Abstract
Salicylic acid (SA) is an important signal molecule in plants. Two pathways of SA biosynthesis have been proposed in plants. Biochemical studies using isotope feeding have suggested that plants synthesize SA from cinnamate produced by the activity of phenylalanine ammonia lyase (PAL). Silencing of PAL genes in tobacco or chemical inhibition of PAL activity in Arabidopsis, cucumber and potato reduces pathogen-induced SA accumulation. Genetic studies, on the other hand, indicate that the bulk of SA is produced from isochorismate. In bacteria, SA is synthesized from chorismate through two reactions catalyzed by isochorismate synthase (ICS) and isochorismate pyruvate lyase (IPL). Arabidopsis contains two ICS genes but has no gene encoding proteins similar to the bacterial IPL. Thus, how SA is synthesized in plants is not fully elucidated. Two recently identified Arabidopsis genes, PBS3 and EPS1, are important for pathogen-induced SA accumulation. PBS3 encodes a member of the acyl-adenylate/thioester-forming enzyme family and EPS1 encodes a member of the BAHD acyltransferase superfamily. PBS3 and EPS1 may be directly involved in the synthesis of an important precursor or regulatory molecule for SA biosynthesis. The pathways and regulation of SA biosynthesis in plants may be more complicated than previously thought.
Key words: salicylic acid biosynthesis, isochorismate synthase, phenylalanine ammonia lyase
Introduction
A wide range of prokaryotic and eukaryotic organisms including plants produces salicylic acid (SA). Studies over the last two decades have shown that SA has important regulatory functions in plants. In thermogenic plants such as voodoo lilies, SA is the natural trigger of heat production by activating alternative respiration, which volatilizes putrid-smelling compounds that attract pollinating insects.1 The most established role of SA is as a signal molecule in plant defense responses. Application of exogenous SA activates expression of plant pathogenesis-related (PR) genes and induces disease resistance.2,3 In resistant tobacco plants, infection of tobacco mosaic virus (TMV) triggers increased SA levels not only in lower infected leaves that develop hypersensitive response (HR) but also in upper uninfected leaves that develop systemic acquired resistance (SAR).4–6 Blocking SA increase through expression of a bacterial salicylate hydroxylase gene in transgenic tobacco nahG plants compromises TMV-induced HR and abolishes SAR.7 The critical role of SA in plant disease resistance has been demonstrated in other plants including Arabidopsis, cucumber and potato.8–12 In addition, SA participates in the regulation of plant responses to a variety of abiotic stresses such as low and high temperature, salts and oxidative conditions.13–15
How plants synthesize SA has been studied for almost half a century. Biochemical studies suggest that SA is synthesized from phenylalanine with benzoate as the immediate precursor.16,17 More recent genetic analyses, on the other hand, indicate that the bulk (>90%) of SA is synthesized from isochorismate.18 While the role of plant isochorismate synthases in SA production has been established, plant enzymes that convert isochorismate to SA have not been identified. Thus, how SA is synthesized in plants is still not fully defined.
Biochemical Studies of the SA Biosynthetic Pathways in Plants
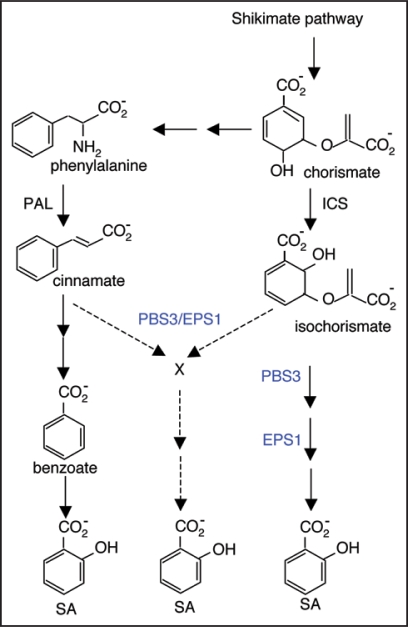
Biochemical studies using isotope feeding have suggested that plants synthesize SA from cinnamate produced by phenylalanine ammonia lyase (PAL) (Fig. 1). PAL is a key regulator of the phenylpropanoid pathway and is induced under a variety of biotic and abiotic stress conditions. SA can be formed from cinnamate via o-coumarate or benzoate depending on whether the hydroxylation of the aromatic ring takes place before or after the chain-shortening reactions. In sunflower, potato and pea, isotope feeding indicated that SA was formed from benzoate, which is synthesized by cinnamate chain shortening reactions most likely through a β-oxidation process analogous to fatty acid β-oxidation.19 Feeding of 14C-labeled phenylalanine and cinnamate to young Primula acaulis and Gaultheria procumbens leaf segments indicated that SA was formed via o-coumarate.20 In the same plants, labeled SA was also formed after treatment with 14C-labeled benzoate,20 suggesting that these plants may use both pathways for SA synthesis. Likewise, in young tomato seedlings, SA appeared to be formed mostly from cinnamate via benzoate but after infection with Agrobacterium tumefaciens, 2-hydroxylation of cinnamate to o-coumarate was favored.21
Figure 1.
Pathways of SA biosynthesis in plants. Isotope feeding experiments suggest that plants synthesize SA from cinnamate produced by PAL. Genetic studies have indicated that the bulk of SA is produced from isochorismate. Given the importance of both PAL and ICS in SA accumulation demonstrated from experiments using genetic mutants, gene silencing and chemical inhibition, it is possible that the PAL and ICS pathways are integrated through a metabolic or regulatory grid in SA biosynthesis. The recently identified PBS3 and EPS1 are important for pathogen-induced SA production and may encode enzymes catalyzing related, and possibly sequential, reactions in the synthesis of an important precursor or regulatory molecule for SA biosynthesis.
In tobacco and rice, several lines of evidence suggest that SA is synthesized from cinnamate via benzoate.22–24 First, infiltration of healthy tobacco leaf discs with 0.1 mM benzoate increased total SA level 14 fold after 18 hours.24 Second, in TMV-infected tobacco, large increases in the levels of benzoate and SA were detected.24 Third, in both TMV-infected tobacco leaves and rice seedlings, labeled benzoate and SA, but not o-coumarate, were detected after feeding with 14C-labeled cinnamate.23,24 More label was incorporated into SA when 14C-labeled benzoate was fed than when 14C-labeled cinnamate was used, consistent with benzoic acid being the immediate precursor of SA.23,24 Similar results were also obtained from the labeling experiments in potato and cucumber.11,25 Furthermore, a benzoic acid 2-hydroxylase (BA2H) activity was detected in plants including tobacco and rice. In tobacco, the BA2H activity was induced by TMV infection and was partially purified as a soluble 160 kDa protein that could be immunoprecipitated by antibodies against the soluble SU2 cytochrome P450 from Streptomyces griseolus.22 Despite the extensive biochemical and molecular evidence, none of the enzymes required for the conversion of SA from cinnamate in the PAL pathway has been isolated from plants. Although partial purification and immunoprecipitation of a tobacco BA2H activity were reported in 1995,22 there has been no further report on its purification or isolation of the corresponding gene(s).
Genetic Studies of the SA Biosynthetic Pathways in Arabidopsis
Some bacteria can synthesize SA from chorismate through two reactions catalyzed by isochorismate synthase (ICS) and isochorismate pyruvate lyase (IPL).18 Arabidopsis contains two ICS genes: ICS1 (also known as SID2) and ICS2.26 In ics1 mutants, total SA accumulation is only about 5–10% of wild-type (WT) levels after infection by the virulent biotroph Erysiphe or avirulent strains of Pseudomonas syringae.26 The residual levels of SA in pathogen-induced ics1 mutants might be synthesized by ICS2 or through another pathway. To examine these possibilities, Garcion et al.27 have generated ics1 ics2 double mutant plants and compared them with the ics1 single mutant plants for UV-induced SA accumulation. Upon UV exposure, the ics1 mutant accumulated roughly 10% and the ics1 ics2 double mutant accumulated about 4% of total SA compared to the wild type.27 Thus, roughly 95% of SA is synthesized from the ICS pathway in UV-treated Arabidopsis plants with the remaining 4% from an alternative pathway.
SA accumulation in Nicotiana benthamiana is also dependent on ICS. The ICS gene from N. benthamiana was cloned and silenced using the well-established tobacco rattle virus (TRV)-based silencing system.28 Three days after UV irradiation treatment, total SA levels increased more than 10 fold in control plants but only 4 fold in plants with silenced ICS expression.28 Two days after infection with the bacterial pathogen P. syringae pv. tomato DC3000, the total SA levels increased strongly in the control wild-type plants.28 By contrast no accumulation of SA could be detected after the pathogen infection in the ICS-silenced plants.28 These results indicate that the bulk of SA after exposure to biotic or abiotic stress is also synthesized from the ICS pathway in N. benthamiana.
Unsolved Puzzles in SA Biosynthesis in Plants
If the PAL and ICS pathways act independently and the ICS pathway is responsible for synthesis of more than 95% of SA, one would expect the PAL pathway to be responsible for synthesis of only a small percentage of SA in pathogen-infected or UV-treated Arabidopsis and N. benthamiana plants. Intriguingly, several studies have suggested that a high PAL activity is important for pathogen-induced SA formation in plants. In tobacco, the levels of free SA produced in both TMV-inoculated and upper systemic leaves of PAL-silenced plants are roughly fourfold lower than those in control plants.29,30 As a result, TMV-induced PR proteins were not accumulated in systemic leaves and TMV-induced SAR was blocked in the PAL-silenced tobacco plants.29,30
Furthermore, PAL inhibitor 2-aminoindan-2-phosphonic acid (AIP) reduced pathogen- or pathogen elicitor-induced SA accumulation in potato, cucumber and Arabidopsis.11,25,31 In Arabidopsis, treatment of the PAL inhibitor made the plants completely susceptible to the downy mildew oomycete Hyaloperonospora parasitica and SA could restore resistance, suggesting that production of SA precursors is a major function of PAL in Arabidopsis downy mildew resistance.31 The nature of the metabolite measured as SA in the AIP-treated Arabidopsis, however, has been questioned recently because the metabolite was determined using HPLC separation and detected by absorption at 280 nm.27 It was indicated that at this wavelength, SA is barely detectable for the amount present in Arabidopsis and fluorescence detection is required for adequate measurement.27 However, fluorescence detection was used in SA quantification in the determination of reduced SA accumulation in PAL-silenced tobacco and AIP-treated potato plants.25,29 If the ICS pathway alone is responsible for synthesis of the bulk of SA in plants, one needs to account for such a strong effect of reduced PAL activity on SA accumulation.
In bacteria, two enzymes catalyze the synthesis of SA from chorismate.18 ICS catalyzes the synthesis of isochorismate from chorismate and IPL catalyzes the conversion of SA from isochorismate. Although plant ICS have been identified and analyzed, no plant IPL has been reported and the sequenced Arabidopsis genome contains no genes encoding proteins similar to the bacterial enzyme (Chen Z, unpublished data). Thus, how isochorismate is converted into SA in plants is still unknown. Plants may contain IPLs that are structurally unrelated to or highly divergent from the bacterial counterparts. Alternatively, conversion of SA from isochorismate in plants might be through a metabolic pathway distinct from that of bacteria and, consequently, catalyzed by enzymes unrelated to IPL.
Insights from other SA-Deficient Mutants
There are other Arabidopsis mutants with altered SA accumulation. Some mutants such as eds1 and pad4 have defects upstream of SA biosynthesis in R gene-mediated disease resistance.32 The npr1 mutants are defective in SA signaling and contain elevated SA levels after pathogen infection presumably due to perturbations in negative feedback regulation of SA biosynthesis in the absence of normal SA signaling.33 The eds5 mutants accumulate very little SA after pathogen infection.34 EDS5 is a member of the MATE (multidrug and toxin extrusion) transporter family and, therefore, could be involved in the transport of certain precursors for SA biosynthesis.34
We have recently isolated an enhanced pseudomonas susceptibility mutant (eps1) in Arabidopsis.35 The eps1 mutants are compromised in resistance to both virulent and avirulent strains of P. syringae and pathogen-induced PR gene expression. In addition, accumulation of total SA is greatly reduced in the eps1 mutants following infection of P. syringae. SA restored resistance to P. syringae and induced PR1 expression in the eps1-1 mutants. These phenotypes of the eps1 mutants are strikingly similar to those of pbs3 (also known as gdg1 or win3) mutants.36–38 These results suggest that PBS3 and EPS1 function upstream of SA in plant defense responses.
EPS1 is a member of the BAHD acyltransferase superfamily,35 which was named based on the first letter of the first four plant enzymes characterized in this family (BEAT, AHCTs, HCBT and DAT) that all catalyze CoA-dependent acylations, a common and significant modification of plant secondary metabolites including small volatile esters, modified anthocyanins, constitutive defense compounds and phytoalexins. PBS3 is a member of the acyl-adenylate/thioester-forming enzyme family (also known as GH3 proteins).39,40 GH3 proteins include JAR1 (GH3.11) that adenylates JA and displayed JA-amido synthetase activity.41 Other GH3 proteins adenylate indoacetic acid (IAA) and catalyze IAA conjugation to amino acids through amide bonds.42 Using a novel high throughput adenylation assay, Okrent et al.43 have found that PBS3 can catalyze conjugation of a benzoate to an amino acid in vitro. 4-Substituted benzoates such as 4-aminobenzoate are favored whereas 2-substituted benzoates including SA are disfavored. In the amino acid conjugation reaction with 4-aminobenzoic acid, Glu was strongly preferred over other amino acids and Glu variants.43 Interestingly, SA specifically and reversibly inhibits PBS3 activity. It is unclear whether 4-substituted benzoates are the physiological substrates of PBS3 and, if so, how the synthesized benzoic conjugates promote SA biosynthesis.
Based on the strikingly similar mutant phenotypes and predicted enzymatic activities, PBS3 and EPS1 may promote SA biosynthesis by catalyzing related, and possibly sequential, reactions in the synthesis of an important precursor for SA biosynthesis. Since the ICS pathway is the major pathway for SA biosynthesis in Arabidopsis, PBS3 and EPS1 might function in the ICS pathway by catalyzing reactions in the conversion of SA from isochorismate (Fig. 1). In P. aeruginosa, IPL catalyzes the elimination of the enolpyruvyl side chain from isochorismate to give SA and pyruvate through a concerted pericyclic pathway, in which the hydrogen atom at C2 is transferred to C9 of the side chain simultaneous with C-O cleavage.44 It is not obvious how the demonstrated activity of PBS3 and the predicted acyltransferase activity of EPS1 can be incorporated into reactions for mere elimination of the enolpryruvyl side chain from isochorismate.
As discussed earlier, silencing or disruption of ICS results in a drastic reduction of pathogen- or UV-induced SA accumulation but silencing or inhibition of PAL also has a major impact on pathogen-induced SA accumulation. The importance of both PAL and ICS for SA biosynthesis raises a possibility that SA synthesis in plants relies on intermediates from both the ISC and PAL pathways. For example, isochorismate from the ICS pathway might not be directly converted into SA as in bacteria but instead might be conjugated with an intermediate from the PAL pathway to produce an unknown SA precursor (Fig. 1), analogous to the way in which intermediates of two different pathways are involved in lignin biosynthesis in the form of the phenylpropanoid intermediate p-coumaroylshikimate.45 The critical role of EPS1, a putative acyltransferase, in pathogen-induced SA accumulation is consistent with an integrated grid through formation of an ester conjugate from intermediates synthesized from two pathways. Alternatively, PBS3 and EPS1 may promote SA biosynthesis by catalyzing synthesis of a regulatory molecule for SA biosynthesis. Since PBS3 can catalyze conjugation of 4-substituted benzoates to an amino acid in vitro,43 the regulatory molecule may be derived from the PAL pathway and positively regulate production or activity of certain enzymes in the ICS pathway for SA biosynthesis. Further analysis of the two mutants and the previously reported ics mutants should provide important insights into how SA is synthesized in Arabidopsis. This information will help understand how this important plant signal molecule is synthesized and regulated in response to biotic and abiotic stresses in other plant species including crop plants.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/8392
References
- 1.Raskin I, Ehmann A, Melander WR, Meeuse BJD. Salicylic acid: a natural inducer of heat production in Arum lilies. Science. 1987;237:1601–1602. doi: 10.1126/science.237.4822.1601. [DOI] [PubMed] [Google Scholar]
- 2.Ward ER, Uknes SJ, Williams SC, Dincher SS, Wiederhold DL, Alexander DC, et al. Coordinate gene activity in response to agents that induce systemic acquired resistance. Plant Cell. 1991;3:1085–1094. doi: 10.1105/tpc.3.10.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White RF. Acetylasalicylic acid (Aspirin) induces resistance to tobacco mosaic virus in tobacco. Virology. 1979;99:410–412. doi: 10.1016/0042-6822(79)90019-9. [DOI] [PubMed] [Google Scholar]
- 4.Malamy J, Carr JP, Klessig DF, Raskin I. Salicylic acid: a likely endogenous signal in the resistance response of tobacco to viral infection. Science. 1990;250:1002–1004. doi: 10.1126/science.250.4983.1002. [DOI] [PubMed] [Google Scholar]
- 5.Yalpani N, Shulaev V, Raskin I. Endogenous salicylic acid levels correlate with accumulation of pathogenesis-related proteins and virus resistance in tobacco. Phytopathol. 1993;83:702–708. [Google Scholar]
- 6.Yalpani N, Silverman P, Wilson TM, Kleier DA, Raskin I. Salicylic acid is a systemic signal and an inducer of pathogenesis-related proteins in virus-infected tobacco. Plant Cell. 1991;3:809–818. doi: 10.1105/tpc.3.8.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaffney TF, Friedrich L, Vernooij L, Negrotto D, Nye G, Uknes S, et al. Requirement of salicylic acid for the induction of systemic acquired resistance. Science. 1993;261:754–756. doi: 10.1126/science.261.5122.754. [DOI] [PubMed] [Google Scholar]
- 8.Delaney TP, Uknes S, Vernooij B, Friedrich L, Weymann K, Negrotto D, et al. A central role of salicylic acid in plant disease resistance. Science. 1994;266:1247–1250. doi: 10.1126/science.266.5188.1247. [DOI] [PubMed] [Google Scholar]
- 9.Halim VA, Eschen-Lippold L, Altmann S, Birschwilks M, Scheel D, et al. Salicylic acid is important for basal defense of Solanum tuberosum against Phytophthora infestans. Mol Plant Microbe Interact. 2007;20:1346–1352. doi: 10.1094/MPMI-20-11-1346. [DOI] [PubMed] [Google Scholar]
- 10.Metraux J-P, Signer H, Ryals JA, Ward E, Wyss-Benz M, et al. Increase in salicylic acid at the onset of systemic acquired resistance in cucumber. Science. 1990;250:1004–1006. doi: 10.1126/science.250.4983.1004. [DOI] [PubMed] [Google Scholar]
- 11.Meuwly P, Molders W, Buchala A, Metraux JP. Local and systemic biosynthesis of salicylic acid in infected cucumber plants. Plant Physiol. 1995;109:1107–1114. doi: 10.1104/pp.109.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu D, Liu Y, Fan B, Klessig D, Chen Z. Is the high basal levels of salicylic acid important for disease resistance in potato? Plant Physiol. 1997;115:343–349. doi: 10.1104/pp.115.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gunes A, Inal A, Alpaslan M, Eraslan F, Bagci EG, et al. Salicylic acid induced changes on some physiological parameters symptomatic for oxidative stress and mineral nutrition in maize (Zea mays L.) grown under salinity. J Plant Physiol. 2007;164:728–736. doi: 10.1016/j.jplph.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Larkindale J, Hall JD, Knight MR, Vierling E. Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol. 2005;138:882–897. doi: 10.1104/pp.105.062257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larkindale J, Knight MR. Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene and salicylic acid. Plant Physiol. 2002;128:682–695. doi: 10.1104/pp.010320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee HI, Leon J, Raskin I. Biosynthesis and metabolism of salicylic acid. Proc Natl Acad Sci USA. 1995;92:4076–4079. doi: 10.1073/pnas.92.10.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ribnicky DM, Shulaev V, Raskin I. Intermediates of salicylic acid biosynthesis in tobacco. Plant Physiol. 1998;118:565–572. doi: 10.1104/pp.118.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serino L, Reimmann C, Baur H, Beyeler M, Visca P, et al. Structural genes for salicylate biosynthesis from chorismate in Pseudomonas aeruginosa. Mol Gen Genet. 1995;249:217–228. doi: 10.1007/BF00290369. [DOI] [PubMed] [Google Scholar]
- 19.Klambt HD. Conversion in plants of benzoic acid to salicylic acid and its b-D-glucoside. Nature. 1962;196:491. [Google Scholar]
- 20.El-Basyouni SZ, Chen d, Ibrahim RK, Neish AC, Towers GHN. The biosynthesis of hydroxybenzoic acids in higher plants. Phytochemistry. 1964;3:485–492. [Google Scholar]
- 21.Chadha KC, Brown SA. Biosynthesis of phenolic acids in tomato plants infected with Agrobacterium tumefaciens. Can J Bot. 1974;52:2041–2047. [Google Scholar]
- 22.Leon J, Shulaev V, Yalpani N, Lawton MA, Raskin I. Benzoic acid 2-hydroxylase, a soluble oxygenase from tobacco, catalyzes salicylic acid biosynthesis. Proc Natl Acad Sci USA. 1995;92:10413–10417. doi: 10.1073/pnas.92.22.10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silverman P, Seskar M, Kanter D, Schweizer P, Metraux JP, et al. Salicylic acid in rice: biosynthesis, conjugation and possible role. Plant Physiol. 1995;108:633–639. doi: 10.1104/pp.108.2.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yalpani N, Leon J, Lawton MA, Raskin I. Pathway of salicylic acid biosynthesis in healthy and virus-inoculated tobacco. Plant Physiol. 1993;103:315–321. doi: 10.1104/pp.103.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coquoz JL, Buchala A, Metraux JP. The biosynthesis of salicylic acid in potato plants. Plant Physiol. 1998;117:1095–1101. doi: 10.1104/pp.117.3.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wildermuth MC, Dewdney J, Wu G, Ausubel FM. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature. 2001;414:562–565. doi: 10.1038/35107108. [DOI] [PubMed] [Google Scholar]
- 27.Garcion C, Lohmann A, Lamodiere E, Catinot J, Buchala A, et al. Characterization and biological function of the ISOCHORISMATE SYNTHASE2 gene of Arabidopsis. Plant Physiol. 2008;147:1279–1287. doi: 10.1104/pp.108.119420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Catinot J, Buchala A, Abou-Mansour E, Metraux JP. Salicylic acid production in response to biotic and abiotic stress depends on isochorismate in Nicotiana benthamiana. FEBS Lett. 2008;582:473–478. doi: 10.1016/j.febslet.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 29.Elkind Y, Edwards R, Mavandad M, Hedrick SA, Ribak O, et al. Abnormal plant development and downregulation of phenylpropanoid biosynthesis in transgenic tobacco containing a heterologous phenylalanine ammonia-lyase gene. Proc Natl Acad Sci USA. 1990;87:9057–9061. doi: 10.1073/pnas.87.22.9057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pallas JA, Paiva NL, Lamb C, Dixon RA. Tobacco plants epigenetically suppressed in phenylalanine ammonia-lyase expression do not develop systemic acquired resistance in response to infection by tobacco mosaic virus. Plant J. 1996;10:281–293. [Google Scholar]
- 31.Mauch-Mani B, Slusarenko AJ. Production of salicylic acid precursors is a major function of phenylalanine ammonia-lyase in the resistance of Arabidopsis to Peronospora parasitica. Plant Cell. 1996;8:203–212. doi: 10.1105/tpc.8.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiermer M, Feys BJ, Parker JE. Plant immunity: the EDS1 regulatory node. Curr Opin Plant Biol. 2005;8:383–389. doi: 10.1016/j.pbi.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 33.Cao H, Glazebrook J, Clarke JD, Volko S, Dong X. The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell. 1997;88:57–63. doi: 10.1016/s0092-8674(00)81858-9. [DOI] [PubMed] [Google Scholar]
- 34.Nawrath C, Heck S, Parinthawong N, Metraux JP. EDS5, an essential component of salicylic acid-dependent signaling for disease resistance in Arabidopsis, is a member of the MATE transporter family. Plant Cell. 2002;14:275–286. doi: 10.1105/tpc.010376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng Z, Qualley A, Fan B, Dudareva N, Chen Z. An important role of a BAHD acyl transferase-like protein in plant innate immunity. Plant J. 2009;57:1040–1053. doi: 10.1111/j.1365-313X.2008.03747.x. [DOI] [PubMed] [Google Scholar]
- 36.Jagadeeswaran G, Raina S, Acharya BR, Maqbool SB, Mosher SL, et al. Arabidopsis GH3-LIKE DEFENSE GENE 1 is required for accumulation of salicylic acid, activation of defense responses and resistance to Pseudomonas syringae. Plant J. 2007;51:234–246. doi: 10.1111/j.1365-313X.2007.03130.x. [DOI] [PubMed] [Google Scholar]
- 37.Lee MW, Lu H, Jung HW, Greenberg JT. A key role for the Arabidopsis WIN3 protein in disease resistance triggered by Pseudomonas syringae that secrete AvrRpt2. Mol Plant Microbe Interact. 2007;20:1192–1200. doi: 10.1094/MPMI-20-10-1192. [DOI] [PubMed] [Google Scholar]
- 38.Nobuta K, Okrent RA, Stoutemyer M, Rodibaugh N, Kempema L, et al. The GH3 acyl adenylase family member PBS3 regulates salicylic acid-dependent defense responses in Arabidopsis. Plant Physiol. 2007;144:1144–1156. doi: 10.1104/pp.107.097691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang KH, Xiang H, Dunaway-Mariano D. Acyl-adenylate motif of the acyl-adenylate/thioester-forming enzyme superfamily: a site-directed mutagenesis study with the Pseudomonas sp. strain CBS3 4-chlorobenzoate:coenzyme A ligase. Biochemistry. 1997;36:15650–15659. doi: 10.1021/bi971262p. [DOI] [PubMed] [Google Scholar]
- 40.Babbitt PC, Kenyon GL, Martin BM, Charest H, Slyvestre M, et al. Ancestry of the 4-chlorobenzoate dehalogenase: analysis of amino acid sequence identities among families of acyl:adenyl ligases, enoyl-CoA hydratases/isomerases and acyl-CoA thioesterases. Biochemistry. 1992;31:5594–5604. doi: 10.1021/bi00139a024. [DOI] [PubMed] [Google Scholar]
- 41.Staswick PE, Tiryaki I, Rowe ML. Jasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic and indole-3-acetic acids in an assay for adenylation. Plant Cell. 2002;14:1405–1415. doi: 10.1105/tpc.000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Staswick PE, Serban B, Rowe M, Tiryaki I, Maldonado MT, et al. Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell. 2005;17:616–627. doi: 10.1105/tpc.104.026690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okrent RA, Brooks MD, Wildermuth MC. Arabidopsis GH3.12 (PBS3) conjugates amino acids to 4-substituted benzoates and is inhibited by salicylate. J Biol Chem. 2009 doi: 10.1074/jbc.M806662200. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DeClue MS, Baldridge KK, Kunzler DE, Kast P, Hilvert D. Isochorismate pyruvate lyase: a pericyclic reaction mechanism? J Am Chem Soc. 2005;127:15002–15003. doi: 10.1021/ja055871t. [DOI] [PubMed] [Google Scholar]
- 45.Hoffmann L, Maury S, Martz F, Geoffroy P, Legrand M. Urification, cloning and properties of an acyltransferase controlling shikimate and quinate ester intermediates in phenylpropanoid metabolism. J Biol Chem. 2003;278:95–103. doi: 10.1074/jbc.M209362200. [DOI] [PubMed] [Google Scholar]