Abstract
Background
Single dose nevirapine and a short course of zidovudine (AZT) are now administered in most hospitals in Uganda to prevent mother-to-child transmission (MTCT) of HIV. The effectiveness of these antiretroviral (ARV) regimens has been shown in the clinical trials but has not been demonstrated outside the clinical trials setting in this country.
Objectives
The study evaluated the effectiveness of short course ARV regimens in a pilot program to prevent mother-to-child transmission of HIV and determined the risk factors for perinatal transmission.
Methods
Cross-sectional study design was used to compare perinatal transmission rates of HIV in two sets of mothers: ARV-treated mothers and ARV-untreated mothers.
Results
109 treated and 90 naïve mother-infant pairs were recruited. HIV transmission rates were similar in the nevirapine (10/61) and AZT (8/48) groups (16.4% vs. 16.7%) respectively but higher in the naïve group (43/90 48%, p= 0.0001). ARV therapy offers a protective effect against MTCT of HIV (Adjusted Odds Ratio 0.22 95%CI 0.09, 0.54) but mothers in Stage 1 and 2 of disease were more likely to benefit from this intervention than mothers in Stage 3 and 4.
Conclusion
In this community-based observational study, ARV reduces the risk of perinatal transmission of HIV but does not eliminate the risk completely. Early screening of asymptomatic pregnant women will identify a group of mothers more likely to benefit from the intervention.
Keywords: HIV-1, mother, child, transmission, antiretroviral therapy
Introduction
Each year 590,000 infants acquire HIV-1 infection from their mothers, mostly in developing countries that are unable to implement antiretroviral interventions now standard in the industrialized world 1. Initial interventions to prevent transmission are expensive and technically complex and not feasible for use in developing countries with limited resources.
A series of randomized clinical trials have been completed to assess the effectiveness of oral antiretroviral therapy (ART) regimens 2–5. More relevant to the situation would be oral regimens at low cost. These clinical trials provide efficacy but do not provide information as to how the intervention will work in practice.
Before 2000, in Uganda, the use of ART in the prevention of mother-to-child transmission (MTCT) of HIV had been carried out only under clinical trial conditions. At the beginning of the year 2000, the Ugandan Ministry of Health and UNICEF began a program to implement therapeutic regimens at 3 hospitals in Kampala. This new program provided an opportunity to assess the effectiveness of antiretroviral (ARV) use among pregnant women outside the clinical trial setting. In this community-based observational study, we estimate and compare the perinatal transmission rates of HIV-1 among mothers who received ART through the implementation program with mothers who did not receive ART. We also determine the modifiable risk factors associated with an increased risk of perinatal HIV transmission. Identification of the factors likely to increase the risk of perinatal transmission of HIV is important in enhancing the therapeutic effect of ARV therapy.
Methods
Study population and setting
Between June and October 2000, we performed a cross ectional study of Ugandan peripartum women to compare the rates of HIV transmission among mothers who were treated with ART compared to mothers who had not received the treatment. Mothers were eligible if they were over 18 years and HIV positive. A consecutive sample of mothers treated with antiretroviral drugs was taken from hospitals participating in the Uganda Ministry of Health UNICEF/Elisabeth Glaser Pediatric AIDS Foundation funded pilot program of nevirapine distribution to prevent perinatal HIV transmission. The hospitals were Mulago, Nsambya and Mengo and are all located in the capital city, Kampala. The program began in March 2000 at these hospitals.
The study enrolled mothers who were 18 years or older attending postnatal clinics and had received a regimen of ART in the prevention of MTCT of HIV. Every mother-infant pair that met the selection criteria and provided informed consent was enrolled as they presented to the clinics.
As a comparison group, we recruited mothers from 3 postpartum clinics around Kampala. At the time of this study, ART was not yet available at these clinics. Thus the mothers recruited from these clinics did not receive ART and are referred to as naïve mothers. Naïve mothers were identified through an existing HIV counseling and screening program at these postnatal clinics. Post-partum mothers and their children report to these clinics for health education and for the vaccination of their infants. A consecutive sample of mothers older than 18 years attending these clinics was enrolled. The inclusion criteria for the naïve mothers were the same as those for treated mothers except for the treatment status. Both groups of mothers had received the standard antenatal care package, which includes folic acid and iron supplementation.
Study design and intervention
As part of the government-sponsored implementation program, treated mothers received either a single dose of nevirapine of 200 mg at the onset of labor or a short course of zidovudine (AZT) at 300 mg twice daily starting at 36 weeks of gestation until delivery and for one week post partum. The nevirapine dosage for the mother was given at 36 weeks of gestation and the mothers were instructed to swallow the medication when labor pains started. The infant received either a single dose of nevirapine syrup at a dosage of 2mg/kg within the first 72 hours after birth or one to two weeks of oral zidovudine therapy postpartum at dosage of 5mg/kg body weight given twice daily.
Patient consent
We obtained informed written consent from all mothers before they were enrolled in the study. The mothers with unknown sero-status also consented for HIV voluntary counseling and testing. The study was reviewed and approved by Institutional Review Boards at Case Western Reserve University, Cleveland, OH, USA and by the AIDS Research Sub-Committee in Uganda.
Measurements
We administered a standard questionnaire to the mother to assess risk factors associated with HIV perinatal transmission. The variables collected included age of mother and infant, breastfeeding status, history of sexually transmitted diseases, number of sexual partners, and condom use. Breast-feeding status was measured by self-report.
We also asked the mothers about prior history of exposure to ARV and whether the father of the infant had used ARV therapy. To assign the stage of maternal HIV disease, we asked the mothers about history of symptoms associated with HIV/AIDS disease and based the staging on the World Health Organization criteria for staging 6. The mothers in stage 3 and 4 were combined into one group since there were only 3 mothers who fell into stage 4 category.
A series of rapid test kits were administered in series to screen mothers for HIV-1 antibodies. The health centers were already using a similar rapid HIV testing protocol in their voluntary counseling and testing program. In a series of 3 rapid kits, the Determine ® (Abbot Laboratories, Abbot Park, IL) test kit was used as the initial test for HIV-1 antibodies. If a mother tested negative on Determine®, she was considered as being negative for HIV-1 antibodies and no further HIV tests were performed. However, If the sample test was positive on the Determine® test, the Unigold ® (Trinity Biotech) test was done as a confirmatory test. The third rapid test available was Serocard ® (Trinity Biotech) and was done only if the results of the first two tests were indeterminate. The results were available to the mothers within an hour after the blood draw was done.
The results were given to the mother after a posttest counseling session. Experienced counselors at each clinic carried out the counseling. We tested the infants for HIV using qualitative Ribonucleic acid polymerase chain reaction (RNA PCR) with the Roche AMPLICOR MONITOR assay (Roche Diagnostics, Indianapolis, IN, US). The infant HIV testing was done at the Makerere University-Johns Hopkins-Case Western Reserve University Core laboratory at Mulago Hospital in Uganda.
Analytic strategy
We determined the descriptive statistics for all the variables and then compared the baseline characteristics in the two treatment categories. HIV transmission rates were estimated separately for the naïve and treated mothers as a proportion of infants with HIV infection. We used unconditional logistic regression to determine the odds ratio (95% confidence interval) for HIV infection in the child according to treatment status of the mother, maternal stage of disease, and breastfeeding status of the infant. We used a stratified analysis and multivariable logistic regression to adjust for confounding and to detect effect modifiers. Data was entered into Epi-Info Version 6 (CDC). Analysis was done using Statistical package for Social Scientists, SPSS ® (Version 10) and Statistical Analysis Software, SAS ® (Version 8)
Results
Study population
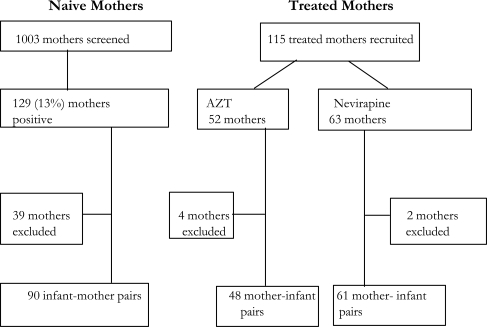
Between June and October 2000, some 1003 naïve mothers were screened for HIV-1. Of these mothers, 129 (13%) mothers tested positive for HIV-1 antibodies. Of the 129 mothers, 90 infant-mother pairs were enrolled in the study (Figure 1). The rest of the mothers (n=39) were not enrolled because they did not give consent to have their infant in the study, or there was failure to draw blood from the infant, in some (n=8) infants the amount of blood was insufficient to perform the RNA PCR.
Figure 1.
Study Population, Screening and Recruitment
Figure 1. Summary of the enrollment of mothers at the clinics.
1003 naïve mothers were screened and of these 129 or 13% were HIV-1 positive. 115 treated mothers were enrolled. 52 of these had received AZT while 63 had received nevirapine. Mothers excluded in the analysis either did not consent to have their child in the study or had HIV results for their infant missing.
During the same time period, 115 HIV-infected mothers treated with either nevirapine or zidovudine as part of the implementation program were screened and all were enrolled in the study. A total of 109 infant-mother pairs were enrolled from the treated group. Of these 109, 48 mothers had received AZT while 61 mothers had received nevirapine. For 6 mother-infant pairs, an HIV status of the infant was not determined for reasons similar to those among naïve mothers.
Among the 45 mother-infant pairs who were excluded or declined to participate, infants were more likely to be born to naïve mothers and were more likely to be breastfed. There were otherwise no significant differences between the mother-infant pairs with and without HIV results of the child in terms of birth weight, gender, and age of infants, or history of maternal sexually transmitted diseases. Because of the absence of HIV results for a proportion of infants, further analysis is based on infants with HIV results available. The final study population comprises 90 naïve mother-infant pairs and 109 treated mother-infant pairs.
Maternal characteristics
The treated mothers were slightly older than the naïve mothers, had older husbands and tended to have had more previous pregnancies compared to the naïve mothers (Table 1). The two groups of mothers were, however, comparable in terms of marital status, place of residence and the number of other wives that their husbands had (Table 1). The naïve mothers were less likely to use condoms than the treated mothers but had had fewer sex partners over the past four years compared to the treated mothers (Table 1). Naïve mothers also had a more advanced stage of HIV disease. The two groups of mothers were comparable in terms of history of symptoms associated with sexually transmitted diseases STDs .
Table 1.
Maternal demographic characteristics, Kampala 2002
| Characteristic | Naive mothers
(n=90) |
Treated mothers
(n=109) |
p value | |
| Mean age of mother (sd) | 25.5 (4.7) | 27.3 (5) | 0.014 | |
| Mean age of husband (sd) | 32.3 (7.5) | 34.5 (7.3)
n(%) |
0.03 | |
| Marital status | ||||
| Single | 13 (15) | 16 (15) | ||
| Married | 71 (80) | 81 (76) | ||
| Widowed | 5 (5) | 10 (9) | 0.61 | |
| Place of residence | ||||
| Urban/town | 37 (41) | 42 (39) | ||
| Trading center | 38 (43) | 52 (49) | ||
| Rural | 14 (16) | 13 (12) | 0.64 | |
| Number of pregnancies (mean) | 3 | 3.5 | 0.06 | |
| STD diagnosis by HW | 35 (40) | 33 (31) | 0.21 | |
| Genital discharge | 30 (34) | 34 (32) | 0.7 | |
| Genital ulcer | 35 (39) | 29 (27) | 0.07 | |
| Stage of HIV disease | ||||
| Stage 1 | 27(30) | 61(56) | ||
| Stage 2 | 30(34) | 28(26) | ||
| Stage 3 | 32(36) | 20(18) | 0.001 | |
| Husband use of ARV | 2(2) | 3(3) | 0.65a | |
| Condom use* | ||||
| Most of the time | 1(1%) | 6(6) | ||
| Sometimes | 26(30%) | 45(43) | ||
| Never | 60(69%) | 54(51) | 0.02a | |
| Number of sexual partners (4years) | ||||
| 1 | 60(69) | 50(51) | ||
| 2 | 25(29) | 41(42) | ||
| 3 or more | 2(2) | 7(7) | 0.03a | |
| Oral thrush | 11(12) | 7(6) | 0.15 | |
| Herps zoster | 4(5) | 8(7) | 0.42 | |
| History of TB tretament | 10(11) | 10(9) | 0.65 | |
Fisher's exact test
Mantel Haenszel chi square test for trend p=0.007
sd = standard deviation
Infant features
There was no significant difference in the birth weights among infants born to treated or naïve mothers (Table 2). The infants of naïve mothers were on average older than those whose mothers were treated (38.5 vs. 8.7 weeks p< 0.001) and were more likely to have breastfed (Table 2). Some infants in the naïve mothers group (4.5%) received antiretroviral therapy postpartum whereas most of the infants in the treated mothers group (90%) received antiretroviral therapy. There were 4 infants who were treated but had mothers who had not received antiretroviral therapy because of lack of drug supply at the time of delivery or mother refusing to swallow the medications before labor.
Table 2.
Infant features startified by maternal treatment status, Kampla 2000
| Charateristics | Naive mothers
(n=90) |
Treated mothers
(n=109) |
p value |
| Infant features | |||
| Birth weight (Kg, mean) | 3.2 | 3.3 | 0.41 |
| Age in weeks (mean) | 38.5 | 8.7 | <0.001 |
| Sex (male) n (%) | 47(56) | 41(38) | 0.02a |
| Infant recieved ARV Treatment n (%) | 4(4) | 99(90) | <0.001 |
| Breastfeeding n (%) | 77(87) | 55(51) | <0.001 |
chi-square with continuity correction
The infants in the two groups were similar in terms of birth weight. The naïve mothers, however had older infants compared to the treated group. Infants in the naïve group were also more likely to be male and to have breastfed compared to the treated group.
Overall transmission rates of HIV
Among the 90 naïve mother-infant pairs, 43 infants (47.8%) were HIV-1 infected whereas among the 109 treated mother-infant pairs, only 18 infants (16.5%) were HIV positive. This gives an unadjusted odds ratio in the treated compared to the naïve mothers of 0.22 (95% CI 0.1–0.4). When the treated mothers were stratified according to the medication received, the proportion of infected infants was comparable between the AZT-treated and nevirapine treated mothers (8/48 or 16.7% and 10/61 or 16.4% in the AZT and nevirapine groups respec tively.
HIV infection rates within each infant-age category were calculated among infants born to naïve mothers to show the trends in the prevalence of HIV (Table 3). The results showed a trend towards increasing proportion of HIV infection rates with increasing infant age (chi square for trend P<0.0002). Among children younger than 20 weeks, 37.5% of infants were HIV infected; this proportion rose to 50% among infants aged between 20–40 weeks. A similar observation was made among children of treated mothers (Table 3). In naïve mothers the proportion of infected children did not increase with a more advanced stage of HIV infection. In contrast, in treated mothers the proportion of infected children rose with the stage of disease. The naïve mothers were more likely to have breast fed their infants compared to the treated mothers (Table 3).
Table 3.
HIV infant infection rate by infant age category, maternal stage of disease and breastfeeding by treatment status of mother, Kampala 2000
| ARV naive mother | ARV treated mother | |
| Number infected/number in column (%) | ||
| Age of infant (weeks) | ||
| <20 | 15/40(38) | 16/101(16) |
| 20–40 | 8/16(50) | 2/8(25) |
| >40- | 20/33(61) | 0 |
| Maternal Satge of HIV | ||
| Stage 1 | 16/27(60) | 7/61(12) |
| Stage 2 | 12/30(40) | 6/28(21) |
| Stage 3 | 15/32(47) | 5/20(25) |
| Breastfeeding | ||
| Yes | 37/77 (48) | 8/55(15) |
| No | 6/12(50) | 10/52(19) |
The table shows a trend towards increasing proportion of infants infected with HIV-1 as age of the infant increases. It also shows increasing proportion of infected infants with advancing stage of disease but only among the treated mothers.
In a univariate logistic regression analysis, maternal antiretroviral therapy was significantly protective of HIV in the infant (OR= 0.21, 95% CI 0.1–0.4) The univariate analysis tended to show an increased risk for HIV transmission to the infant with presence of genital ulcer in the last 4 years (OR=1.71; 95% CI 0.91–3.2), history of an STD in the past four years (OR=1.67; 95% CI 0.89–3.14), and among male infants (OR=1.47; 95% CI 0.79–2.74) but none was statistically significant. The age of infants was associated with HIV infection as older infants and children were more likely to be infected compared to the younger ones. Breastfeeding was associated with an increased risk for transmission (OR=1.5, 95% CI 0.78–3.0), but this risk was not statistically significant.
A stratified analysis was done according to the breastfeeding status of the mothers and showed a protective effect of ARV in each stratum. Among the women who were breastfeeding, the odds ratio (OR) for infection among treated mothers compared with naïve mothers was 0.18 (95% CI 0.08–0.44) and among the mothers who did not breastfeed their infants, the OR was 0.24 (95 % CI 0.06–0.9). There was an increasing risk of HIV transmission with the advancing stage of HIV disease with the risk increasing from 1.27 times (95% CI 0.61–2.64) among mothers in stage 2 to 1.76 times (95% CI 0.85–3.68) among mothers in stage 3/4. In a multivariable logistic regression analysis adjusting for stage of disease, breastfeeding and age of the infant, the protective effect of antiretroviral therapy in the prevention of HIV infection in the child persisted (OR= 0.22; 95%CI 0.09, 0.54).
Because of the significant differences in age of the infants in the naïve and treated groups, we did a separate analysis only including infants less than 25 weeks of age. Among the 106 children of treated mothers in this group, 17 (16%) were HIV infected compared to 16 of 43 (37.2%) in the naïve group. The adjusted risk of infection in the infant was 0.32 (95% CI 0.14–0.72).
Discussion
This observational study shows the effectiveness of antiretroviral prophylaxis in the prevention of perinatal transmission of HIV in a government-sponsored program in a developing country. The results show that a brief course of oral ARV therapy can successfully reduce mother-to-child transmission of HIV. In a pilot program that implements ARV in pregnant mothers in Uganda, antiretroviral therapy reduced the risk of perinatal transmission of HIV by 78%. The benefit of ARV remains even after adjusting for the confounding effect of breastfeeding, maternal stage of HIV disease and age of infant. Furthermore, the level of reduction in transmission was similar for nevirapine and zidovudine treatment groups. The degree of protection found in this observational study was greater than that found in the HIVNET 012 and Thai CDC clinical trials. The observational nature of the study and also the significant baseline differences observed between the treated and naïve groups in our study could explain this difference. In particular, the naïve group in this study had a high level of transmission in part because of the older age of infants and the associated longer duration of breastfeeding. Therefore, the protective effect may be exaggerated because of the high level of transmission in the naïve group through breastfeeding.
As was observed in the HIVNET 012 study from Uganda , ARV therapy reduces but does not eliminate the risk of perinatal transmission of HIV. Indeed, the transmission of HIV still remains high (17%) despite administration of ARV therapy in this observational study. What are the potential reasons for this treatment failure? First, transmission may occur in-utero prior to the administration of ARV 7–11.
The frequency of early in-utero HIV infection appears to be low, compared with perinatal transmission rates in infants born to HIV-1-infected mothers 12. The strategy of peripartum therapy targets the high-risk period of transmission during labor and delivery. Second, in more advanced stages of HIV disease, brief courses of ARV may not suppress viral replication sufficiently to prevent transmission. Third, after delivery breastfeeding may promote transmission of HIV 13–19. Finally, in the absence of these factors, intrinsic drug resistance to antiretroviral therapy may account for the continued transmission 20–24. There are no large scale studies done to-date to accurately estimate the proportion of HIV transmission to the infant attributable to de novo resistance in the mother despite ART therapy. However some case studies and other small studies have shown evidence of mother-to-child transmission of HIV drug resistant strains 25–26. In the first case ever reported of mother-to-child transmission of drug resistant HIV 25, the AZT resistance was not attributable to AZT given at the end of pregnancy or during labor rather AZT resistance was present before gestation. In the French Perinatal Cohort study, sequence analysis was carried out on 34 infected children and mutations related to AZT resistance were found in 7 of the 34 children 27. Evidence of mother to child transmission of AZT resistant HIV-1 was found in 4 cases. However, in the assessment of mothers and infants from the HIVNET 012 study, the authors found little evidence for transmission of nevirapine-resistant variants from women to infants who were infected by 6–8 weeks of age 40.
In this study, the infants benefited from the antiretroviral therapy regardless of their stage of HIV disease. Among the treated mothers, mothers in stage one of disease, however, have a lower risk of transmission when compared to the mothers in the more advanced stages of disease. This finding is consistent with findings from a study of maternal viral load and the risk of perinatal transmission of HIV-1 in New York 28, Kenya 29 and West Africa 30. The mothers in the early stage of disease seem to benefit more from the treatment because of the significant association between levels of HIV RNA and transmission among AIDS-free women with CD4 count >500×106/l. Another study from Uganda also showed lower transmission rates for all women receiving single dose nevirapine 31, however the best results were seen in a subgroup of among mothers with the lowest CD4 counts.
Breastfeeding poses a significant risk of transmission of HIV and the greatest risk occurs in the first 2 months of life 13. Our study shows that the risk of HIV sero-conversion increases with the age of the infant, probably attributed to the cumulative effect of exposure to infected breast milk. However, this trend is most evident among the infants whose mothers were naïve to ARV because these infants had a wider age range as compared to those who were treated. Breastfeeding may therefore nullify the effects of the antiretroviral intervention given to prevent perinatal transmission of HIV. This is consistent with findings from the Petra Study Team 32 where benefits from short-course ARV regimens given in the peripartum period diminished considerably after 18 months of follow up.
The mothers who received ARV treatment in our study were less likely to have breastfed their infants compared to those who did not receive ARV therapy. This could be attributed to the counseling given to the treated mothers during antenatal clinic attendance. In this study, breastfeeding is a classic confounder as it is associated with the use of ARV, the exposure of intent, and with the outcome of interest. This confounding effect of breastfeeding was dealt with by1 adjusting for breastfeeding in a multivariable model and 2 restricting the analysis to infants under 25 weeks of age. The protective effect of ARV therapy still persists.
Currently there are campaigns in developing countries for scaling up the implementation of short regimens to prevent vertical transmission of HIV. These campaigns have been accepted 33 and successfully implemented 34–37. Despite the public health mandate for this treatment, there is concern that widespread use of short course antiretroviral regimens will result in the selection of drug resistant variants that may be sexually or vertically transmitted 38. Single dose nevirapine has been shown to result in the selection of drug resistant variants 39–42. Presence of drug resistant strains of HIV may be responsible for the continued transmission despite the ARV therapy. Genotypic testing is being done to examine this hypothesis. Plans should be made to incorporate drug resistance monitoring as part of the treatment campaigns. The World Health Organization has already recognized the need to develop a global antiretroviral resistance monitoring program and held a consultative meeting in which the experts suggested a review of the existing regional and national networks to identify sentinel sites and reference laboratories to participate in ARV drug surveillance 43. The sentinel surveillance for drug resistance will be cost effective because not all sites implementing ARV programs are able to monitor drug resistance individually particularly the programs in resource-limited countries.
Limitations of the study include the observational nature of the study. This is shown by the differences in some key variables like age of the infant and breastfeeding status. Therefore, the age of the infant and breastfeeding are major biases in this study. The small size of the study does not allow us to demonstrate the significant risk attributable to STDs in the perinatal transmission of HIV. It should be noted that women in the study from private hospitals like Nsambya and Mengo may be significantly socially different from women at Mulago, the national referral Hospital and the postpartum clinics around Kampala.
In this operational study, we confirm the findings from clinical trials showing the effectiveness of a short antiretroviral therapy regimen in preventing mother-to-child transmission of HIV. There is therefore an urgent need to expand access to the program countrywide to prevent new pediatric HIV infections. There is also a need to address the continued high transmission rates resulting from treatment failures.
Acknowledgements
We thank the mothers in Uganda for accepting to participate in the study. We would like to thank the members of staff at Kawempe, Kiswa and Naguru Hospitals for the assistance in the recruitment, counseling and HIV testing of the study participants. We would also like to thank the hospital staff of Mulago, Nsambya and Mengo Hospitals for their assistance in the recruitment of study participants.
The AIDS International Training and Research Program of the Fogarty Center (National Institute of Health) at Case Western Reserve University funded this study through grant number TW00011.
References
- 1.De Cock KM, Fowler MG, Mercier E, de Vincenzi I, Saba J, Hoff E, et al. Prevention of mother-to-child HIV transmission in resource-poor countries: translating research into policy and practice. JAMA. 2000;283(9):1175–1182. doi: 10.1001/jama.283.9.1175. [DOI] [PubMed] [Google Scholar]
- 2.Sperling RS, Shapiro DE, Coombs RW, Todd JA, Herman SA, McSherry GD, et al. Maternal viral load, zidovudine treatment, and the risk of transmission of human immunodeficiency virus type from mother to infant. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1996;335(22):1621–1629. doi: 10.1056/NEJM199611283352201. [DOI] [PubMed] [Google Scholar]
- 3.Guay LA, Musoke P, Fleming T, Bagenda D, Allen M, Nakabiito C, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354(9181):795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 4.Shaffer N, Chuachoowong R, Mock PA, Bhadrakom C, Siriwasin W, Young NL, et al. Short-course zidovudine for perinatal HIV-1 ransmission in Bangkok, Thailand: a randomised controlled trial. Bangkok Collaborative Perinatal HIV Transmission Study Group. Lancet. 1999;353(9155):773–780. doi: 10.1016/s0140-6736(98)10411-7. [DOI] [PubMed] [Google Scholar]
- 5.Wiktor SZ, Ekpini E, Karon JM, Nkengasong J, Maurice C, Severin ST, et al. Short-course oral zidovudine for prevention of mother-to-child transmission of HIV-1 in Abdijan, Cote d'Ivoire: a randomised trial. Lancet. 1999;353(9155):781–785. doi: 10.1016/S0140-6736(98)10412-9. [DOI] [PubMed] [Google Scholar]
- 6.Katabira E, Kamya MR, Mubiru FX, Bakyaita NN. HIV Infection:Diagnostic and Treatment Strategies For Health Care Workers. 2nd Edition. Republic of Uganda, P.O. Box 7272, Kampala, Uganda: Published by The STD/AIDS Control Programme, Ministry of Health; 2000. pp. 4–5. [Google Scholar]
- 7.Peckham CS. Human immunodeficiency virus infection and pregnancy. Sex Transm Dis. 1994;21(2 Suppl):S28–S31. [PubMed] [Google Scholar]
- 8.Toth FD, Bacsi A, Beck Z, Szabo J. Vertical transmission of human immunodeficiency virus. Acta Microbiol Immunol Hung. 2001;48(3–4):413–427. doi: 10.1556/AMicr.48.2001.3-4.10. [DOI] [PubMed] [Google Scholar]
- 9.Mandelbrot L. Timing of in utero HIV infection: implications for prenatal diagnosis and management of pregnancy. AIDS Patient Care STDS. 1997;11(3):139–147. doi: 10.1089/apc.1997.11.139. [DOI] [PubMed] [Google Scholar]
- 10.Santmyire BR. Vertical transmission of HIV from mother to child in sub-Saharan Africa: modes of transmission and methods for prevention. Obstet Gynecol Surv. 2001;56(5):306–312. doi: 10.1097/00006254-200105000-00026. [DOI] [PubMed] [Google Scholar]
- 11.McGowan JP, Shah SS. Prevention of perinatal HIV transmission during pregnancy. J Antimicrob Chemother. 2000;46(5):657–668. doi: 10.1093/jac/46.5.657. [DOI] [PubMed] [Google Scholar]
- 12.Brossard Y, Aubin JT, Mandelbrot L, Bignozzi C, Brand D, Chaput A, et al. Frequency of early in utero HIV-1 infection: a blind DNA polymerase chain reaction study on 100 fetal thymuses. AIDS. 1995;9(4):359–366. [PubMed] [Google Scholar]
- 13.Miotti PG, Taha TE, Kumwenda NI, Broadhead R, Mtimavalye LA, Van der Hoeven L, et al. HIV transmission through breastfeeding: a study in Malawi. JAMA. 1999;282(8):744–749. doi: 10.1001/jama.282.8.744. [DOI] [PubMed] [Google Scholar]
- 14.Semba RD, Kumwenda N, Hoover DR, Taha TE, Quinn TC, Mtimavalye L, et al. Human immunodeficiency virus load in breast milk, mastitis, and mother-to-child transmission of human immunodeficiency virus type 1. J Infect Dis. 1999;180(1):93–98. doi: 10.1086/314854. [DOI] [PubMed] [Google Scholar]
- 15.John GC, Richardson BA, Nduati RW, Mbori-Ngacha D, Kreiss JK. Timing of breast milk HIV-1 transmission: a meta analysis. East Afr Med J. 2001;78(2):75–79. doi: 10.4314/eamj.v78i2.9092. [DOI] [PubMed] [Google Scholar]
- 16.John GC, Nduati RW, Mbori-Ngacha DA, Richardson BA, Panteleeff D, Mwatha A, et al. Correlates of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission: association with maternal plasma HIV-1 RNA load, genital HIV-1 DNA shedding, and breast infections. J Infect Dis. 2001;183(2):206–212. doi: 10.1086/317918. [DOI] [PubMed] [Google Scholar]
- 17.Fowler MG, Newell ML. Breast-feeding and HIV-1 transmission in resource-limited settings. J Acquir Immune Defic Syndr. 2002;30(2):230–299. doi: 10.1097/00042560-200206010-00012. [DOI] [PubMed] [Google Scholar]
- 18.Nduati R, John G, Mbori-Ngacha D, Richardson B, Overbaugh J, Mwatha A, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA. 2000;283(9):1167–1174. doi: 10.1001/jama.283.9.1167. [DOI] [PubMed] [Google Scholar]
- 19.Nduati R. Breastfeeding and HIV-1 infection. A review of current literature. Adv Exp Med Biol. 2000;478:201–210. doi: 10.1007/0-306-46830-1_18. [DOI] [PubMed] [Google Scholar]
- 20.Kijak GH, Avila MM, Salomon H. Mother-to-child transmission of drug-resistant HIV. Drug Resist Updat. 2001;4(1):29–37. doi: 10.1054/drup.2001.0182. [DOI] [PubMed] [Google Scholar]
- 21.Johnson VA, Petropoulos CJ, Woods CR, Hazelwood JD, Parkin NT, Hamilton CD, et al. Vertical transmission of multidrug-resistant human immunodeficiency virus type 1 (HIV-1) and continued evolution of drug resistance in an HIV-1 infected infant. J Infect Dis. 2001;183(11):1688–1693. doi: 10.1086/320697. [DOI] [PubMed] [Google Scholar]
- 22.Brenner BG, Wainberg MA. The role of antiretrovirals and drug resistance in vertical transmission of HIV-1 infection. Ann N Y Acad Sci. 2000;918:9–15. doi: 10.1111/j.1749-6632.2000.tb05467.x. [DOI] [PubMed] [Google Scholar]
- 23.Colgrove RC, Pitt J, Chung PH, Welles SL, Japour AJ. Selective vertical transmission of HIV-antiretroviral resistance mutations. AIDS. 1998;12(17):2281–2288. doi: 10.1097/00002030-199817000-00009. [DOI] [PubMed] [Google Scholar]
- 24.McIntosh K. Antiretroviral resistance and HIV vertical transmission. Acta Paediatr Suppl. 1997;421:29–32. doi: 10.1111/j.1651-2227.1997.tb18316.x. [DOI] [PubMed] [Google Scholar]
- 25.Siegrist CA, Yerly S, Kaiser L, et al. Mother-to-Child Transmission of Zidovudine-resistant HIV-1. Lancet. 1994;344:1771–1772. [PubMed] [Google Scholar]
- 26.Desai N, Mathur M. Selective transmission of multidrug resistant HIV to a newborn related to poor maternal adherence. Sex Trans Infect. 2003 Oct;79(5):419–421. doi: 10.1136/sti.79.5.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masquelier B, Chaix ML, Burgard M, Lechenadec J, Doussin A, Simon F, et al. Zidovudine genotypic resistance in HIV-1 infected newborns in the French Perinatal cohort. J Acquir Immune Defic Syndr. 2001 Jun 1;27(2):99–104. doi: 10.1097/00126334-200106010-00001. [DOI] [PubMed] [Google Scholar]
- 28.Thea DM, Steketee RW, Pliner V, Bornschlegel K, Brown T, Orloff S, et al. The effect of maternal viral load on the risk of perinatal transmission of HIV-1. New York City Perinatal HIV Transmission Collaborative Study Group. AIDS. 1997;11(4):437–444. doi: 10.1097/00002030-199704000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Temmerman M, Nyong'o AO, Bwayo J, Fransen K, Coppens M, Piot P. Risk factors for mother-to-child transmission of human immunodeficiency virus-1 infection. Am J Obste Gynecol. 1995 Feb;172(2 Pt 1):700–705. doi: 10.1016/0002-9378(95)90597-9. [DOI] [PubMed] [Google Scholar]
- 30.O'Donovan D, Ariyoshi K, Milligan P, Ota M, Yamuah L, et al. Maternal Plasma viral RNA levels determine marked differences in mother-to-child transmission rates of HIV-1 and HIV-2 in The Gambia. MRC/Gambia Government/University College London Medical School working group on mother-to-child transmission of HIV. AIDS. 2000 Mar 10;14(4):441–448. doi: 10.1097/00002030-200003100-00019. [DOI] [PubMed] [Google Scholar]
- 31.Nakabiito C, Guay LA, Musoke P, Fleming T, Jackson JB, Miiro F, et al. Effect of Nevirapine for Perinatal HIV prevention appears strong among women with advanced disease: Subgroup analyses of HIVNET 012. Abstract TuOrB1174; XIV International AIDS Conference; July 7–12 2002; Barcelona, Spain. [Google Scholar]
- 32.The Petra Study Team, author. Efficacy of three short course regimens of Zidovudine and Lamivudine in preventing early and late transmission of HIV from mother to child in Tanzania, South Africa, and Uganda (Petra Study): a randomized, double blind, placebo controlled trial. Lancet. 2002;359:1178–1186. doi: 10.1016/S0140-6736(02)08214-4. [DOI] [PubMed] [Google Scholar]
- 33.Meda N, Leroy V, Viho I, Msellati P, Yaro S, Mandelbrot L, et al. Field acceptability and effectiveness of the routine utilization of zidovudine to reduce mother-to-child transmission of HIV-1 in West Africa. AIDS. 2002;16(17):2323–2328. doi: 10.1097/00002030-200211220-00013. [DOI] [PubMed] [Google Scholar]
- 34.Kanshana S, Simonds RJ. National program for preventing mother-to-child HIV transmission in Thailand: successful implementation and lessons learned. AIDS. 2002;16(7):953–959. doi: 10.1097/00002030-200205030-00001. [DOI] [PubMed] [Google Scholar]
- 35.Etiebet MA, Fransman D, Forsyth B, Coetzee N, Hussy G. Integrating prevention of mother-to-child transmission into antenatal care: learning from the experiences of women in South Africa. AIDS Care. 2004 Jan;16(1):37–46. doi: 10.1080/09540120310001633958. [DOI] [PubMed] [Google Scholar]
- 36.Urban M, Chersich M. Acceptability and utilization of voluntary HIV testing and nevirapine to reduce mother-to-child transmission of HIV-1 integrated into routine clinical care. S Afr Med J. 2004;94(5):362–366. [PubMed] [Google Scholar]
- 37.Perez F, Mukotekwa T, Miller A, Orne-Gliemann J, Glenshaw M, Chitsike I, et al. Implementing a rural programme of prevention of mother-to-child transmission of HIV in Zimbabwe. Trop Med Int Health. 2004 Jul;9(7):774–783. doi: 10.1111/j.1365-3156.2004.01264.x. [DOI] [PubMed] [Google Scholar]
- 38.Wainberg MA, Friedland G. Public health implications of antiretroviral therapy and HIV drug resistance. JAMA. 1998;279(24):1977–1983. doi: 10.1001/jama.279.24.1977. [DOI] [PubMed] [Google Scholar]
- 39.Jackson JB, Becker-Pergola G, Guay LA, Musoke P, Mracna M, Fowler MG, et al. Identification of the K103N resistance mutation in Ugandan women receiving nevirapine to prevent HIV-1 vertical transmission. AIDS. 2000;14(11):F111–F115. doi: 10.1097/00002030-200007280-00001. [DOI] [PubMed] [Google Scholar]
- 40.Eshleman SH, Mracna M, Guay LA, Deseyve M, Cunningham S, Mirochnick M, et al. Selection and fading of resistance mutations in women and infants receiving nevirapine to prevent HIV-1 vertical transmission (HIVNET 012) AIDS. 2001;15(15):1951–1957. doi: 10.1097/00002030-200110190-00006. [DOI] [PubMed] [Google Scholar]
- 41.Eshleman SH, Jackson JB. Nevirapine resistance after single dose prophylaxis. AIDS Rev. 2002;4(2):59–63. [PubMed] [Google Scholar]
- 42.Morris L, Pillay C, Gray G, McIntyre J. HIV-1 drug resistance and mother-to-child transmission. SADJ. 2001;56(12):614–616. [PubMed] [Google Scholar]
- 43. [August 29, 2004];Internet Web site. http://www.who.int/emc-documents/antimicrobial_resistance/docs/whocdscsrdrs200111web.pdf.