Abstract
Soil inoculation with the ACC deaminase-containing rhizobacterium Variovorax paradoxus 5C-2 increased pea (Pisum sativum) growth and yield in both well watered and drying soil, with an attenuation of systemic ACC signaling likely key in the latter case.1 However, inoculated plants also had increased xylem ABA concentrations (which may also promote growth) in drying soil. Possible mediation of ABA levels by V. paradoxus 5C-2 was investigated in two experiments in which maize (Zea mays) growth was promoted. Xylem ABA concentration of both inoculated and uninoculated plants increased similarly as leaf water potential decreased. Furthermore, hormone flow modeling showed a decreased phloem flow of ABA back to the root. Thus Variovorax paradoxus 5C-2 does not intensify ABA signaling in planta.
Key words: abscisic acid, ACC deaminase, flow modelling, rhizobacteria, soil drying, Zea mays
Plant growth promoting rhizobacteria are commonly found in the rhizosphere (on the root surface) and promote plant growth via several diverse mechanisms, including alteration of plant hormone signaling. Different strains can produce auxins2 and cytokinins3 or mediate plant ethylene levels via the bacterial enzyme ACC deaminase (ACCd) that degrades the immediate ethylene precursor.4 Distinguishing the specific mechanism(s) of plant growth promotion is challenging since a single strain can alter several plant hormone signaling pathways. Thus soil inoculation with the cytokinin-producing bacterium Bacillus subtilis approximately doubled both shoot cytokinin and ABA concentration.5 To establish that plant responses are due to the action of a specific hormone, mutant bacterial strains have been created that do not alter plant hormone signaling. ACCd-containing bacteria stimulated plant growth while mutants in the same genetic background but lacking ACCd did not.1,6,7
The ACCd-containing bacterium Variovorax paradoxus 5C-2 attenuated a drought-induced increase in xylem ACC concentration in pea.1 Since xylem ACC delivery can account for shoot ethylene evolution,8 and ethylene often acts as a growth inhibitor, this systemic mechanism likely was partially responsible for the improved growth and yield of inoculated plants. However, in drying soil, inoculated plants also had a higher xylem ABA concentration than control plants, correlating with improved shoot and root growth. Plants inoculated with a mutant bacterial strain with decreased ACCd activity had a similar xylem ABA concentration to control plants and shoot growth promotion was not observed.1 Since under some circumstances ABA can promote rather than inhibit growth,9 the increase in ABA may have been causally related to the promotion of shoot and root growth (perhaps by suppressing the production of ACC and/or ethylene) in the inoculated plants. However, this difference in xylem ABA concentration may not be a direct impact of rhizobacteria as increased shoot growth (and hence transpiration) of inoculated plants likely caused additional soil drying.1 We report here two additional experiments with maize that more closely investigate any possible relationship between ACCd-containing rhizobacteria and plant ABA relations.
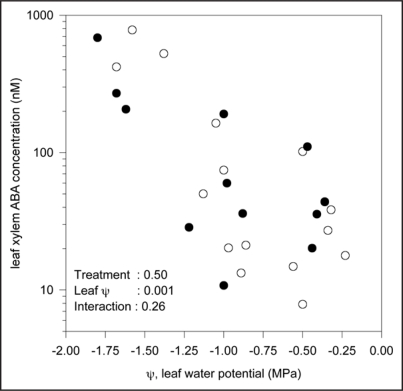
A two (±V. paradoxus 5C-2) by two (±soil drying) experiment was performed in a greenhouse at Lancaster with plants grown in 3 L pots filled with non-sterile potting compost (John Innes No. 2, J, Arthur Bowers, UK). A concentrated rhizobacterial suspension (1010 cells mL-1 to reach a final concentration of 106 cells g-1 soil) was placed at the base of half the plants 25 days after planting, then two watering regimes (100% and 50% of plant evapotranspiration) initiated one week later. At weekly intervals, leaf water potential was measured using a Scholander-type pressure chamber, an overpressure applied to collect xylem sap for subsequent ABA determination, and plants harvested to determine shoot biomass. Two way analysis of variance indicated that deficit irrigation decreased shoot growth by 49%, rhizobacteria stimulated (p = 0.02) shoot growth by 19%, and that rhizobacterial effects were independent of soil water status (p Value for interaction term was 0.15). Leaf xylem ABA concentration increased as leaf water potential decreased (Fig. 1), but there was no significant effect of rhizobacterial inoculation (p Value for interaction term was 0.26). Thus V. paradoxus 5C-2 did not affect leaf xylem ABA concentration of maize across a wide range of soil water availabilities.
Figure 1.
The relationship between leaf xylem ABA concentration and leaf water potential for control maize (Zea mays L.) plants (○) and those inoculated with V. paradoxus 5C-2 (●). Each point represents a single plant and p Values determined by two-way ANOVA for each main effect (inoculation and leaf water potential) and their interaction are reported.
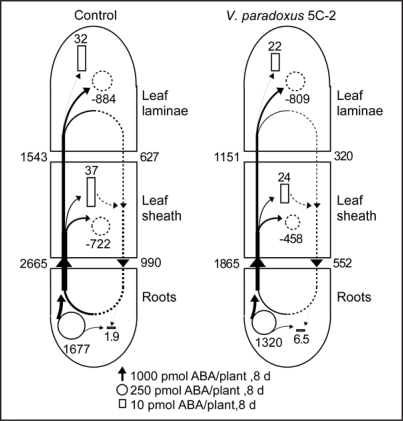
A more comprehensive approach to determine plant ABA relations is hormone flow modelling.10 Maize plants were grown in well-watered, well washed (but not sterile) quartz sand in 1 L pots in a greenhouse in Wurzburg and 5 mL of V. paradoxus 5C-2 suspension was washed into the substrate by 10 mL of nutrient solution every other day. Plants were harvested and xylem and phloem saps collected 8 days apart. V. paradoxus 5C-2 increased dry weight of the leaf laminae and sheaths by approximately 20% and decreased ABA concentrations of these organs also by 20%. Inoculated plants showed a tendency of slightly lower root ABA biosynthesis and xylem transport, but phloem flow back to the root was clearly decreased (Fig. 2).
Figure 2.
Flow profiles for metabolism, transport and deposition of ABA in control maize (Zea mays L.) plants and those inoculated with V. paradoxus 5C-2 over 8 d of the study period starting 6 d after planting. After treatment with V. paradoxus, the surface of the maize roots appeared slightly yellow, indicative of successful bacterial colonisation (V. paradoxus has characteristic yellow colonies in vitro). Based on the assumption that mass flow occurs both in xylem and phloem, the net K flows (obtained as described in16 and the ratios of ABA:K in the transport fluids were used to estimate the net flows of ABA in the two transport pathways over the study period. The increments of ABA in the maize tissues were calculated from two harvests (6 d and 14 d after planting). The metabolism of ABA in maize tissue was calculated according to.17 Dotted arrows indicate flows in the phloem, black arrows flows in the xylem. The width of the arrows (flows), the areas of the squares (deposition), and of the circles (complete circles, net synthesis; dotted circles, net degradation) are drawn in relation to the rates of flows. The numbers beneath the arrows, squares and circles indicate the net flows in nmol plant-1 8 d-1. Further details will be reported elsewhere (Jiang et al. manuscript in preparation).
It has been shown repeatedly that ethylene impacts on ABA biosynthesis and vice versa11,12 thus inoculation with ACCd-containing rhizobacteria should affect plant ABA levels. Since one of the functions of ABA is to restrict ethylene synthesis,9 the decreased ethylene evolution of plants inoculated with ACCd-containing rhizobacteria13 might cause feedback regulation of ABA levels. The ABA flow-modelling experiment partially supports this contention, but further work is required to examine the expression of genes involved in ABA biosynthesis in inoculated plants.
Species differences may account for the different impacts of V. paradoxus 5C-2 on ABA signaling in pea1 and maize (Figs. 1 and 2). In this context, the absence of a root exodermis (which forms in well aerated roots of other species) in legumes such as pea may enhance the exchange of molecules (such as plant hormones) between the rhizosphere and the plant xylem. Much more work is required with a range of plant/rhizobacteria combinations to substantiate this hypothesis. Although V. paradoxus 5C-2 does not stimulate ABA signaling in maize in either well-watered (Fig. 2) or drying soil (Fig. 1), it will be interesting to determine whether other rhizosphere organisms that synthesise ABA14,15 do.
Acknowledgements
BBSRC (Grant ref: BB/DO12821/1), and the Russian Foundation of Basic Research (grants 03-04-48252-a and 06-04-49486-a) supported this work. R.G.T. thanks HDC for a studentship.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/8574
References
- 1.Belimov AA, Dodd IC, Hontzeas N, Theobald JC, Safronova VI, Davies WJ. Rhizosphere bacteria containing ACC deaminase increase yield of plants grown in drying soil via both local and systemic hormone signalling. New Phytol. 2009;181:413–423. doi: 10.1111/j.1469-8137.2008.02657.x. [DOI] [PubMed] [Google Scholar]
- 2.Costacurta A, Vanderleyden J. Synthesis of phytohormones by plant-associated bacteria. Crit Rev Microbiol. 1995;21:1–18. doi: 10.3109/10408419509113531. [DOI] [PubMed] [Google Scholar]
- 3.Timmusk S, Nicander B, Granhall U, Tillberg E. Cytokinin production by Paenibacillus polymyxa. Soil Biol Biochem. 1999;31:1847–1852. [Google Scholar]
- 4.Glick BR, Penrose DM, Li JP. A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J Theoretical Biol. 1998;190:63–68. doi: 10.1006/jtbi.1997.0532. [DOI] [PubMed] [Google Scholar]
- 5.Arkhipova TN, Prinsen E, Veselov SU, Martynenko EV, Melentiev AI, Kudoyarova GR. Cytokinin producing bacteria enhance plant growth in drying soil. Plant Soil. 2007;292:305–315. [Google Scholar]
- 6.Glick BR, Jacobson CB, Schwarze MMK, Pasternak JJ. 1-aminocyclopropane-1-carboxylic acid deaminase mutants of the plant-growth promoting rhizobacterium Pseudomonas putida GR12-2 do not stimulate canola root elongation. Can J Microbiol. 1994;40:911–915. [Google Scholar]
- 7.Belimov AA, Dodd IC, Safranova VI, Hontzeas N, Davies WJ. Pseudomonas brassicacearum strain AM3 containing 1-aminocyclopropane-1-carboxylate deaminase can show both pathogenic and growth-promoting properties in its interaction with tomato. J Exp Bot. 2007;58:1485–1495. doi: 10.1093/jxb/erm010. [DOI] [PubMed] [Google Scholar]
- 8.Else MA, Jackson MB. Transport of 1-aminocyclopropane-1-carboxylic acid (ACC) in the transpiration stream of tomato (Lycpersicon esculentum) in relation to foliar ethylene production and petiole epinasty. Aust J Plant Physiol. 1998;25:453–458. [Google Scholar]
- 9.Sharp RE. Interaction with ethylene: changing views on the role of abscisic acid in root and shoot growth responses to water stress. Plant Cell Environ. 2002;25:211–222. doi: 10.1046/j.1365-3040.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- 10.Jiang F, Hartung W. Long-distance signalling of abscisic acid (ABA): the factors regulating the intensity of the ABA signal. J Exp Bot. 2008;59:37–43. doi: 10.1093/jxb/erm127. [DOI] [PubMed] [Google Scholar]
- 11.Chiwocha SDS, Cutler AJ, Abrams SR, Ambrose SJ, Yang J, Ross ARS, et al. The etr1-2 mutation in Arabidopsis thaliana affects the abscisic acid, auxin, cytokinin and gibberellin metabolic pathways during maintenance of seed dormancy, moist-chilling and germination. Plant J. 2005;42:35–48. doi: 10.1111/j.1365-313X.2005.02359.x. [DOI] [PubMed] [Google Scholar]
- 12.Grossmann K, Hansen H. Ethylene triggered abscisic acid: a principle in plant growth regulation? Physiol Plant. 2001;113:9–14. [Google Scholar]
- 13.Mayak S, Tirosh T, Glick BR. Plant growth-promoting bacteria that confer resistance to water stress in tomatoes and peppers. Plant Sci. 2004;166:525–530. [Google Scholar]
- 14.Cohen AC, Bottini R, Piccoli PN. Azospirillum brasilense Sp 245 produces ABA in chemically-defined culture medium and increases ABA content in Arabidopsis plants. Plant Growth Reg. 2008;54:97–103. [Google Scholar]
- 15.Forchetti G, Masciarelli O, Alemano S, Alvarez D, Abdala G. Endophytic bacteria in sunflower (Helianthus annuus L.): isolation, characterization and production of jasmonates and abscisic acid in culture medium. App Microbiol Biotech. 2007;76:1145–1152. doi: 10.1007/s00253-007-1077-7. [DOI] [PubMed] [Google Scholar]
- 16.Jeschke WD, Klagges S, Hilpert A, Bhatti AS, Sarwar G. Partitioning and flows of ions and nutrients in salt-treated plants of Leptochloa fusca L. Kunth I. Cations and chloride. New Phytol. 1995;130:23–35. [Google Scholar]
- 17.Jiang F, Jeschke WD, Hartung W. Abscisic acid (ABA) flows from Hordeum vulgare to the hemiparasite Rhinanthus minor and the influence of infection on host and parasite abscisic acid relations. J Exp Bot. 2004;55:2323–2329. doi: 10.1093/jxb/erh240. [DOI] [PubMed] [Google Scholar]