Abstract
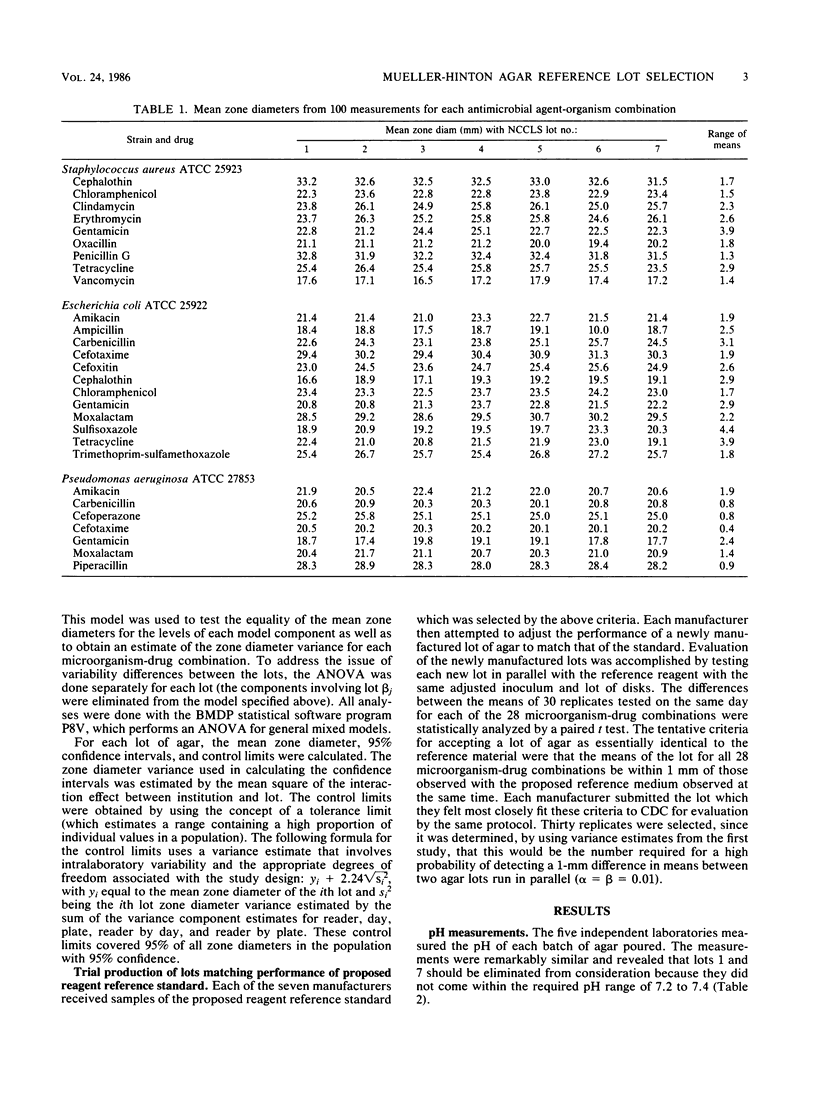
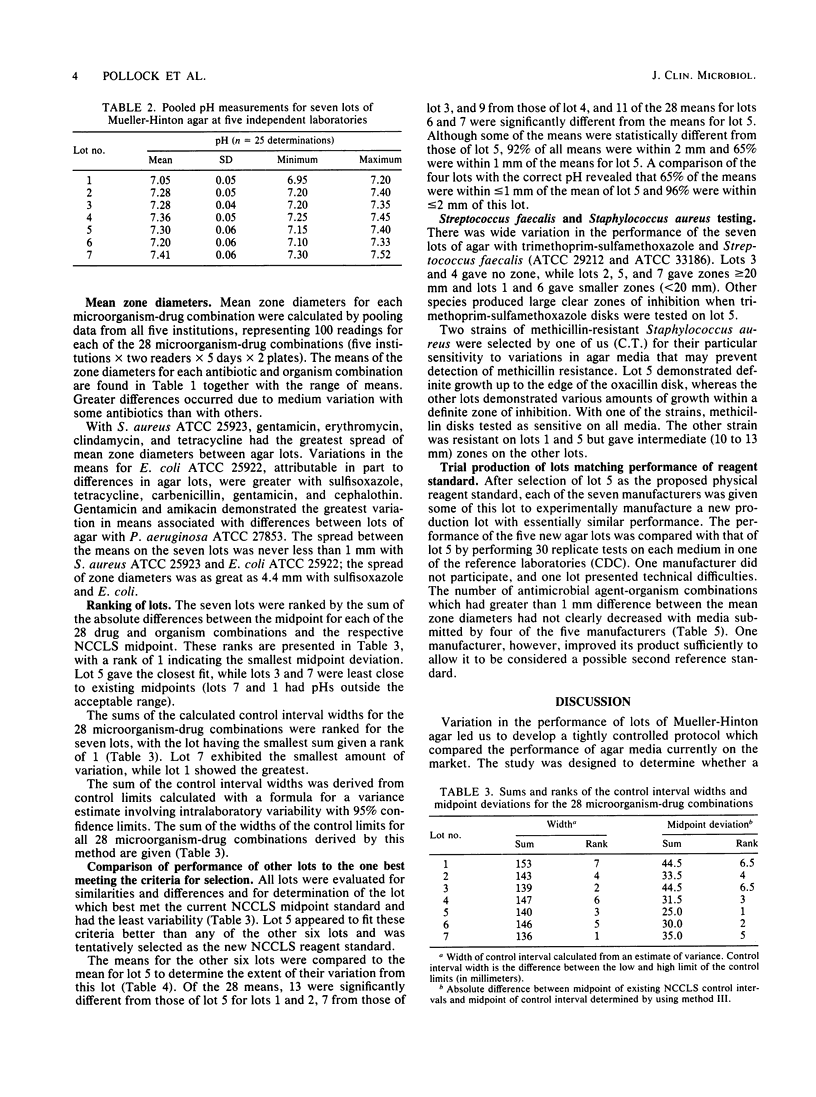
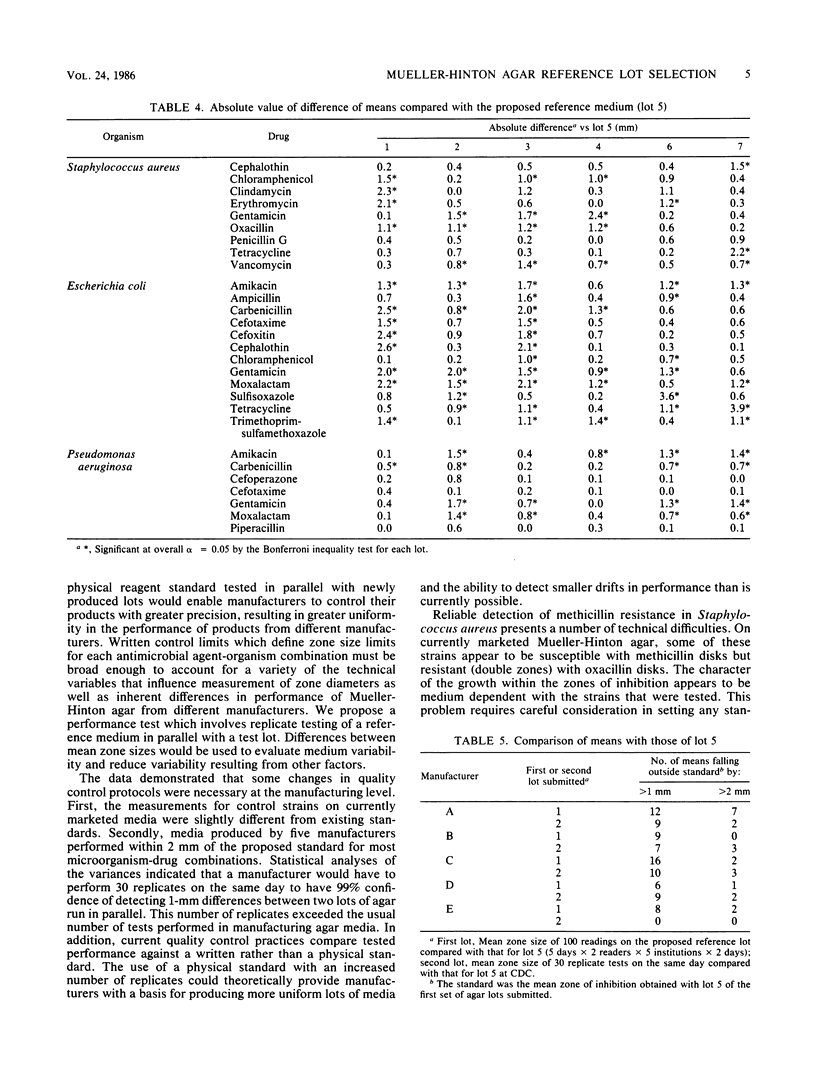
A collaborative study was undertaken to evaluate the performance of currently marketed Mueller-Hinton agars from seven manufacturers by replicate disk diffusion tests with standard quality control strains. Identification of the manufacturers was concealed, and the resulting data were evaluated for the selection of a physical reagent standard against which the performance of future production lots would be tested and made to conform. A medium was selected which was sufficiently close to existing National Committee for Clinical Laboratory Standards quality control limits that current interpretive criteria would require minimum modification. Two of the seven lots were eliminated from further consideration because the final pHs were outside acceptable limits. The remaining four lots had 96% of mean zone diameters less than or equal to 2 mm from those of the chosen lot and 65% of the means were less than or equal to 1 mm from those of the chosen lot for all 28 antimicrobial agent-organism combinations. Manufacturers then attempted to produce new lots of Mueller-Hinton agar which performed within the prescribed limits of the chosen lot. One lot performed in close conformity with the selected standard, but the overall performance of the media was essentially the same as that of the randomly chosen lots in the initial study. It was concluded that one of the original seven lots demonstrated properties which made it a tentative candidate for a physical reagent standard and that the use of a physical reagent standard in evaluating production lots might aid in stabilizing the performance of Mueller-Hinton agar.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Brenner V. C., Sherris J. C. Influence of different media and bloods on results of diffusion antibiotic susceptibility tests. Antimicrob Agents Chemother. 1972 Feb;1(2):116–122. doi: 10.1128/aac.1.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'amato R. F., Thornsberry C., Baker C. N., Kirven L. A. Effect of calcium and magnesium ions on the susceptibility of Pseudomonas species to tetracycline, gentamicin polymyxin B, and carbenicillin. Antimicrob Agents Chemother. 1975 May;7(5):596–600. doi: 10.1128/aac.7.5.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew W. L., Barry A. L., O'Toole R., Sherris J. C. Reliability of the Kirby-Bauer disc diffusion method for detecting methicillin-resistant strains of Staphylococcus aureus. Appl Microbiol. 1972 Aug;24(2):240–247. doi: 10.1128/am.24.2.240-247.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson H. M., Sherris J. C. Antibiotic sensitivity testing. Report of an international collaborative study. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;217(Suppl):1+–1+. [PubMed] [Google Scholar]
- Garrod L. P., Waterworth P. M. Effect of medium composition on the apparent sensitivity of Pseudomonas aeruginosa to gentamicin. J Clin Pathol. 1969 Sep;22(5):534–538. doi: 10.1136/jcp.22.5.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanus F. J., Sands J. G., Bennett E. O. Antibiotic activity in the presence of agar. Appl Microbiol. 1967 Jan;15(1):31–34. doi: 10.1128/am.15.1.31-34.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny M. A., Pollock H. M., Minshew B. H., Casillas E., Schoenknecht F. D. Cation components of Mueller-Hinton agar affecting testing of Pseudomonas aeruginosa susceptibility to gentamicin. Antimicrob Agents Chemother. 1980 Jan;17(1):55–62. doi: 10.1128/aac.17.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles R. C., Gilmore B. F. Quality control of agar diffusion susceptibility tests. Data from the Quality Assurance Service Microbiology Program of the College of American Pathologists. Am J Clin Pathol. 1981 Oct;76(4 Suppl):590–596. [PubMed] [Google Scholar]
- Kunin C. M., Edmondson W. P. Inhibitor of antibiotics in bacteriologic agar. Proc Soc Exp Biol Med. 1968 Oct;129(1):118–122. doi: 10.3181/00379727-129-33264. [DOI] [PubMed] [Google Scholar]
- Pollock H. M., Minshew B. H., Kenny M. A., Schoenknecht F. D. Effect of different lots of Mueller-Hinton agar on the interpretation of the gentamicin susceptibility of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1978 Sep;14(3):360–367. doi: 10.1128/aac.14.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reller L. B., Schoenknecht F. D., Kenny M. A., Sherris J. C. Antibiotic susceptibility testing of Pseudomonas aeruginosa: selection of a control strain and criteria for magnesium and calcium content in media. J Infect Dis. 1974 Nov;130(5):454–463. doi: 10.1093/infdis/130.5.454. [DOI] [PubMed] [Google Scholar]
- Thornsberry C., McDougal L. K. Successful use of broth microdilution in susceptibility tests for methicillin-resistant (heteroresistant) staphylococci. J Clin Microbiol. 1983 Nov;18(5):1084–1091. doi: 10.1128/jcm.18.5.1084-1091.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterworth P. M. Uniformity in sensitivity test media. J Antimicrob Chemother. 1978 Jan;4(1):4–6. doi: 10.1093/jac/4.1.4. [DOI] [PubMed] [Google Scholar]
- Yousef R. T., el-Nakeeb M. A., el-Masri M. H. Binding of antibiotics to agar and its relation to the inhibition zone. Acta Pharm Suec. 1967 Sep;4(4):253–260. [PubMed] [Google Scholar]


