Abstract
Background
Patients with end-stage renal disease (ESRD) undergoing hemodialysis (HD) are apparently exposed to enhanced oxidative stress and to inflammation. It was the aim of this study to characterize the state of systemic oxidative stress of ESRD patients before and following HD using highly specific biomarkers, F2-isoprostanes and 4-hydroxynonenal (HNE). Furthermore the question should be answered, if there are associations between inflammation and systemic oxidative stress and/or between systemic oxidative stress and renal anemia, which is more or less typical for HD patients.
Patients and methods
Concentrations of F2-isoprostanes, HNE, C-reactive protein (CRP) as marker of inflammation, and hemoglobin were measured in serum samples of patients with ESRD before and after HD and of healthy control persons for comparison. Total (esterified plus free) F2-isoprostanes were quantified by highly sensitive gas chromatography/mass spectrometry technique, HNE by thin layer chromatography and HPLC/UV detection, CRP by immunoturbidimetry and hemoglobin by clinico-chemical routine assay.
Results
1. HD patients showed significantly higher serum concentrations of F2-isoprostanes and HNE than healthy human control subjects. 2. Total (esterified plus free) F2-isoprostane levels before HD were not significantly different from those after HD, whereas HNE levels were significantly decreased in patients after HD. 3. F2-isoprostane concentrations in HD patients correlated with the levels of CRP, whereas HNE concentrations inversely correlated with the content of hemoglobin.
Conclusion
Both, F2-isoprostanes and HNE serum concentrations are useful oxidative stress parameters in ESRD patients undergoing HD. Whereas HNE strongly correlates with the severity of renal anemia, leading to left heart insufficiency, F2-isoprostanes (sum of free plus esterified) highly correlate with the degree of inflammation.
Keywords: F2-isoprostanes, end-stage renal disease (ESRD), hemodialysis patients, C-reactive protein, 4-hydroxynonenal, renal anemia
Introduction
Hemodialysis (HD) in end-stage renal disease (ESRD) patients is associated with the development of oxidative stress, caused by the impairment of antioxidant defences and the bioincompatibility of the dialysis technique contributing to cardiotoxic effects (Canaud et al. 1999).
Reactive oxygen species (ROS) are known to play a role in the pathogenesis and progression of chronic renal failure (CRF). Apart from the kidney disease itself and the uremia, numerous other factors appear to contribute to oxidative stress (Trachtman et al. 1992), including HD via contact activation of neutrophils by the artificial surfaces of the dialysis membrane and the tubing system (Westhuyzen et al. 1995).
Increased levels of oxidants (e.g. malonic dialdehyde) and lower levels of antioxidants (glutathione, α-tocopherol) in CRF patients play an important role in the development of endothelial dysfunction, atherogenesis and cardiovascular disease (Loughrey et al. 1994; Delmas Beauvieux et al. 1995; Ceballos-Picot et al. 1996; Cavdar et al. 1997; Peuchant et al. 1997; Sommerburg et al. 1998; Ludat et al. 2000; Siems et al. 2002a; Siems et al. 2002b). A more specific biomarker of oxidative stress represents HNE, one of the major end products of lipid peroxidation generated mainly from arachidonic acid. HNE has cytotoxic and mutagenic properties, induces apoptosis, NF-κB activation and inhibition of enzyme activities (Esterbauer et al. 1991; Sommerburg et al. 1993; Grune et al. 1994; Siems et al. 1996; Sommerburg et al. 1998; Uchida et al. 1999; Ruef et al. 2001).
Previously, first reports on F2-isoprostanes as indicators of lipid peroxidation in renal diseases were published (Spittle et al. 2001; Handelman et al. 2001; Ikizler et al. 2002; Lim et al. 2002; Danielski et al. 2003; Ferraro et al. 2003; Kim et al. 2004; Wiswedel et al. 2005). F2-Isoprostanes have been established as chemically stable, highly specific and reliable biomarkers of in vivo oxidative stress, which can be very sensitively measured by gas chromatography/mass spectrometry (Morrow et al. 2002). Measurements in dialysis patients before dialysis showed higher plasma levels of F2-isoprostanes than in control subjects (Spittle et al. 2001; Handelman et al. 2001). But the data are not unique: in contrast to the data of Spittle et al. (2001) and of Handelman et al. (2001), Dogra et al. (2001) did not find an increase in plasma and urinary F2-isoprostanes in patients with nephrotic syndrome.
Both arachidonic acid oxidation products, F2-isoprostanes and HNE, are known to be involved in atherogenesis and myocardial injury (Blasig et al. 1995; Ruef et al. 2001; Morrow et al. 2002), which is the most critical point of life quality and mortality in CRF (Foley et al. 1995; Silverberg et al. 2000). There are also strong interactions between both types of cardiovascular risk factors, e.g. HNE induces the formation of 8-iso-PGF2α in vascular smooth muscle cells (Ruef et al. 2001).
HD patients are not only exposed to enhanced oxidative stress, but also to inflammation (Spittle et al. 2001). Cardiovascular mortality remains high in ESRD patients, particularly in the group with evidence for inflammation (Yeun and Kaysen 2000). Inflammation may enhance oxidative stress through increased free radical generation via activation of NADPH oxidase and may directly modify proatherogenic factors.
The aim of this paper is 1. to determine F2-isoprostanes and HNE in the serum of ESRD patients before and following HD and for comparison in healthy control persons and 2. to examine if the markers of oxidative stress are correlated to the degree of inflammation and/or to the degree of renal anemia.
Patients and Methods
Patients
Patients and probands were required to provide written informed consent before the start of the evaluation. The place of dialysis was at the dialysis centre of Scorrano, Italy. Inclusion criteria of patients were as follows: presence of renal anemia (hemoglobin concentration<11 g/dl, time on dialysis >6 months, treatment in the center >3 months, normal values of GOT, GPT, LDH and γ-GT. Among the egligible patients the anamnesis about their life style required to exclude 12 persons because of smoking. Further criteria of exclusion were: patients taking antioxidants, patients with acute infection, with considerable iron overload, with an acute phase of rheumatological disorders and with neoplasiae. Furthermore, HD patients without well-functioning vascular excess and patients treated by vitamin E-bounded membranes were excluded. Only one patient was excluded at the end of the dialysis study because of wrong sampling. 14 patients came with hypertension. We aimed a strict control of hypertension with a target blood pressure ≤130/80 mm Hg (β-receptor blocker, ACE-I inhibitors, angiotensin II receptor blocker). For the investigated patients the “dry” weight was defined by impedenziometry and the “dry” weight was reached at the end of the HD session. 12 patients had intima media thickness and five of them had also documented one or more carotid plaques measured by B-mode ultrasonography. For 11 patients a left ventricular hypertrophy was documented, 16 patients were treated with erythropoietin. All patients were treated three times/week. High flux dialysis filters consisting of polysulphone/polyacrylonitrile were used. The evaluation was done at the midweek dialysis session. The participants of the control group were healthy persons from the dialysis centers in Scorrano and Berlin (nurses, doctors, technicians etc.). F2-isoprostane concentrations (8-iso-PGF2α and 9α,11α-PGF2α) and HNE in correlation to CRP and hemoglobin content were analysed in 14 non-diabetic HD patients with chronic renal failure (6 male, 8 female, middle age 59 ± 14 years). F2-isoprostanes before and following hemodialysis were compared in 19 non-diabetic ESRD patients (5 additional patients: 2 male, 3 female, from the same age group). There were also control groups for analysis of F2-isoprostanes with 65 (30 male, 35 female, middle age 55 ± 15 years) and for analysis of HNE with 131 healthy probands (77 male and 54 female, middle age 60 ± 10 years).
Sample extraction, hydrolysis and purification of F2-isoprostanes
The determination of the concentrations of F2-isoprostanes (sum of esterified and non-esterified isomers) was carried out as described previously (Wiswedel et al. 2004) with some modifications. To hydrolyze esterified lipids, the serum samples (1.0 mL) were treated with 320 μL of KOH (1 mol/L) at 40 °C for 30 min. Thereafter, the samples were neutralized by addition of 3 mL HCl (0.1 mol/L), and the pH was adjusted to 2 with HCl (1 mol/L). 9α,11α-PGF2α-d4 (1 ng in 20 μL ethanol) was added as internal standard. The samples were centrifuged at 5,000 × g for 15 min, and the supernatant was applied onto a C18-cartridge, pre-washed with 5 mL of methanol and 5 mL of water. The cartridge was then washed with 10 mL HCl (0.1 mol/L) and 10 mL of acetonitrile/water (15/85, v/v). Isoprostanes were eluted from the column with 5 mL of n-hexane/ethyl acetate/2-propanol (30/65/5, v/v/v). The prostanoid extract was applied then onto a NH2-cartridge, pre-washed with n-hexane (10 mL). The column was successively washed with 10 mL of n-hexane/ethyl acetate (30/70, v/v), acetonitrile/water (90/10, v/v) and acetonitrile. Finally, F2-isoprostanes were eluted with 5 mL of ethyl acetate/methanol/acetic acid (10/85/5, v/v/v).
Derivatization and GC-MS/negative ion chemical ionization analysis
The extracts from the NH2-chromatography step were evaporated to dryness under a stream of argon at 45 °C. The residues were reconstituted with 40 μL of pentafluorobenzyl-bromide (10% in acetonitrile) and 20 μL of N,N-diisopropylethylamine (10% in acetonitrile) and incubated at 45 °C for 30 min. Thereafter, 50 μL of bis-(trimethylsilyl) trifluoroacetamide (BSTFA) and 5 μL of N,N-diisopropylethylamine were added to the dried sample. The samples were kept at 45 °C for 45 min, the solvents were removed, and the samples were reconstituted in 40 μL isooctane containing 0.1% BSTFA. F2-isoprostanes were separated by GC/MS using the following temperature program: initial temperature of 175 °C for 2 min; with a rate of 30 °C/min to a final temperature of 270 °C maintained for 20 min; total run time: 25.2 min. Quantitative analysis was performed with ammonia as reagent gas using selected ion monitoring (SIM) of the carboxylate anion [M-181]− at m/z 569 and 573 for F2-isoprostanes and 9α,11α-PGF2α-d4, respectively. The m/z 569 ion current chromatogram showed the occurrence of two major peaks in serum samples, peak I co-eluting with authentic 8-iso-PGF2α and peak II co-eluting with authentic 9α,11α-PGF2α. Peak I is expected to contain exclusively F2-isoprostanes, whereas peak II may contain both, mainly F2-isoprostanes, but additionally cyclooxygenase-derived PGF2α (Wiswedel et al. 2004).
The response factors for the isoprostane isomers were 1.00 and 0.55 for 9α,11α- and 8-iso-PGF2α, respectively (means of three separate determinations).
F2-isoprostane analyses were performed in triplicate throughout for each plasma sample.
Determination of CRP and hemoglobin
CRP was measured by immunoturbidimetry (Grützmeier and von Schenck, 1989), and hemoglobin with spectrophotometric methods within the clinical laboratory routine parameters. CRP and hemoglobin concentrations were 16.6 ± 3.8 mg/L serum resp. 9.75 ± 0.64 g/dl (mean ± SEM n = 14) for HD patients and 3.3 ± 0.1 resp. 15.1 ± 0.4 (mean ± SEM, n = 131; p < 0.05 resp. p < 0.01; patients versus controls) for healthy control persons.
4-Hydroxynonenal (HNE) analysis
The measurement of HNE includes the modification of this aldehyde with dinitrophenylhydrazine, the thin layer chromatographic analysis of the dinitrophenylhydrazones in three groups, and finally the isocratic analysis of the dinitrophenylhydrazine derivatives of the 4-hydroxyalkenales (Esterbauer et al. 1982; Grune et al. 1994; Sommerburg et al. 1993). During the HPLC-analysis a mixture of methanol and water (4:1, v:v) was used as an eluent. The detection-wavelength was set to 378 nm. The HNE-standard was produced from the diacetale which was stored in a chloroform solution at −20 °C.
Statistical analyses
All data are presented as mean ± SEM. Comparisons between independent groups (patients versus controls) have been performed with the two-sample t test, comparisons between dependent groups (before and after dialysis) with the t test for paired data. Correlations between variables have been investigated with linear regression and Pearson’s correlation coefficient. All tests have been carried out two-sided and P values below 0.05 were considered to be significant.
Results
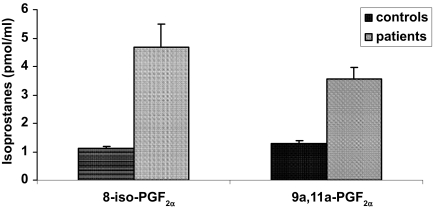
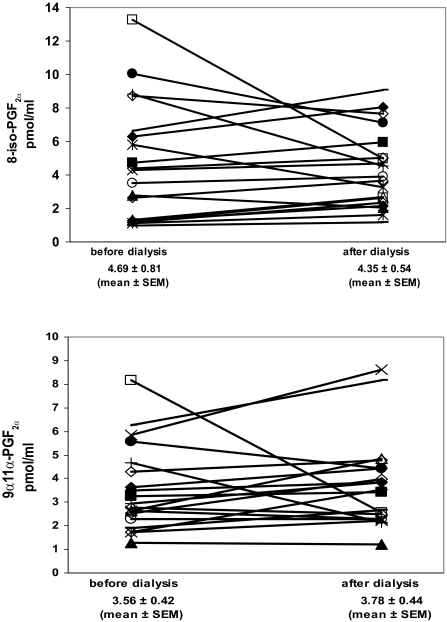
Data concerning serum concentrations of F2-isoprostanes as biomarkers of the oxidative status of HD patients and normal control persons are shown in Figure 1. It can be seen that HD patients (4.69 ± 0.81 and 3.56 ± 0.42 pmol/mL) exhibited 4.2-fold (8-iso-PGF2α) and 2.8-fold (9α,11α-PGF2α) higher F2-isoprostane serum levels than healthy controls (1.12 ± 0.05 and 1.28 ± 0.11 pmol/mL). Furthermore, F2-isoprostane concentrations did not significantly change in patients before and following hemodialysis, as was shown in Figure 2a and 2b.
Figure 1.
F2-Isoprostane concentrations (8-iso-PGF2α and 9α,11α-PGF2α) in serum samples of patients (n = 19) and control persons (n = 65) Mean values ± SEM; two-sample t test; p < 0.001 versus control.
Figure 2.
F2-Isoprostane concentrations [8-iso-PGF2α (a) and 9α,11α-PGF2α (b)] in serum samples of patients before and after hemodialysis (n = 19). The differences are not significant (t test for paired data).
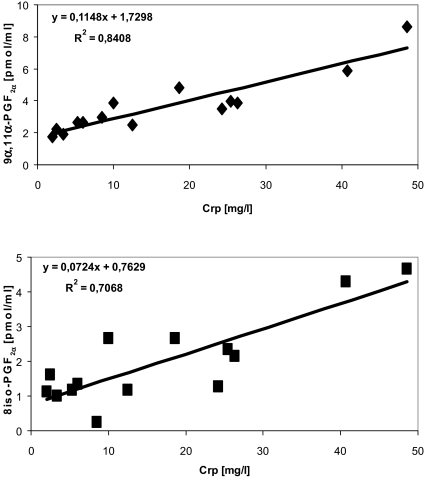
For 8-iso-PGF2α and 9α,11α-PGF2α, correlations with a representative parameter of inflammation (CRP) and with a representative parameter of the degree of renal anemia (hemoglobin concentration) were examined. Figure 3 shows the correlations of F2-isoprostane concentrations [9α,11α-PGF2α (Fig. 3a) and 8-iso-PGF2α (Fig. 3b)] and CRP as parameter for inflammatory processes in the patients. We demonstrated a positive correlation of total (esterified plus free) F2-isoprostane isomers with the degree of inflammation, whereas no correlation between F2-isoprostanes and the degree of renal anemia (hemoglobin content) was found (not documented here).
Figure 3.
Correlation between levels of F2-isoprostanes [8-iso-PGF2α (a) and 9a,11a-PGF2α (b)] and C-reactive protein; n = 14 patients. Correlations have been investigated with linear regression analysis.
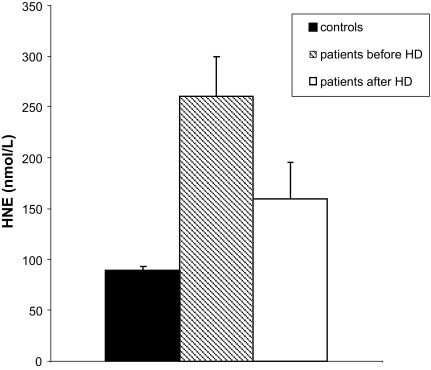
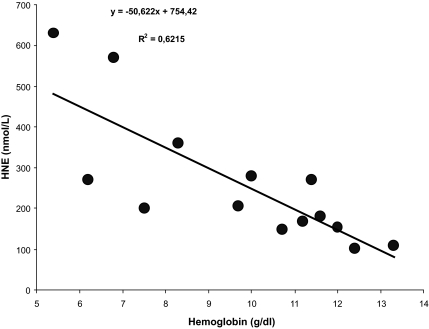
In contrast, HNE, formed as one of the main degradation products of arachidonic acid, which is significantly increased in serum samples of ESRD patients (Fig. 4a), changed in dependence on hemoglobin concentrations, i.e. the HNE levels were higher with decreasing hemoglobin. It showed, therefore, an inverse relationship (significant negative correlation) with the degree of renal anemia (Fig. 4b). However, no significant correlation between the levels of HNE and the C-reactive protein (degree of inflammation) was observed (not shown).
Figure 4a.
Serum concentrations of 4-hydroxynonenal in patients with chronic renal failure before and after hemodialysis (n = 14) compared to control persons (n = 131). Mean ± SEM, two-sample t test for comparison between patients and controls (p < 0.001; before HD vs. control and p = 0.002; after HD vs. control) and t test for paired data for comparison between patients before and after dialysis (p < 0.001).
Figure 4b.
Correlation between HNE concentrations and hemoglobin content (n = 14 patients).
Correlations have been investigated with linear regression analysis.
Discussion
Chronic inflammation in HD patients
Many of ESRD and HD patients are in the state of chronic inflammation. The reason why renal failure is a source of inflammation has not been fully elucidated. A multifactorial origin of inflammation may be taken in consideration (Stenvinkel and Alvestrand, 2002). These include renal insufficiency (leading in the end stage to uremia) and its complications; as already established atherosclerosis, diabetes, hypertension; consequences of renal replacement treatment; back-leakage of endotoxins from contaminated dialysate and frequent infections (Tsirpanlis, 2005). Less biocompatible HD methods may contribute to the enhanced stimulation of neutrophiles and monocytes (through dialysis membrane interaction), the induction of cytokines, as IL-1, IL-6, TNFα and the increase of CRP (Morena et al. 2005). Even at initial stages of chronic renal failure, the CRP level is elevated (Tsirpanlis, 2005). CRP could be a sensitive risk index of the overall morbidity and mortality in CRF patients. As an acute phase protein with high sensitivity and low specificity, CRP is mainly synthesized in the liver and may be regulated largely by circulating levels of IL-6 (Thaunat et al. 2005; Vollenbroeker et al. 2005).
CRP has many features that may contribute to an inflammatory process: it binds to damaged cells and activates the complement system. It binds to atherogenic lipoproteins and induces aggregation of LDL and VLDL in vitro; and stimulates thrombus formation (Galle et al. 2003). Bergstrom et al. (2000) and Galle et al. (2003) reported that CRP is 5–10-fold higher in HD patients than in healthy controls (16.3 vs. 1.8 mg/L), which is associated with an increased cardiovascular risk. In the group of patients which we investigated the values were 16.6 vs. 3.3 mg/L, i.e. quite similar.
Inflammation may be mainly responsible for oxidative stress
Inflammation is discussed as a well-documented factor influencing the development of oxidative stress in dialysis patients (Wratten et al. 2002). The way by which inflammation can contribute to oxidative stress in chronic renal failure is via activation of NADPH oxidase by interleukins and anaphylatoxins produced during HD sessions and/or myeoloperoxidase (Morena et al. 2005). Further sources of ROS are the mitochondrial respiratory chain, lipoxygenases, cyclooxygenases, xanthin oxidase, and NO synthase.
Both ROS and their secondary products are involved in the pathophysiological processes in ESRD. Oxidative stress, resulting from imbalance between oxidant production and antioxidative defence mechanisms, has been documented in ESRD patients using lipid, protein, nucleic acid and carbohydrate oxidative markers (Paleschi et al. 2007; Rutkowski et al. 2007; Coskun et al. 2007; Piroddi et al. 2007; Pai et al. 2007).
A highly significant positive correlation was observed between logCRP and reactive oxygen metabolites in HD patients by Samouilidou et al. 2007. F2-isoprostanes, associated with the inflammation process in patients on HD, positively correlated with CRP (Handelman et al. 2001; Ikizler et al. 2002) as also described in this paper. HNE, however, as readily diffusible molecule upon dialysis doesn’t follow the increase of CRP in chronic renal failure (Siems et al. 2002b and this study). Interestingly, an inverse correlation between HNE and hemoglobin serum concentrations could be demonstrated and therefore a role of HNE as predictor of hemolytic anemia was suggested.
Esterified F2-isoprostanes may be not only reliable biomarkers of oxidative stress, but also mediators of inflammation
Measurement of F2-isoprostanes has advantages over other quantitative markers of oxidative stress: they are chemically stable, specific products of lipid peroxidation, they are formed in vivo and are present in all normal tissues and biological fluids (Montuschi et al. 2004). GC-MS is the reference analytical method for isoprostane measurement in biological fluids and tissues. F2-isoprostanes are widely accepted to correlate with the cardiovascular risk in human beings (Ikizler et al. 2002). This is an additional argument for the use of F2-isoprostanes in ESRD, which is closely connected with cardiac insufficiency.
F2-isoprostanes have been found to exert potent biological actions and therefore may participate as pathophysiological mediators of disease (Montuschi et al. 2004). In patients with lung diseases, 8-iso-PGF2α concentrations in exhaled breath condensate reflect the degree of airway inflammation (Montuschi et al. 2000) with high levels reported in patients with chronic obstructive pulmonary disease and in smokers. 8-iso-PGF2α is not only known as a pulmonary, but also as a potent renal vasoconstrictor. In the rat, F2-isoprostanes reduce glomerular filtration rate and renal blood flow by 40%–45% in the low nanomolar range (Takahashi et al. 1992). F2-isoprostanes have important in vitro activities that could be relevant to the pathophysiology of atherosclerosis. They promote platelet activation (Patrono and Fitzgerald, 1997) and induce mitogenesis in vascular smooth muscle cells (Fukunaga et al. 1993). Moreover, F2-isoprostane formation is increased during LDL oxidation in vitro and F2-isoprostanes are major contributors to the proadhesive effect induced by minimally oxidatively modified LDL on neutrophils (Fontana et al. 2002). F2-isoprostanes exert their biological effects by receptor-mediated interactions (acting as full or partial agonists at thromboxane receptors) and also by receptor-independent mechanisms (Montuschi et al. 2004).
F2-isoprostane serum levels of HD patients in this study were significantly increased versus healthy control persons. This is in agreement with other reports (Walter et al. 2000; Handelman et al. 2001; Lim et al. 2002). But, there were no significant differences of F2-isoprostane levels before and after HD, as was also found by Handelman et al. (2001), and in contrast to Kim et al. (2004), who observed 4-fold higher levels post hemodialysis.
Both human and experimental studies have indicated associations of F2-isoprostanes and severe inflammatory conditions, diabetes and atherosclerosis. Recently it was shown that intravenous administration of 8-iso-PGF2α induced cyclooxygenase-mediated prostaglandin (PGF2α) formation and activation of inflammatory responses (Basu, 2004). In another recent study, 8-iso-PGF2α induced IL-8 expression in human macrophages, a chemokine involved in inflammation and atherogenesis through mitogen-activated protein kinases (Scholz et al. 2003). These studies emphasize that F2-isoprostranes might be mediators of inflammation involving cyclooxygenases and/or cytokines and promote a possible link between oxidative stress and inflammation.
Increased concentrations of F2-isoprostanes are related with high concentrations of CRP. As in our measurements also in those of Spittle et al. (2001) and Handelman et al. (2001) was found that F2-isoprostanes correlate to the concentration of C-reactive protein as one of the most important factors in predicting cardiovascular mortality, which is by far the main cause of mortality in ESRD patients with cardio-renal anemia syndrome (Silverberg et al. 2003; 2004a, 2004b).
Conclusions and outlook
In this study it was shown that ESRD patients undergoing hemodialysis are exposed to enhanced oxidative stress and to inflammation. The main limitations of this study were the relatively small number of patients and the obviously incomplete consideration of all parameters influencing inflammation and/or oxidative stress (e.g. lipid metabolism, blood pressure, cardiovascular disorders).
Biomarkers of oxidative stress, F2-isoprostanes and HNE, are significantly increased in the serum of patients and are highly correlated either to the degree of inflammation or renal anemia as well. Inflammation and oxidative stress may act synergistically to increase cardiovascular morbidity and mortality risk in the patients. The correction of anemia (e.g. by erythropoietin) reduces the oxidative stress to some extent, and also the cardiovascular risk. The prevention of oxidative stress in HD might, however, also focus on improving the hemocompatibility of the dialysis system, the supplementation of antioxidants and the modulation of NADPH oxidase by pharmacological approaches. In order to prove the effectiveness of therapeutic interventions, further efforts are obligatory. These include the enlargement and proper selection of suitable biomarkers that can be related to the pathogenesis and the development of the disease. Concerning F2-isoprostanes and partially HNE, most of the strict criteria for validation of the use of a biomarker in vivo (Biomarker Definition Working Group, 2001) are appropriate, but they only do reflect the ROS-mediated oxidation of arachidonic acid. Further limitations are 1. that the analysis of the used biomarkers (in particular F2-isoprostanes) is labor-intensive and requires expensive equipment and 2. an increase in F2-isoprostanes locally in tissues is not detected by measuring systemic oxidant stress. For HD patients it should be taken into account that small molecules, as HNE and possibly free F2-isoprostanes (but not esterified) are washed out during dialysis. It would be necessary, therefore, to follow up the serum or plasma concentrations of esterified F2-isoprostanes and those of other biomarkers, which have to be defined, very thoroughly in longitudinal studies.
Acknowledgment
We gratefully acknowledge the important help and advice of the expert statistician PD Dr. Siegfried Kropf (Institute of Biometry und Informatics, Medical Faculty, Otto-von-Guericke University Magdeburg, Germany).
Footnotes
Disclosure
The authors report no conflicts of interest.
References
- Basu S. Isoprostanes: Novel bioactive products of lipid peroxidation. . Free Radic. Res. 2004;38:105–22. doi: 10.1080/10715760310001646895. [DOI] [PubMed] [Google Scholar]
- Beauchamp MH, Martinez-Bermudez AK, Gobeil F, et al. Role of thromboxane in retinal microvascular degeneration in oxygen-induced retinopathy. . J. Appl. Physiol. 2001;90:2279–99. doi: 10.1152/jappl.2001.90.6.2279. [DOI] [PubMed] [Google Scholar]
- Bergstrom J, Lindhol B, Lacson E, et al. What are the causes and consequences of the chronic inflammatory state in chronic dialysis patients? Seminars Dialysis. 2000;13:163–75. doi: 10.1046/j.1525-139x.2000.00044.x. [DOI] [PubMed] [Google Scholar]
- Blasig IE, Grune T, Schoenheit K, et al. 4-Hydroxynonenal, a novel indicator of lipid peroxidation for reperfusion injury of the myocardium. Am. J. Physiol., 269 (Heart Circ. Physiol) 1995;38:H14–22. doi: 10.1152/ajpheart.1995.269.1.H14. [DOI] [PubMed] [Google Scholar]
- Canaud B, Cristol J, Morena M, et al. Imbalance of oxidants and antioxidants in haemodialysis patients. . Blood Purif. 1999;17:99–106. doi: 10.1159/000014381. [DOI] [PubMed] [Google Scholar]
- Cavdar C, Camsari T, Semin I, et al. Lipid peroxidation and antioxidant activity in chronic hemodialysis patients with recombinant human erythropoietin. . Scand J. Urol. Nephrol. 1997;31:371–75. doi: 10.3109/00365599709030622. [DOI] [PubMed] [Google Scholar]
- Ceballos-Picot I, Witko-Sarsat V, Merad-Boudia M, et al. Glutathione antioxidant system as a marker of oxidative stress in chronic renal failure. . Free Radic. Biol. Med. 1996;21:845–53. doi: 10.1016/0891-5849(96)00233-x. [DOI] [PubMed] [Google Scholar]
- Coskun C, Kural A, Doventas Y, et al. Hemodialysis and protein oxidation products. . Ann. N. Y. Acad. Sci. 2007;1100:404–8. doi: 10.1196/annals.1395.045. [DOI] [PubMed] [Google Scholar]
- Danielski M, Ikizler TA, McMonagle E, et al. Linkage of hypoalbuminemia, inflammation, and oxidative stress in patients receiving maintenance hemodialysis therapy. . Am. J. Kidney Dis. 2003;42:286–94. doi: 10.1016/s0272-6386(03)00653-x. [DOI] [PubMed] [Google Scholar]
- Delmas Beauvieux MC, Combe C, Peuchant E. Evaluation of red blood cell lipid peroxidation in hemodialyzed patients during erythropoietin therapy supplemented or not with iron. Nephron. 1995;31:406–10. doi: 10.1159/000188510. [DOI] [PubMed] [Google Scholar]
- Dogra G, Ward N, Croft KD, et al. Oxidant stress in nephrotic syndrome: comparison of F(2)-isoprostanes and plasma antioxidant potential. . Nephrol. Dial. Transplant. 2001;16:1626–30. doi: 10.1093/ndt/16.8.1626. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Cheeseman KH, Dianzani MU, et al. Separation and characterization of the aldehydic products of lipid peroxidation stimulated by ADP-Fe2+ in rat liver microsomes. . J. Biochem. 1982;208:129–40. doi: 10.1042/bj2080129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. . Free Radic. Biol. Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Ferraro B, Galli F, Frei B, et al. Peroxynitrite-induced oxidation of plasma lipids is enhanced in stable hemodialysis patients. . Kidney Int. 2003;63:2207–13. doi: 10.1046/j.1523-1755.2003.00008.x. [DOI] [PubMed] [Google Scholar]
- Foley RN. The prognostic importance of left ventricular geometry in uremic cardiomyopathy. . J. Am. Soc. Nephrol. 1995;5:2024–31. doi: 10.1681/ASN.V5122024. [DOI] [PubMed] [Google Scholar]
- Fontana L, Giagulli C, Cominacini L, et al. β2 Integrin-dependent neutrophil adhesion induced by minimally modified low-density lipoproteins is mainly mediated by F2-isoprostanes. Circulation. 2002;106:2434–41. doi: 10.1161/01.cir.0000037223.92135.38. [DOI] [PubMed] [Google Scholar]
- Fukunaga M, Makita M, Roberts LJ, et al. Evidence for the existence of F2-isoprostane receptors on rat vascular smooth muscle cells. . Am. J. Physiol. 1993;264:1619–24. doi: 10.1152/ajpcell.1993.264.6.C1619. [DOI] [PubMed] [Google Scholar]
- Galle J, Seibold S, Wanner C. Inflammation in uremic patients: What is the link? . Kidney Blood Press. Res. 2003;26:65–75. doi: 10.1159/000070986. [DOI] [PubMed] [Google Scholar]
- Grützmeier S, von Schenck H. Four immunochemical methods for measuring C-reactive protein in plasma compared. . Clin. Chem. 1989;35:461–63. [PubMed] [Google Scholar]
- Grune T, Siems WG, Zollner H, Esterbauer H. Metabolism of 4-hydroxynonenal, a cytotoxic lipid peroxidation product, in Ehrlich mouse ascites cells at different proliferation stages. . Cancer Res. 1994;54:5231–35. [PubMed] [Google Scholar]
- Handelman GJ, Walter MF, Adhikarla R, et al. Elevated plasma F2-isoprostanes in patients on long-term hemodialysis. . Kidney Int. 2001;59:1960–66. doi: 10.1046/j.1523-1755.2001.0590051960.x. [DOI] [PubMed] [Google Scholar]
- Ikizler TA, Morrow JD, Roberts LJ, et al. Plasma F2-isoprostane levels are elevated in chronic hemodialysis patients. . Clin. Nephrol. 2002;58:190–97. doi: 10.5414/cnp58190. [DOI] [PubMed] [Google Scholar]
- Kalantar-Zadeh K, Brennan ML, Hazen SL. Serum myelo-peroxidase and mortality in maintenance hemodialysis patients. . Am. J. Kidney Dis. 2006;48:59–68. doi: 10.1053/j.ajkd.2006.03.047. [DOI] [PubMed] [Google Scholar]
- Kim KM, Jung BH, Paen, et al. Alteration of F2-isoprostanes before and after hemodialysis in end-stage renal disease patients. Prostaglandins, Leukot Essent Fatty Acids. 2004;70:475–78. doi: 10.1016/j.plefa.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Lim PS, Chang YM, Thien LM, et al. 8-iso-Prostaglandin F2 as a useful clinical biomarker of oxidative stress in ESRD patients. . Blood Purif. 2002;20:537–42. doi: 10.1159/000066962. [DOI] [PubMed] [Google Scholar]
- Loughrey CM, Young IS, Lightbody JH, et al. Oxidative stress in hemodialysis. . Q. J. Med. 1994;87:679–83. [PubMed] [Google Scholar]
- Ludat K, Sommerburg O, Grune T, et al. Oxidation parameters in complete correction of renal anemia. . Clin. Nephrol. 2000;53(1):S30–5. [PubMed] [Google Scholar]
- Montuschi P, Collins JV, Ciabattoni G, et al. Exhaled 8-isoprostane as an in vivo biomarker of lung oxidative stress in patients with COPD and healthy smokers. . Am. J. Respir. Crit. Care Med. 2000;162:1175–77. doi: 10.1164/ajrccm.162.3.2001063. [DOI] [PubMed] [Google Scholar]
- Montuschi P, Barnes PJ, Roberts LJ. Isoprostanes: markers and mediators of oxidative stress. . FASEB J. 2004;18:1791–800. doi: 10.1096/fj.04-2330rev. [DOI] [PubMed] [Google Scholar]
- Morena M, Delbosc S, Dupuy AM, et al. Overproduction of reactive oxygen species in end-stage renal disease patients: a potential component of hemodialysis-associated inflammation. . Hemodial Int. 2005;9:37–46. doi: 10.1111/j.1492-7535.2005.01116.x. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Zackert WE, van der Ende DS, et al. Cadenas E, Packer L. Handbook of Antioxidants. New York, Basel. 2nd edition. Marcel Dekker, Inc; 2002. Qantification of isoprostanes as indicators of oxidant stress in vivo; pp. 57–74. [Google Scholar]
- Pai AB, Boyd AV, McQuade CR, et al. Comparison of oxidative stress markers after intravenous administration of iron dextran, sodium ferric gluconate, and iron sucrose in patients undergoing hemodialysis. Pharmacotherapy. 2007;27:343–50. doi: 10.1592/phco.27.3.343. [DOI] [PubMed] [Google Scholar]
- Palleschi S, De Angelis S, Diana L, et al. Reliability of oxidative stress biomarkers in hemodialysis patients: a comparative study. . Clin. Chem. Lab. Med. 2007;45:1211–18. doi: 10.1515/CCLM.2007.266. [DOI] [PubMed] [Google Scholar]
- Patrono C, FitzGerald GA. Isoprostanes: potential markers of oxidant stress in atherothrombotic disease. . Arterioscler. Thromb. Vasc. Biol. 1997;17:2309–15. doi: 10.1161/01.atv.17.11.2309. [DOI] [PubMed] [Google Scholar]
- Peuchant E, Delmas-Beauvieux MC, Dubourg L, et al. Antioxidant effects of a supplemented very low protein diet in chronic renal failure. . Free Radic. Biol. Med. 1997;22:313–20. doi: 10.1016/s0891-5849(96)00282-1. [DOI] [PubMed] [Google Scholar]
- Piroddi M, Depunzio I, Calabrese V, et al. Oxidatively-modified and glycated proteins as candidate pro-inflammatory toxins in uremia and dialysis patients. Amino Acids. 2007;32:573–92. doi: 10.1007/s00726-006-0433-8. [DOI] [PubMed] [Google Scholar]
- Ruef J, Moser M, Bode C, et al. 4-Hydroxynonenal induces apoptosis, NF-KB-activation and formation of 8-isoprostane in vascular smooth muscle cells. . Basic Res. Cardiol. 2001;96:143–50. doi: 10.1007/s003950170064. [DOI] [PubMed] [Google Scholar]
- Rutkowski P, Slominska EM, Szolkiewicz M, et al. Relationship between uremic toxins and oxidative stress in patients with chronic renal failure. . Scand J. Urol. Nephrol. 2007;41:243–8. doi: 10.1080/00365590601017170. [DOI] [PubMed] [Google Scholar]
- Salomon RG, Batyreva E, Kaur K, et al. Isolevuglandin-protein adducts in humans: products of free radical-induced lipid oxidation through the isoprostane pathway. . Biochim. Biophys. Acta. 2000;1485:225–35. doi: 10.1016/s1388-1981(00)00038-x. [DOI] [PubMed] [Google Scholar]
- Samoulidou E, Grapsa E, Kakavas I, et al. Oxidative stress markers and C-reactive protein in end-stage renal failure patients on dialysis. . Int. Urol. Nephrol. 2003;35:393–97. doi: 10.1023/b:urol.0000022846.83505.3f. [DOI] [PubMed] [Google Scholar]
- Samoulidou E, Grapsa E, Karpouza A, et al. Reactive oxygen metabolites: a link between oxidative stress and inflammation in patients on hemodialysis. . Blood Purif. 2007;25:175–78. doi: 10.1159/000098521. [DOI] [PubMed] [Google Scholar]
- Scholz H, Yndestad A, Damas JK, et al. 8-isoprostane increases expression of interleukin-8 in human macrophages through activation of mitogen-activated protein kinases. . Cardiovasc. Res. 2003;59:945–54. doi: 10.1016/s0008-6363(03)00538-8. [DOI] [PubMed] [Google Scholar]
- Siems W, Hapner SJ, van Kuijk FJGM. 4-Hydroxynonenal inhibits Na+-K+-ATPase. . Free Radic. Biol. Med. 1996;20:215–23. doi: 10.1016/0891-5849(95)02041-1. [DOI] [PubMed] [Google Scholar]
- Siems W, Quast S, Carluccio F, et al. Oxidative stress in chronic renal failure as a cardiovascular risk factor. . Clin. Nephrol. 2002a;58(1):12–19. [PubMed] [Google Scholar]
- Siems W, Carluccio F, Grune T, et al. Elevated serum concentration of cardiotoxic lipid peroxidation products in chronic renal failure in relation to severity of renal anemia. . Clin. Nephrol. 2002b;58(1):20–5. [PubMed] [Google Scholar]
- Siems W, Quast S, Carluccio F, et al. Oxidative stress in cardiorenal anemia syndrome: correlations and therapeutic possibilities. . Clin. Nephrol. 2003;60:22–30. [PubMed] [Google Scholar]
- Siems W, Carluccio F, Radenkovic S, Grune T, Hampl H. Oxidative stress in renal anemia of hemodialysis patients is mitigated by epoetin treatment. . Kidney Blood Press. Res. 2005;28:295–301. doi: 10.1159/000090184. [DOI] [PubMed] [Google Scholar]
- Silverberg DS, Wexler D, Blum M, et al. The use of subcutaneous erythropoietin and intravenous iron for the treatment of the anemia of severe, resistant congestive heart failure improves cardiac and renal function and functional cardiac class, and markedly reduces hospitalizations. J Am Coll Cardiology (JACC) 2000;35:1737–44. doi: 10.1016/s0735-1097(00)00613-6. [DOI] [PubMed] [Google Scholar]
- Silverberg DS, Wexler D, Blum M, et al. The cardio renal anemia syndrome: correcting anemia in patients with resistant congestive heart failure can improve both cardiac and renal function and reduce hospitalizations. . Clin. Nephrol. 2003;60:93–102. [PubMed] [Google Scholar]
- Silverberg DS, Wexler D, Iaina A. The role of anemia in congestive heart failure and chronic kidney insufficiency: the cardiorenal anemia syndrome. . Perspect. Biol. Med. 2004a;47:575–89. doi: 10.1353/pbm.2004.0072. [DOI] [PubMed] [Google Scholar]
- Silverberg DS, Wexler D, Blum M, et al. The interaction between heart failure, renal failure and anemia—the cardiorenal anemia syndrome. . Blood Purif. 2004b;22:277–84. doi: 10.1159/000078698. [DOI] [PubMed] [Google Scholar]
- Sommerburg O, Grune T, Klee S, et al. Formation of 4-hydroxynonenal and further aldehydic mediators of inflammation during bromotrichloromethane treatment of rat liver cells. Mediators Inflammation. 1993;2:27–31. doi: 10.1155/S0962935193000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommerburg O, Grune T, Hampl H, et al. Does long-term treatment of renal anaemia with recombinant erythropoietin influence oxidative stress in haemodialysed patients. . Nephrol. Dial. Transplant. 1998;13:2583–87. doi: 10.1093/ndt/13.10.2583. [DOI] [PubMed] [Google Scholar]
- Spittle MA, Hoenich NA, Handelman GJ, et al. Oxidative stress and inflammation in hemodialysis patients. . Am. J. Kidney Dis. 2001;38:1408–13. doi: 10.1053/ajkd.2001.29280. [DOI] [PubMed] [Google Scholar]
- Stenvinkel P, Alvestrand A. Inflammation in end-stage renal disease: sources, consequences, and therapy. Seminars Dialysis. 2002;15:329–37. doi: 10.1046/j.1525-139x.2002.00083.x. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Nammour TM, Fukunaga M, et al. Glomerular actions of a free-radical generated novel prostaglandin, 8-epi-prostaglandin F2α in the rat. Evidence for interaction with thromboxane A2 receptors. . J. Clin. Invest. 1992;90:136–41. doi: 10.1172/JCI115826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaunat G, Beaumont C, Chatenoud L, et al. Anemia after late introduction of sirolimus may correlate with biochemical evidence of a chronic inflammatory state. Transplantation. 2005;80:1212–19. doi: 10.1097/01.tp.0000179106.07382.6a. [DOI] [PubMed] [Google Scholar]
- Trachtman H, Wilson D, Rao PS. The role of oxygen-free radicals in the development of chronic renal failure. . Life Sci. 1992;50:1877–83. doi: 10.1016/0024-3205(92)90548-4. [DOI] [PubMed] [Google Scholar]
- Tsirpanlis G. The pattern of inflammation and a potential new clinical meaning and usefulness of C-reactive protein in end-stage renal failure patients. . Kidney Blood Press. Res. 2005;28:55–61. doi: 10.1159/000082165. [DOI] [PubMed] [Google Scholar]
- Uchida K, Shiraishi M, Naito Y, et al. Activation of stress signaling pathway by the end product of lipid peroxidation. 4-hydroxynonenal is a potential inducer of intracellular peroxide production. . J. Biol. Chem. 1999;274:2234–42. doi: 10.1074/jbc.274.4.2234. [DOI] [PubMed] [Google Scholar]
- Vollenbroeker B, Koch JH, Fobker M, Suwelack B, Hohage H, Muller U. Determination of cyclosporine and its metabolites in blood via HPLC-MS and correlation to clinically important parameters. . Transplant. Proc. 2005;37:1741–44. doi: 10.1016/j.transproceed.2005.03.149. [DOI] [PubMed] [Google Scholar]
- Walter MF, Blumberg JB, Dolnikowski GG, et al. Streamlined F2-isoprostane analysis in plasma and urine with high-performance liquid chromatography and gas chromatography/mass spectroscopy. . Anal. Biochem. 2000;280:73–9. doi: 10.1006/abio.1999.4476. [DOI] [PubMed] [Google Scholar]
- Wanner C, Metzger T. C-reactive protein a marker for all-cause and cardiovascular mortality in haemodialysis patients. . Nephrol. Dial. Transplant. 2002;17(suppl 8):29–32. doi: 10.1093/ndt/17.suppl_8.29. [DOI] [PubMed] [Google Scholar]
- Westhuyzen J, Adams CE, Fleming SJ. Evidence for oxidative stress during in vitro dialysis. Nephron. 1995;70:49–54. doi: 10.1159/000188543. [DOI] [PubMed] [Google Scholar]
- Wiswedel I, Hirsch D, Kropf S, et al. Flavanol-rich cocoa drink lowers plasma F2-isoprostane concentrations in humans. . Free Radic. Biol. Med. 2004;37:411–21. doi: 10.1016/j.freeradbiomed.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Wiswedel I, Hirsch D, Carluccio F, et al. F2-Isoprostanes as biomarkers of lipid peroxidation in patients with chronic renal failure. Biofactors. 2005;24:201–8. doi: 10.1002/biof.5520240124. [DOI] [PubMed] [Google Scholar]
- Wratten ML, Galaris D, Tetta C, et al. Evolution of oxidative stress and inflammation during hemodialysis and their contribution to cardiovascular disease. Antioxidants Redox Signaling. 2002;4:935–44. doi: 10.1089/152308602762197470. [DOI] [PubMed] [Google Scholar]
- Yeun JY, Kaysen GA. C-reactive protein, oxidative stress, homocysteine, and troponine as inflammatory and metabolic predictors of atherosclerosis in ESRD. . Curr. Opin. Nephrol. Hypertens. 2000;9:621–30. doi: 10.1097/00041552-200011000-00006. [DOI] [PubMed] [Google Scholar]
- Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. . Clin. Pharmacol. Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]