Abstract
Receptor tyrosine kinases (RTKs) are the second largest family of membrane receptors and play a key role in the regulation of vital cellular processes, such as control of cell growth, differentiation, metabolism, and migration. The production of whole-length RTKs in large quantities for biophysical or structural characterization, however, is a challenge. In this study, a cell engineering strategy using the anti-apoptotic Bcl-2 family protein, Bcl-xL, was tested as a potential method for increasing stable expression levels of a recombinant RTK membrane protein in Chinese hamster ovary (CHO) cells. Wild type and CHO cells stably overexpressing heterologous Bcl-xL were transformed with the gene for a model RTK membrane protein, ErbB2, on a plasmid also containing the Zeocin resistance gene. While CHO cells exhibited a gradual decrease in expression with passaging, CHO-bcl-xL cells offered an increased and sustained level of ErbB2 expression following continuous passaging over more than 33 days in culture. The increased ErbB2 expression in CHO-bcl-xL cells was evident both in stable transfected pools and in clonal isolates, and demonstrated both in western blot analysis and flow cytometry. Furthermore, the sustained high-level protein expression in CHO-bcl-xL cells does not alter the correct membrane localization of the ErbB2 protein. Our results demonstrate that cellular engineering, specifically anti-apoptosis engineering, can provide increased and stable ErbB2 membrane protein expression in mammalian cells. This approach may also be useful for other membrane proteins in which large quantities are needed for biophysical and structural studies.
Keywords: membrane protein, epidermal growth factor receptors, ErbB2, Neu, Zeocin, Bcl-xL, anti-apoptosis engineering, CHO, mammalian culture, metabolic engineering
Introduction
The human proteome encodes nearly 1000 integral membrane proteins, representing nearly 30% of all proteins in the cell. These large numbers of membrane proteins are involved in a myriad of cell signaling pathways and processes within the cell [1, 2]. Therefore, it is not surprising that membrane proteins represent nearly 50% of all potential pharmacological targets [3, 4]. Understanding how these membrane proteins function is critical to the molecular medicine and biotechnology fields.
The second largest family of membrane receptors is the receptor tyrosine kinase (RTK) family. RTK-mediated signaling plays a key role in the regulation of vital cellular processes, such as control of cell growth, differentiation, metabolism, and migration [5]. Defects in RTK signaling lead to various developmental abnormalities and cancers, and the mechanism behind the pathologies is under intense investigation [6]. RTKs are single-pass membrane proteins with extracellular ligand-binding domains and intracellular kinase domains. They all signal via lateral dimerization in the membrane plane. Once a dimer is formed (usually in the presence of a ligand), the two cytoplasmic domains come into contact. The contact stimulates catalytic activity, and results in the intermolecular autophosphorylation of the receptor subunits. This activates the catalytic domains for the phosphorylation of cytoplasmic substrates and triggers signaling cascades [7].
Members of the RTK family have been classified based on their structural and ligand-affinity properties. One of the best studied subfamilies is the epidermal growth factor receptors (EGFRs or ErbBs), which has a demonstrated role in many human cancers [8, 9]. Crystal structures of solved extracellular and catalytic domains of ErbB receptors have provided valuable insights into the process of ligand-induced dimerization [10–13]. Yet, many questions remain about the mechanism of receptor activation, and in particular about the transduction of the signal from the extracellular domains to the catalytic domains. The transmembrane domains have been shown to play a role in the process [14–16], and thus a complete understanding of the activation processes requires biochemical and crystallographic studies of whole-length receptors. The production of ErbB whole-length receptors in large quantities, however, is a challenge.
The main methods for determining the molecular structure of a protein, X-ray crystallography and nuclear magnetic resonance (NMR) spectroscopy, require significant quantities of purified sample. While some stable, high abundance membrane proteins such as the G-protein-coupled-receptor (GPCR) rhodopsin have been purified and characterized from native tissues [17], most membrane proteins exhibit very low natural expression, thus prohibiting purification of sufficient amounts of protein from these sources. Therefore, researchers have looked at a variety of heterologous expression systems for the production of membrane proteins for structural and biophysical characterization.
Mammalian cells may particularly be useful for the expression of complex proteins due to their capacity to perform post-translational modifications and, thus, they are routinely used for production of large, recombinant biotherapeutics such as antibodies in commercial applications [18]. As a result, researchers have successfully expressed functional membrane proteins in baby hamster kidney [19], HEK 293 [19], COS-1 [20], and CHO [21, 22] cells among others. Unfortunately, the expression level of recombinant membrane proteins from such systems is often lower than that of secreted proteins. Such difficulties on production may partly explain why membrane proteins account for a relatively small percentage of available protein structures.
A great effort has been made to increase the recombinant protein expression from mammalian cells, particularly in the area of secreted monoclonal antibodies. Cell engineering strategies, in particular, have targeted mammalian cell bottlenecks, including secretion [23], cell cycle [24], and apoptosis activation [25, 26], all from within the cell. Anti-apoptosis cell engineering has the ability to maintain viability of cells in the stressful conditions of bioreactors, allowing for increased production run times and higher product yields. Expression of membrane proteins may represent one such stressful condition, especially since membrane protein overexpression has been shown to initiate the unfolded protein response [27]. As the need to understand the action and interactions of membrane proteins for medical and pharmacological efforts increases, the requirement for high-level membrane protein production systems becomes critical. Here, we examine the application of the anti-apoptosis gene bcl-xL as a potential method for increasing stable expression levels of recombinant membrane proteins using ErbB2 as a model.
Materials and Methods
Cell Lines
Wild-type CHO and CHO-bcl-xL cell lines have been described previously [28]. Cells were maintained in DMEM (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Invitrogen), non-essential amino acids (Invitrogen), and L-Glutamine (Invitrogen) in a humidified 5% CO2 incubator at 37°C.
DNA Constructs
The plasmid pSV2_neu, encoding rat ErbB2 protein (Neu), was obtained from the laboratory of Prof. Daniel Donoghue (UC San Diego). The cDNA encoding the erbB2 gene was excised from the pSV2_neu plasmid using the HindIII and SalI sites and inserted directionally into the pcDNA3.1/zeo vector (Invitrogen, Carlsbad, CA) using the HindIII and XhoI sites to generate pcDNA3.1/zeo_erbB2.
Transfections
Transfection of the vectors was performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) in OptiMEM reduced-serum medium (Invitrogen) according to the manufacturer’s recommendations. For selection of CHO cells stably expressing the gene, Zeocin (Invitrogen) was added to a concentration of 0.250 mg/mL 24 hours post transfection.
Western Blot Analysis
Cells were lysed with lysis buffer containing 1% Triton X-100, 25 mM Tris-HCl, 1 mM EDTA, 150 mM NaCl, 1 mM NaVO4, and complete-mini protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany). Cellular debris and insoluble proteins were removed by centrifugation, and the protein concentration of the cell extracts was measured using a BCA protein assay kit (Pierce, Rockford, IL). Equal total protein amounts of clarified cell lysates were denatured and loaded into 3–8% NuPAGE Tris-acetate gels (Invitrogen, Carlsbad, CA) and separated by electrophoresis at 150V for 80 minutes. Separated proteins were then blotted onto nitrocellulose membranes (Biorad, Richmond, CA) at 75 V for 90 minutes. Membranes were blocked with 3% milk in Tris buffer saline (TBS) for one hour. For detection of Bcl-xL protein, blots were incubated with an anti-Bcl-xS/L antibody (clone S-18, Santa Cruz Biotechnology, Santa Cruz, CA) at a dilution of 1:200. For detection of ErbB2 receptor, blots were incubated with a primary α-C-ErbB2 IgG antibody (Neu-C-18, Santa Cruz Biotechnology, Santa Cruz, CA) at a dilution of 1:500 in 3% milk/TBS overnight at 4°C. A secondary anti-rabbit IgG HRP conjugate (w402B, Promega, Madison, WI) was used at a dilution of 1:2500, and incubated at room temperature for 90 minutes. Similarly, for detection of ErbB2 activation, blots were incubated with a primary α-phospho-ErbB2 antibody (2243P, Cell Signaling Technology, Beverly, MA). For detection of actin, blots were incubated with an anti-actin antibody (Sigma Chemicals, St. Louis, MO). Chemiluminescent detection of the HRP-conjugated antibody was achieved using the ECL western blotting detection kit (GE Healthcare, Piscataway, NJ) and BioMax Light film (Kodak, Rochester, NY).
Immunofluorescence
Cells were transiently-transfected with pcDNA3.1/zeo_erbB2. Twenty-four hours after transfection, cells were fixed with 3% paraformaldehyde for 30 minutes at 4°C, and blocked for one hour at room temperature with 3% BSA in TBS. Cells were then incubated with the primary antibody, α-C-ErbB2 (Millipore, Billerica, MA) at a concentration of 1:500 in 1% BSA overnight. For fluorescence detection, an α-mouse IgG fluorescein conjugate (401214, Calbiochem, Los Angeles, CA) at a concentration of 1:100 in 1% BSA/TBS was used. Fluorescence imaging was carried out using a Nikon confocal microscope equipped with an imaging system.
Flow Cytometry Analysis
Cells were seeded in 6-well plates, incubated for 24 hours, and resuspended in 5 mM EDTA. Resuspended cells were washed with 1% FBS in PBS, followed by incubation in a solution of α-c-ErbB2 (OP16, Calbiochem) in 1% FBS/PBS for 30 minutes. For fluorescence detection, an α-mouse IgG fluorescein conjugate (401214, Calbiochem) in 1% FBS/PBS was used. Cell staining was quantified using a FACSCalibur flow cytometer (Beckon Dickinson).
Results
Transient Expression Studies
Our goal was to determine the impact of Bcl-xL overexpression on the production of ErbB2 in mammalian cells. To determine if ErbB2 protein could be expressed in CHO-bcl-xL cells at the plasma membrane, we transiently-transfected wild-type and Bcl-xL-expressing CHO cells with a strong mammalian expression vector encoding the erbB2 gene. Immunofluorescence staining of these transiently-transfected cells showed strong immunoreactivity to the cells. At the periphery of the cell, a strong reactivity owing to the plasma membrane localization of the protein was evident, and the membrane protein was distributed homogeneously on the cell surface for both the wild-type and Bcl-xL-expressing CHO cell lines (Figure 1A and 1B, respectively). No background fluorescence was detected in untransfected CHO cells (Figure 1C). Similarly, CHO and CHO-bcl-xL cells that were transfected with the empty vector also showed no membrane staining (data not shown).
Figure 1.
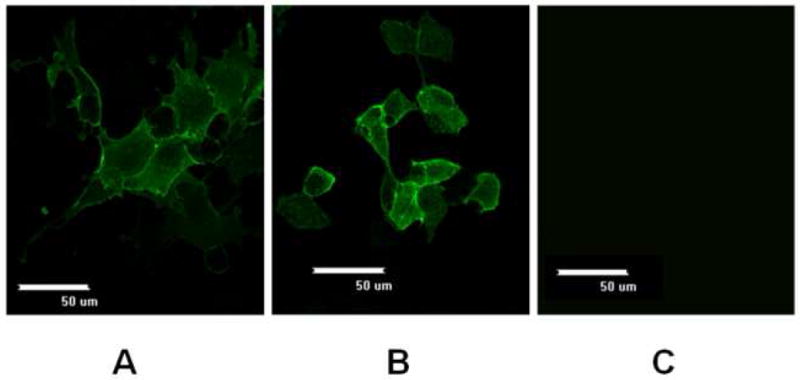
Immunofluorescence images of ErbB2 expression in wild-type (A) and Bcl-xL-expressing (B) CHO cells after transient transfection with pcDNA3.1/zeo_erbB2. Images were captured using a 60X objective at 24 hours post transfection. (C) There was no fluorescence detected in untrasfected CHO cells. Similar results were obtained with cells transfected with the empty vector pcDNA3.1/zeo (not shown).
We next sought to confirm overexpression of Bcl-xL in the CHO-bcl-xL cell line chosen for this study. The relative levels of Bcl-xL as determined by Western blot analysis are shown in Figure 2. An anti-Bcl-xL antibody showed a reactive band at approximately 28 kDa, which corresponds to the size of full length Bcl-xL protein. While there was a low but detectable level of endogenous hamster Bcl-xL in the wild-type CHO cells, a much stronger band was evident in the CHO cells overexpressing human Bcl-xL chosen for this study. To ensure that the relative band intensities reflected the actual expression levels in the cells, each lane was loaded with equal total cellular protein, and the samples were analyzed on the same gel and Western blot. Thus, we can confirm the overexpression of the Bcl-xL protein in our CHO-bcl-xL cell line.
Figure 2.

Western blot of Bcl-xL in CHO and CHO-bcl-xL cell lines. Equal total cellular protein (50 μgrams) was loaded per lane and membranes were probed with an anti-Bcl-xL antibody. All samples were run on the same Western blot; non-relevant lanes have been removed for clarity. The arrows on the left indicate where the corresponding molecular weight marker from the protein ladder ran.
We next addressed whether there were any differences in expression of the ErbB2 receptor in the CHO and CHO-bcl-xL cell lines under transient conditions. CHO and CHO-bcl-xL cells were transfected with equivalent amounts of DNA encoding the erbB2 gene and harvested 24 hours after transfection before ErbB2 expression analysis by Western blot. Western blot detection of ErbB2 was evident in a band corresponding to the full length ErbB2 (~180 kDa) in both the CHO and CHO-bcl-xL cell lines (Figure 3). While the CHO-bcl-xL cell line did show slightly-higher band intensity, Western blots of replicate transient transfections showed a range of relative expression levels between the two cell lines (data not shown).
Figure 3.
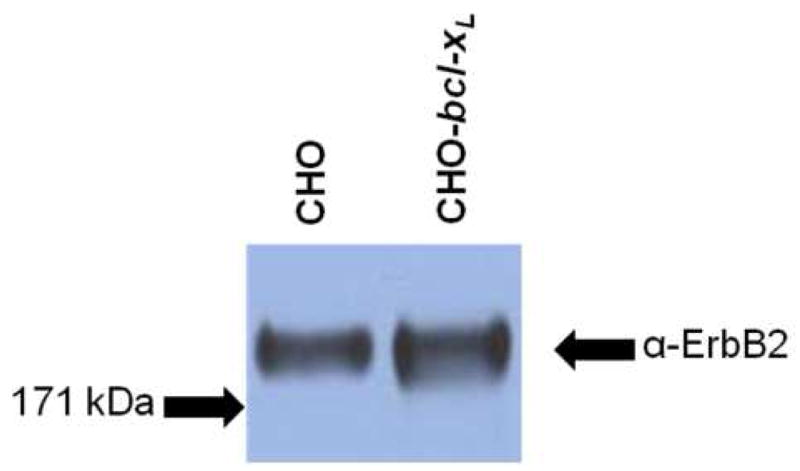
Western blot analysis of ErbB2 transient expression in CHO and CHO-bcl-xL cells transfected with pcDNA3.1/zeo_erbB2. Cells were harvested 24 hours post transfection and equivalent masses of total cellular protein were loaded onto the gel for analysis. Membranes were probed with an anti-ErbB2 antibody. The arrows on the left indicate where the corresponding molecular weight marker from the protein ladder ran.
Stable ErbB2 Expression
While transient transfection conditions are ideal for production of small amounts of recombinant protein, stable expression systems allow for protein production from a much larger percentage of cells which can also be expanded for large-scale production. Therefore, we transfected CHO and CHO-bcl-xL cells with equivalent amounts of DNA encoding ErbB2 on a plasmid which also contains a gene encoding for antibiotic resistance. The transfected cells were then subjected to selection with the antibiotic Zeocin. Western blot assessment at 24 hours after transfection showed that the wild-type CHO and CHO-bcl-xL cells both exhibited detectable levels of ErbB2 receptor (Figure 4A). In this experiment, the expression of ErbB2 is higher in CHO cells, confirming the variation in the transient expression of ErbB2 between the CHO and CHO-bcl-xL cells seen previously (Figure 3).
Figure 4.
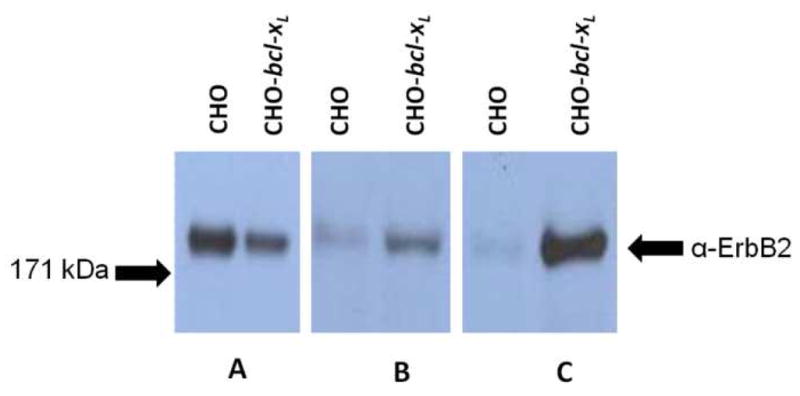
Western blot analysis of ErbB2 expression in CHO and CHO-bcl-xL cells. Samples containing equal amounts of total cellular protein were analyzed from 1 day (A), 13 days (B), and 33 days (C) post transfection. Equal total cellular amounts of 10 μg (A), 75 μg (B), or 40 μg (C) were loaded per lane to ensure band intensities reflect relative expression levels. Membranes were probed with an anti-ErbB2 antibody. All samples were run on the same Western blot; non-relevant lanes have been removed for clarity. The arrows on the left indicate where the corresponding molecular weight marker from the protein ladder ran.
After 13 days of selection in Zeocin, transfected pools were again analyzed for expression of ErbB2. Unlike early time points, this time the CHO and CHO-bcl-xL cells showed dramatically different levels of ErbB2 expression (Figure 4B). While CHO cells exhibited low levels of ErbB2 protein, the CHO-bcl-xL cells retained significant levels of ErbB2 expression as detected by Western blot. This increased expression of ErbB2 in CHO-bcl-xL pool cells after 2 weeks of selection was confirmed in a total of three independent transfection experiments (data for other two experiments not shown).
In order to determine if the enhancement in ErbB2 expression in CHO-bcl-xL cells was maintained during subsequent passaging and maintenance of the cells, stable pools of CHO and CHO-bcl-xL cells expressing the ErbB2 protein were subcultured for a total passaging of 33 days with media containing Zeocin. After this time period, we again analyzed the ErbB2 protein expression by Western blot (Figure 4C). While the ErbB2 protein was barely detectable in the CHO cells, the CHO-bcl-xL cells exhibited a significantly higher level of sustained ErbB2 protein expression. Replicate, independent transfection experiments showed a similar enhancement in ErbB2 expression levels for the CHO-bcl-xL cells relative to the wild-type CHO control at this same time point after selection (data not shown).
To address if the expressed ErbB2 in the stable pools of CHO and CHO-bcl-xL cells were functional, equal amounts of cellular protein mass from the transfected cells were loaded and probed for protein expression. The nitrocellulose membrane was also stripped and stained for phosphorylated ErbB2, an indicator of protein activity. As shown in Figure 5, the full-length ErbB2 receptor is expressed only in the stable pool of CHO-bcl-xL cells transfected with pcDNA3.1/zeo_erbB2 (lane 2), and the receptors are phosphorylated and functional. No detectable expression or activity was observed in CHO pools transfected with pcDNA3.1/zeo_erbB2 (lane 1) or CHO and CHO-bcl-xL pools transfected with the empty vector pcDNA3.1/zeo (lanes 3 and 4). Equivalent mass loadings were verified by probing the amount of actin in each lane.
Figure 5.
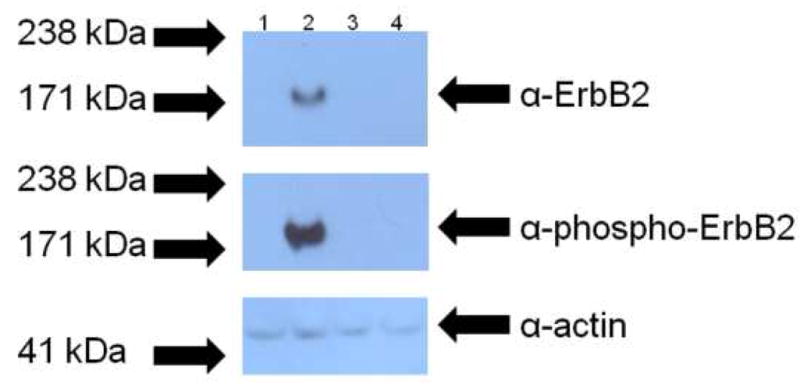
Western blot analysis of ErbB2 activity in stable pools of CHO and CHO-bcl-xL cells. Samples containing equal amounts of total cellular protein from stable pools of CHO and CHO-bcl-xL cells transfected with pcDNA3.1/zeo_erbB2 (lanes 1 and 2, respectively) and transfected with the empty vector (lanes 3 and 4, respectively) were analyzed to determine if the expressed ErbB2 was functional. The top panel shows the expression of ErbB2, and the middle panel shows the activity of the protein as assessed by the receptor’s phosphorylation. The bottom panel shows the corresponding anti-actin blot to ensure that equal cellular protein was loaded in each lane. All samples were run on the same Western blot. The arrows on the left indicate where the corresponding molecular weight marker from the protein ladder ran.
To determine the distribution of the expression level of ErbB2 in the stable pools, cells were immunostained with an α-ErbB2 primary antibody and a fluorescein-conjugated secondary antibody. Flow cytometry measurements of stable pools of CHO-bcl-xL cells transfected with pcDNA3.1/zeo (negative control, Figure 6A) and of CHO cells transfected with pcDNA3.1/zeo_erbB2 (Figure 6B) are similar, with the bulk of the fluorescence signal (~90%) arising from autofluorescence and background signal (101 – 102). Thus, the expression level of ErbB2 in the stable pool of CHO cells is either undetectable or very low. In contrast, the flow cytometry data shows that, in the stable pool of CHO-bcl-xL cells transfected with pcDNA3.1/zeo_erbB2, the majority of the cells (~80%) exhibited much higher fluorescence intensities (102 – 104) and, therefore, were expressing ErbB2 (Figure 6C). The observed range of fluorescence intensity distribution is relatively broad as expected for a stable pool of cells with different expression levels of the protein.
Figure 6.
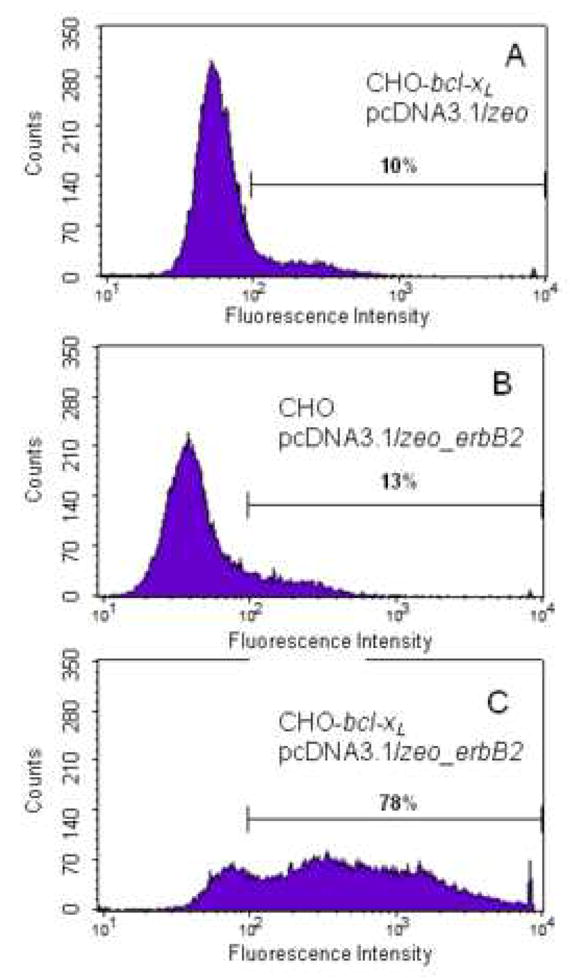
Flow cytometry data of (A) stable pool of CHO-bcl-xL cells transfected with pcDNA3.1/zeo (empty vector, used as negative control), (B) stable pool of CHO cells transfected with pcDNA3.1/zeo_erbB2, and (C) stable pool of CHO-bcl-xL cells transfected with pcDNA3.1/zeo_erbB2. Cells were stained with anti-ErbB2 as the primary antibody, and a FITC-conjugated secondary antibody. Equal number of cells (25,000) was sorted from each sample. Panel A is the negative control, panel B shows the low expression of ErbB2 in CHO cells, and panel C shows the higher number of cells expressing ErbB2 in CHO-bcl-xL cells. Autofluorescence and background signals are detected at intensities below 102. The percentage of cells with fluorescence intensities between 102 and 104 is shown for each set.
While CHO-bcl-xL cells showed prolonged and enhanced expression of the ErbB2 protein, we wanted to know whether the plasma membrane localization of the receptor was affected after prolonged culture. Immunostaining of the stable pools of CHO-bcl-xL cells expressing ErbB2 (from day 33 of the experiment) showed a plasma membrane fluorescence distribution as in the transient transfections (Figure 7), thus confirming that the ErbB2 receptor was still expressed at the plasma membrane after many passages.
Figure 7.
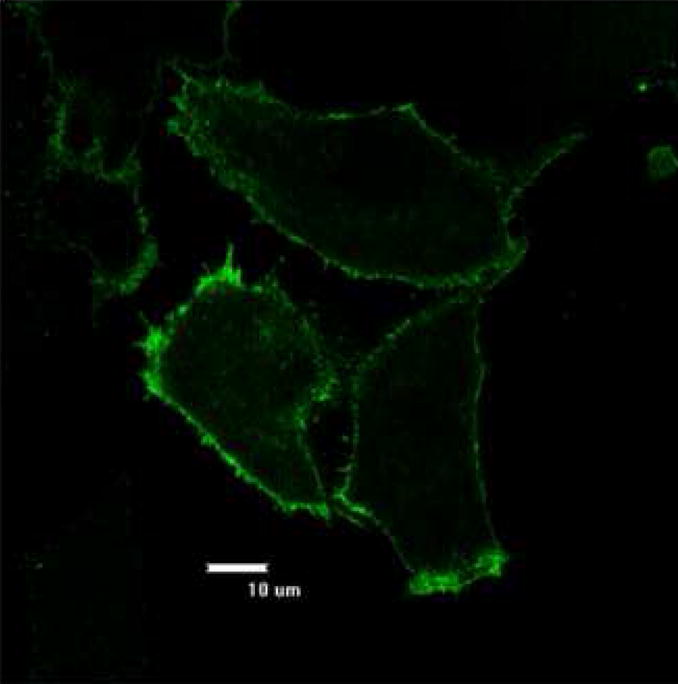
Anti-ErbB2 immunostaining of CHO-bcl-xL stably expressing the ErbB2 protein. Images were taken using a 60X objective after 33 days of culture with the antibiotic Zeocin. There was no fluorescence detected under microscopy for the control cells that were not transfected with pcDNA3.1/zeo_erbB2.
To verify that the sustained expression of ErbB2 is attributed to the overexpression of Bcl-xL, individual clones were selected from the stable pools of CHO and CHO-bcl-xL cells transfected with pcDNA3.1/zeo_erbB2. Western blots from these clones show a higher expression level of ErbB2 in cells overexpressing Bcl-xL (Figure 8) as compared to the levels from the CHO controls. As expected, clones selected from a stable pool of CHO-bcl-xL cells transfected with pcDNA3.1/zeo (empty vector) do not display any expression of ErbB2.
Figure 8.

Western blot analysis of ErbB2 expression in CHO and CHO-bcl-xL clones. Clones were selected from stable pools of CHO and CHO-bcl-xL cells transfected with pcDNA3.1/zeo_erbB2 and CHO-bcl-xL cells transfected with pcDNA3.1/zeo (empty vector, control). Equal total cellular amounts of 20 μg were loaded per lane to ensure that band intensities reflect relative expression levels. Membranes were probed with anti-ErbB2 and anti-Bcl-xL antibodies. All samples were run on the same Western blot; non-relevant lanes have been removed for clarity. The arrows on the left indicate where the corresponding molecular weight marker from the protein ladder ran.
Discussion
The membrane protein ErbB2 plays important roles in mediating growth factor signaling, and thus, has been implicated in numerous pathologies, including cancers [6]. Increasing the stable expression levels of ErbB2 in transfected mammalian cells is a critical step that will further advance the biophysical characterization, structure determination, and high-throughput screening studies for the design of effective therapeutics. The problem with ErbB2, which is common for many membrane proteins, is that low endogenous levels of expression hinder the analysis of these proteins. Thus, a variety of heterologous expression systems for membrane proteins have been examined. Comparisons [19, 29] and reports on the use of a variety of expression systems exist, including bacterial [Error! Reference source not found., 30], yeast [31, 32], insect [33, 34], mammalian cells [29, 35], and even cell-free systems [36]. While each system has its benefits, mammalian cells in particular have shown promise for the production of functional membrane proteins in previous studies [37,38–40]. Yet, lower expression levels in mammalian cells means that optimization of the expression system is important for implementing a robust platform for large-scale production, purification, and analysis. Here we examine the expression of the integral membrane protein ErbB2 in CHO cells, which are widely used for the production of secreted recombinant proteins in commercial applications [18]. Functional expression of membrane proteins in CHO cells has previously been demonstrated for adrenergic [22], neurokinin [41], and adenosine [42] GPCRs as well as RTKs [43–45].
We chose to compare expression of the ErbB2 receptor in a wild-type CHO cell line with one that was also expressing the heterologous human bcl-xL gene. This cell line has been employed to improve survival in the presence of apoptotic insults [28, 46, 47] and also used to improve production of antibodies and secreted proteins by other researchers [48, 49]. Indeed, the CHO-bcl-xL cell line shows overexpression of the anti-apoptotic Bcl-xL protein compared to the endogenous CHO Bcl-xL protein (Figure 2). We were able to detect endogenous CHO (hamster) Bcl-xL protein in our cell lines using an anti-human Bcl-xL antibody. This is not surprising since it has been demonstrated previously for CHO Bcl-xL [50] and can be expected due to the high sequence similarity of the two Bcl-xL species isoforms.
While higher ErbB2 expression in CHO-bcl-xL cells was not evident during transient expression, stable transfection of the receptor in CHO-bcl-xL cells resulted in sustained ErbB2 expression in stable pools as well as clonal isolates. It is likely that Bcl-xL exerts a positive effect during the stable pool and clonal isolation selection process. Bcl-xL may provide for higher stable expression of the ErbB2 receptor through its anti-apoptotic function in CHO cells. Although the exact mechanism of how Bcl-xL works is still under investigation, the anti-apoptosis protein Bcl-xL is known to prevent the release of cytochrome c and Smac from the mitochondria, leading to the inhibition of caspase-mediated apoptosis [28]. Expression of large, complex recombinant proteins, such as ErbB2, has the potential to initiate internal stresses on the cell that may affect growth and survival. This was seen during stable expression of the rat serotonin transporter, where HEK 293 cells expressing the transporter grew slowly even in the presence of a transporter inhibitor [29]. High level membrane protein expression may lead to protein accumulation in the endoplasmic reticulum and activation of the unfolded protein response (UPR) in mammalian cells [27]. While the UPR is a type of survival mechanism, the process can also lead to apoptotic [51] or autophagic cell death [52]. Anti-apoptotic Bcl-2 family members such as Bcl-xL are ideal pro-survival molecules in that they can protect against both forms of cell death [53, 54]. Hence, Bcl-xL overexpression may be protecting the CHO cells from apoptosis and/or autophagy triggered by ErbB2 expression.
The overexpression of Bcl-xL in the engineered CHO cell line may also allow the survival of cell lines that would normally not endure the cell line selection processes. It has been shown that Zeocin, one of the bleomycin/phleomycin family of antibiotics, will bind DNA and induce apoptosis [55]. Interestingly, the antibiotic initiates release of cytochrome c and down-regulates expression of Bcl-xL [55] along with other anti-apoptotic genes. Thus, it is possible that the enhanced expression of Bcl-xL facilitates cellular survival and proliferation in CHO pools and stable clones during the lengthy Zeocin selection process.
Thus, the presence of Bcl-xL in CHO-bcl-xL may imbue the cells with properties that allow continued survival and proliferation of high-producing cells during extend passaging. Although the exact mechanism is still unclear, it is evident that overexpression of Bcl-xL provides inherent advantages to CHO cells expressing the ErbB2 membrane protein. Indeed, the combination of stresses manifested both by expressing a complex membrane protein in CHO cells in combination with apoptotic stresses caused by antibiotic selection may require enhanced expression of anti-apoptotic genes in order to ensure survival of CHO cells over extended passaging.
In addition to maintaining expression during long term culture of stable cell lines, expression of the ErbB2 protein in a CHO cell line expressing Bcl-xL did not alter the localization of the protein as analyzed by immunofluorescence confocal microscopy. Previously, Bcl-xL expression has also been shown to increase secreted recombinant protein production [26,56] with no detectable changes in product quality [49]. Thus, Bcl-xL appears to offer significant advantages for increasing production of both secreted and membrane recombinant proteins in mammalian expression systems.
In summary, we have shown a method for increasing the stable production of the integral membrane protein ErbB2 in CHO cells by enhancing the expression of the anti-apoptotic gene bcl-xL. This work provides an excellent foundation for subsequent cell engineering strategies that can lead to higher production of ErbB2 for structural or biochemical studies. In the future, the use of anti-apoptotic engineering should be considered for other members of the RTK family (e.g., ErbB, FGFR, VEGFR, etc.), G-protein coupled receptors and other integral membrane proteins. Yet, Bcl-xL cell engineering represents only one of many cell engineering strategies that may lead to increased production of heterologous proteins. While most previous cell engineering studies have focused on the production of secreted recombinant proteins, similar bottlenecks may exist in production of recombinant membrane proteins. Future strategies to increase membrane protein production may involve enhancing cellular organelles [23], codon usage [57], and media formulation [58], all of which have been implemented successfully for increased expression of secreted recombinant proteins. The true test of these methods will be in their ability to increase production of a variety of membrane proteins, leading to successful purification of large quantities of membrane proteins for subsequent biophysical and biochemical characterization.
Acknowledgments
This work was supported by NIH Grant GM068619 and Research Scholar Grant RSG-04-201-01 from the American Cancer Society to K.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wallin E, von Heijne G. Genome-wide analysis of integral membrane proteins from eubacterial, archaean, and eukaryotic organisms. Protein Sci. 1998;7:1029–1038. doi: 10.1002/pro.5560070420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu JF, Rost B. Comparing function and structure between entire proteomes. Protein Sci. 2001;10:1970–1979. doi: 10.1110/ps.10101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bane SE, Velasquez JE, Robinson AS. Expression and purification of milligram levels of inactive G-protein coupled receptors in E. coli. Protein Expr Purif. 2007;52:348–355. doi: 10.1016/j.pep.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grisshammer R, Tate G. Preface: overexpression of integral membrane proteins. Biophys Acta. 2003;1610:1–2. [Google Scholar]
- 5.Fantl WJ, Johnson DE, Williams LT. Signalling by receptor tyrosine kinases. Annu Rev Biochem. 1993;62:453–481. doi: 10.1146/annurev.bi.62.070193.002321. [DOI] [PubMed] [Google Scholar]
- 6.van der Geer P, Hunter T, Lindberg RA. Receptor protein-tyrosine kinases and their signal transduction pathways. Annu Rev Cell Biol. 1994;10:251–337. doi: 10.1146/annurev.cb.10.110194.001343. [DOI] [PubMed] [Google Scholar]
- 7.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 8.Holbro T, Civenni G, Hynes NE. The ErbB receptors and their role in cancer progression. Exp Cell Res. 2003;284:99–110. doi: 10.1016/s0014-4827(02)00099-x. [DOI] [PubMed] [Google Scholar]
- 9.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 10.Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW, Jr, Leahy DJ. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature. 2003;421:756–760. doi: 10.1038/nature01392. [DOI] [PubMed] [Google Scholar]
- 11.Ferguson KM, Berger MB, Mendrola JM, Cho HS, Leahy DJ, Lemmon MA. EGF activates its receptor by removing interactions that autoinhibit ectodomain dimerization. Mol Cell. 2003;11:507–517. doi: 10.1016/s1097-2765(03)00047-9. [DOI] [PubMed] [Google Scholar]
- 12.Cho HS, Leahy DJ. Structure of the extracellular region of HER3 reveals an interdomain tether. Science. 2002;297:1330–1333. doi: 10.1126/science.1074611. [DOI] [PubMed] [Google Scholar]
- 13.Bouyain S, Longo PA, Li S, Ferguson KM, Leahy DJ. The extracellular region of ErbB4 adopts a tethered conformation in the absence of ligand. Proc Natl Acad Sci U S A. 2005;102:15024–15029. doi: 10.1073/pnas.0507591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li E, Hristova K. Role of receptor tyrosine kinase transmembrane domains in cell signaling and human pathologies. Biochemistry. 2006;45:6241–6251. doi: 10.1021/bi060609y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li E, You M, Hristova K. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and forster resonance energy transfer suggest weak interactions between fibroblast growth factor receptor 3 (FGFR3) transmembrane domains in the absence of extracellular domains and ligands. Biochemistry. 2005;44:352–360. doi: 10.1021/bi048480k. [DOI] [PubMed] [Google Scholar]
- 16.Li E, You M, Hristova K. FGFR3 dimer stabilization due to a single amino acid pathogenic mutation. J Mol Biol. 2006;356:600–612. doi: 10.1016/j.jmb.2005.11.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 18.Wurm FM. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol. 2004;22:1393–1398. doi: 10.1038/nbt1026. [DOI] [PubMed] [Google Scholar]
- 19.Eifler N, Duckely M, Sumanovski LT, Egan TM, Oksche A, Konopka JB, Luthi A, Engel A, Werten PJ. Functional expression of mammalian receptors and membrane channels in different cells. J Struct Biol. 2007;159:179–193. doi: 10.1016/j.jsb.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Chelikani P, Reeves PJ, Rajbhandary UL, Khorana HG. The synthesis and high-level expression of a beta2-adrenergic receptor gene in a tetracycline-inducible stable mammalian cell line. Protein Sci. 2006;15:1433–1440. doi: 10.1110/ps.062080006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prather PL, McGinn TM, Claude PA, Liu-Chen LY, Loh HH, Law PY. Properties of a kappa-opioid receptor expressed in CHO cells: interaction with multiple G-proteins is not specific for any individual G alpha subunit and is similar to that of other opioid receptors. Brain Res Mol Brain Res. 1995;29:336–346. doi: 10.1016/0169-328x(94)00264-f. [DOI] [PubMed] [Google Scholar]
- 22.Fraser CM, Arakawa S, McCombie WR, Venter JC. Cloning, sequence analysis, and permanent expression of a human alpha 2-adrenergic receptor in Chinese hamster ovary cells. Evidence for independent pathways of receptor coupling to adenylate cyclase attenuation and activation. J Biol Chem. 1989;264:11754–11761. [PubMed] [Google Scholar]
- 23.Tigges M, Fussenegger M. Xbp1-based engineering of secretory capacity enhances the productivity of Chinese hamster ovary cells. Metab Eng. 2006:264–272. doi: 10.1016/j.ymben.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Ifandi V, Al-Rubeai M. Regulation of cell proliferation and apoptosis in CHO-K1 cells by the coexpression of c-Myc and Bcl-2. Biotechnol Prog. 2005;21:671–677. doi: 10.1021/bp049594q. [DOI] [PubMed] [Google Scholar]
- 25.Figueroa B, Jr, Sauerwald TM, Oyler GA, Hardwick JM, Betenbaugh MJ. A comparison of the properties of a Bcl-xL variant to the wild-type anti-apoptosis inhibitor in mammalian cell cultures. Metab Eng. 2003;5:230–245. doi: 10.1016/s1096-7176(03)00044-2. [DOI] [PubMed] [Google Scholar]
- 26.Majors BS, Betenbaugh MJ, Pederson NE, Chiang GG. Enhancement of transient gene expression and culture viability using Chinese hamster ovary cells overexpressing Bcl-xL. Biotechnology and Bioengineering. 2008;101:567–578. doi: 10.1002/bit.21917. [DOI] [PubMed] [Google Scholar]
- 27.Cudna RE, Dickson AJ. Endoplasmic reticulum signaling as a determinant of recombinant protein expression. Biotechnol Bioeng. 2003;81:56–65. doi: 10.1002/bit.10445. [DOI] [PubMed] [Google Scholar]
- 28.Sauerwald TM, Figueroa B, Jr, Hardwick JM, Oyler GA, Betenbaugh MJ. Combining caspase and mitochondrial dysfunction inhibitors of apoptosis to limit cell death in mammalian cell cultures. Biotechnol Bioeng. 2006;94:362–372. doi: 10.1002/bit.20874. [DOI] [PubMed] [Google Scholar]
- 29.Tate CG, Haase J, Baker C, Boorsma M, Magnani F, Vallis Y, Williams DC. Comparison of seven different heterologous protein expression systems for the production of the serotonin transporter. Biochim Biophys Acta. 2003;1610:141–153. doi: 10.1016/s0005-2736(02)00719-8. [DOI] [PubMed] [Google Scholar]
- 30.Tate CG, Grisshammer R. Heterologous expression of G-protein-coupled receptors. Trends Biotechnol. 1996;14:426–430. doi: 10.1016/0167-7799(96)10059-7. [DOI] [PubMed] [Google Scholar]
- 31.Niebauer RT, Robinson AS. Exceptional total and functional yields of the human adenosine (A2a) receptor expressed in the yeast Saccharomyces cerevisiae. Protein Expr Purif. 2006;46:204–211. doi: 10.1016/j.pep.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 32.O’Malley MA, Lazarova T, Britton ZT, Robinson AS. High-level expression in Saccharomyces cerevisiae enables isolation and spectroscopic characterization of functional human adenosine A2a receptor. J Struct Biol. 2007;159:166–178. doi: 10.1016/j.jsb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bouvier M, Menard L, Dennis M, Marullo S. Expression and recovery of functional G-protein-coupled receptors using baculovirus expression systems. Curr Opin Biotechnol. 1998;9:522–527. doi: 10.1016/s0958-1669(98)80040-2. [DOI] [PubMed] [Google Scholar]
- 34.Dolby V, Collen A, Lundqvist A, Cronet P. Overexpression and functional characterisation of the human melanocortin 4 receptor in Sf9 cells. Protein Expr Purif. 2004;37:455–461. doi: 10.1016/j.pep.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 35.Ames R, Nuthulaganti P, Fornwald J, Shabon U, van-der-Keyl H, Elshourbagy N. Heterologous expression of G protein-coupled receptors in U-2 OS osteosarcoma cells. Receptors Channels. 2004;10:117–124. doi: 10.1080/10606820490515012. [DOI] [PubMed] [Google Scholar]
- 36.Klammt C, Lohr F, Schafer B, Haase W, Dotsch V, Ruterjans H, Glaubitz C, Bernhard F. High level cell-free expression and specific labeling of integral membrane proteins. Eur J Biochem. 2004;271:568–580. doi: 10.1111/j.1432-1033.2003.03959.x. [DOI] [PubMed] [Google Scholar]
- 37.Sarramegna V, Talmont F, Demange P, Milon A. Heterologous expression of G-protein-coupled receptors: comparison of expression systems fron the standpoint of large-scale production and purification. Cell Mol Life Sci. 2003;60:1529–1546. doi: 10.1007/s00018-003-3168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker P, Munoz M, Combe MC, Grouzmann E, Herzog H, Selbie L, Shine J, Brunner HR, Waeber B, Wittek R. High level expression of human erbB2ropeptide Y receptors in mammalian cells infected with a recombinant vaccinia virus. Mol Cell Endocrinol. 1993;91:107–112. doi: 10.1016/0303-7207(93)90261-h. [DOI] [PubMed] [Google Scholar]
- 39.Lundstrom K, Mills A, Buell G, Allet E, Adami N, Liljestrom P. High-level expression of the human erbB2rokinin-1 receptor in mammalian cell lines using the Semliki Forest virus expression system. Eur J Biochem. 1994;224:917–921. doi: 10.1111/j.1432-1033.1994.00917.x. [DOI] [PubMed] [Google Scholar]
- 40.Ingi T, Kitajima Y, Minamitake Y, Nakanishi S. Characterization of ligand-binding properties and selectivities of three rat tachykinin receptors by transfection and functional expression of their cloned cDNAs in mammalian cells. J Pharmacol Exp Ther. 1991;259:968–975. [PubMed] [Google Scholar]
- 41.Turcatti G, Ceszkowski K, Chollet A. Biochemical characterization and solubilization of human NK2 receptor expressed in Chinese hamster ovary cells. J Recept Res. 1993;13:639–652. doi: 10.3109/10799899309073684. [DOI] [PubMed] [Google Scholar]
- 42.Townsend-Nicholson A, Shine J. Molecular cloning and characterisation of a human brain A1 adenosine receptor cDNA. Brain Res Mol Brain Res. 1992;16:365–370. doi: 10.1016/0169-328x(92)90248-a. [DOI] [PubMed] [Google Scholar]
- 43.Engelman JA, Janne PA, Mermel C, Pearlberg J, Mukohara T, Fleet C, Cichowski K, Johnson BE, Cantley LC. ErbB-3 mediates phosphoinositide 3-kinase activity in gefitinib-sensitive non-small cell lung cancer cell lines. Proc Natl Acad Sci U S A. 2005;102:3788–3793. doi: 10.1073/pnas.0409773102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harari D, Tzahar E, Romano J, Shelly M, Pierce JH, Andrews GC, Yarden Y. ErbB2regulin-4: a novel growth factor that acts through the ErbB-4 receptor tyrosine kinase. Oncogene. 1999;18:2681–2689. doi: 10.1038/sj.onc.1202631. [DOI] [PubMed] [Google Scholar]
- 45.Tzahar E, Waterman H, Chen X, Levkowitz G, Karunagaran D, Lavi S, Ratzkin BJ, Yarden Y. A hierarchical network of interreceptor interactions determines signal transduction by ErbB2 differentiation factor/erbB2regulin and epidermal growth factor. Mol Cell Biol. 1996;16:5276–5287. doi: 10.1128/mcb.16.10.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mastrangelo AJ, Hardwick JM, Bex F, Betenbaugh MJ. Part I. Bcl-2 and Bcl-x(L) limit apoptosis upon infection with alphavirus vectors. Biotechnol Bioeng. 2000;67:544–554. [PubMed] [Google Scholar]
- 47.Mastrangelo AJ, Hardwick JM, Zou S, Betenbaugh MJ. Part II. Overexpression of bcl-2 family members enhances survival of mammalian cells in response to various culture insults. Biotechnol Bioeng. 2000;67:555–564. doi: 10.1002/(sici)1097-0290(20000305)67:5<555::aid-bit6>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 48.Meents H, Enenkel B, Eppenberger HM, Werner RG, Fussenegger M. Impact of coexpression and coamplification of sICAM and antiapoptosis determinants bcl-2/bcl-x(L) on productivity, cell survival, and mitochondria number in CHO-DG44 grown in suspension and serum-free media. Biotechnol Bioeng. 2002;80:706–716. doi: 10.1002/bit.10449. [DOI] [PubMed] [Google Scholar]
- 49.Chiang GG, Sisk WP. Bcl-x(L) mediates increased production of humanized monoclonal antibodies in Chinese hamster ovary cells. Biotechnol Bioeng. 2005;91:779–792. doi: 10.1002/bit.20551. [DOI] [PubMed] [Google Scholar]
- 50.Peterson NC, Servinsky M. Characterization of the effects of Bcl-2 and Bcl-xl deletion mutant expression in cell lines used for antibody production. Hybridoma (Larchmt) 2005;24:275–282. doi: 10.1089/hyb.2005.24.275. [DOI] [PubMed] [Google Scholar]
- 51.Knowlton AA. Life, death, the unfolded protein response and apoptosis. Cardiovasc Res. 2007;73:1–2. doi: 10.1016/j.cardiores.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 52.Bernales S, McDonald KL, Walter P. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol. 2006;4:e423. doi: 10.1371/journal.pbio.0040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levine B, Sinha S, Kroemer G. Bcl-2 family members: Dual regulators of apoptosis and autophagy. Autophagy. 2008;4:600–606. doi: 10.4161/auto.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 55.Hwang J, Kim YY, Hug S, Shim J, Park C, Kimm K, Choi DK, Park TK, Kim S. The time-dependent serial gene response to Zeocin treatment involves caspase-dependent apoptosis in HeLa cells. Microbiol Immunol. 2005;49:331–342. doi: 10.1111/j.1348-0421.2005.tb03737.x. [DOI] [PubMed] [Google Scholar]
- 56.Meents H, Enenkel B, Eppenberger HM, Werner RG, Fussenegger M. Impact of coexpression and coamplification of sICAM and antiapoptosis determinants bcl-2/bcl-x(L) on productivity, cell survival, and mitochondria number in CHO-DG44 grown in suspension and serum-free media. Biotechnol Bioeng. 2002;80:706–716. doi: 10.1002/bit.10449. [DOI] [PubMed] [Google Scholar]
- 57.Kim CH, Oh Y, Lee TH. Codon optimization for high-level expression of human erythropoietin (EPO) in mammalian cells. Gene. 1997;199:293–301. doi: 10.1016/s0378-1119(97)00384-3. [DOI] [PubMed] [Google Scholar]
- 58.Schroder M, Matischak K, Friedl P. Serum- and protein-free media formulations for the Chinese hamster ovary cell line DUKXB11. J Biotechnol. 2004;108:279–292. doi: 10.1016/j.jbiotec.2003.12.005. [DOI] [PubMed] [Google Scholar]


