Abstract
Saliva is an easy accessible plasma ultra-filtrate. Therefore, saliva can be an attractive alternative to blood for measurement of diagnostic protein markers. Our aim was to determine stability and protein composition of saliva. Protein stability at room temperature was examined by incubating fresh whole saliva with and without inhibitors of proteases and bacterial metabolism followed by Surface Enhanced Laser Desorption/Ionization (SELDI) analyses. Protein composition was determined by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) fractionation of saliva proteins followed by digestion of excised bands and identification by liquid chromatography tandem mass spectrometry (LC-MS/MS). Results show that rapid protein degradation occurs within 30 minutes after sample collection. Degradation starts already during collection. Protease inhibitors partly prevented degradation while inhibition of bacterial metabolism did not affect degradation. Three stable degradation products of 2937 Da, 3370 Da and 4132 Da were discovered which can be used as markers to monitor sample quality. Saliva proteome analyses revealed 218 proteins of which 84 can also be found in blood plasma. Based on a comparison with seven other proteomics studies on whole saliva we identified 83 new saliva proteins. We conclude that saliva is a promising diagnostic fluid when precautions are taken towards protein breakdown.
Keywords: saliva, sample stability, biomarkers, proteomics, mass spectrometry, protein breakdown
Introduction
Saliva is a plasma ultra-filtrate that includes specific salivary proteins produced by three major salivary glands (parotid, sub-mandibular and sub-lingual) and other smaller glands (Baum, 1993). Salivary glands produce around 750 ml of fluid each day (Chicharro, 1998). After secretion in the mouth cavity, the fluid is mixed with bacteria, lining cells, nasal secretions and bronchial secretions and is termed whole saliva (Kaufman, 2000; Kaufman, 2002). Whole saliva is easy to collect in a non-invasive way. This makes saliva an attractive alternative to blood testing (Kaufman, 2002; Lawrence, 2002). Compared to blood sampling, whole saliva collection requires no specially trained personnel, can reduce discomfort and anxiety and may simplify serial sample collection. Saliva tests are also safer than blood tests regarding the risk for hepatitis and HIV. As a diagnostic fluid, saliva has been studied in pilot experiments for several pathological conditions, such as celiac disease (Lanander-Lumikari, 2000), rheumatoid arthritis (Helenius, 2005), HIV (Holmstrom, 1990; Malamud, 1992; Matsuda, 1993; Frerichs, 1994), diabetes mellitus (Belazi, 1998; Lopez, 2003), preterm birth (Heine, 2000; Ramsey, 2003), breast cancer (Streckfus, 2005; Streckfus, 2006), sjögren’s syndrome (Ryu, 2006) and for evaluation of hematopoietic stem cell transplantation (Imanguli, 2007). Saliva composition is influenced by several factors, e.g. circadian rhythms, oral health status and exercise (Dawes, 1993; Chicharro, 1998) but also micro organisms and proteases may have a considerable effect on sample stability/protein degradation. Before saliva can be used as a diagnostic fluid for protein markers in the clinic, its stability should be determined. At present there are only three studies on protein stability in saliva samples (Morris, 2002; Ng, 2003; Schipper, 2007). Two of the studies report on the stability of specific proteins i.e. IgA, Lysozyme (Ng, 2003) and IgG (Morris, 2002). One recent study determined overall protein stability of saliva samples stored on ice, at −20 °C and at −80 °C (Schipper, 2007). In the current study we evaluated in detail the overall protein stability of saliva at room temperature over the first four hours after sample collection since this is a critical period where protein breakdown could be expected. The effect of sample handling, inhibition of bacterial growth and inhibition of protease activity on saliva protein stability was examined by comparative profiling with Surface Enhanced Laser Desorption/Ionization Time of Flight Mass Spectrometry (SELDI-TOF-MS) (Merchant, 2000). In addition we studied whole saliva composition. Whole saliva protein composition has been studied using different proteomic strategies (Huang, 2004; Vitorino, 2004; Wilmarth, 2004; Hu, 2005; Xie, 2005; Guo, 2006; Walz, 2006). Xie et al. (Xie, 2005) identified 437 proteins in saliva using free flow electrophoreses. Guo et al. (Guo, 2006) could identify 1381 proteins employing a capillary electrophoresis approach. However, because of the complexity of whole saliva, each proteomics strategy leads to partial overlapping subsets of saliva proteins (Guo, 2006). Therefore, different proteomics strategies contribute to a comprehensive view of the whole saliva proteome. We analyzed whole saliva composition by fractionating saliva proteins on SDS-PAGE followed by LC-MS/MS analyses of digests from cut-out sections of the gel lane. This proteomics approach has not been applied to saliva before. The results of this approach are compared to previous proteomics studies on whole saliva and discussed in terms of protein origin and function.
Materials and Methods
Chemicals
Ammonium bicarbonate, triton X-100, azide, phenylmethylsulphonylfluoride (PMSF), EDTA, ditiothreitol (DTT), iodoacetamide, α-cyano-4-hydroxy cinnamic acid diethylamine salt (CHCA), formic acid (FA) and trifluoroacetic acid (TFA) were purchased from Sigma-Aldrich (Steinheim, Germany). Acetonitrile (ACN) and acetone were obtained from Biosolve (Valkenswaard, The Netherlands), leupeptin from Roche (Mannheim, Germany) and ammonium acetate and 2-propanol from Merck (Darmstadt, Germany). MES running buffer and SeeBlue Pre-Stained standard for SDS-PAGE were obtained from Invitrogen (Breda, The Netherlands). Coomassie staining (PageBlue Staining Solution) was from Fermentas (Vilnius, Lithuania). Seq. grade modified trypsin porcine was purchased from Promega (Madison, WI, U.S.A.).
Saliva collection
Whole saliva was collected from healthy subjects, four male and three female, between 08:00 a.m. and 10:00 a.m. after overnight fasting, to minimize the influence of circadian rhythms and food debris. Subjects were asked to rinse their mouths with water and discard this before sample collection. Saliva was allowed to accumulate in the floor of the mouth. The accumulated saliva was then spit into a polypropylene test tube and this was repeated until enough saliva was collected (Navazesh, 1993). During the collection process the sample tubes were kept on ice.
Sample pretreatment
Samples were processed according to Hu et al. (Hu, 2005). Briefly, samples were centrifuged for 5 minutes at 1300 g at 4 °C. The pellet was discarded (debris) and the supernatant was centrifuged for 15 minutes at 14000 g at 4 °C. After centrifugation, the supernatant was stored at −20 °C until analysis. Samples were analyzed the same day.
Sample stability studies
In the first experiment, sample stability was determined in saliva obtained from seven healthy volunteers (four male, three female). Freshly collected saliva samples were either directly processed (time point 0) or left at room temperature for four hrs before processing. Aliquots of the 0 and 4 hrs time points were then analyzed by SELDI-TOF-MS in duplicate (see below).
In a second experiment a unique saliva sample freshly collected from one healthy male volunteer was divided in 3 aliquots of 1.2 ml and incubated for 0, 0.5, 1 and 4 hrs at room temperature with either a) 40 μl of 100 mM sodium azide to inhibit bacterial activity, b) protease inhibitors: 60 μl of 2 mg/ml PMSF in 2-propanol, 1.2 μl of 1 mg/ml leupeptin in water, 12 μl of 100 mM EDTA, or c) no additives (control). Water was added to a final volume of 1.273 ml for all 3 conditions. At each time point an aliquot was taken and treated as described above (sample pre-treatment section). Thereafter, samples were analyzed in triplicate by SELDI-TOF-MS for protein profiling.
CM10 weak cation exchange proteinchip arrays (Ciphergen biosystems, Fremont, CA, U.S.A.) were assembled in a 96 well bioprocessor and the spots were washed two times with 200 μl binding buffer (100 mM NH4Ac pH 4.0, 0.05% Triton X-100) for 5 minutes with vigorous shaking. After removing the buffer from the wells, 90 μl binding buffer and 10 μl saliva sample were randomly applied to the spots (in duplicate or triplicate as detailed above). Samples were allowed to incubate for 30 minutes with continuous shaking. Then, they were removed and spots were washed 3 times with 200 μl binding buffer for 5 minutes and once with 200 μl de-ionized water for 5 minutes. The chips were removed from the bioprocessor and air-dried for 15 minutes, followed by two additions (1 μl each) of a 20% solution of CHCA prepared in 50% ACN and 0.5% TFA. Spots were analyzed using the ProteinChip Reader (model PBS II, Ciphergen Biosystems). The mass spectrometer was calibrated using the All-in-One peptide calibration kit (Ciphergen Biosystems) with a focus mass of 6000 Da. Spectra from the saliva samples were collected with the proteinchip software 3.1 (Ciphergen Biosystems) in the mass range 1–20 kDa. Laser intensity was 190, ionsource voltage 20000 V and detector voltage 2150 V. Cluster analysis was performed by Ciphergen Express 3.0 software (Ciphergen Biosystems): a) between samples collected at 0 hr and 4 hr, combining spectra of all seven volunteers measured in duplicate (28 spectra in total) and b) between different time points (0 hrs, 0.5 hrs, 1 hr and 4 hrs) for every condition (control, in presence of azide and in presence of protease inhibitors) measured in triplicate. Before cluster analyses, spectra to be compared were selected, the baseline was subtracted and profiles were normalized using total ion current. Peaks with a signal to noise ratio higher than 5 were selected and were clustered with peaks with similar masses (mass deviation 0.3%) in other profiles with signal to noise ratios higher than 2. The percentage of spectra in which a peak must appear in order to form a cluster was set to 20%. Significant differences (p < 0.05) in peak height of particular masses were calculated.
Saliva protein composition
A saliva sample freshly obtained from a healthy volunteer was processed immediately after collection as described in the sample pretreatment section. 10 μl of processed saliva were mixed with NuPAGE LDS sample buffer (Invitrogen, Carlsbad, CA, U.S.A.) according to standard protocol from the manufacturer. SDS-PAGE was then performed on a NuPAGE 12% Bis-Tris gel (Invitrogen) run at 200 V for 50 minutes with MES buffer (Invitrogen). Proteins were visualized with Coomassie staining. For protein identification, the whole lane was excised in 30 bands. Each band was cut into small pieces and stored at −20 °C until analysis. Then, they were washed in water and dehydrated in ACN. Reduction was performed by covering gel pieces with 10 mM DTT in 100 mM ammonium bicarbonate for 1 hr at 60 °C. DTT solution was then replaced by 55 mM iodoacetamide in 100 mM ammonium bicarbonate and gel pieces were incubated at room temperature in the dark for 45 minutes. After washing in water and dehydration in ACN, 0.1 μg of trypsin (in 50 mM ammonium bicarbonate) was added and gel pieces were allowed to rehydrate on ice for 20 minutes. Digestion was carried out overnight at 37 °C. Peptides were extracted by treating the gel pieces with 0.1%FA for 30 minutes with continuous shaking. Peptide mixtures were then stored at −20 °C until LC-MS/MS analysis was performed.
Separation of the resulting tryptic peptide mixtures was performed by nano-scale reversed-phase LC-MS/MS. The Agilent 1100 nanoflow/capillary LC system (Agilent, Paolo Alto, CA, U.S.A.) was equipped with a trapping column (5 × 0.3 mm C18RP) (Dionex/LC Packings, Amsterdam, The Netherlands) and a nanocolumn (150 × 0.075 mm, C18Pepmap) (Dionex/LC Packings). Peptides mixtures were injected into the trapping column at a flow rate of 10 μl/min (3%ACN/0.1%TFA). After 10 minutes the trapping column was switched into the nanoflow system and the trapped peptides were separated using the nanocolumn at a flow rate of 0.3 μl/min in a linear gradient elution from 95%A (3%ACN/0.1%TFA) to 50%B (97%ACN/0.1%TFA) in 70 minutes, followed by an increase up to 80%B in 5 minutes. The eluting peptides were on-line electrosprayed into the QStar XL Hybrid ESI Quadrupole time-of-flight tandem mass spectrometer, ESI-QTOF-MS/MS (Applied Biosystems, Framingham, MA; MDS Sciex, Concord, Ontario, Canada) provided with a nanospray source equipped with a New Objective ESI needle (10 μm tip diameter). Typical values for needle voltage were 2 kV in positive ion mode. The mass spectrometer was set to perform data acquisition in the positive ion mode, typically with a selected mass range of 300–1500 m/z. Peptides with +2 to +4 charge states were selected for tandem mass spectrometry, and the time of summation of MS/MS events was set to be 2 s. The three most abundant charged peptides above a 30 count threshold were selected for MS/MS and dynamically excluded for 60 s with 50 amu mass tolerance.
ProID software (Applied Biosystems) was used to identify proteins from the mass spectrometric datasets according to UniProt database (May 2005, 181,000 entries). Mass tolerance was set to 0.15 Da (MS) and 0.1 Da (MS/MS) and carboxamido-methylation and methionine oxidation were chosen as modifications for database search.
Results
Sample stability
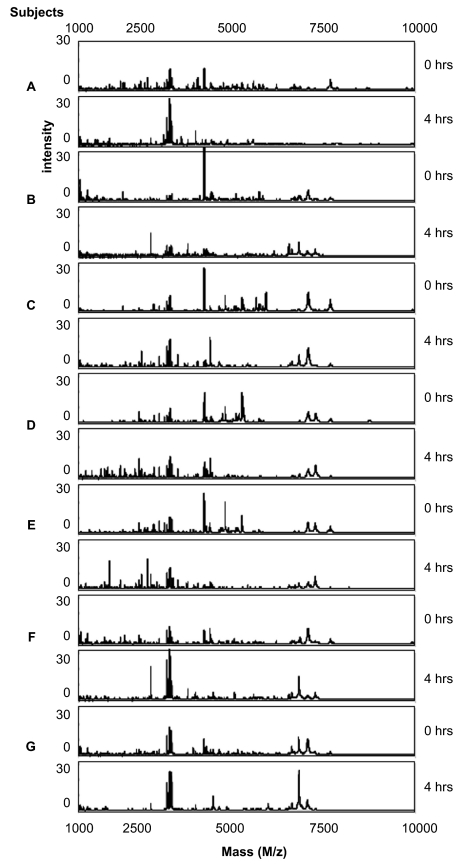
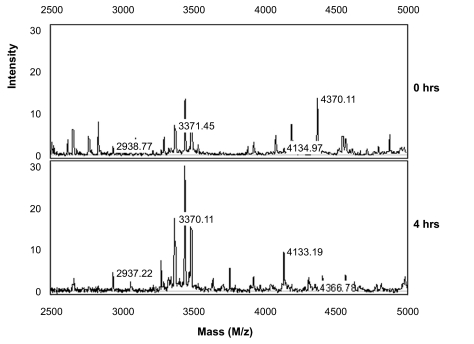
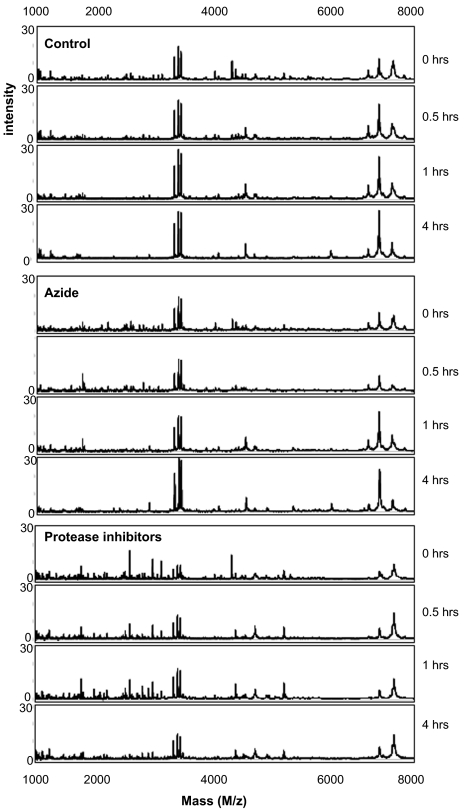
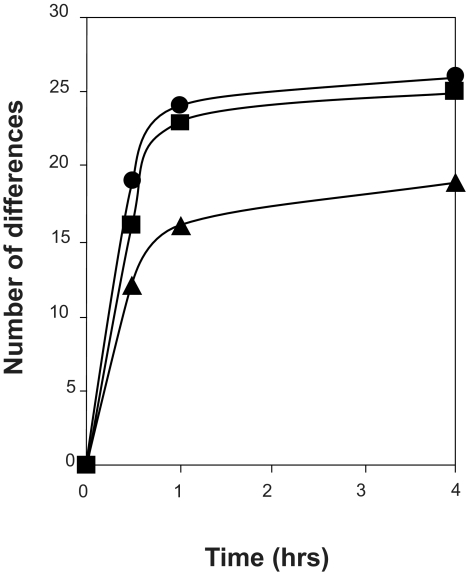
The stability of saliva at room temperature after sample collection from four male and three female volunteers was evaluated. Freshly collected saliva samples were either directly processed (time point 0) or left at room temperature for 4 hrs and processed as described in the sample pretreatment section. Aliquots taken at the two time points were then spotted in duplicate on CM10 weak cation exchange chips and protein profiles were obtained by SELDI-TOF-MS. Representative spectra obtained from the 1 to 10 kDa range are shown in Figure 1. In this mass range degradation products of larger proteins can be expected. When comparing the protein profiles of fresh samples (0 hrs) of the seven volunteers (A–G) it is evident that there is already considerable variation, especially in the mass range of 1 to 5 kDa between the different individuals. This may be due to biological variation and/or may indicate different degrees of protein degradation between individuals. Also over the period of 4 hrs at room temperature many changes in the spectra can be observed (Fig. 1). To find common peaks that were changed over the 4 hrs period in all seven samples we performed a cluster analyses on the acquired spectra. In total 11 differences were detected and listed in Table 1 together with their fold change in peak intensity between the two conditions. Most peptides are decreased in abundance at 4 hrs, probably because they are further degraded into single amino acids during this period. However, 3 peptides with masses 2937 Da, 3370 Da and 4132 Da are increasing in time which indicates that they are relatively stable breakdown products of larger proteins. Although SELDI technology allows rapid comparison of sample composition, protein/peptide identification is troublesome because it involves purification of each degradation product. Therefore, we attempted to identify the 2937 Da breakdown product by direct SELDI-MS/MS which is possible for peptides with masses below 3000 Da. However, the 2937 Da peptide could not be fragmented by MS/MS even with the highest energy settings and argon as collision gas. This indicates that it is very stable, possibly due to a high degree of post-translational modifications such as glycosylation which may also explain its stability in vivo. As Figure 2 indicates, the three marker peptides can already be detected in “fresh” samples (0 hrs), although with lower intensities. This indicates that breakdown already starts during sample collection and suggests that these markers may be useful indicators of protein breakdown in saliva samples. We examined the protein degradation in saliva in detail to obtain more knowledge on the time frame of the degradation process and whether it was possible to inhibit degradation. Protein degradation could be caused by bacteria in the mouth cavity and/or by proteases present in saliva. Therefore, we studied the influence of sodium azide, an inhibitor of bacterial energy metabolism, and of a protease inhibitor cocktail consisting of PMSF, leupeptin (both serine and cysteine protease inhibitors) and EDTA, an inhibitor of metallo-proteases. Saliva samples were incubated for 0, 0.5, 1 and 4 hrs in the presence and absence of the above mentioned inhibitors. Subsequently, protein profiles were generated (Fig. 3) and compared for differences by cluster analyses as described above. In Figure 4 the number of significant differences is depicted for the different conditions and the different time points compared to 0 hrs (control). For saliva without inhibitors already 19 differences were observed in the first 30 minutes incubation. Thereafter, the number of differences stabilizes. This can be explained by assuming that equilibrium has been reached at this point between the formation of peptides from the breakdown of larger proteins and the degradation of these peptides into single amino acids which are in the mass range of the matrix peaks and therefore are not detected. At 4 hrs 26 differences were observed compared to 0 hrs. This indicates that protein degradation in saliva is a relatively rapid process. It is also clear from Figure 4 that protein breakdown is almost not affected by the addition of azide to the samples, indicating that bacterial metabolism is not contributing much to the protein degradation process, at least for the first hour after collection. The protease inhibitor cocktail is more effective in slowing down the degradation process (Fig. 4). At 4 hrs, 19 differences were observed in the presence of protease inhibitors compared to 26 differences in the control sample. Nevertheless, protein degradation is still substantial with the inhibitor cocktail used. Different inhibitors or combinations of inhibitors need to be evaluated to determine their effectiveness.
Figure 1.
Sample stability at room temperature. Protein profiles of saliva samples of seven volunteers (A–G) taken at 0 and 4 hrs of incubation at room temperature are shown for the mass range of 1000 to 10000 Da. Protein profiles were generated using CM10 proteinchips and 100 mM NH4Ac pH 4.0 as binding and washing buffer. CHCA was used as matrix.
Table 1.
Masses with significantly different peak intensities between 0 and 4 hrs of incubation of whole saliva at room temperature.
| Peak (m/z) | p-value | Fold change |
|---|---|---|
| 2937 | 0.0017 | 6.8 |
| 3370 | 0.025 | 2.0 |
| 4132 | 0.047 | 3.0 |
| 4368 | 0.0017 | −6.1 |
| 4928 | 0.0017 | −17.5 |
| 5210 | 0.018 | −3.7 |
| 5376 | 0.0017 | −16.5 |
| 5839 | 0.018 | −2.8 |
| 7751 | 0.0017 | −6.1 |
| 10422 | 0.0088 | −5.0 |
| 15495 | 0.0017 | −4.9 |
Clusters were determined using S/N > 5 (first pass) and S/N > 2 (second pass). Differences were considered significant if p < 0.05.
Figure 2.
Detailed view of the SELDI profile of volunteer A (see also Fig. 1) in the mass range of 2500 to 5000 Da for saliva samples taken at 0 and 4 hrs of incubation at room temperature. The labeled peaks are discovered degradation markers. The complete list of markers is shown in Table 1.
Figure 3.
Representative SELDI spectra of a saliva sample incubated at 0, 0.5, 1 and 4 hrs at room temperature in the absence (control) and presence of sodium azide, an inhibitor of bacterial metabolism, and a protease inhibitor cocktail.
Figure 4.
Number of significant differences in peak intensity between the different conditions (• control, + azide and ▴ protease inhibitors) and the different time points compared to 0 hrs. Differences were calculated from the spectra (Fig. 3) for the mass ranges of 1000 to 20000 Da using cluster analyses of triplicate measurements of the samples. Clusters were defined using S/N > 5 (first pass) and S/N > 2 (second pass). Differences were considered significant if p < 0.05.
The saliva proteome
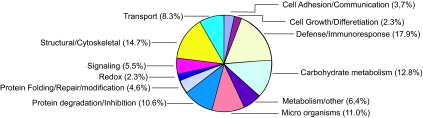
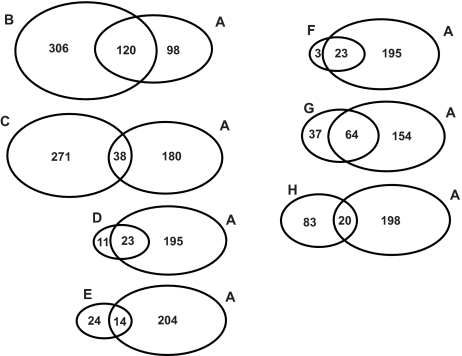
To determine the saliva composition, saliva proteins were fractionated by SDS-PAGE (Fig. 5). The whole lane was sliced into 30 bands and digested by trypsin. The digests of the bands were subjected to LC-MS/MS for protein identification, as described in detail in the Materials and Methods section. In total we identified 218 proteins, 182 with 99% confidence and 36 with 95% confidence. A complete list of identified proteins is shown in Table 2. Proteins were classified into 12 functional categories based on information from Swiss Prot, Source and Human Protein Reference Database. For each protein also the functional category is listed. In Figure 6 an overview of the different categories is given. The largest category (19.2%) consists of enzymes involved in metabolism, mainly in carbohydrate metabolism (12.8%). This includes enzymes such as α-amylase, lactate dehydrogenase, malate dehydrogenase and fructose-biphosphate aldolase. Another important category (17.9%) includes proteins that are involved in immune response and defense against bacteria. In this group there is a large cluster of IgG chains besides antibactericidal peptides such as dermcidin and bactericidal permeability-increasing protein. Also many proteins from bacterial origin were identified (11% of total). 10.6% of the proteins are involved in degradation. Six proteases were identified in this group e.g. kallikrein, cathepsin D and lysozyme C. Thirteen protease inhibitors are also part of this category such as cystatins, alpha-2-macroglobulin and TIMP-1. The proteases are likely to contribute to the rapid breakdown of saliva proteins that was described above. Also many structural proteins (14.7% of total) were found which are probably derived from cells lining the mouth cavity together with other intracellular proteins that were identified. The transport proteins (8.3% of total) are mainly serum-derived such as albumin, apolipoprotein A-1, transferrin, and ceruloplasmin. Minor categories of proteins are signaling (5.5%), protein modification (4.6%), cell growth and differentiation (2.3%), cell adhesion (3.7%), and proteins involved in maintaining redox status (2.3%). We also compared our results, listed in Table 2, to the HUPO plasma proteome initiative list of 3020 plasma proteins, identified with at least two peptides by LC-MS/MS (www.bioinformatics.med.umich.edu/hupo/ppp). According to the HUPO list, 84 proteins (38.5%) identified in the current study and indicated in Table 2 are also found in plasma. To determine the relevance of the identified proteins, we compared our results to seven other proteomics studies (Huang, 2004; Wilmarth, 2004; Vitorino, 2004; Hu, 2005; Xie, 2005; Walz, 2006; Guo, 2006) on whole saliva composition. Figure 7 shows that there is only partial overlap with our study. Based on this analyses 83 new saliva proteins were identified in our study which are indicated in Table 2.
Figure 5.
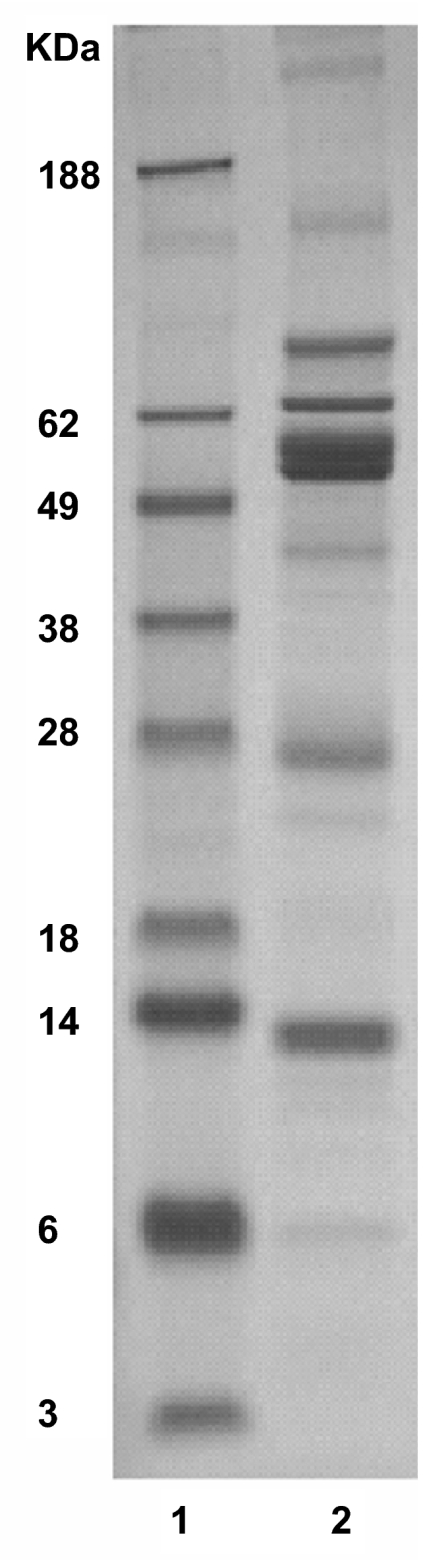
SDS-PAGE of saliva proteins. Lane 1 represents the molecular weight markers. Lane 2 represents proteins present in 10 μl of processed saliva. This lane was cut into 30 bands for further identification by LC-MS/MS.
Table 2.
List of proteins identified with 99% and 95% confidence in human saliva.
| Protein name | Accession nr | Function | Mass (Da) |
|---|---|---|---|
| 99% confidence: | |||
| 14-3-3 protein beta/alpha | P31946 | Signalling | 27951 |
| 14-3-3 protein zeta/delta§ | P63104 | Signalling | 27745 |
| 6-phosphogluconate dehydrogenase, decarboxylating | P52209 | Energy/metabolism | 53009 |
| 78 kDa glucose-regulated protein precursor§ | P11021 | Protein Folding/Repair | 72333 |
| Actin§ | P60709 | Structural/Cytoskeletal | 41737 |
| Actin-like protein 3# | P61158 | Structural/Cytoskeletal | 47240 |
| Actin-related protein 2/3 complex subunit 4# | P59998 | Structural/Cytoskeletal | 19536 |
| Adenine phosphoribosyltransferase | P07741 | Energy/metabolism | 19477 |
| Adenosylhomocysteinase# | P23526 | Energy/metabolism | 47585 |
| Alcohol dehydrogenase [NADP+]# | P14550 | Energy/metabolism | 36442 |
| Alcohol dehydrogenase class IV mu/sigma chain# | P40394 | Energy/metabolism | 40006 |
| Aldehyde dehydrogenase, dimeric NADP-preferring# | P30838 | Energy/metabolism | 50379 |
| Aldo-keto reductase family1 member B10 | O60218 | Energy/metabolism | 36021 |
| Alpha enolase§ | P06733 | Energy/metabolism | 47038 |
| Alpha-1-acid glycoprotein 1 precursor§,# | P02763 | Defense/Immunoresponse | 23512 |
| Alpha-1-antitrypsin precursor§ | P01009 | Protein Degradation/Inhibitor | 46737 |
| Alpha-actinin 1§ | P12814 | Structural/Cytoskeletal | 103058 |
| Apolipoprotein A-I precursor§ | P02647 | Transport | 30778 |
| Arginase 1§ | P05089 | Energy/metabolism | 34735 |
| ATPase 4, plasma membrane-type# | Q9SU58 | Micro organism | 105718 |
| Bactericidal permeability-increasing protein precursor# | P17213 | Transport | 53396 |
| Calgranulin B§ | P06702 | Cell Adhesion/Communication | 13242 |
| Carbonic anhydrase VI precursor§ | P23280 | Energy/metabolism | 35365 |
| Carboxylesterase 2 precursor | O00748 | Energy/metabolism | 61807 |
| Cathepsin D precursor | P07339 | Protein Degradation/Inhibitor | 44552 |
| Ceruloplasmin precursor§ | P00450 | Transport | 122205 |
| Chaperone protein dnaK# | Q7NXI3 | Micro organism | 69122 |
| Chitinase 3-like protein 2 precursor# | Q15782 | Cell Growth/Differentiation | 43501 |
| Chloride intracellular channel protein 1 | O00299 | Transport | 26792 |
| Clusterin precursor§ | P10909 | Cell Growth/Differentiation | 52495 |
| Cofilin, non-muscle isoform | P23528 | Structural/Cytoskeletal | 18371 |
| Complement C3 precursor§ | P01024 | Signalling | 187164 |
| Complement C4 precursor§ | P01028 | Defense/Immunoresponse | 192771 |
| Complement factor H precursor§,# | P08603 | Energy/metabolism | 139070 |
| Coronin-1A | P31146 | Structural/Cytoskeletal | 50895 |
| Cystatin A§ | P01040 | Protein Degradation/Inhibitor | 11006 |
| Cystatin B§ | P04080 | Protein Degradation/Inhibitor | 11140 |
| Cystatin C precursor§ | P01034 | Protein Degradation/Inhibitor | 15799 |
| Cystatin D precursor | P28325 | Protein Degradation/Inhibitor | 16080 |
| Cystatin S precursor | P01036 | Protein Degradation/Inhibitor | 16214 |
| Cystatin SA precursor | P09228 | Protein Degradation/Inhibitor | 16445 |
| Cystatin SN precursor | P01037 | Protein Degradation/Inhibitor | 16362 |
| Dermcidin precursor§,# | P81605 | Defense/Immunoresponse | 11284 |
| Desmocollin-2 precursor | Q02487 | Cell Adhesion/Communication | 99962 |
| Desmoglein-3 precursor | P32926 | Cell Adhesion/Communication | 107503 |
| Diablo homolog, mitochondrial precursor# | Q9NR28 | Signalling | 27131 |
| Dihydroxy-acid dehydratase# | Q8XWR1 | Micro organism | 58965 |
| Dipeptidyl peptidase IV§,# | P27487 | Protein Degradation/Inhibitor | 88279 |
| DNA polymerase IV# | Q9JYS8 | Micro organism | 35966 |
| Elongation factor 1-alpha§ | P68104 | Protein Synthesis | 50141 |
| Elongation factor 1-gamma | P26641 | Protein Synthesis | 49988 |
| Ezrin§,# | P15311 | Cell Growth/Differentiation | 69268 |
| F-actin capping protein beta subunit | P47756 | Structural/Cytoskeletal | 31219 |
| Farnesyl pyrophosphate synthetase# | P14324 | Energy/metabolism | 40532 |
| Fatty acid-binding protein, epidermal | Q01469 | Energy/metabolism | 15033 |
| Fibrinogen gamma chain precursor§ | P02679 | Protein Modification/Polymerization | 51512 |
| FixC protein# | Q8Z9K9 | Micro organism | 45687 |
| Fructose-bisphosphate aldolase A§ | P04075 | Energy/metabolism | 39289 |
| Fructose-bisphosphate aldolase C | P09972 | Energy/metabolism | 39325 |
| Galectin-3 binding protein precursor§ | Q08380 | Cell Adhesion/Communication | 65331 |
| Galectin-7§ | P47929 | Cell Adhesion/Communication | 14944 |
| Gelsolin precursor§ | P06396 | Structural/Cytoskeletal | 85698 |
| Genome polyprotein# | P17593 | Micro organism | 255497 |
| Glucose-6-phosphate isomerase§ | P06744 | Energy/metabolism | 63016 |
| Glutaminyl-tRNA synthetase# | Q8EG26 | Micro organism | 64103 |
| Glutathione S-transferase P | P09211 | Signalling | 23225 |
| Glyceraldehyde-3-phosphate dehydrogenase 1 | P04406 | Energy/metabolism | 35922 |
| Haptoglobin precursor | P00738 | Transport | 45205 |
| Heat shock 70 kDa protein 1§ | P08107 | Protein Folding/Repair | 70052 |
| Heat shock cognate 71 kDa protein§ | P11142 | Protein Folding/Repair | 70898 |
| Hemoglobin alpha chain | P69905 | Transport | 15126 |
| Hemoglobin beta chain | Q9UK54 | Transport | 13964 |
| Hemopexin precursor§ | P02790 | Transport | 51676 |
| Hurpin | Q9UIV8 | Protein Degradation/Inhibitor | 44276 |
| Hypothetical 84.6 kDa protein# | Q04263 | Micro organism | 84602 |
| Ig alpha-1 chain C region | P01876 | Defense/Immunoresponse | 37655 |
| Ig alpha-2 chain C region§ | P01877 | Defense/Immunoresponse | 36508 |
| Ig gamma-1 chain C region§ | P01857 | Defense/Immunoresponse | 36106 |
| Ig gamma-2 chain C region§ | P01859 | Defense/Immunoresponse | 35885 |
| Ig heavy chain V region MOPC 47A# | P01786 | Defense/Immunoresponse | 12975 |
| Ig heavy chain V-II region NEWM# | P01825 | Defense/Immunoresponse | 12790 |
| Ig heavy chain V-III region GAL§,# | P01781 | Defense/Immunoresponse | 12731 |
| Ig heavy chain V-III region HIL# | P01771 | Defense/Immunoresponse | 13566 |
| Ig heavy chain V-III region TUR§,# | P01779 | Defense/Immunoresponse | 12431 |
| Ig heavy chain V-III region VH26 precursor# | P01764 | Defense/Immunoresponse | 12582 |
| Ig kappa chain C region# | P01834 | Defense/Immunoresponse | 11609 |
| Ig kappa chain V-I region CAR§ | P01596 | Defense/Immunoresponse | 11608 |
| Ig kappa chain V-I region WEA§,# | P01610 | Defense/Immunoresponse | 11704 |
| Ig kappa chain V-I region | P01611 | Defense/Immunoresponse | 11840 |
| Ig kappa chain V-III region B6§,# | P01619 | Defense/Immunoresponse | 11636 |
| Ig kappa chain V-III region GOL§ | P04206 | Defense/Immunoresponse | 11830 |
| Ig kappa chain V-IV region Len§ | P01625 | Defense/Immunoresponse | 12640 |
| Ig lambda chain C regions# | P01842 | Defense/Immunoresponse | 11237 |
| Ig lambda chain V-I region NEW# | P01701 | Defense/Immunoresponse | 11453 |
| Ig lambda chain V-I region WAH§,# | P04208 | Defense/Immunoresponse | 11725 |
| Ig lambda chain V-III region LOI§ | P80748 | Defense/Immunoresponse | 11935 |
| Ig lambda chain V-III region SH§ | P01714 | Defense/Immunoresponse | 11393 |
| Ig lambda chain V-IV region Hil | P01717 | Defense/Immunoresponse | 11517 |
| Ig mu chain C region | P01871 | Defense/Immunoresponse | 49557 |
| Immunoglobulin J chain§ | P01591 | Defense/Immunoresponse | 15594 |
| Interleukin-1 receptor antagonist prec. | P18510 | Defense/Immunoresponse | 20055 |
| Kallikrein 1 precursor | P06870 | Protein Degradation/Inhibitor | 28890 |
| Keratin, type I cuticular HA3-II§,# | Q14525 | Structural/Cytoskeletal | 46214 |
| Keratin, type I cytoskeletal 10 | P13645 | Structural/Cytoskeletal | 59519 |
| Keratin, type I cytoskeletal 14§,# | P02533 | Structural/Cytoskeletal | 51490 |
| Keratin, type I cytoskeletal 16§ | P08779 | Structural/Cytoskeletal | 51137 |
| Keratin, type I cytoskeletal 9§ | P35527 | Structural/Cytoskeletal | 62129 |
| Keratin, type I microfibrillar 48 kDa# | P02534 | Structural/Cytoskeletal | 46674 |
| Keratin, type II cuticular HB4# | Q9NSB2 | Structural/Cytoskeletal | 64895 |
| Keratin, type II cytoskeletal 1# | P04104 | Structural/Cytoskeletal | 65092 |
| Keratin, type II cytoskeletal 1§ | P04264 | Structural/Cytoskeletal | 65886 |
| Keratin, type II cytoskeletal 2 epidermal§ | P35908 | Structural/Cytoskeletal | 65865 |
| Keratin, type II cytoskeletal 4§ | P19013 | Structural/Cytoskeletal | 57285 |
| Keratin, type II cytoskeletal 5§ | P13647 | Structural/Cytoskeletal | 62447 |
| Keratin, type II cytoskeletal 6A | P02538 | Structural/Cytoskeletal | 59914 |
| Keratin, type II cytoskeletal 6D# | P48667 | Structural/Cytoskeletal | 42468 |
| Keratin, type II cytoskeletal 6E | P48668 | Structural/Cytoskeletal | 59894 |
| Keratin, type II microfibrillar, component 7C# | P15241 | Structural/Cytoskeletal | 53682 |
| Lactoperoxidase precursor | P22079 | Redox | 80288 |
| Lactotransferrin precursor§ | P02788 | Transport | 78182 |
| Leukotriene A-4 hydrolase | P09960 | Energy/metabolism | 69154 |
| L-lactate dehydrogenase A chain§ | P00338 | Energy/metabolism | 36558 |
| L-lactate dehydrogenase B chain§ | P07195 | Energy/metabolism | 36507 |
| Long palate, lung and nasal epith. carc.ass. prot.1prec. | Q8TDL5 | Defense/Immunoresponse | 52442 |
| L-plastin§ | P13796 | Structural/Cytoskeletal | 70158 |
| Lysozyme C precursor | P61626 | Protein Degradation/Inhibitor | 16537 |
| Macrophage capping protein | P40121 | Structural/Cytoskeletal | 38518 |
| Malate dehydrogenase, cytoplasmic | P40925 | Energy/metabolism | 36295 |
| Maspin precursor | P36952 | Protein Degradation/Inhibitor | 42138 |
| Matrix metalloproteinase-9 precursor§ | P14780 | Protein Degradation/Inhibitor | 78427 |
| Maturase K# | Q9GI85 | Micro organism | 61017 |
| Metalloproteinase inhibitor 1 prec.§ | P01033 | Protein Degradation/Inhibitor | 23171 |
| Moesin | P26038 | Structural/Cytoskeletal | 67689 |
| Monocyte differentiation antigen CD14 precursor§,# | P08571 | Defense/Immunoresponse | 40076 |
| Mucin 5B precursor | Q9HC84 | Cell Adhesion/Communication | 590499 |
| Myeloperoxidase precursor | P05164 | Defense/Immunoresponse | 83869 |
| Myoglobin# | P02144 | Transport | 17053 |
| Myosin heavy chain, non-muscle type A§ | P35579 | Structural/Cytoskeletal | 226401 |
| N-acetylglucosamine kinase# | Q9UJ70 | Energy/metabolism | 37244 |
| Neutrophil gelatinase-associated lipocalin prec.§ | P80188 | Transport | 22588 |
| Outer membrane usher protein pefC precursor# | P37868 | Micro organism | 86370 |
| Peptidyl-prolyl cis-trans isomerase A | P62937 | Protein Folding/Repair | 17881 |
| Peroxiredoxin 5, mitochondrial precursor | P30044 | Redox | 22026 |
| Peroxiredoxin 6 | P30041 | Redox | 24904 |
| Phosphatidylethanolamine-binding protein | P30086 | Protein Degradation/Inhibitor | 20926 |
| Phosphoglucomutase§,# | P36871 | Energy/metabolism | 61318 |
| Phosphoglycerate kinase 1 | P00558 | Energy/metabolism | 44483 |
| Phosphoglycerate mutase 1 | P18669 | Energy/metabolism | 28673 |
| Phospholipid transfer protein prec.§,# | P55058 | Energy/metabolism | 54739 |
| Plasminogen activator inhibitor-2 prec.# | P05120 | Signalling | 46596 |
| Polymeric-immunoglobulin receptor precursor | P01833 | Defense/Immunoresponse | 83314 |
| Proactivator polypeptide precursor | P07602 | Protein Degradation/Inhibitor | 58113 |
| Profilin-1§ | P07737 | Structural/Cytoskeletal | 14923 |
| Prolactin-inducible protein precursor§ | P12273 | Defense/Immunoresponse | 16572 |
| Proline-rich protein 3 precursor peptide P-B) | P02814 | Unknown | 8188 |
| Prominin 1 precursor# | O43490 | Signalling | 97202 |
| Protein-glutamine glutamyltransferase E prec.§ | Q08188 | Energy/metabolism | 76632 |
| Purine nucleoside phosphorylase | P00491 | Energy/metabolism | 32118 |
| Pyruvate kinase, isozymes M1/M2§ | P14618 | Energy/metabolism | 57806 |
| Rab GDP dissociation inhibitor beta | P50395 | Signalling | 50663 |
| Ras-related C3 botulinum toxin substrate 2# | P15153 | Transport | 21429 |
| Rho GDP-dissociation inhibitor 2 | P52566 | Signalling | 22857 |
| S100 calcium-binding protein A7§ | P31151 | Cell Growth/Differentiation | 11326 |
| Salivary alpha-amylase precursor | P04745 | Energy/metabolism | 57768 |
| Serine/threonine-protein kinase BRI1- like 2 precursor# | Q9ZPS9 | Energy/metabolism | 39280 |
| Serine/threonine-protein kinase RIPK4# | P57078 | Energy/metabolism | 91611 |
| Serotransferrin precursor§ | P02787 | Transport | 77050 |
| Short palate, lung and nasal epith.carc.ass.prot.2prec. | Q96DR5 | Transport | 27011 |
| Small proline-rich protein 3 | Q9UBC9 | Structural/Cytoskeletal | 18154 |
| SPARC-like protein 1 precursor | Q14515 | Protein Degradation/Inhibitor | 75216 |
| Squamous cell carcinoma antigen 1§ | P29508 | Protein Degradation/Inhibitor | 44565 |
| Sugar fermentation stimulation protein homolog# | Q97VP5 | Micro organism | 27830 |
| Thioredoxin§ | P10599 | Redox | 11606 |
| Transaldolase | P37837 | Energy/metabolism | 37540 |
| Transcobalamin I precursor | P20061 | Transport | 48195 |
| Transgelin-2 | P37802 | Structural/Cytoskeletal | 22260 |
| Transketolase§ | P29401 | Energy/metabolism | 67878 |
| Triosephosphate isomerase isomerise§ | P60174 | Protein Folding/Repair | 26538 |
| Tyrosine recombinase xerC | Q8UC70 | Micro organism | 34532 |
| Ubiquitin# | O46543 | Protein Degradation/Inhibitor | 8583 |
| Von Ebner’s gland protein precursor | P31025 | Transport | 19250 |
| Zinc-alpha-2-glycoprotein precursor§ | P25311 | Energy/metabolism | 33872 |
| 95% confidence proteins | |||
| 14-3-3 protein sigma§ | P31947 | Signalling | 27774 |
| 30S ribosomal protein S20# | Q7VQL2 | Micro organism | 10264 |
| 50S ribosomal protein L5# | Q8CRH2 | Micro organism | 20236 |
| Acetyl-CoA acetyltransferase# | P45359 | Energy/metabolism | 41241 |
| Alanyl-tRNA synthetase# | Q971J4 | Micro organism | 103675 |
| Alpha-2-macroglobulin precursor§ | P01023 | Protein Degradation/Inhibitor | 163278 |
| Bact.l/permeability-increasing protein- like 1 prec. | Q8N4F0 | Transport | 49172 |
| Beta crystallin B1 (Beta-35)# | P53674 | Cell Growth/Differentiation | 27892 |
| Carbonyl reductase [NADPH] 1# | P16152 | Energy/metabolism | 30244 |
| Carcinoembryonic antigen-related cell adh. mol.5prec.§,# | P06731 | Defense/Immunoresponse | 76796 |
| Catalase§ | P04040 | Redox | 59625 |
| CD9 antigen (p24)# | P21926 | Cell Adhesion/Communication | 25285 |
| Chaperone protein htpG | P61185 | Micro organism | 73731 |
| Cystatin B# | Q862Z5 | Protein Degradation/Inhibitor | 11103 |
| Dihydrolipoyllysine-residue succinyltransferase# | P36957 | Energy/metabolism | 48640 |
| Ethanolamine utilization protein# | P41793 | Micro organism | 49174 |
| F-actin capping protein alpha-2 subunit# | P47755 | Structural/Cytoskeletal | 32818 |
| Ferredoxin II# | P00237 | Micro organism | 9962 |
| Genome polyprotein# | P17594 | Micro organism | 255428 |
| Glucose-6-phosphate 1-dehydrogenase# | P11413 | Energy/metabolism | 59135 |
| Heat shock protein HSP 90-beta# | P08238 | Protein Folding/Repair | 83133 |
| Hut operon positive regulatory protein# | P10943 | Micro organism | 16064 |
| Hypothetical protein ynaA# | P77658 | Micro organism | 37060 |
| Hypothetical UPF0135 protein CPn0137# | Q9Z946 | Micro organism | 27236 |
| Ig heavy chain V region UPC10§,# | P01811 | Defense/Immunoresponse | 13001 |
| Ig kappa chain V-II region TEW§ | P01617 | Defense/Immunoresponse | 12316 |
| Ig kappa chain V-III region NG9 precursor§,# | P01621 | Defense/Immunoresponse | 10729 |
| Myeloblastin precursor | P24158 | Protein Degradation/Inhibitor | 27807 |
| Potential phospholipid-transporting ATPase VA# | O60312 | Energy/metabolism | 167688 |
| Probable Na(+)/H(+) antiporter nhx-9 | P35449 | Micro organism | 75281 |
| Probable serine/threonine-protein kinase# | P28966 | Micro organism | 65248 |
| Pyruvate kinase, isozymes R/L# | P30613 | Energy/metabolism | 61830 |
| Rho GDP-dissociation inhibitor 1 | P52565 | Signalling | 23076 |
| Serum albumin precursor§ | P02768 | Transport | 69367 |
| Vinculin# | P18206 | Cell adhesion/Communication | 123668 |
| Zinc finger protein 446# | Q9NWS9 | Signalling | 48957 |
Proteins that are also found in plasma according to the HUPO Plasma Proteome Initiative list of plasma proteins (www.bioinformatics.med.umich.edu/app/hupo/ppp/).
Novel saliva proteins identified in this study compared to seven previous studies (see Fig. 7).
Figure 6.
Functional categories of identified proteins, based on information from Swiss Prot, Source and Human Protein Reference Database.
Figure 7.
Venn diagrams comparing the proteome results obtained with this study (A) versus those achieved by Xie et al. (Xie, 2005) (B), Hu et al. (Hu, 2005) (C), Vitorino et al. (Vitorino, 2004) (D), Walz et al. (Walz, 2006) (E), Huang et al. (Huang, 2004) (F), Wilmarth et al. (Wilmarth, 2004) (G), Guo et al. (Guo, 2006) (H). Only part of the data of the study by Guo et al. is publicly available and was used in this comparison.
Discussion
There is growing interest in using saliva as a diagnostic fluid because of its relatively simple and non-invasive collection procedures. A prerequisite for measuring diagnostic protein markers in saliva is that these proteins are stable in saliva. In this study we show that relatively rapid protein degradation occurs in whole saliva samples at room temperature. We developed a SELDI-based assay to quickly monitor sample integrity. With this assay we show that protein degradation in saliva at room temperature is rapid and starts already during sample collection and handling. Three degradation products with masses of 2937 Da, 3370 Da and 4132 Da were discovered that can be used to monitor the degradation process and to determine the quality of a saliva sample before protein analyses. These markers increase 2 to 7-fold over a period of 4 hrs storage at room temperature, suggesting they are stable breakdown products of larger proteins. The proteome analyses indicates that there are at least six proteases present in saliva (see Table 2) which are probably involved in the observed protein degradation. However, also 13 protease inhibitor proteins were identified which may counteract protease activity. Nevertheless, the overall balance is clearly in favour of degradation. Protein breakdown in saliva could be partly inhibited by a protease inhibitor cocktail targeting serine, cysteine and metallo-proteases. Also in another study, that investigated storage of saliva samples at different temperatures, only partially prevention of degradation was observed with a different inhibitor cocktail (Schipper, 2007). More research is clearly needed to find more effective protease inhibitor cocktails to prevent degradation. A complicating factor in such studies will be that many protease inhibitors are peptides themselves or covalently bind to proteins thereby changing their masses. Both will interfere with proteomics measurements in biomarker discovery studies but may not interfere with ELISA-based measurements of individual proteins. Based on the results of our study we recommend to freeze samples immediately after collection, e.g. in liquid nitrogen, to minimize protein breakdown. Sample processing at 4 °C as well as the use of protease inhibitors can help to reduce degradation. Based on a study by Schipper et al. (Schipper, 2007) long time storage at −80 °C is recommended.
Several different strategies have been employed to analyze the saliva proteome such as 2 D gel (Huang, 2004; Wilmarth, 2004; Vitorino, 2004; Hu, 2005; Walz, 2006), capillary iso-electric focusing (Guo, 2006), and free flow electrophoresis (Xie, 2005). Our approach was to fractionate saliva proteins by SDS-PAGE followed by LC-MS/MS for protein identification. Overall 218 proteins were identified by this proteomics strategy, not applied to saliva before. From the identified proteins we deduced the main functions. These are: carbohydrate-breakdown, immune response/defence against bacteria, and protein degradation. By comparing our results with seven previous proteomics studies (Fig. 7) on whole saliva composition we find only partial overlap with our study. 83 new saliva proteins from our study which were not previously identified are indicated in Table 2. These results show that with each proteomics strategy, partial overlapping subsets of saliva proteins are identified. Therefore, different proteomic approaches will contribute to a more comprehensive view of the saliva proteome. Many of the identified proteins are also found in plasma. Comparison with the HUPO plasma proteome database learned that 38.5% of the identified proteins can also be found in plasma. This relatively high percentage of plasma proteins in saliva illustrate the possibilities for use of saliva as an alternative to blood for diagnosis and biomarker discovery. However, protein breakdown in saliva samples poses a serious problem for quantitative measurements. We conclude that saliva can be a promising diagnostic fluid when precautions are taken towards protein breakdown.
Acknowledgments
This work was supported by the Netherlands Proteomic Centre (project 6.3.)
References
- Baum BJ. Principles of saliva secretion. . Ann. N. Y. Acad. Sci. 1993;694:17–23. doi: 10.1111/j.1749-6632.1993.tb18338.x. [DOI] [PubMed] [Google Scholar]
- Belazi MA, Galli-Tsinopoulou A, Drakoulakos D, et al. Salivary alterations in insulin-dependent diabetes mellitus. Int. J. Paediatr. Dent. 1998;8:29–33. doi: 10.1046/j.1365-263x.1998.00057.x. [DOI] [PubMed] [Google Scholar]
- Chicharro JL, Lucia A, Perez M, et al. Saliva composition and exercise. . Sports Med. 1998;26:17–27. doi: 10.2165/00007256-199826010-00002. [DOI] [PubMed] [Google Scholar]
- Dawes C. Considerations in the development of diagnostic tests on saliva. . Ann. N. Y. Acad. Sci. 1993;694:265–9. doi: 10.1111/j.1749-6632.1993.tb18359.x. [DOI] [PubMed] [Google Scholar]
- Frerichs RR, Silarug N, Eskes N, et al. Saliva-based HIV-antibody testing in Thailand. AIDS. 1994;8:885–94. doi: 10.1097/00002030-199407000-00004. [DOI] [PubMed] [Google Scholar]
- Guo T, Rudnick PA, Wang W, et al. Characterization of the human salivary proteome by capillary isoelectric focusing/nanoreversed-phase liquid chromatography coupled with ESI-tandem MS. . J. Proteome. Res. 2006;5:1469–78. doi: 10.1021/pr060065m. [DOI] [PubMed] [Google Scholar]
- Heine RP, McGregor JA, Goodwin TM, et al. Serial salivary estriol to detect an increased risk of preterm birth. . Obstet. Gynecol. 2000;96:490–7. doi: 10.1016/s0029-7844(00)01004-8. [DOI] [PubMed] [Google Scholar]
- Helenius LM, Meurman JH, Helenius I, et al. Oral and salivary parameters in patients with rheumatic diseases. Acta. Odontol. Scand. 2005;63:284–93. doi: 10.1080/00016350510020043. [DOI] [PubMed] [Google Scholar]
- Holmstrom P, Syrjanen S, Laine P, et al. HIV antibodies in whole saliva detected by ELISA and western blot assays. . J. Med. Virol. 1990;30:245–8. doi: 10.1002/jmv.1890300403. [DOI] [PubMed] [Google Scholar]
- Hu S, Xie Y, Ramachandran P, et al. Large-scale identification of proteins in human salivary proteome by liquid chromatography/mass spectrometry and two-dimensional gel electrophoresis-mass spectrometry. Proteomics. 2005;5:1714–28. doi: 10.1002/pmic.200401037. [DOI] [PubMed] [Google Scholar]
- Huang CM. Comparative proteomic analysis of human whole saliva. . Arch. Oral. Biol. 2004;49:951–62. doi: 10.1016/j.archoralbio.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Imanguli MM, Atkinson JC, Harvey KE, et al. Changes in salivary proteome following allogeneic hematopoietic stem cell transplantation. . Exp. Hematol. 2007;35:184–192. doi: 10.1016/j.exphem.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman E, Lamster IB. Analysis of saliva for periodontal diagnosis—a review. . J. Clin. Periodontol. 2000;27:453–65. doi: 10.1034/j.1600-051x.2000.027007453.x. [DOI] [PubMed] [Google Scholar]
- Kaufman E, Lamster IB. The diagnostic applications of saliva—a review. . Crit. Rev. Oral Biol. Med. 2002;13:197–212. doi: 10.1177/154411130201300209. [DOI] [PubMed] [Google Scholar]
- Lawrence HP. Salivary markers of systemic disease: noninvasive diagnosis of disease and monitoring of general health. . J. Can. Dent. Assoc. 2002;68:170–4. [PubMed] [Google Scholar]
- Lenander-Lumikari M, Ihalin R, Lahteenoja H. Changes in whole saliva in patients with coeliac disease. . Arch. Oral Biol. 2000;45:347–54. doi: 10.1016/s0003-9969(00)00008-x. [DOI] [PubMed] [Google Scholar]
- Lopez ME, Colloca ME, Paez RG, et al. Salivary characteristics of diabetic children. . Braz. Dent. J. 2003;14:26–31. doi: 10.1590/s0103-64402003000100005. [DOI] [PubMed] [Google Scholar]
- Malamud D. Saliva as a diagnostic fluid. . BMJ. 1992;305:207–8. doi: 10.1136/bmj.305.6847.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda S, Oka S, Honda M, et al. Characteristics of IgA antibodies against HIV-1 in sera and saliva from HIV-seropositive individuals in different clinical stages. . Scand J. Immunol. 1993;38:428–34. doi: 10.1111/j.1365-3083.1993.tb02584.x. [DOI] [PubMed] [Google Scholar]
- Merchant M, Weinberger SR. Recent advancements in surface-enhanced laser desorption/ionization-time of flight-mass spectrometry. Electrophoresis. 2000;21:1164–77. doi: 10.1002/(SICI)1522-2683(20000401)21:6<1164::AID-ELPS1164>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Morris M, Cohen B, Andrews N, et al. Stability of total and rubella-specific IgG in oral fluid samples: the effect of time and temperature. J. Immunol. Methods. 2002;266:111–116. doi: 10.1016/s0022-1759(02)00114-x. [DOI] [PubMed] [Google Scholar]
- Navazesh M. Methods for collecting saliva. . Ann. N. Y. Acad. Sci. 1993;694:72–7. doi: 10.1111/j.1749-6632.1993.tb18343.x. [DOI] [PubMed] [Google Scholar]
- Ng V, Koh D, Fu Q, et al. Effects of storage time on stability of salivary immunoglobulin A and lysozyme. . Clin. Chim. Acta. 2003;338:131–134. doi: 10.1016/j.cccn.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Ramsey PS, Andrews WW. Biochemical predictors of preterm labor: fetal fibronectin and salivary estriol. Clin. Perinatol. 2003;30:701–33. doi: 10.1016/s0095-5108(03)00109-x. [DOI] [PubMed] [Google Scholar]
- Ryu OH, Atkinson JC, Hoehn GT, et al. Identification of parotid salivary biomarkers in Sjogren’s syndrome by surface-enhanced laser desorption/ionization time-of-flight mass spectrometry and two-dimensional difference gel electrophoresis. Rheumatology. 2006;45:1077–86. doi: 10.1093/rheumatology/kei212. [DOI] [PubMed] [Google Scholar]
- Schipper R, Loof A, de Groot J, et al. SELDI-TOF-MS of saliva: Methodology and pre-treatment effects. . J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2007;847:45–53. doi: 10.1016/j.jchromb.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Streckfus C, Bigler L. The use of soluble, salivary c-erbB.-2 for the detection and post-operative follow-up of breast cancer in women: the results of a five-year translational research study. . Adv. Dent. Res. 2005;18:17–24. doi: 10.1177/154407370501800105. [DOI] [PubMed] [Google Scholar]
- Streckfus CF, Bigler LR, Zwick M. The use of surface-enhanced laser desorption/ionization time-of-flight mass spectrometry to detect putative breast cancer markers in saliva: a feasibility study. . J. Oral Pathol. Med. 2006;35:292–300. doi: 10.1111/j.1600-0714.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- Vitorino R, Lobo MJ, Ferrer-Correira AJ, et al. Identification of human whole saliva protein components using proteomics. Proteomics. 2004;4:1109–15. doi: 10.1002/pmic.200300638. [DOI] [PubMed] [Google Scholar]
- Walz A, Stuhler K, Wattenberg A, et al. Proteome analysis of glandular parotid and submandibular-sublingual saliva in comparison to whole human saliva by two-dimensional gel electrophoresis. Proteomics. 2006;6:1631–39. doi: 10.1002/pmic.200500125. [DOI] [PubMed] [Google Scholar]
- Wilmarth PA, Riviere MA, Rustvold DL, et al. Two-dimensional liquid chromatography study of the human whole saliva proteome. . J. Proteome. Res. 2004;3:1017–23. doi: 10.1021/pr049911o. [DOI] [PubMed] [Google Scholar]
- Xie H, Rhodus NL, Griffin RJ, et al. A catalogue of human saliva proteins identified by free flow electrophoresis-based peptide separation and tandem mass spectrometry. Mol. Cell. Proteomics. 2005;4:1826–30. doi: 10.1074/mcp.D500008-MCP200. [DOI] [PubMed] [Google Scholar]