Abstract
Plants implement differential cell growth as an adaptation process in order to direct their development in a way that allow them to better cope with the environmental conditions. This process requires the complex integration of multiple hormone signalings, though, a lot remain to be known about the mechanisms and the molecular actors that take part in this hormonal dialogue. We have previously shown that Sl-IAA3, an Aux/IAA gene, is a molecular link between auxin and ethylene responses in tomato plants. We show here that the expression of Sl-IAA3 in etiolated seedlings is restricted to the inner side of the apical hook, opposite to that of the HOOKLESS gene whose loss-of-function mutation results in the absence of hook formation. We propose a model on how auxin and ethylene modulate the expression of Auxin Response Factor 2 (ARF2) via IAA3 and HLS protein to regulate hypocotyl bending.
Key words: apical hook, hormone signaling, auxin/ethylene cross-talk, tomato, transcription factors
The coordination of plant developmental processes and growth adaptation rely on complex interplay between individual hormone and non-hormone signalings. The phytohormones auxin and ethylene are essential regulators of plant development and the mechanisms governing the interactions between these two hormones are becoming better understood even though, so far, only few molecular players of this cross-talk have been identified.1–3
The ethylene-mediated regulation of auxin biosynthesis occurs through the activation of WEI2/ASA1 and WEI7/ASB1, anthranilate synthase subunits that catalyse the first step in tryptophane biosynthesis. The reciprocal effect of auxin on ethylene biosynthesis through the activation of several ACC synthases has also been described.4 A comprehensive study combining physiological, genetic and genomic approaches uncovered a simple mechanistic model for the interaction between the two hormones in roots. Indeed, in addition of acting independently on the same target genes, ethylene and auxin can reciprocally regulate each other's biosynthesis and influence each other's response pathways.5 This model provides a likely explanation for the strong ethylene response defects observed in auxin mutants. In accordance with these data we have recently shown that phenotypic responses to the downregulation of Sl-IAA3 gene in tomato include alterations to the classical auxin-regulated processes of apical dominance and hypocotyl elongation as well as to typical ethylene responses such as apical hook formation in etiolated seedlings and leaf epinasty in light-grown plants.6 These results suggest that Sl-IAA3 is an integral regulator of auxin and ethylene responses in tomato plants.
The induction of apical hook formation in Arabidopsis represents one of the best described examples of auxin-ethylene cross-talk in plants and the hook is a result of differential cell elongation on opposite sides of the hypocotyls.7–9 An Arabidpsis mutant lacking differential growth in the apical region of the hypocothyl (hookless1) has been identified and it was proposed that the mutated gene encodes a key regulator that integrates ethylene and auxin signaling pathways during apical hook formation.7 Subsequently, a partial suppressor of hls1 phenotype resulting from a loss-of-function mutation in the ARF2 gene (Auxin Reponse Factor) was isolated.9 Interestingly, our recent data indicate that antisense-mediated inhibition of Sl-AA3, a tomato Aux/IAA gene, results in exaggerated hook formation associated with a downregulation of ARF2 expression. By contrast, accumulation of Sl-HLS transcripts is not altered in the AS-IAA3 plants suggesting that the exaggerated hook formation in the transgenic lines does not involve an alteration in Sl-HLS expression.
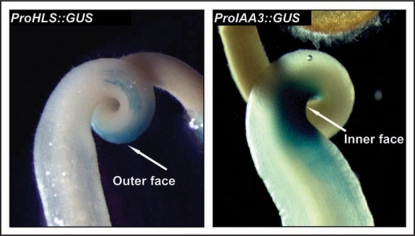
To further investigate potential interactions between Sl-HLS and Sl-IAA3 in controlling hook formation we analyzed the spatial expression of the Sl-HLS and Sl-IAA3 in the apical hook by native promoter-reporter constructs. We generated transgenic lines expressing a 1.3 kb fragment of the Sl-HLS promoter fused to the GUS reporter gene and assessed the GUS staining in etiolated tomato seedlings treated with ethylene. Remarkably, ProHLS::GUS staining was restricted to the outer side of the hook curvature, whereas the Sl-IAA3 promoter drove GUS staining exclusively on the inner side (Fig. 1). These data suggest that Sl-IAA3 acts as a repressor of auxin/ethylene-mediated cell elongation on the inner surface of the apical hook and/or conversely that SI-HLS1 is involved in promoting cell elongation on the outer surface. Sl-IAA3 and Sl-HLS genes provide therefore tissue-specific markers for the inner and outer sides of the apical hook, respectively, and the corresponding promoters could be useful to target the ectopic expression of transgenes to a specific side of the hook.
Figure 1.
The expression of Sl-IAA3 and Sl-HLS genes takes place on opposite sides of the hook. Tomato ProHLS::GUS and ProIAA3::GUSseedlings were dark-grown for 5 days and then treated for 48 h with 10 µl l−1 of ethylene. The images are representative of at least three independent experiments with n > 30 seedlings per experiment.
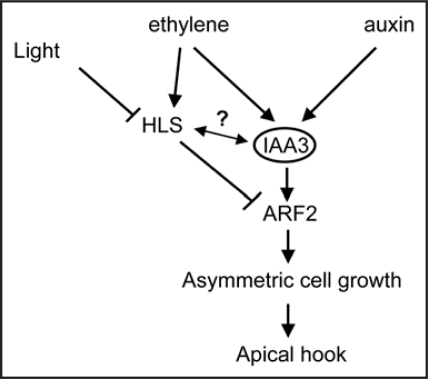
We postulate that Sl-IAA3 and Sl-HLS may act in parallel pathways both of them involving ARF2 as a downstream component, or Sl-HLS may act upstream of Sl-IAA3 to downregulate its expression which might explain why Sl-IAA3 is not expressed in the upper side of the hook where the expression of Sl-HLS is high. Figure 2 depicts a model mechanism on how the convergence of ethylene and auxin signaling impacts differential growth of etiolated seedlings in an IAA3-dependent manner. IAA3 serves as central integrator of ethylene and auxin. In this model, HLS-dependent and IAA3-dependent regulation of ARF2, a negative regulator of the differential auxin response, leads to enhanced differential growth and exaggerated hook curvature. In conclusion, we suggest that the process of hook formation requires an interplay between HLS, IAA3 and ARF2 proteins.
Figure 2.
A model mechanism describing the different players of the auxin/ethylene interplay leading to differential growth in etiolated seedlings.
Continued efforts are now engaged to determine the post-translational regulation of IAA3 by auxin and ethylene and to test the potential interaction between IAA3 and ARF2 proteins.
The Universal Genome Walker Kit (Clontech Laboratories, Inc., Palo Alto, CA, USA) was used to isolate 1.3 kb of the Sl-HLS gene promoter region. The Sl-HLS promoter was then fused to the β-glucuronidase (GUS) reporter gene in the plp100 binary vector10 and used for stable tomato transformation [cv. MicroTom]. Growth conditions were performed as described previously.6 For histochemical GUS analysis, ProHLS::GUS and ProIAA3::GUS transgenic lines were incubated at 37°C for 5 h with GUS-staining solution (100 mM sodium phosphate buffer, pH 7.2, 10 mM EDTA, 0.1% Triton and 1 mM 5-bromo-4-chloro-3-indolyl-b-D-glucuronic acid). Following GUS staining, samples were washed several times to extract chlorophyll using a graded ethanol series.
Footnotes
Previously published online as a Plant Signaling & Behavior E-publication: http://www.landesbioscience.com/journals/psb/article/8748
References
- 1.Alonso JM, Stepanova AN, Solano R, Wisman E, Ferrari S, Ausubel FM, Ecker JR. Five components of the ethylene-response pathway identified in a screen for weak ethylene insensitive mutants in Arabidopsis. Proc Natl Acad Sci USA. 1993;100:2992–2997. doi: 10.1073/pnas.0438070100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chilley PM, Casson SA, Tarkowski P, Wang KL-C, Hawkins N, Hussey PJ, et al. The POLARIS peptide of Arabidopsis regulates auxin transport and root growth via effects on ethylene signaling. Plant Cell. 2006;18:3058–3072. doi: 10.1105/tpc.106.040790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swarup R, Perry P, Hagenbeek D, van der Straeten D, Beemster GTS, Sandberg G, et al. Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. Plant Cell. 2007;19:2186–2196. doi: 10.1105/tpc.107.052100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stepanova AN, Hoyt JM, Hamilton AA, Alonso JM. A link between ethylene and auxin uncovered by the characterization of two root-specific ethylene-insensitive mutants in Arabidopsis. Plant Cell. 2005;17:2230–2242. doi: 10.1105/tpc.105.033365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stepanova AN, Yun J, Likhacheva AV, Alonso JM. Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell. 2007;19:2169–2185. doi: 10.1105/tpc.107.052068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaabouni S, Jones B, Delalande C, Wang H, Li Z, Mila I, et al. Sl-IAA3, a tomato Aux/IAA at the crossroads of auxin and ethylene signalling involved in differential growth. J Exp Bot. 2009;60:1349–1362. doi: 10.1093/jxb/erp009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lehman A, Black R, Ecker JR. HOOKLESS1, an ethylene response gene, is required for differential cell elongation in the Arabidopsis hypocotyl. Cell. 1996;85:183–194. doi: 10.1016/s0092-8674(00)81095-8. [DOI] [PubMed] [Google Scholar]
- 8.Raz V, Ecker JR. Regulation of differential growth in the apical hook of Arabidopsis. Development. 1999;126:3661–3668. doi: 10.1242/dev.126.16.3661. [DOI] [PubMed] [Google Scholar]
- 9.Li H, Johnson P, Stepanova A, Alonso JM, Ecker JR. Convergence of signaling pathways in the control of differential cell growth in Arabidopsis. Dev Cell. 2004;7:193–204. doi: 10.1016/j.devcel.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Szabados L, Charrier B, Kondorosi A, de Bruijn FJ, Ratet P. New plant promoter and enhancer testing vectors. Molec Breed. 1995;1:419–423. [Google Scholar]