1− DX-5+ | CD3+ | B220+ |
|---|---|---|---|---|---|---|
| BM transfer | ||||||
| wt | wt | 4 | 2.9 ± 0.7 | ND | 29 ± 3 | 62 ± 2 |
| wt | LTα−/− | 4 | 0.5 ± 0.2* | ND | 31 ± 2 | 62 ± 3 |
| LTα−/− | wt | 4 | 0.6 ± 0.3* | ND | 40 ± 9 | 52 ± 3 |
| LTα−/− | LTα−/− | 4 | 0.7 ± 0.3* | ND | 34 ± 5 | 51 ± 3 |
| Splenocyte transfer | ||||||
| wt | wt | 2 | 2.9 | ND | ||
| wt | LTα−/− | 2 | 2.8 | ND | ||
| Donor BM mixed 1:1 | ||||||
| C57BL/6:129/Sv | 129/Sv | 4 | 2.0 ± 0.8 | 1.2 ± 0.5 | 33 ± 9 | 52 ± 9 |
| B6LTα−/−:129/Sv | 129/Sv | 4 | 0.6 ± 0.3† | 1.1 ± 0.5 | 24 ± 4 | 61 ± 5 |
Values reported represent cells with the indicated phenotype expressed as percentages ± SD of total splenocytes (n = 4, except for splenocyte transfers where n = 2). The analyses were performed 4–8 weeks after BM transfer or 10 days after splenocyte transfer. The number of NK cells resulting from each transplant was determined by double staining splenocytes with PK136 PE (anti-NK1.1) and DX-5 FITC (pan NK). NK cells from C57BL/6 mice are NK1.1+DX-5+, whereas NK cells from 129/Sv mice are NK1.1−DX-5+. In C57BL/6 mice, there are essentially no NK1.1−DX-5+ cells. ND, not determined.
, Statistically significant difference compared to wt control (P < 0.01 by Student’s t test).
, Statistically significant difference compared to C57BL/6:129/Sv (P < 0.01 by paired t test).
Many aspects of the splenic microarchitecture are grossly altered in LTα−/− mice (2). To explore whether this altered microenvironment retained the ability to support the localization of NK cells into the spleen, we transferred splenocytes from wt mice into sublethally irradiated LTα−/− or wt recipients. Five to ten days after transfer, the numbers of NK cells in the spleens of reconstituted LTα−/− mice were similar to those in the spleens of reconstituted wt mice (Table 2). Although it is still possible that defective migration of mature NK cells to the spleen could underlie the reduced numbers of splenic NK cells in LTα−/− mice, the results suggest that this is unlikely. Rather, these data support defective NK development as the etiology of the LTα−/− NK phenotype.
Membrane LT on BM-Derived Cells Is Essential for NK Cell Development.
To explore whether BM-derived cells must be competent to produce LTα to support the development of NK cells, BM cells from either wt or LTα−/− donors were transferred into lethally irradiated adult wt recipients. The number of NK cells in mice reconstituted with LTα−/− BM remained low 4–8 weeks after BM transfer, at a time when T and B cells were restored and when NK cells had been restored by transfer of wt BM (Table 2). These data indicate that there is an intrinsic defect in NK development in LTα−/− mice. LTα−/− NK cell precursors cannot, even in the microenvironment of a wt mouse, develop normally into NK cells. These data are consistent with a model in which membrane LT on NK cells themselves or on NK cell precursors may be required for LTβR signaling of stromal cells, which in return promotes further NK cell development.
Requirement for an LTα-Dependent Interaction Between NK Precursors and LT-Responsive Stromal Cells.
To investigate further the characteristics of the interaction between developing NK cells and their stromal microenvironment, we created mixed BM chimeras. BM cells from wt C57BL/6J (B6) or B6 LTα−/− mice (both NK1.1+) were mixed at a 1:1 ratio with BM cells from wt 129/SvJ mice (NK1.1−) and transferred into lethally irradiated 129/SvJ recipients. If bystander cells expressing membrane LT can provide a signal to stromal cells that can support NK cell development in trans, then NK1.1+ cells should be generated following transfer of a mixture of B6 LTα−/− and 129/SvJ BM. Although NK1.1+ cells were formed when 129/Sv mice were reconstituted with a mixture of B6 and 129/Sv BM, no NK1.1+ cells were formed when a mixture of B6 LTα−/− and 129/Sv BM was used (Table 2). In contrast, the number of NK1.1− NK cells (DX-5+ cells) was comparable in both groups, indicating that the requisite interactions for development of 129/Sv NK cells were present. Thus, although the membrane LT on 129/Sv-derived BM cells could signal the development of NK cells from the 129/Sv precursors, it could not act in trans to support the development of NK1.1+ cells from LTα−/− precursors. This suggests that the membrane LT-sensitive stromal cell may only be competent to support the development of a membrane LT-expressing precursor that it contacts directly.
IL-15 Can Bypass the Block in NK Cell Development in LTα−/− BM.
We suggest that the cells in the developing NK lineage deliver a membrane LT signal to BM stromal cells and that this LT-stimulated stromal cell provides an environment that supports the development of NK cells. This model is consistent with recent studies suggesting that IRF-1-stimulated IL-15 production by the microenvironment is also required for NK cell development (14, 15). Of interest, we have found that when BM cells from LTα−/− mice are cultured in vitro with exogenously added IL-15 large numbers of NK1.1+ cells are formed (Fig. 3A). These NK1.1+ cells show natural killing activity against YAC-1 cells (Fig. 3B). Additionally, unstimulated total BM cells from LTα−/− mice demonstrate low levels of IL-15 mRNA expression similar to wt BM cells; however, unlike IRF-1−/− mice, BM cells from LTα−/− mice up-regulate IL-15 mRNA expression similarly to BM cells from wt mice after stimulation for 6 h with lipopolysaccharide and IFN-γ (Fig. 3C). These data suggest that the action of LTα in NK development is either independent of IL-15/IRF-1 or upstream in the IL-15/IRF-1 pathway. It is possible that in the normal BM microenvironment membrane, LT on NK cell precursors interacts with LTβR on stromal cells, which then leads to the development of stromal cells. Therefore, membrane ligands, such as LT, may occupy a unique position in the development of NK cells.
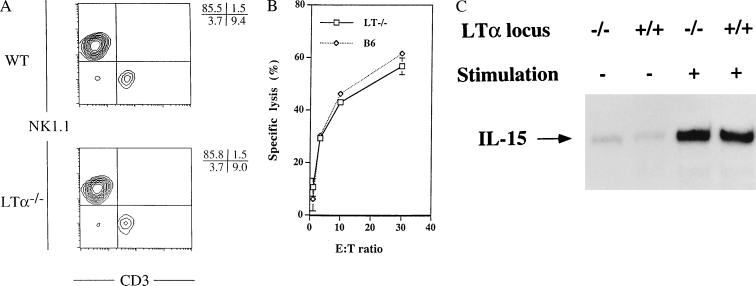
Figure 3.
IL-15 can bypass the developmental block of NK cells in LTα−/− mice. (A) When BM cells are cultured in vitro for 7 days with recombinant IL-15, equal numbers of NK1.1+CD3− cells are induced from wt and LTα−/− BM (5.4–5.6 × 106 cells). (B) NK1.1+CD3− cells induced in vitro from LTα−/− (□) and wt (⋄) BM show similar natural killing activity against YAC-1 cells. (C) Expression of IL-15 mRNA in BM cells. Total RNA was prepared from freshly isolated BM cells or BM cells stimulated in vitro with lipopolysaccharide and IFN-γ. IL-15 mRNA was quantitatively analyzed by RNase protection assay. Equivalent amounts of mRNA for glyceraldehyde-3-phosphate dehydrogenase were observed in all four samples.
Our data suggest a reciprocal interaction between NK cell precursors and the microenvironment in which they develop. This direct, reciprocal interplay for cellular maturation may not be unique for the NK cell lineage. We have previously shown that membrane LT-expressing B lymphocytes are essential and sufficient for the induction of clusters of follicular dendritic cells. The microenvironment represented by the follicular dendritic cells then supports the terminal maturation of the B cells permitting the development of a high-affinity, isotype-switched antibody response (28). It appears, therefore, that membrane LT-dependent signaling by BM-derived cells to the supporting cells of the microenvironment may be generally necessary for maturation or activation of these stromal cells, which then support the development and function of the membrane LT-expressing cells. Further investigation of the reciprocal cellular collaborations resulting from direct ligand–receptor interactions will lead to detailed understanding of the molecular mechanisms by which the BM microenvironment supports lymphoid cell development.
Acknowledgments
The authors gratefully acknowledge the technical assistance of Guangming Huang and Liping Yang. The authors thank Dr. J. Browning for anti-LTβ antibody and LTβR–Ig fusion protein, Drs. M. Marino and L. Old for TNFα−/− mice, and Dr. J. Peschon for TNFR−/− mice. K. Iizuka is supported by a fellowship from the Eastern Missouri Chapter of the Arthritis Foundation. These studies were supported in part by National Institutes of Health grants (to D.D.C., Y.-X.F., and W.M.Y.), and National Multiple Sclerosis Society grant (to Y.-X.F.). D.C. and W.M.Y. are investigators of the Howard Hughes Medical Institute.
ABBREVIATIONS
- BM
bone marrow
- IRF-1
interferon regulatory factor-1
- LT
lymphotoxin
- LTβR
LTβ receptor
- NK
natural killer
- TNFα
tumor necrosis factor α
- TNFR-I
55-kDa type I TNF receptor
- TNFR-II
75-kDa type II TNF receptor
- wt
wild type
References
- 1.Ware C F, VanArsdale T L, Crowe P D, Browning J L. Curr Top Microbiol Immunol. 1995;198:175–218. doi: 10.1007/978-3-642-79414-8_11. [DOI] [PubMed] [Google Scholar]
- 2.Chaplin D D, Fu Y-X. Curr Opin Immunol. 1998;10:289–297. doi: 10.1016/s0952-7915(98)80167-2. [DOI] [PubMed] [Google Scholar]
- 3.Browning J L, Ngam-ek A, Lawton P, DeMarinis J, Tizard R, Chow E P, Hession C, O’Brine-Greco B, Foley S F, Ware C F. Cell. 1993;72:847–856. doi: 10.1016/0092-8674(93)90574-a. [DOI] [PubMed] [Google Scholar]
- 4.Browning J L, Sizing I D, Lawton P, Bourdon P R, Rennert P D, Majeau G R, Ambrose C M, Hession C, Miatkowski K, Griffiths D A, et al. J Immunol. 1997;159:3288–3298. [PubMed] [Google Scholar]
- 5.Crowe P D, VanArsdale T L, Walter B N, Ware C F, Hession C, Ehrenfels B, Browning J L, Din W S, Goodwin R G, Smith C A. Science. 1994;264:707–710. [PubMed] [Google Scholar]
- 6.De Togni P, Goellner J, Ruddle N H, Streeter P R, Fick A, Mariathasan S, Smith S C, Carlson R, Shornick L P, Strauss-Schoenberger J, et al. Science. 1994;264:703–707. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- 7.Banks T A, Rouse B T, Kerley M K, Blair P J, Godfrey V L, Kuklin N A, Bouley D M, Thomas J, Kanangat S, Mucenski M L. J Immunol. 1995;155:1685–1693. [PubMed] [Google Scholar]
- 8.Koni P A, Sacca R, Lawton P, Browning J L, Ruddle N H, Flavell R A. Immunity. 1997;6:491–500. doi: 10.1016/s1074-7613(00)80292-7. [DOI] [PubMed] [Google Scholar]
- 9.Alimzhanov M B, Kuprash D V, Kosco-Vilbois M H, Luz A, Turetskaya R L, Tarakhovsky A, Rajewsky K, Nedospasov S A, Pfeffer K. Proc Natl Acad Sci USA. 1997;94:9302–9307. doi: 10.1073/pnas.94.17.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Futterer A, Mink K, Luz A, Kosco-Vilbois M H, Pfeffer K. Immunity. 1998;9:59–70. doi: 10.1016/s1074-7613(00)80588-9. [DOI] [PubMed] [Google Scholar]
- 11.Fu Y-X, Molina H, Matsumoto M, Huang G, Min J, Chaplin D D. J Exp Med. 1997;185:2111–2120. doi: 10.1084/jem.185.12.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rennert P D, Browning J L, Mebius R, Mackay F, Hochman P S. J Exp Med. 1996;184:1999–2006. doi: 10.1084/jem.184.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rennert P D, Browning J L, Hochman P S. Int Immunol. 1997;9:1627–1639. doi: 10.1093/intimm/9.11.1627. [DOI] [PubMed] [Google Scholar]
- 14.Ohteki T, Yoshida H, Matsuyama T, Duncan G S, Mak T W, Ohashi P S. J Exp Med. 1998;187:967–972. doi: 10.1084/jem.187.6.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogasawara K, Hida S, Azimi N, Tagaya Y, Sato T, Yokochi-Fukuda T, Waldmann T A, Taniguchi T, Taki S. Nature (London) 1998;391:700–703. doi: 10.1038/35636. [DOI] [PubMed] [Google Scholar]
- 16.Williams N S, Moore T A, Schatzle J D, Puzanov I J, Sivakumar P V, Zlotnik A, Bennett M, Kumar V. J Exp Med. 1997;186:1609–1614. doi: 10.1084/jem.186.9.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohteki T, Shirley H, Suzuki H, Mak T W, Ohashi P S. J Immunol. 1997;159:5931–5935. [PubMed] [Google Scholar]
- 18.Peschon J J, Torrance D S, Stocking K L, Glaccum M B, Otten C, Willis C R, Charrier K, Morrissey P J, Ware C B, Mohler K M. J Immunol. 1998;160:943–952. [PubMed] [Google Scholar]
- 19.Marino M W, Dunn A, Grail D, Inglese M, Noguchi Y, Richards E, Jungbluth A, Wada H, Moore M, Williamson B, Basu S, Old L J. Proc Natl Acad Sci USA. 1997;94:8093–8098. doi: 10.1073/pnas.94.15.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Idris A H, Iizuka K, Smith H R C, Scalzo A A, Yokoyama W M. J Exp Med. 1998;188:2243–2256. doi: 10.1084/jem.188.12.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bix M, Liao N-S, Zijlstra M, Loring J, Jaenisch R, Raulet D. Nature (London) 1991;349:329–331. doi: 10.1038/349329a0. [DOI] [PubMed] [Google Scholar]
- 22.Yokoyama W. In: Fundamental Immunology. Paul W E, editor. Philadelphia: Lippincott; 1999. pp. 575–603. [Google Scholar]
- 23.Yoshimoto T, Paul W E. J Exp Med. 1994;179:1285–1295. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bendelac A, Rivera M N, Park S H, Roark J H. Annu Rev Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 25.Leite-de-Moraes M C, Dy M. Eur Cytokine Network. 1997;8:229–237. [PubMed] [Google Scholar]
- 26.Mauri D N, Ebner R, Montgomery R I, Kochel K D, Cheung T C, Yu G-L, Ruben S, Murphy M, Eisenberg R J, Cohen G H, Spear P G, Ware C F. Immunity. 1998;8:21–30. doi: 10.1016/s1074-7613(00)80455-0. [DOI] [PubMed] [Google Scholar]
- 27.Dorshkind K, Pollack S B, Bosma M J, Phillips R A. J Immunol. 1985;134:3798–3801. [PubMed] [Google Scholar]
- 28.Fu Y-X, Huang G, Wang Y, Chaplin D D. J Exp Med. 1998;187:1009–1018. doi: 10.1084/jem.187.7.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]