Abstract
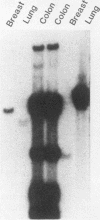
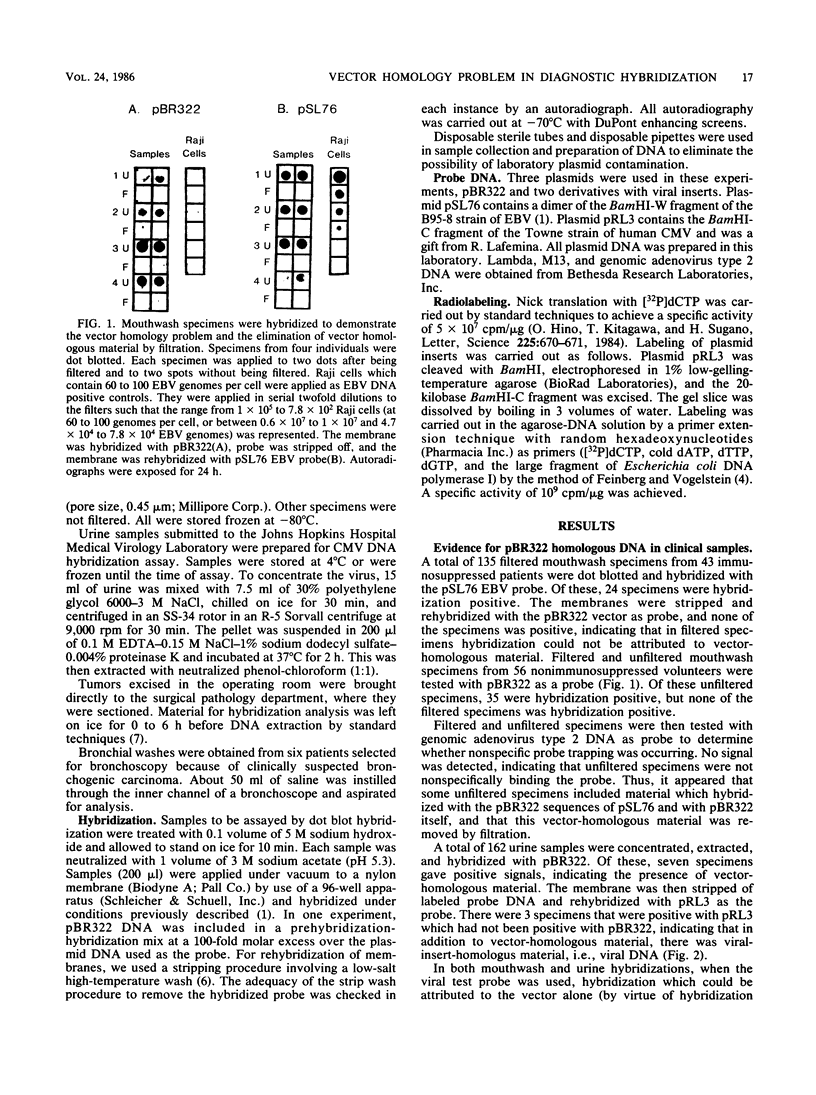
Nucleic acid hybridization techniques using cloned probes are finding application in assays of clinical specimens in research and diagnostic laboratories. The probes that we and others have used are recombinant plasmids composed of viral inserts and bacterial plasmid vectors such as pBR322. We suspected that there was material homologous to pBR322 present in many clinical samples. because hybridization occurred in samples which lacked evidence of virus by other techniques. If the presence of this vector-homologous material was unrecognized, hybridization in the test sample might erroneously be interpreted as indicating the presence of viral sequences. In this paper we demonstrate specific hybridization of labeled pBR322 DNA with DNA from various clinical samples. Evidence is presented that nonspecific probe trapping could not account for this phenomenon. In mixing experiments, it is shown that contamination of clinical samples with bacteria would explain such a result. Approaches tested to circumvent this problem included the use of isolated insert probes, alternate cloning vectors, and cold competitor pBR322 DNA in prehybridization and hybridization mixes. None proved entirely satisfactory. We therefore emphasize that it is essential that all hybridization detection systems use a control probe of the vector alone in order to demonstrate the absence of material with vector homology in the specimen tested.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambinder R. F., Wingard J. R., Burns W. H., Hayward S. D., Saral R., Perry H. R., Santos G. W., Hayward G. S. Detection of Epstein-Barr virus DNA in mouthwashes by hybridization. J Clin Microbiol. 1985 Mar;21(3):353–356. doi: 10.1128/jcm.21.3.353-356.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andiman W., Gradoville L., Heston L., Neydorff R., Savage M. E., Kitchingman G., Shedd D., Miller G. Use of cloned probes to detect Epstein-Barr viral DNA in tissues of patients with neoplastic and lymphoproliferative diseases. J Infect Dis. 1983 Dec;148(6):967–977. doi: 10.1093/infdis/148.6.967. [DOI] [PubMed] [Google Scholar]
- Chou S., Merigan T. C. Rapid detection and quantitation of human cytomegalovirus in urine through DNA hybridization. N Engl J Med. 1983 Apr 21;308(16):921–925. doi: 10.1056/NEJM198304213081603. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saemundsen A. K., Purtilo D. T., Sakamoto K., Sullivan J. L., Synnerholm A. C., Hanto D., Simmons R., Anvret M., Collins R., Klein G. Documentation of Epstein-Barr virus infection in immunodeficient patients with life-threatening lymphoproliferative diseases by Epstein-Barr virus complementary RNA/DNA and viral DNA/DNA hybridization. Cancer Res. 1981 Nov;41(11 Pt 1):4237–4242. [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Shmookler Reis R. J., Lumpkin C. K., Jr, McGill J. R., Riabowol K. T., Goldstein S. Amplification of inter-Alu extrachromosomal DNA during cellular ageing: retraction and explanation. Nature. 1985 Jul 11;316(6024):167–167. doi: 10.1038/316167a0. [DOI] [PubMed] [Google Scholar]
- Spector S. A., Rua J. A., Spector D. H., McMillan R. Detection of human cytomegalovirus in clinical specimens by DNA-DNA hybridization. J Infect Dis. 1984 Jul;150(1):121–126. doi: 10.1093/infdis/150.1.121. [DOI] [PubMed] [Google Scholar]
- Virtanen M., Syvänen A. C., Oram J., Söderlund H., Ranki M. Cytomegalovirus in urine: detection of viral DNA by sandwich hybridization. J Clin Microbiol. 1984 Dec;20(6):1083–1088. doi: 10.1128/jcm.20.6.1083-1088.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]