Abstract
Glycolipids are major components of the plasma membrane, interacting with themselves, other lipids, and proteins to form an array of heterogeneous domains with diverse biological properties. Considerable effort has been focused on identifying protein binding partners for glycolipids and the glycan specificity for these interactions, largely achieved through assessing interactions between proteins and homogenous, single species glycolipid preparations. This approach risks overlooking both the enhancing and attenuating roles of heterogeneous glycolipid complexes in modulating lectin binding. Here we report a simple method for assessing lectin–glycolipid interactions. An automatic thin-layer chromatography sampler is employed to create easily reproducible arrays of glycolipids and their heterodimeric complexes immobilized on a synthetic polyvinyl-difluoride membrane. This array can then be probed with much smaller quantities of reagents than would be required using existing techniques such as ELISA and thin-layer chromatography with immuno-overlay. Using this protocol, we have established that the binding of bacterial toxins, lectins, and antibodies can each be attenuated, enhanced, or unaffected in the presence of glycolipid complexes, as compared with individual, isolated glycolipids. These findings underpin the wide-ranging influence and importance of glycolipid–glycolipid cis interactions when the nature of protein–carbohydrate recognition events is being assessed.
Keywords: complexes, ganglioside, glycoarray, glycolipid, lectin
Introduction
Protein–carbohydrate recognition events are central to many biological processes encompassing cell–cell interactions and signaling, microbial adherence, virulence factor binding, and immune recognition (Karlsson 2001; Schiavo and van der Goot 2001; Hakomori 2002; Lalli et al. 2003; Miller-Podraza et al. 2004; Avril, Wagner, et al. 2006; Crocker and Redelinghuys 2008; Todeschini et al. 2008). Numerous protein–carbohydrate domains have been mapped and structurally solved (Paulson et al. 2006; DeMarco and Woods 2008). Powerful resources have been developed to screen glycan libraries for new protein binding partners, with a major emphasis on identifying one-to-one interactions using immobilized oligosaccharides (http://www.functionalglycomics.org/static/index.shtml, The Functional Glycomics Gateway).
The characterization of a new class of neuropathy-associated anti-glycolipid autoantibodies has added an unexpected dimension to this field. Surprisingly, these autoantibodies only interact with pairs of glycolipids in complex, whilst failing to interact with either glycolipid alone (Kaida et al. 2004, 2006, 2007; Willison 2005, 2006; Kusunoki et al. 2008). This finding is consistent with the increasing awareness that, in the living membrane of cells, glycolipids do not exist in isolation but instead closely interact in cis with other lipids, thereby forming heterogeneous microdomains (Simons and Ikonen 1997). Glycolipid cis interactions have also been shown to be important in negatively regulating monoclonal autoantibody binding to the ganglioside GM1 (Greenshields et al. 2009) and in modulating cancer cell motility via the CD82-GM2:GM3 complex interaction (Todeschini and Hakomori 2008), attesting their functional significance. Furthermore, gangliosides have been shown to spontaneously form heterodimers in solution by electrospray ionization mass spectrometry (Todeschini et al. 2008).
These findings impact profoundly upon our understanding of carbohydrate recognition by lectins. Reductionist studies assessing binding to single immobilized glycans risk overlooking interactions between proteins and glycolipid complexes. Importantly, the number of potential glycan ligands is dramatically increased when their heterogeneous association is considered. Thus, from 20 single glycolipid molecules alone, 190 distinct pairings can be generated, making current low-throughput techniques, such as multiwell enzyme-linked immunosorbent assays (ELISAs), impractical, not least for their handling-intensive nature, insensitivity, and lack of parsimony for scarce reagents. Here we report a miniaturized combinatorial glycoarray based on a synthesis of previously published concepts and methods (Kaida et al. 2004; Kanter et al. 2006) that allows for the simple and rapid assessment of lectin binding to glycolipid complexes and illustrate the ability of the technique to identify previously unknown complexes bound by bacterial toxins, siglecs, and antibodies.
Results
The glycan binding of bacterial toxins, siglecs, and antibodies is modulated by glycolipid complexes
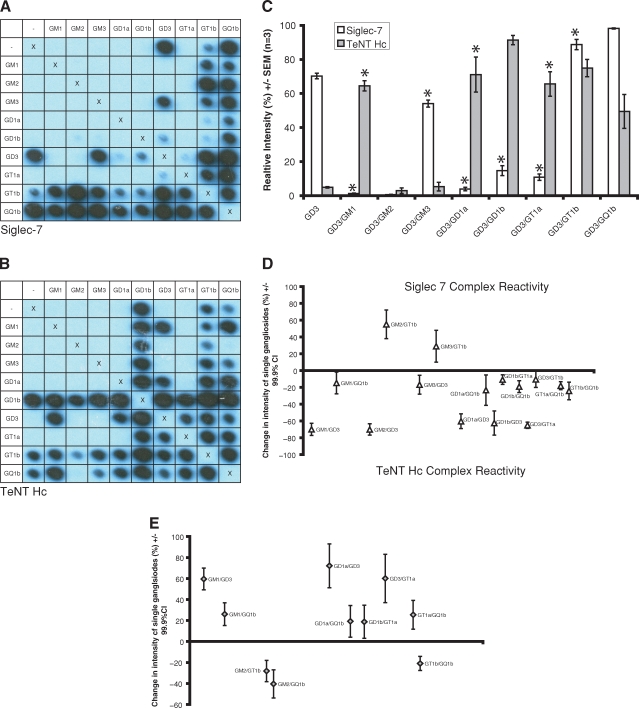
The glycoarray method was developed to allow various lectins to be simultaneously assayed against a large number of complexes and their component single glycolipids. A TLC autosampler was used to create reproducible grids of glycolipids and their complexes in duplicate on polyvinylidene difluoride (PVDF) membranes. These were then probed with the proteins of interest. Inter- (n = 5) and intra-assay (n = 9) coefficients of variation were measured at 4.1% and 8.6%, respectively (see supplementary data online for further details). We used this technique to map the ganglioside complex specificity of proteins previously reported to display a lectin-like activity, such as the horseradish peroxidase-conjugated binding fragment of tetanus neurotoxin (TeNT HC-HRP) (Deinhardt et al. 2006), cholera toxin B subunit (CTB), recombinant chimaeras containing the extracellular region of Siglecs fused to the Fc domain of human IgG1 (Siglec-Fc) (Crocker et al. 2007), anti-ganglioside monoclonal antibodies (Goodyear et al. 1999; Bowes et al. 2002; Boffey et al. 2004, 2005), and human neuropathy sera. Figure 1 compares the specificity of TeNT HC and Siglec-7 for different gangliosides and ganglioside complexes. Significantly in this context, the binding of these lectins to GD3 and GD3:GM1 is markedly different. Siglec-7 (Figure 1A, C and D) reacts with GD3 in isolation, yielding a mean relative signal intensity of 70.3%, yet the intensity for complexes of GD3 with any of GM1, GM2, GD1a, GD1b, and GT1a is reduced at between 0.62% and 14.7% (P < 0.0001, GLM ANOVA with Dunnett correction, family error rate 0.05, n = 3). Likewise, TeNT HC (Figure 1B and E) binding to GQ1b is massively reduced in the presence of GM2 (P = 0.002, GLM ANOVA with Dunnett correction, family error rate 0.05, n = 3). We have termed this behavior “complex attenuated”. Conversely, TeNT HC does not bind to the single gangliosides GD3, GM1, GD1a or GT1a, yet reacts strongly with heterodimers of these gangliosides containing GD3 (“complex enhanced”, Figure 1C). A confounding factor in attributing this effect to the formation of a neo-epitope is that lower degrees of binding to individual glycolipids could simply summate. To take into account this possibility, we have expanded the previous ELISA definition (that for true complex reactivity, the optical density (OD) for the complex must exceed the sum of the individual ODs) (Kaida et al. 2007) to include an additional degree of statistical rigor. We have defined “complex modulated” binding as the state in which the signal intensity of the complex minus the sum of the signal intensities of the isolated glycolipids (to a maximum of 100%) is significantly different from zero. Interactions meeting this definition are marked with an asterisk on the histogram (Figure 1C). The magnitude of the effect along with confidence intervals is depicted in Figure 1D–E.
Fig. 1.
Row and column headings reveal the complex at each location. ‘X's represent negative controls (methanol only) and act as a line of symmetry for duplicate spots within the same membrane. (A) Siglec-7-Fc reacts with GD3 in isolation, yet the signal intensity for most complexes of GD3 is either abolished or much reduced (“complex attenuated”). (B) TeNT HC does not bind to the single gangliosides GD3, GM1, GD1a or GT1a, yet reacts strongly with heterodimers of these gangliosides containing GD3 (“complex enhanced”). (C) Quantification of the modulatory effects of GD3 series complexes on siglec-7 and TeNT HC binding. Asterisks denote complexes displaying binding levels significantly different from the sum of the two individual components. Complex modulated binding was defined as the sum of intensity readings for the single gangliosides (to a maximum of 100%) subtracted from the signal generated by the complex being significantly different to zero, shown by the 99.9% confidence interval for this difference failing to cross zero. Complexes fitting this definition, along with the magnitude of the effect, are plotted for Siglec-7 (D) and TeNT HC (E). Ganglioside complexes where reactivity was not significantly different to that of the component glycolipids are not shown in these graphs.
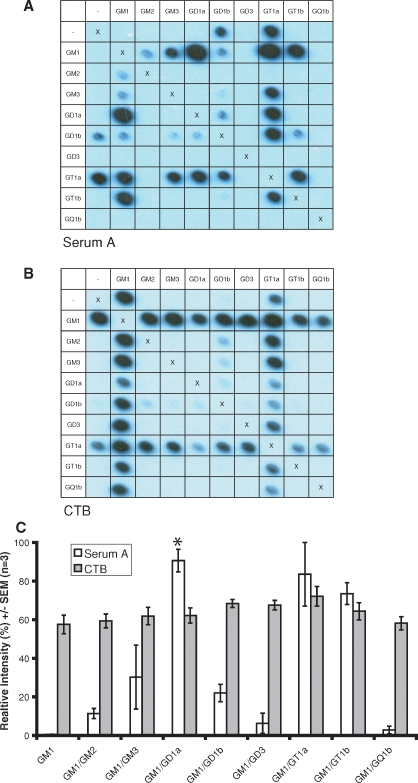
Figure 2 compares the serum (serum A, Figure 2A and C) obtained from a patient with the acute inflammatory polyradiculoneuropathy, Guillain–Barré syndrome (GBS), in which antiganglioside antibodies are sometimes found (Rinaldi and Willison 2008), with HRP-labeled CTB subunit (Figure 2B and C). There is no significant difference in CTB binding to GM1 compared with any of the GM1 series complexes (“complex independent”) at a 1:20,000 dilution, suggesting that the amount of GM1 remaining bound to the membrane with the complexes is not significantly reduced or increased compared with GM1 alone (Figure 2C). Furthermore, to demonstrate that the apparent uniformity of CTB binding in this situation was not simply a result of saturation, a higher concentration of 1:10,000 was also assayed. The absolute intensity for each complex with the higher concentration was increased, showing that the signal is not saturated at the lower concentration. Results for the 1:20,000 dilution have been normalized to the most intense spot at 1:10,000 to reflect this. In contrast, serum A binding to the complexes GM1:GD1a and GM1:GT1b is increased by 87.3% and 68.9% (P = 0.037 and P = 0.056, two-sided two-sample t-test with Bonferroni's correction, family error rate 0.05, n = 3) compared to the sum of the intensities of the two component gangliosides.
Fig. 2.
(A) Serum A binding to GM1:GD1a is enhanced compared with the individual component gangliosides. There is no significant difference in CTB binding (B) to GM1 compared with any of the GM1 series complexes (“complex independent”). The average signal intensities (n = 3) are quantified (C) with asterisks denoting complexes displaying binding levels significantly different from the sum of the two component glycolipids.
Taken as a whole, these results also indicated that the attenuation and enhancement of lectin binding seen is not simply a result of dilution of one lipid by another. For example, for tentanus toxin, simply diluting the GD3 with GM2 or GM3 has no effect on binding, whereas GD3 plus GM1 leads to a significantly enhanced signal. The same argument can be made for serum A reactivity with the GM1:GD1a complex. If GM1 is instead “diluted” with GD3 or GQ1b, or GD1a with GM3, GM3, GD3, GT1b or GQ1b, no binding results. Likewise, the inhibitory effect of GM1 on GD3 binding by siglec-7 is not replicated by dilution of GD3 with GM3.
The display platform for glycolipids and their complexes influences lectin binding
Although the above effects were also observed on ELISA (results not shown), this is not always the case. The method of immobilization and charge of the array surface may modulate lectin binding by altering the orientation and spacing of the target carbohydrates, especially when a heterogeneous mixture of glycolipids is used. We therefore compared the binding of a variety of lectins to ganglioside complexes displayed on both the PVDF array and polystyrene ELISA plates.
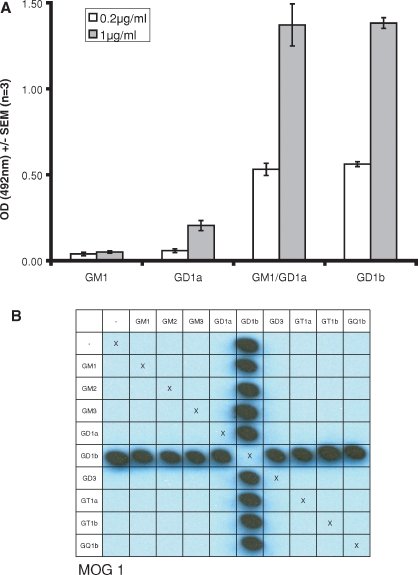
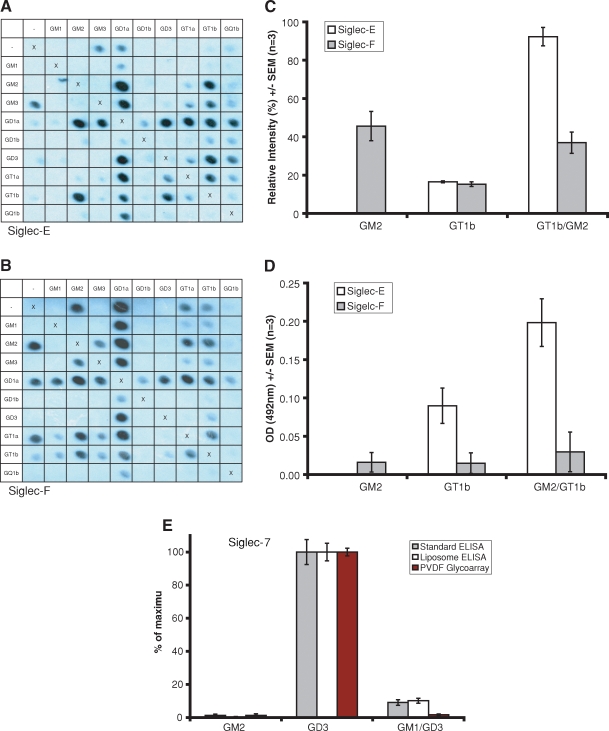
Binding of lectins to complexes is dependent on the display platform employed. As shown in Figure 3A, the monoclonal antibody MOG1, raised against GD1b containing liposomes, binds equally as well on ELISA to the GM1:GD1a complex as it does to GD1b. However, GM1:GD1a reactivity is not seen using the PVDF glycoarray (Figure 3B), even when the concentration of the mAb is increased 10-fold. Siglec-E displays enhanced binding to the complex GM2:GT1b in both systems (Figure 4A, C and D). Conversely, on PVDF, Siglec-F-Fc (Figure 4B and C) binds GM2 and remains able to bind the GM2:GT1b mixture, yet on ELISA the binding of this siglec to the same target glycolipids is not significantly elevated above background (Figure 4D, filled bars). The inhibitory effect of GM1 on the binding of Siglec-7-Fc to GD3 is observed to occur on both surfaces and in liposome-based assays, as shown in Figure 4E.
Fig. 3.
The mAb MOG1 binds the GM1:GD1a complex on ELISA (A). On PVDF glycoarrays (B), only GD1b containing complexes are bound by the antibody, whilst no signal is seen with the GM1:GD1a complex.
Fig. 4.
Siglec-E binds to the neo-epitope formed by GM2:GT1b complex on the PVDF glycoarray, but not to GM2 alone, (A,C) a finding which is also replicated on ELISA (D). Conversely, siglec-F binds to GM2 and to the GM2:GT1b complex on PVDF (B,C), whereas by ELISA reactivity to these target glycolipids is not significantly elevated above background (D). Siglec-7 binding to GD3 is inhibited by GM1 on PVDF-glycoarray, by ELISA and in liposome based assays (E). The average absolute values from each set of three independent experiments have been normalized to 100% to allow comparison on the same histogram. The uncorrected maximum values were 0.34, 1.14 and 70.3% for standard ELISA, liposome ELISA, and PVDF-glycoarray respectively. Error bars represent SEM, corrected for the normalization.
Discussion
In comparison with protein–protein interactions, protein– carbohydrate interactions are generally of low affinity, being more dependent on the high avidity conferred by multivalency and ligand clustering (Raman et al. 2005; Paulson et al. 2006). Furthermore, subtle changes in the carbohydrate structure can greatly affect protein binding. It is not surprising that cis-interactions among glycolipids modulate lectin binding, as these could potentially alter clustering, spacing, orientation, or tertiary structure. The implications of ligand-enhancing and -attenuating complexes are potentially wide as illustrated in the following examples drawn from the current study. Siglec-7 is a CD33-related human natural killer cell receptor (Avril, North, et al. 2006), and siglecs-E and -F are mouse lectins found on neutrophils, monocytes, dendritic cells, and eosinophils, respectively. Siglecs are involved in self-/non-self-recognition, yet their ligands are present on host cells and as well as many pathogens (Crocker et al. 2007). The further level of complexity introduced by cis-interacting glycolipids could allow fine-tuning of siglec-dependent recognition relevant to host immunity (Avril, Wagner, et al. 2006). Secondly, the ability of neuropathy-associated anti-GM1 autoantibodies to induce neuronal injury is variable, depending upon their attenuated binding in the presence of complexed GM1. Thus, in separate studies we have recently shown that the pathological potential of a pair of anti-GM1 antibodies in vivo can be predicted from their ability to bind GM1 complexes in vitro. One antibody was prevented from binding GM1 in solid phase assays and in live neural tissue by the presence of a cis-interaction between GM1 and GD1a, and was therefore “non-pathogenic”. Conversely, the second antibody was able to bind GM1:GD1a complexes in assays and live tissue, and thereby exert pathogenic effects (Greenshields et al. 2009). Thirdly, TeNT HC initially binds to GD1b on the axonal surface, yet when the toxin is internalized, the ganglioside remains on the plasma membrane (Deinhardt et al. 2006). During the internalization at the plasma membrane, GD1b might be sequestered by a different complex, reducing its affinity for TeNT HC and allowing the dissociation of the toxin from the ganglioside prior to internalization. Although we have not demonstrated a dramatic on–off effect for TeNT HC binding to GD1b series complexes, the statistically significant differences in signal intensity between a number of different GD1b complexes may have biological relevance. Alternatively, other GD1b complexes not studied in the current array format may prove important in vivo. Conversely, CTB displays complex-independent binding to GM1 and enters cells bound to GM1 (Lencer et al. 1999; Lencer 2004), reinforcing the notion that glycolipid complexes cannot modulate dissociation of CTB from GM1 prior to internalization. These examples highlight the subtle ways in which different glycolipid interactions could modify lectin binding and thereby modulate any subsequent functional or pathological effects. Furthermore, many membrane proteins are glycosylated, and these oligosaccharides interact with other protein and carbohydrate molecules, with functional importance in processes such as cell–cell interaction (Hakomori 2002). It is possible that the heterogenous clustering of oligosaccharides in this paradigm might also influence such processes. To date, the only direct evidence for the existence of ganglioside complexes in nature is detailed in the studies already discussed (Todeschini et al. 2008; Todeschini and Hakomori 2008; Greenshields et al. 2009). In light of these observations, however, it would seem that looking at other glycolipid complexes and assessing their modulatory effects in vivo will prove to be a fruitful area of future research.
Although current considerations of the modulatory effects of ganglioside complexes, including this one, have dealt with only heterodimers, it is possible that even more intricate interactions, involving three or more glycolipids, might prove equally as important. Investigating such situations becomes increasingly more difficult as the number of component glycolipids increases, making the ability to automatically array ligands described here even more valuable.
At present, there is a conceptual mismatch between assessing lectin interactions in vivo, where many accessory factors in plasma membranes will influence binding, and in vitro, where investigations have almost exclusively focused on assessing reactivity to isolated, purified oligosaccharides in artificial systems (Blixt et al. 2004; Byres et al. 2008). Single ganglioside dot-blot on PVDF was first described in 1993 (Chabraoui et al. 1993). More recently, single lipids and glycolipids have been automatically arrayed onto PVDF membranes and probed with cerebrospinal fluid from patients with multiple sclerosis (Kanter et al. 2006). Ganglioside complex reactivity has so far only been assessed by ELISA and thin-layer chromatography (TLC) with immuno-overlay. (Kaida et al. 2004) This, however, is the first description of an automated, combinatorial system which has the capacity to assess the influence of interactions between greater numbers of glycolipids and other accessory molecules on lectin binding. This technique builds upon these previously described methodologies, and has allowed us to demonstrate that, for several types of lectin studied, binding to complexes can be enhanced, attenuated, or unaffected compared with reactivity against single glycolipids. This concept, and its practical demonstration, reveals new horizons in the study of diverse processes including cell–cell recognition, toxin binding, autoimmunity, and microbial invasion.
Material and methods
PDVF glycoarray
A detailed protocol for this method is provided as supplementary data. In brief, working solutions of glycolipids were made at 0.1 μg/mL. For complexes, a 1:1 (v/v) mixture was created and sonicated for 3 min before use. A TLC autosampler (Camag, Switzerland) was then used to spot single gangliosides and their complexes at predetermined locations on PVDF membranes affixed to glass slides. Printed membranes were dried for 20 min in a fume hood then kept at 4°C overnight prior to use. Membranes were blocked in 2%BSA/PBS, incubated with the primary sample diluted in 1%BSA/PBS, and then washed. For samples requiring a secondary antibody, this was then applied diluted in 1% BSA before a further wash cycle. Detection was via an ECL plus (Amersham/GE Healthcare, UK) chemiluminescent reaction, rendered on radiographic film. Films were digitized by flatbed scanning and the images quantified by ImageQuant TL software (Amersham Biosciences, UK).
Ganglioside complex ELISA
Working solutions for ELISA were made by diluting gangliosides in methanol to 2 μg/mL. Complexes were then generated as for the PVDF glycoarray. As a negative control, 100 μL of methanol only was added to a number of wells per ELISA plate (Immulon 2HB). Subsequently, 100 μL of the single or complex ganglioside solution was added per well and allowed to air dry for 40 h in the fume hood. Plates were kept at 4°C for at least 1 h prior to further use.
Plates were blocked with 2% BSA/PBS for 1 h at 4°C. Primary samples were diluted as for PVDF glycoarray. Then 100 μL of the diluted solution was applied to each coated well of the ELISA plate. Incubation was for 2 h (mAbs, siglecs, sera) or 2 min (cholera toxin B-subunit) at 4°C.
The primary solution was discarded, and the plates immersed in cold PBS were then discarded for five cycles. For directly labeled bacterial toxins and siglecs, the detection buffer was then applied. For mAbs and sera, 100 μL of the appropriate secondary antibody, diluted 1:3000 in 1% BSA, was applied to the wells and incubated for 1 h at 4°C. The plates then underwent the same wash protocol as for the primary solution. Detection was performed with an o-phenylenediamine dihydrochloride solution. The reaction was terminated with 50 μL of 4 M H2SO4. Optical density at 492 nm was detected by an automated plate reader (Ascent Multiscan, Labsystems, GMI, USA).
Liposome production
Liposomes were generated using a sequential sonication, freeze-thaw, and extrusion method. Cholesterol, sphingomyelin, dicetylphosphate, and gangliosides were dissolved in a 1:1 mixture of chloroform:methanol to 1 μg/mL. These lipids were mixed in a 5:4:1:1 ratio respectively, or 5:4:1:1:1 when a second ganglioside was included to create ganglioside complexes. Blank liposomes contained no ganglioside. The lipid mixture was dried under a nitrogen steam to form a film on the wall of a 15 mL tube. The lipids were then resuspended in 1 mL of PBS by vortexing and sonication alternately for 15 min in total. The mixture was subjected to five freeze-thaw cycles by immersion in liquid nitrogen and then thawing in a water bath at 37°C. Unilamellar liposomes were then created by repeated extrusion (11 times) through a 0.4 μm pore size membrane using a hand-driven extruder. Hundred microliters of the appropriate final liposome preparation was added per well on an Immulon 2HB ELISA plate and incubated overnight at 4°C. Blank liposomes were used as a negative control. Following washing in PBS, these liposome plates then underwent the same protocol as for the ganglioside complex ELISA described above.
Supplementary Data
Supplementary data for this article is available online at http://glycob.oxfordjournals.org/.
Funding
The Wellcome Trust (085225/Z/08/Z to S.R. and 077041/Z/05/Z to H.J.W.) and a Wellcome Trust Senior Fellowship WT081882MA (to P.R.C.).
Conflict of interest statement
SR, KMB, CSG, and HJW are co-applicants on a patent pending submission regarding this methodology.
Glossary
Abbreviations
- BSA
bovine serum albumin
- CTB
cholera toxin B-subunit
- ELISA
enzyme-linked immunosorbent assay
- GLM ANOVA
general linear model analysis of variance
- PBS
phosphate buffered saline
- PVDF
polyvinylidene difluoride
- Siglec
sialic acid binding immunoglobulin-like lectin
- TeNT HC
tetanus neurotoxin binding fragment
- TLC
thin-layer chromatography
References
- Avril T, North SJ, Haslam SM, Willison HJ, Crocker PR. Probing the cis interactions of the inhibitory receptor Siglec-7 with alpha2,8-disialylated ligands on natural killer cells and other leukocytes using glycan-specific antibodies and by analysis of alpha2,8-sialyltransferase gene expression. J Leukoc Biol. 2006;80(4):787–796. doi: 10.1189/jlb.1005559. [DOI] [PubMed] [Google Scholar]
- Avril T, Wagner ER, Willison HJ, Crocker PR. Sialic acid-binding immunoglobulin-like lectin 7 mediates selective recognition of sialylated glycans expressed on Campylobacter jejuni lipooligosaccharides. Infect Immun. 2006;74(7):4133–4141. doi: 10.1128/IAI.02094-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blixt O, Head S, Mondala T, Scanlan C, Huflejt ME, Alvarez R, Bryan MC, Fazio F, Calarese D, Stevens J, et al. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc Natl Acad Sci USA. 2004;101(49):17033–17038. doi: 10.1073/pnas.0407902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boffey J, Nicholl D, Wagner ER, Townson K, Goodyear C, Furukawa K, Furukawa K, Conner J, Willison HJ. Innate murine B cells produce anti-disialosyl antibodies reactive with Campylobacter jejuni LPS and gangliosides that are polyreactive and encoded by a restricted set of unmutated V genes. J Neuroimmunol. 2004;152(1-2):98–111. doi: 10.1016/j.jneuroim.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Boffey J, Odaka M, Nicoll D, Wagner ER, Townson K, Bowes T, Conner J, Furukawa K, Willison HJ. Characterisation of the immunoglobulin variable region gene usage encoding the murine anti-ganglioside antibody repertoire. J Neuroimmunol. 2005;165(1-2):92–103. doi: 10.1016/j.jneuroim.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Bowes T, Wagner ER, Boffey J, Nicholl D, Cochrane L, Benboubetra M, Conner J, Furukawa K, Furukawa K, Willison HJ. Tolerance to self gangliosides is the major factor restricting the antibody response to lipopolysaccharide core oligosaccharides in Campylobacter jejuni strains associated with Guillain–Barre syndrome. Infect Immun. 2002;70(9):5008–5018. doi: 10.1128/IAI.70.9.5008-5018.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byres E, Paton AW, Paton JC, Lofling JC, Smith DF, Wilce MC, Talbot UM, Chong DC, Yu H, Huang S, et al. Incorporation of a non-human glycan mediates human susceptibility to a bacterial toxin. Nature. 2008;456(7222):648–652. doi: 10.1038/nature07428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabraoui F, Derrington EA, Mallie-Didier F, Confavreux C, Quincy C, Caudie C. Dot-blot immunodetection of antibodies against GM1 and other gangliosides on PVDF-P membranes. J Immunol Methods. 1993;165(2):225–230. doi: 10.1016/0022-1759(93)90348-b. [DOI] [PubMed] [Google Scholar]
- Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7(4):255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- Crocker PR, Redelinghuys P. Siglecs as positive and negative regulators of the immune system. Biochem Soc Trans. 2008;36(Pt 6):1467–1471. doi: 10.1042/BST0361467. [DOI] [PubMed] [Google Scholar]
- Deinhardt K, Berninghausen O, Willison HJ, Hopkins CR, Schiavo G. Tetanus toxin is internalized by a sequential clathrin-dependent mechanism initiated within lipid microdomains and independent of epsin1. J Cell Biol. 2006;174(3):459–471. doi: 10.1083/jcb.200508170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarco ML, Woods RJ. Structural glycobiology: A game of snakes and ladders. Glycobiology. 2008;18(6):426–440. doi: 10.1093/glycob/cwn026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyear CS, O’Hanlon GM, Plomp JJ, Wagner ER, Morrison I, Veitch J, Cochrane L, Bullens RW, Molenaar PC, Conner J, et al. Monoclonal antibodies raised against Guillain–Barre syndrome-associated Campylobacter jejuni lipopolysaccharides react with neuronal gangliosides and paralyze muscle-nerve preparations. J Clin Invest. 1999;104(6):697–708. doi: 10.1172/JCI6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenshields KN, Halstead SK, Zitman FM, Rinaldi S, Brennan KM, O’Leary C, Chamberlain LH, Easton A, Roxburgh J, Pediani J, et al. The neuropathic potential of anti-GM1 autoantibodies is regulated by the local glycolipid environment. J Clin Invest. 2009;119(3):595–610. doi: 10.1172/JCI37338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakomori SI. Inaugural article: The glycosynapse. Proc Natl Acad Sci USA. 2002;99(1):225–232. doi: 10.1073/pnas.012540899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaida K, Kanzaki M, Morita D, Kamakura K, Motoyoshi K, Hirakawa M, Kusunoki S. Anti-ganglioside complex antibodies in Miller Fisher syndrome. J Neurol Neurosurg Psychiatry. 2006;77(9):1043–1046. doi: 10.1136/jnnp.2006.087940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaida K, Morita D, Kanzaki M, Kamakura K, Motoyoshi K, Hirakawa M, Kusunoki S. Ganglioside complexes as new target antigens in Guillain–Barre syndrome. Ann Neurol. 2004;56(4):567–571. doi: 10.1002/ana.20222. [DOI] [PubMed] [Google Scholar]
- Kaida K, Morita D, Kanzaki M, Kamakura K, Motoyoshi K, Hirakawa M, Kusunoki S. Anti-ganglioside complex antibodies associated with severe disability in GBS. J Neuroimmunol. 2007;182(1-2):212–218. doi: 10.1016/j.jneuroim.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Kanter JL, Narayana S, Ho PP, Catz I, Warren KG, Sobel RA, Steinman L, Robinson WH. Lipid microarrays identify key mediators of autoimmune brain inflammation. Nat Med. 2006;12(1):138–143. doi: 10.1038/nm1344. [DOI] [PubMed] [Google Scholar]
- Karlsson KA. Pathogen-host protein–carbohydrate interactions as the basis of important infections. Adv Exp Med Biol. 2001;491:431–443. doi: 10.1007/978-1-4615-1267-7_28. [DOI] [PubMed] [Google Scholar]
- Kuijf ML, Van Doorn PA, Tio-Gillen AP, Geleijns K, Ang CW, Hooijkaas H, Hop WCJ, Jacobs BC. Diagnostic value of anti-GM1 ganglioside serology and validation of the INCAT-ELISA. J Neurol Sci. 2005;239(1):37–44. doi: 10.1016/j.jns.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Kusunoki S, Kaida K, Ueda M. Antibodies against gangliosides and ganglioside complexes in Guillain–Barre syndrome: New aspects of research. Biochim Biophys Acta. 2008;1780(3):441–444. doi: 10.1016/j.bbagen.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Lalli G, Bohnert S, Deinhardt K, Verastegui C, Schiavo G. The journey of tetanus and botulinum neurotoxins in neurons. Trends Microbiol. 2003;11(9):431–437. doi: 10.1016/s0966-842x(03)00210-5. [DOI] [PubMed] [Google Scholar]
- Lencer WI. Retrograde transport of cholera toxin into the ER of host cells. Int J Med Microbiol. 2004;293(7-8):491–494. doi: 10.1078/1438-4221-00293. [DOI] [PubMed] [Google Scholar]
- Lencer WI, Hirst TR, Holmes RK. Membrane traffic and the cellular uptake of cholera toxin. Biochim Biophys Acta. 1999;1450(3):177–190. doi: 10.1016/s0167-4889(99)00070-1. [DOI] [PubMed] [Google Scholar]
- Miller-Podraza H, Johansson P, Angstrom J, Larsson T, Longard M, Karlsson KA. Studies on gangliosides with affinity for Helicobacter pylori: Binding to natural and chemically modified structures. Glycobiology. 2004;14(3):205–217. doi: 10.1093/glycob/cwh028. [DOI] [PubMed] [Google Scholar]
- Paulson JC, Blixt O, Collins BE. Sweet spots in functional glycomics. Nat Chem Biol. 2006;2(5):238–248. doi: 10.1038/nchembio785. [DOI] [PubMed] [Google Scholar]
- Raman R, Raguram S, Venkataraman G, Paulson JC, Sasisekharan R. Glycomics: An integrated systems approach to structure–function relationships of glycans. Nat Methods. 2005;2(11):817–824. doi: 10.1038/nmeth807. [DOI] [PubMed] [Google Scholar]
- Rinaldi S, Willison HJ. Ganglioside antibodies and neuropathies. Curr Opin Neurol. 2008;21(5):540–546. doi: 10.1097/WCO.0b013e32830b84b7. [DOI] [PubMed] [Google Scholar]
- Schiavo G, Van Der Goot FG. The bacterial toxin toolkit. Nat Rev Mol Cell Biol. 2001;2(7):530–537. doi: 10.1038/35080089. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387(6633):569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Todeschini AR, Dos Santos JN, Handa K, Hakomori SI. Ganglioside GM2/GM3 complex affixed on silica nanospheres strongly inhibits cell motility through CD82/cMet-mediated pathway. Proc Natl Acad Sci USA. 2008;105(6):1925–1930. doi: 10.1073/pnas.0709619104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todeschini AR, Hakomori SI. Functional role of glycosphingolipids and gangliosides in control of cell adhesion, motility, and growth, through glycosynaptic microdomains. Biochim Biophys Acta. 2008;1780(3):421–433. doi: 10.1016/j.bbagen.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townson K, Boffey J, Nicholl D, Veitch J, Bundle D, Zhang P, Samain E, Antoine T, Bernardi A, Arosio D, et al. Solid phase immunoadsorption for therapeutic and analytical studies on neuropathy-associated anti-GM1 antibodies. Glycobiology. 2007;17(3):294–303. doi: 10.1093/glycob/cwl074. [DOI] [PubMed] [Google Scholar]
- Willison HJ. Ganglioside complexes: New autoantibody targets in Guillain–Barre syndromes. Nat Clin Pract Neurol. 2005;1(1):2–3. doi: 10.1038/ncpneuro0001. [DOI] [PubMed] [Google Scholar]
- Willison HJ. Ganglioside complexes as targets for antibodies in Miller Fisher syndrome. J Neurol Neurosurg Psychiatry. 2006;77(9):1002–1003. doi: 10.1136/jnnp.2006.094441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willison HJ, Veitch J, Swan AV, Baumann N, Comi G, Gregson NA, Illa I, Zielasek J, Hughes RA. Inter-laboratory validation of an ELISA for the determination of serum anti-ganglioside antibodies. Eur J Neurol. 1999;6(1):71–77. doi: 10.1046/j.1468-1331.1999.610071.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.