Summary
Little is known on the functional differences of the human skin myeloid DC subsets, epidermal CD207+ Langerhans cells (LCs) and dermal CD14+ DCs. We show that CD14+ DCs prime CD4+ T cells into cells that induce naïve B cells to switch isotype and become plasma cells. LCs preferentially induce the differentiation of CD4+ T cells secreting Th2 cytokines and are remarkably efficient at priming and crosspriming naïve CD8+ T cells. A third DC population, CD14-CD207-CD1a+ DC population, which resides in the dermis can activate CD8+ T cells better than CD14+ DCs but less efficiently than LCs. Thus, human skin display three DC subsets, two of them i.e. CD14+ DCs and LCs, display functional specializations; the preferential activation of humoral or cellular immunity respectively.
Introduction
Pioneering studies in the late 19th and early 20th century led to the understanding that the immune system has two primary effector modalities: a cellular arm whose function was first described in Metchnikoff’s studies of phagocytes and a humoral arm whose properties were detailed by the work of Behring, Kitasato and Ehrlich on antitoxins (Silverstein, 2003). Decades of debate between the “humoralists” and “cellularists” about the basis of immune protection eventually led to the present view that both arms are critical for vertebrate’s survival. B cells and CD8+ T cells are the primary effectors of the adaptive immune system while CD4+ T cells both regulate the functions of these other lymphocyte types and have effector activities of their own (Germain, 2004). Each of these cell types is composed of subsets with specialized functions. The CD4+ T cell compartment is particularly complex as it includes Th1, Th2, Th17 (Weaver et al., 2007), and follicular helper T cells (Tfh) (King et al., 2008), that arise by polarized differentiation of naïve precursors, as well as regulatory T cells (Sakaguchi et al., 2006; Shevach, 2006). T cells are under the control of dendritic cells (DCs) which stimulate immunologically naïve T cells following the efficient formation and presentation of specific peptide-MHC complexes (Banchereau and Steinman, 1998; Steinman and Banchereau, 2007). More than that, DCs control the “polarization” of T cell responses, by delivering a variety of signals that differentially skew effector T cell development. DCs also play a critical role maintaining peripheral tolerance by down-regulating T cell responses to self antigens. The ability of DCs to induce specific types of T cell responses reflects the type of maturation signals they receive at the time of antigen encounter. In addition, it is becoming clear that distinct DC subsets exist, which have been associated with specific T cell outcomes, in addition to the stimulation of B cell and NK cell responses. Two major DC subtypes are recognized: the myeloid DCs (mDCs) and the plasmacytoid DCs (pDCs) (Banchereau et al., 2000; Shortman and Liu, 2002).
In mice, splenic mDCs were originally shown to be composed of two major mDC subsets with marked differences in biological function; CD8α+ CD11b– ‘lymphoid’ DCs and CD8α–CD11b+ ‘myeloid’ DCs. CD8α+ DCs are able to produce large amount of IL-12, and polarize naïve CD4+ T cells towards the Th1 phenotype, while CD8α– DCs preferentially induce Th2 responses (Maldonado-Lopez et al., 1999; Pulendran et al., 1999; Soares et al., 2007). Acquiring a better understanding of the role of mDC subsets in activation of distinct arms of the adaptive immune system is critical to the generation of new vaccines that address chronic diseases such as HIV-mediated AIDS, malaria, or Hepatitis C for which no vaccines are available (Pulendran and Ahmed, 2006). While the study of mouse DC subsets can make important contributions in this regard, it is crucial to do such studies using human cells, as major differences exist between the human and mouse immune systems (Mestas and Hughes, 2004). Thus, to successfully generate human vaccines, we need to understand the diversity and biology of human DC subsets.
In human skin, at least two different mDC subsets have been characterized: epidermal Langerhans cells (LCs) and dermal interstitial DCs (dermal DCs) (Nestle et al., 1993; Valladeau and Saeland, 2005). Over the years, dermal DCs were further subdivided into at least two subsets. The presence of two dermal DC subsets was also recently reported in mice which display a Langerin/CD207 subset in the dermis (Bursch et al., 2007; Ginhoux et al., 2007; Poulin et al., 2007). Detailed functional studies of these different mDC populations have progressed slowly, mostly because of the difficulties involved in isolating purified cells from tissues. However, such studies were in part feasible when we found that culturing CD34+ hematopoietic progenitor cells (CD34-HPC) with GM-CSF and TNFα gives rise to both CD1a+CD14– LCs, and CD14+CD1a– DCs. CD14+ DCs were found to be unique in their ability to induce the differentiation of naïve B cells into IgM-secreting plasma cells (Caux et al., 1997). No unique functions could, however, be identified for LCs. Here, we report our detailed study of the biological functions of two of the three DCs subsets of human skin, dermal CD14+ DCs and LCs.
Results
Generation and isolation of human mDC subsets
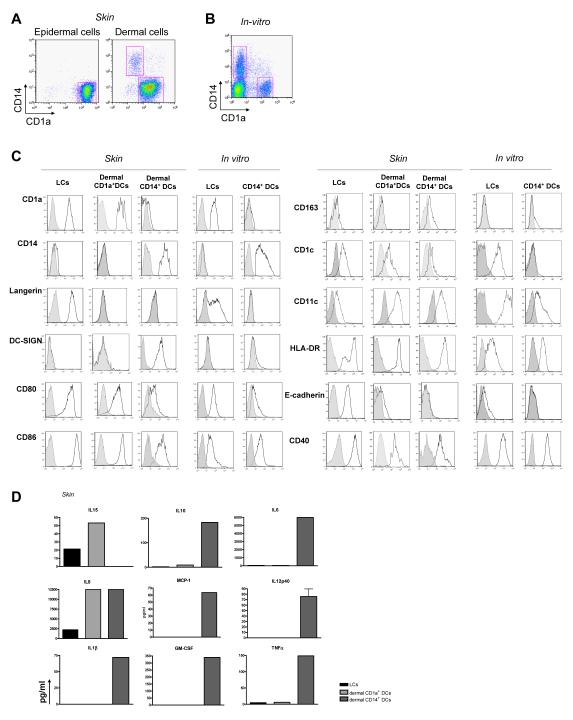
We have analyzed the biological functions of human mDC subsets that are either isolated from dermis and epidermis or generated in vitro by culturing blood CD34+ HPCs with GM-CSF, Flt3-L and TNFα. Briefly, a dispase treatment of skin samples enabled us to separate the epidermis from the dermis and cells were allowed to migrate for two days before purification using cell sorting. Epidermal sheets yielded CD1ahighCD14–HLA-DR+ cells expressing Langerin/CD207, a marker of LCs (Figures 1A). Dermis yielded two distinct populations: CD1a–CD14+HLA-DR+ cells (dermal CD14+ DCs) and CD1adimCD14—HLA-DR+ cells (dermal CD1a+ DCs) (Figure 1A). Studies performed with twelve skin samples revealed that LCs represented 58% of all the DCs that were isolated, while dermal CD1a+ DCs CD14+ DCs represent respectively 30% and 12% of all isolated DCs (Figure S1A). In situ analysis show that dermal CD1a+ DCs are mostly located in the upper dermis (Figure S1B).
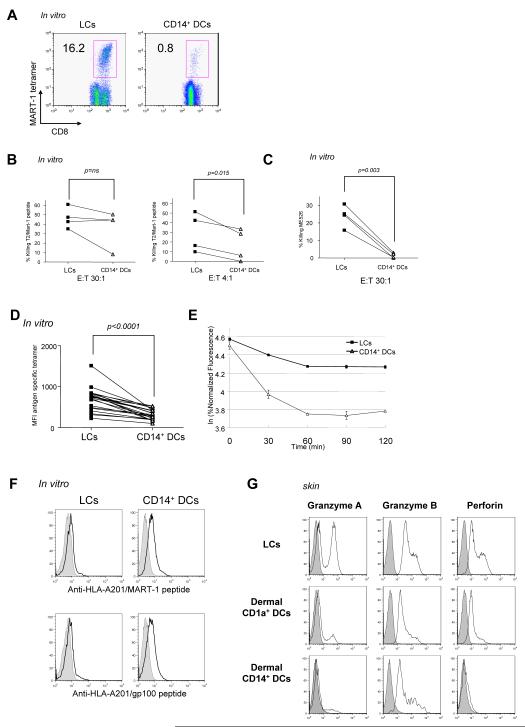
Figure 1.
Purification and characterization of dermal and epidermal DCs obtained from human skin and of in vitro CD34-HPCs derived mDC subsets
(A) Expression of CD1a and CD14 on purified skin DCs defines epidermal CD1a+ LCs, dermal CD1a+ DCs, and dermal CD14+ DCs subpopulations.
(B) Expression of CD1a and CD14 on CD34-HPC cultured with GM-CSF and TNFα for 9 days defines two subpopulations of mDCs, LCs and CD14+ DCs.
(C) Expression of surface antigens on skin and in vitro-generated DC subsets. Data show similarity of in vitro LCs to skin LCs, and of the in vitro CD14+ DCs to skin dermal CD14+ DCs. Dermal CD1a+ DCs show an intermediate phenotype compared to LCs and dermal CD14+ DCs.
(D) Cytokine production by CD40L-activated skin DC subsets. Data indicate the expression of IL-15 by LCs and dermal CD1a+ DCs and IL-10 production by dermal CD14+ DCs. Production of IL-6, IL12p40, MCP-1, GM-CSF, IL-1β and TNF-α is restricted to CD14+ DCs.
DCs generated in vitro by culturing CD34+ HPCs for 9 days were sorted into CD1a+CD14—LCs (LCs) and CD1a—CD14+ DCs (CD14+ DCs) (Figure 1B).
Both skin LCs and in vitro-generated LCs expressed Langerin/CD207 and high levels of costimulatory molecules (CD80, CD86 and CD40, Figure 1C). The three skin DC populations expressed CD11c. Neither of the two dermal DC populations expresses Langerin/CD207 (Figures 1C, S1C and S1D), in contrast with the recent description of mouse Langerin/CD207+ dermal DCs. CD14+ DCs uniquely express DC-SIGN, as well as CD163 and transcribe Factor XIIIa (Figures S1E and S1F) but do not express CD1c. The two other CD1a+ DC populations express CD1c (Figure 1C).
Upon stimulation through CD40, different cytokines are secreted by the different DCs. CD14+ DCs secrete IL-10, MCP-1, IL-1β, IL-6, TNFα and GM-CSF (Figure 1D) and transcribe TGFβ and IL-10 (Figures S2A-B), while epidermal LCs and dermal CD1a+ DCs do not. In contrast, epidermal LCs and dermal CD1a+ DCs transcribe (Figure S2C) and secrete IL-15 (Figure 1D). The two dermal DC subsets secrete large amounts of IL-8 while LCs secrete much less. In vitro-generated CD14+ DCs produce the same cytokines as skin dermal CD14+ DCs (de Saint-Vis et al., 1998). IL-15 is produced by in vitro-generated LCs (Figure S2D).
Thus, LCs and CD14+ DCs generated in vitro show a phenotype remarkably similar to that of LCs and CD14+ DCs isolated from skin respectively.
LCs prime CD4+ T cells to secrete Type 2 cytokines
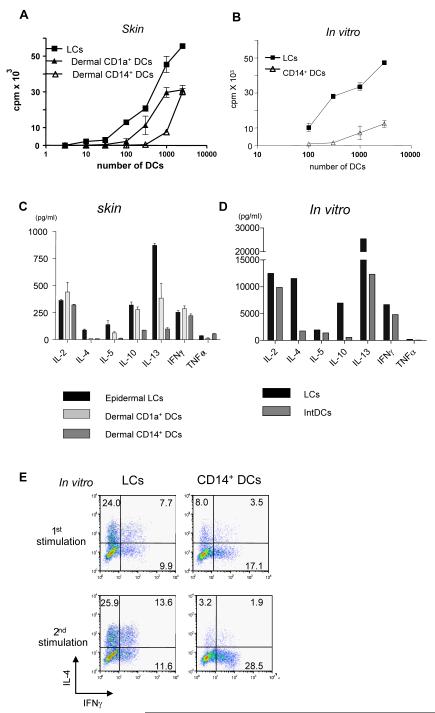
A function unique to DCs is their capacity to induce allogeneic naïve CD4+ T cells to proliferate (Mixed Lymphocytes Reaction or MLR). Indeed, LCs from skin were powerful stimulators of naïve CD4+ T cell proliferation (Figure 2A) while dermal CD14+ DCs were weaker. Dermal CD1a+ DCs showed intermediate activity In vitro-generated LCs were also strong stimulators of naive CD4+ T cell proliferation (Figures 2B and S3). In contrast, CD14+ DCs were weaker stimulators and MLR performed with LCs yielded higher numbers of CD4+ T cells than those made with CD14+ DCs (Figure S3).
Figure 2.
LCs are superior to CD14+ DCs at inducing the proliferation of allogeneic naïve CD4+ T cells and the differentiation of Type 2 cytokines secreting CD4+ T cells
(A) Skin-LCs induce more robust proliferation of allogeneic naïve CD4+ T cells than do dermal CD1a+ DCs or dermal CD14+ DCs. [H3]-thymidine incorporation. One of four experiments.
(B) In vitro-generated LCs stimulate stronger proliferation of allogeneic naïve CD4+ T cells than do CD14+ DCs. One five experiments.
(C) Naive CD4+ T cells primed with skin LCs secrete more Type 2 cytokines than those primed with dermal DC subsets. Proliferating naïve CD4+ T cells, primed by skin mDC subsets, were sorted and re-stimulated with CD3/CD28 mAbs overnight followed by cytokine analysis. One of three experiments.
(D) Naive CD4+ T cells primed with in vitro-generated LCs secrete more Type 2 cytokines upon restimulation. One of five experiments.
(E) LCs promote T cell differentiation towards IFNγ-IL-4+ Type 2 T cells. Naïve CD4+ T cells cultured for 6 d with each in vitro-generated mDC subsets were stimulated with PMA and ionomycin in the presence of monensin. Intracytoplasmic cytokines were analyzed using anti-IFNγ and anti-IL-4 mAbs (upper panel). Some CD4+ T cells were restimulated with the same DC subset for 3 days before the cytokine analysis (lower panel). One of ten experiments.
The purified skin DC subsets were co-cultured for 6 days with allogeneic naive CD4+ T cells to examine their capacity to polarize cytokine secretion. Activated CD4+ T cells were re-stimulated overnight with CD3/CD28 microbeads. The three skin DC subsets induced CD4+ T cells that produced comparable amounts of IL-2 and IFNγ (Figure 2C). However, LCs were remarkably efficient at inducing CD4+ T cells to secrete Type 2 cytokines: IL-4, IL-5 and IL-13. Dermal CD1a+ DCs primed CD4+ T cells to secrete less IL-5 and IL-13 when compared to LCs-primed CD4+ T cells but more than those primed by CD14+ DCs. No IL-4 was detected by CD4+ T cells primed by dermal CD1a+ DCs. The in vitro-generated mDC subsets behaved as did their in vivo counterparts, with LCs being more efficient inducers of Type 2 cytokine secretion by CD4+ T cells compared to CD14+ DCs (Figure 2D). Both mDC subsets induced the generation of IFNγ- and IL-4-producing polarized CD4+ T cells. Two cycles of T cell stimulation with the same DC subset yielded a more profound skewing of T cell differentiation by LCs towards Th2 (Figure 2E). The differential ability of the DC subsets to trigger CD4+ T cell proliferation and polarization was upheld upon maturation with either LPS or CD40L (Figure S4).
Thus, LCs are more efficient than CD14+ DCs in inducing the polarization of naïve CD4+ T cells into Type 2 cytokines secreting cells.
CD14+ DCs polarize naïve CD4+ T cells into follicular helper T cells
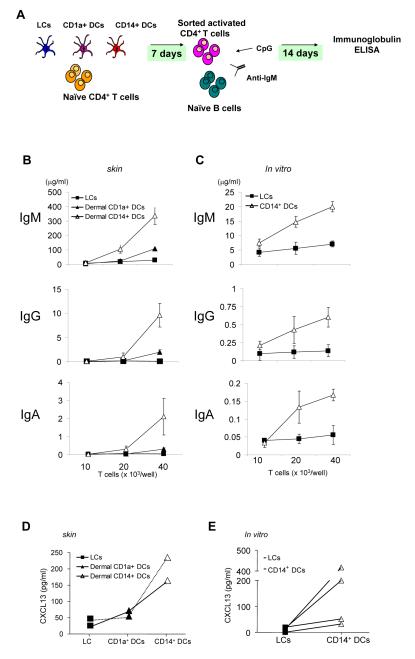
Type 2 T cells induce mouse naïve B cells to switch towards IgG1 and IgE in an IL-4-dependent manner, while Type 1 T cells induce IFNγ-dependent switch towards IgG2a. It is currently thought that these T cells are involved in the extrafollicular pathway of B-cell differentiation to induce short-lived plasma cells outside the germinal center. Another subset of CD4+ T cells, follicular helper T cells (Tfh) promotes the differentiation of B cells into high-affinity antibody secreting cells within germinal centers (King et al., 2008). Thus, allogeneic naïve CD4+ T cells, cultured for one week with the three purified skin DC subsets, were tested for their ability to activate naïve B cells (Figure 3A). Activated CD4+ T cells were sorted free of DCs at day 7 and cultured for 10 days with naïve IgM+IgD+ B cells preactivated with CpG2006 (a TLR-9 ligand) and anti-IgM mAb. CD4+ T cells cultured with dermal CD14+ DCs induced naïve B cells to produce large amounts of IgM (Figure 3B). CD4+ T cells cultured with LCs or dermal CD1a+ DCs can also induce naïve B cells to secrete IgM but to a lower extent. However, most remarkably, CD4+ T cells cultured with dermal CD14+ DCs induce naïve B cells to switch isotype secretion towards IgG and IgA. In contrast, CD4+ T cells cultured with LCs did not induce naïve B cells to switch isotypes. The dermal CD1a+ DCs were slightly more efficient than the LCs but much less than the dermal CD14+ DCs (Figures 3B and S5). In vitro-generated CD14+ DCs, but not in vitro-generated LCs, were also able to induce the differentiation of naïve CD4+ T cells into cells that could induce naïve B cells to switch isotype (Figure 3C). Consistent with a Tfh phenotype, the CD4+ T cells primed with CD14+ DCs purified from dermis (Figure 3D) or from DC cultures (Figure 3E) secrete high levels of CXCL13, a chemokine that promotes follicular homing of T and B cells.
Figure 3.
CD14+ DCs but not LCs or dermal CD1a+ DCs polarize CD4+ T cells into Tfh cells
(A) Experimental protocol. Allogeneic naïve CD4+ T cells were cultured for 7 days with sorted purified mDC subsets. Activated CD4+ T cells (FSChighCD11c-CD4+ T cells) were sorted and cultured with naïve B cells that were preactivated with CpG and anti-IgM. After 14 days, Ig levels were measured by ELISA.
(B) Ig production by naïve B cells cocultured with CD4+ T cells primed with distinct skin mDC subsets. Naïve B cells were cultured with indicated number of CD4+ T cells stimulated by each skin mDC subset.
(C) Ig production assay with in vitro-generated mDC subsets.
(D-E) Production of CXCL13 by CD4+ T cells primed by skin mDC subsets (D) or in vitro-generated mDC subsets (E). Primed CD4+ T cells (day 7) were restimulated overnight with CD3/CD28 mAbs before CXCL13 was measured.
Thus, dermal CD14+ DCs but not LCs or dermal CD1a+ DCs, induce the differentiation of naïve CD4+ T cells into T cells that can induce naïve B cells to switch isotype and differentiate into cells secreting large amounts of Igs.
LCs efficiently prime allogeneic naïve CD8+ T cells
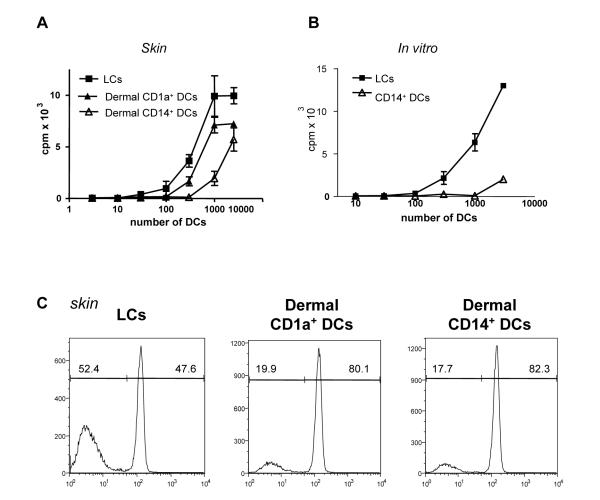
The capacity of the different mDC subsets to activate allogeneic naïve CD8+ T cells (CCR7+CD45RA+CD45RO-) was assessed using [3H]-labeled thymidine (Figure 4A) and CFSE dilution (Figure 4C). Indeed, LCs are powerful stimulators of naïve CD8+ T cell proliferation, while dermal CD14+ DCs were weak stimulators and dermal CD1a+ DCs showed intermediate activity, though significantly lower compared to LCs. In vitro-generated LCs were also inducing the proliferation of alloreactive CD8+ T cells more efficiently than CD14+ DCs did (Figures 4B and S6). Thus, LCs are powerful inducers of naïve CD8+ T cell proliferation.
Figure 4.
LCs are more efficient than CD14+ DCs at priming allogeneic naïve CD8+ T cells
(A) Skin LCs stimulate stronger proliferation of allogeneic naïve CD8+ T cells than do dermal CD1a+ DCs or dermal CD14+ DCs. [H3]-thymidine incorporation. One of four experiments.
(B) In vitro-generated LCs stimulate stronger proliferation of allogeneic naïve CD8+ T cells than do CD14+ DCs. One of four experiments.
(C) Skin LCs stimulate stronger proliferation of allogeneic naïve CD8+ T cells than do dermal CD1a+ DCs or dermal CD14+ DCs as indicated by dilution of CFSE dye. One of three experiments.
LCs preferentially select and expand antigen specific CTLs
To assess the capacity of mDC subsets to prime antigen-specific CD8+ T cell responses, we analyzed the priming of CD8+ T cells specific for the melanoma differentiation antigen MART-1. The purified mDC subsets were incubated with an HLA-A201-restricted MART-1 decamer peptide, and then cultured with purified naïve CD8+ T cells for 9 days, with soluble CD40L to ensure DC activation. LCs were able to induce considerable expansion of MART-1-specific CD8+ T cells as illustrated by the binding of MART-1-HLA-A201 tetramers to cultured lymphocytes (Figures 5A and S7A). CD14+ DCs were far less efficient at inducing priming and expansion of MART-1-specific naïve CD8+ T cells (Figures 5A and S7A p<0.0001, paired Student t-test). The lower efficiency of CD14+ DCs was not due to T cell death resulting from an overly vigorous response (data not shown and Figure S6). LCs were also more potent than CD14+ DCs at inducing the expansion of naïve CD8+ T cells specific for another melanoma-associated antigen, gp100 when pulsed as an HLA-A201-binding nonamer peptide (Figures S7B and S7C). CD8+ T cells primed with MART-1 peptide loaded-LCs and CD14+ DCs were able to kill HLA-A201+ T2 cells loaded with MART-1 peptide at high E:T ratio (Figure 5B, left panel). However, LC-activated CD8+ T cells were always more efficient than CD14+ DC-induced CD8+ T cells, as demonstrated by their higher cytotoxic function at a low effector to target ratio (Figure 5B, right panel). More interestingly, CD8+ cytotoxic T cells activated by LCs were able to kill HLA-A201+ tumor targets such as ME526 in the absence of added peptide antigen (Figure 5C). In contrast, CD8+ T cells generated with CD14+ DCs were unable to do so.
Figure 5.
LCs are more efficient than CD14+ DCs at priming antigen specific high avidity effector CD8+ T cells
(A) LCs from an HLA-A201+ donor, loaded with an HLA-A201-restricted MART-1 peptide, induce higher expansion of specific CD8+ T cells when compared to peptide loaded-CD14+ DCs (staining with MART-1-HLA-A201 tetramer).
(B) MART-1-specific CD8+ T cells primed by LCs and CD14+ DCs are cytotoxic against T2 cells loaded with 10-8 M MART-126-35 peptide at high Effector to Target (E:T) ratio (30:1, left panel). At a lower E:T ratio (4:1. right panel), LCs-primed CTLs are more cytotoxic than CD14+ DCs-primed CTLs.
(C) MART-1-specific CD8+ T cells primed by LCs, but not by CD14+ DCs, can kill the HLA A201+ melanoma cell line MEL526 expressing MART-1.
(D) MART-1-HLA-A201 tetramer fluorescence intensity is higher on CD8+ T cells primed by LCs than on those primed by CD14+ DCs. fifteen experiments. Paired Student t-test.
(E) MART-1-HLA-A201 tetramers dissociate at a slower rate from CD8+ T cells primed by LCs than from those primed by CD14+ DCs. CD8+ T cells primed by MART-126-35-loaded mDC subsets were incubated with MART-1/HLA-A201 tetramer, and then an excess of unlabeled anti-HLA-A201 Ab was added to prevent rebinding of the tetramer after dissociation. The intensity of tetramer fluorescence was analyzed at various time points. Data are shown as the natural logarithm of the percentage of maximum fluorescence (corresponding to mean fluorescence at t0) plotted against time.
(F) Staining peptide-loaded DCs with monoclonal antibodies endowed with TCR-like specificity, revealed that CD14+ DCs present higher levels of peptide-HLA-A201 complexes than LCs upon loading with HLA-A201 peptides (MART-126-35 upper panel, gp100209-217 in lower panel).
(G) LCs-primed CD8+ T cells express higher level of the effector molecules; granzyme A, Granzyme B and perforin compared to dermal DC subsets CD1a+-and CD14+ DCs.
Taken together, these data indicate that LCs are more efficient than CD14+ DCs at priming naïve CD8+ T cells to become potent cytotoxic effector cells able to respond functionally to naturally presented levels of TCR ligand.
LCs induce high avidity CTLs
The superior cytotoxic effector properties of LC-primed CD8+ T cells as compared to CD14+ DC-primed CD8+ T cells might be in part due to the selection of high avidity T cells. Indeed, the intensity of MART-1-HLA-A201 tetramer binding to MART-1-specific CD8+ T cells revealed that LC-primed CD8+ T cells bound more tetramers than CD14+ DC-primed CD8+ T cells (Figures 5A and 5D). The two CD8+ T cell populations showed the same level of fluorescence after staining with anti-CD3 Ab, indicating that the difference in tetramer staining was not merely due to higher levels of TCR expression on the LC-induced T cells (data not shown). Indeed, LC-primed CD8+ T cells released bound tetramer at a slower pace than did CD14+ DC-primed CD8+ T cells (Figure 5E) (Molldrem et al., 2003). This indicates that the LC-primed antigen-specific CD8+ T cells express TCRs with a higher avidity for the peptide-MHC (pMHC) complexes in comparison to CD14+ DC-primed antigen-specific CD8+ T cells.
The limited CD8+ T cell priming ability of CD14+ DCs could be due to low levels of pMHC complexes presented on their cell surface after incubation with the MART-1 peptide. However, staining with monoclonal antibodies that specifically bind to MART-1-HLA-A201 pMHC complexes (Noy et al., 2005) revealed that after peptide loading, CD14+ DCs presented considerably higher levels of specific pMHC complexes than LCs (Figure 5F, upper panel). The higher expression of pMHC complexes by CD14+ DCs cannot be simply explained by higher overall expression of MHC-Class I molecules on CD14+ DCs as compared to LCs, as both subsets displayed comparable expression of surface MHC class I molecules (data not shown). Similarly, CD14+ DCs loaded with a HLA-A201-restricted gp100 peptide display higher levels of pMHC complexes on their surface (Figure 5F, lower panel). To determine whether the higher levels of pMHC complexes on CD14+ DCs had a negative effect on the growth of specific CD8+ T cells, CD14+ DCs were loaded with lower peptide concentrations (0.3 μM) to approximate the level of pMHC found on LCs. This did not result in the generation of higher numbers of MART-1-specific CD8+ T cells with higher avidity (data not shown). Thus, independent of their level of TCR ligand, LCs display a preferential ability to expand high avidity antigen-specific naive CD8+ T cells.
CD14+ DCs induce naive CD8+ T cells to express low levels of Granzymes
The low killing properties of CD8+ T cells expanded with CD14+ DCs might be explained by an incomplete maturation of the naïve CD8+ T cells into cytotoxic effectors. Indeed CD8+ T cells primed with skin-derived LCs were found to express high levels of effector molecules granzyme A, granzyme B and perforin (Figure 5G, upper row). In contrast CD8+ naïve T cells primed with dermal CD14+ DCs (Figure 5G, lower row) expressed little Granzyme A and B and little Perforin, thereby providing an explanation for their limited killing properties. Dermal CD1a+ DCs induced naïve CD8+ T cells to express higher levels of effector molecules when compared to CD14+ DCs but were less efficient in comparison to LCs (Figure 5G, middle row). A similar phenotype was seen with in vitro-generated DCs, as CD8+ T cells primed by LCs expressed higher level of effector molecules compared to CD14+ DCs (Figure S7D).
Thus LCs efficiently induce the generation of cytotoxic T cells expressing high levels of cytotoxic proteins while CD14+ DCs generate T cells with low cytotoxic effector molecules.
LCs efficiently cross-present antigens to CD8+ T cells
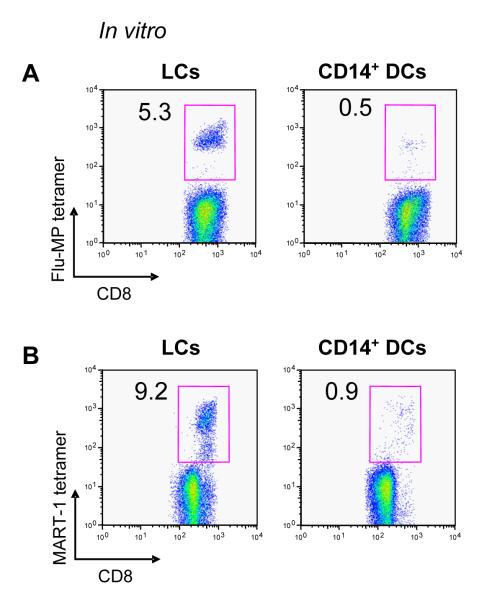
We examined the capacity of each DC subset to present external unprocessed protein antigens to CD8+ T cells (cross-presentation). In vitro-cultured LCs and CD14+ DCs were incubated with a recombinant influenza virus matrix protein (Flu-MP), and cultured with autologous CD8+ T cells. Expansion of HLA-A201-restricted Flu-MP58-66-specific CD8+ T cells was analyzed after 7-9 d. LCs efficiently induced proliferation of Flu-MP58-66-specific CD8+ T cells (Figures 6A and S8A). By contrast, CD14+ DCs barely induced Flu-MP58-66-specific CD8+ T cell population even after the maturation with LPS (data not shown). To assess crosspriming, in vitro-cultured LCs and CD14+ DCs were incubated with a 15 mer peptide (MART-121-35 YTTAEEAAGIGILTV) containing the HLA-A201-restricted MART-1 decamer epitope (MART-126-35 EAAGIGILTV) and cultured for 9 d with autologous naïve CD8+ T cells and soluble CD40L. In vitro LCs were far more potent than CD14+ DCs at cross-priming MART-126-35-specific CD8+ T cells, a highly reproducible finding (Figure 6B and S8B). Thus, LCs are more potent than CD14+ DCs at cross-presenting soluble antigens.
Figure 6.
LCs are more efficient than CD14+ DCs at cross-presenting antigens to CD8+ T cells
(A) LCs loaded with soluble Flu-MP efficiently cross-present and expand Flu-MP58-66-specific CD8+ T cells when compared to CD14+ DCs.
(B) LCs cross-prime MART-1-specific CD8+ T cells more efficiently than CD14+ DCs when loaded with a 15-mer MART-1 peptide (MART-121-35) as analysed by MART-126-35-HLA-A201 tetramer staining.
Both CD14+ DCs and LCs are able to activate memory T cells
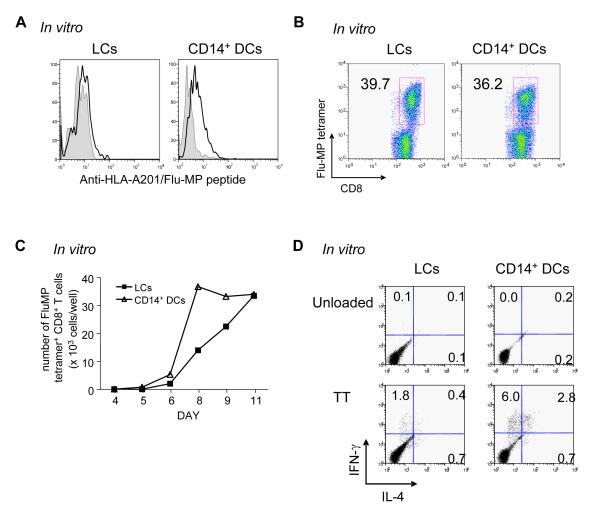
The poor capacity of CD14+ DCs loaded with Flu-MP to stimulate Flu-MP-specific CD8+ T cells might be due to their limited ability to stimulate memory T cells when compared to LCs (Belz et al., 2007). Thus, we investigated whether CD14+ DCs are able to stimulate memory Flu-MP58-66-specific CD8+ T cells when loaded with the immunodominant Flu-MP58-66 peptide. As observed earlier with the MART-1 and gp100 peptides (Figure 5F), CD14+ DCs displayed more Flu-MP-HLA-A201 complexes than LCs (Figure 7A). CD14+ DCs and LCs loaded with the Flu-MP peptide were equally potent at inducing the proliferation of Flu-MP-specific CD8+ T cells (Figures 7B and 7C). Thus, both LCs and CD14+ DCs are equally capable of presenting MHC Class I restricted peptides and induce comparable expansion of memory CD8+ T cells.
Figure 7.
Both CD14+ DCs and LCs efficiently activate memory T cells
(A) Flu-MP58-66 peptide-loaded CD14+ DCs express more Flu-MP58-66/HLA-A201 complexes on the cell surface than peptide-loaded LCs, as determined with a Flu-MP58-66/HLA-A201-specific tetramerized monoclonal antibody.
(B) Frequency of Flu-MP58-66-specific CD8+ T cells analyzed with Flu-MP58-66-HLA-A201 tetramer 9 days after activation of CD8+ T cells with Flu-MP peptide-loaded LCs or CD14+ DCs from an HLA-A201+ donor.
(C) Kinetics of Flu-MP58-66-specific CD8+ T cell expansion in response to stimulation with peptide-loaded LCs or CD14+ DCs.
(D) CD14+ DCs efficiently stimulate autologous TT-specific memory CD4+ T cells. CD4+ T cells were stimulated with each autologous DC subset loaded with TT or none. At day 7, T cells were restimulated with CD34-DCs loaded with TT for 5 h, and intracytoplasmic IFNγ and IL-4 production was analyzed by flow cytometry. One of four experiments.
Since CD14+ DCs had not efficiently cross-presented the Flu-MP protein to CD8+ T cells, we analyzed whether CD14+ DCs loaded with a soluble protein antigen can stimulate antigen-specific memory CD4+ T cells. To this end, both DC subsets were incubated overnight with tetanus toxin (TT), and then cultured for 7 days with autologous CD4+ T cells. To detect TT-specific CD4+ T cells, activated CD4+ T cells were restimulated with TT-loaded CD34-HPCs-derived DCs for 6 h in the presence of monensin. CD14+ DCs loaded with TT were able to stimulate TT-specific IL-4 and IFNγ producing memory CD4+ T cells even more efficiently than LCs (Figures 7D and S9). Thus, CD14+ DCs appear to be more efficient than LCs at processing and presenting soluble proteins in the MHC Class II-restricted pathway.
Discussion
The biological raison d’être for separate mDC subsets in skin, which includes at least two dermal DC subsets, and the epidermal Langerhans Cells has remained a puzzle until now. Our present detailed analysis of human skin DCs, which builds up on other earlier studies (Caux et al., 1997; Morelli et al., 2005; Nestle et al., 1998; Nestle et al., 1993; Zaba et al., 2007), reveals their different immunological functions.
CD14 dermal DCs Preferentially Initiate Humoral Immunity
One of the major findings of the present study is the demonstration that CD14+ DCs display a unique ability to induce the generation of Tfh, CD4+ T cells able to induce naïve B cells to switch isotype and produce large amounts of Igs (King et al., 2008). Indeed, the CD14+ DC-polarized T cells secrete the cytokine CXCL13, a feature unique to the CD4+ T cell population isolated from tonsil GCs . This newly identified property of CD14+ DCs, together with our earlier demonstration of the capacity of CD14+ DCs from cultures of CD34-DCs to induce naive B cells to secrete IgM (Caux et al., 1997), indicate that CD14+ DCs specialize in the control of mature B cell differentiation. This novel function discovered with human DCs might also apply to mouse dermal DCs which migrate into the outer paracortex, just beneath the B cell follicles, whereas LCs migrate into the T cell rich inner paracortex (Kissenpfennig et al., 2005). While able to induce naïve CD4+ T cells to potently help B cells, CD14+ DCs, unlike LCs, are not inducing CD4+ T cells to secrete typical Type 2 cytokines.
When considering naïve CD8+ T cells, dermal CD14+ DCs show a very poor ability to induce their differentiation into potent CTL effectors. This is not due to the inability to generate peptide-MHC Class I complexes but rather to the inability to induce the expression of the cytotoxic effector molecules (Granzymes A/B, perforin) on the differentiating T cells. The limited ability of CD14+ DCs to cross-present proteins such as Influenza Matrix Protein is not due to a lower ability to process proteins. Rather, as recently documented with mouse lymphoid organ resident DC subsets (Dudziak et al., 2007), it represents a functional specialization as they are more potent at processing MHC Class II restricted peptides from tetanus toxoid.
The complexity of the dermal Antigen-Presenting Cells, which was identified in studies from the 90’s (Nestle et al., 1998; Nestle et al., 1993) was recently reanalyzed (Zaba et al., 2007), demonstrating the presence of CD11c+ CD1c(BDCA-1)+ dermal DCs and CD11c- CD163+FXIIIa+ macrophages in dermis. The dermal macrophages are thus a further component in the dermal immune system. The immediate question arises as to whether the dermal CD14+ DCs described in this study are equivalent to the dermal CD11c- CD163+FXIIIa+ macrophages. A comprehensive analysis of the DCs subsets migrated out of the dermis show that both CD1a+ and CD14+ DCs express CD11c. Thus our CD14+ DCs which crawl out of the dermis are distinct from the CD11c-CD14+ macrophages which stay within the dermis. Indeed, our preliminary studies using collagenase induced digestion of dermis, as used by Zaba et al, yields a heterogeneous population of CD14+ cells that include both DCs and macrophages and will necessitate a detailed immunological analysis of the various components. Our current view is that dermal CD163+FXIIIa+ cells include both non-migrating CD11-macrophages and CD11c+CD14+ migratory DCs, the later is indeed able to prime naïve T cells into follicular helper T cells. It is possible that these macrophages and CD14+ DCs cells might transform into each other as was shown earlier with monocyte-derived DCs and macrophages in response to a balance of IL-6 and TNF (Chomarat et al., 2003).
Thus, our current data together with our previous observation that CD14+ DCs can induce B cell priming (Caux et al., 1997), suggest that CD14+ DCs have a special capacity to both directly and indirectly, through T cell polarization, affect B cell differentiation towards antibody secretion. Thus we conclude that CD14+ DCs preferentially control humoral immunity.
LCs Preferentially Initiate Cellular Immunity
Our detailed studies on naïve CD8+ T cell priming with Class I binding peptides as well as cross-priming with proteins and polypeptides which require processing, indicate that LCs are strong inducers of the differentiation of naive CD8+ T cells into potent cytotoxic effectors. Indeed naïve CD8+ T cells primed by LCs acquire high levels of Granzyme A and B as well as perforin. Studies performed with CD34-HPCs derived DCs loaded with killed tumor cells (Cao et al., 2007; Ratzinger et al., 2004) also indicated that LCs and CD14+ DCs were able to induce some priming of CD8+ T cells provided several culture cycles were performed. Under these conditions, LCs were not significantly more potent than CD14+ DCs, in a variance with our current findings which might come from a differential processing of dead tumor cells and proteins. Our data therefore extend the earlier demonstration that generated LCs are better at priming IFNγ-secreting CD8+ T cells than monocyte-derived DCs (Ratzinger et al., 2004).
Although LCs are less efficient in MHC Class II peptide presentation than CD14+ DCs, as shown by the activation of Tetanus toxoid-specific memory T cells, they are able to polarize naïve CD4+ T cells into cells secreting Type 2 cytokines. Indeed, our findings might explain the preferential skewing towards Th2 responses that was observed in T cells from mice receiving antigen in the epidermis (rich in LCs) using antigen-loaded gold particles delivered with a gene gun (Alvarez et al., 2005). Both LCs and CD14+DC s are capable of inducing IFNγ-secreting CD4+ T cells. However, further studies are necessary to establish whether the IFNγ secreting CD4+ T cells generated in response to CD14+ DCs are biologically equivalent to those made in the presence of LCs. In contrast, LCs are not capable of inducing Tfh development, nor are they capable of inducing naïve B cells to secrete IgM (Caux et al., 1997). Indeed, the localization of LCs to the inner paracortex is consistent with their function ability to prime CD8+ T cells and CD4+ T cells.
For many years, LCs have been viewed as a paradigmatic population in DC biology, whereby DCs survey and sense pathogens, become activated, and migrate to the draining lymph node where they present antigens to lymphocytes, resulting in pathogen-specific immune responses . Recent studies in mice have challenged this concept, however. Studies with Herpes Simplex viruses (HSV) suggested that LCs had no role in the development of an immune response against the virus (Allan et al., 2003; Zhao et al., 2003). Rather, dermal DCs appeared to transport the viral antigens into the draining lymph nodes and deliver them to the resident DCs (Allan et al., 2006). Genetic depletion of LCs in mice resulted in either normal (Kissenpfennig et al., 2005), increased (Kaplan et al., 2005) or decreased (Bennett et al., 2005) hypersensitivity reactions. Our findings are however in line with studies performed in mice that involved peptide-loaded epidermal DCs injected subcutaneously (Celluzzi and Falo, 1997) or lentiviral vectors delivered to LCs by genetic immunization (He et al., 2006). Furthermore, consistent with our data, another recent mouse study actually shows that LCs actually cross present antigens to CD8+ T cells in vivo (Stoitzner et al., 2006). Thus, while studies in mice have not yet settled with respect to the role of LCs, our data indicate that human LCs preferentially activate cell-mediated immunity.
Dermal CD1a+ DCs might be the equivalent to the mouse Langerin+ Dermal DCs
Our study shows that dermal CD1a+ DCs represent a significant population of DCs which are present in the upper layer of the dermis. They are most likely the dermal BDCA1 cells described by Zaba et al as they express CD1c, the antigen recognized by the BDCA-1 antibody. This population is a potent inducer of the proliferation of allogeneic CD4+T cells (this study and (Zaba et al., 2007)) and CD8+ T cells though less efficient that LCs. Indeed, their phenotype is closer to that of LCs than that of CD14+ DCs. A major exception is the lack of Langerin and E-Cadherin, characteristic of LCs. Indeed, Langerin expression could be detected solely on LCs in the epidermis in situ. Similar to LCs, dermal CD1a+ DCs essentially produce IL-15 and little of the proinflammatory molecules expressed by dermal CD14+ DCs with the possible exception of IL-8. They are, however, less potent than LCs in inducing the polarization of CD4+ T cells into Th2 cells and that of naïve CD8+T cells into highly potent Granzyme A/B and Perforin positive CTLs. they might represent the precursors of LCs, and might also correspond to the recently identified mouse Langerin+ dermal DCs that are capable of participating in skin immune response (Bursch et al., 2007; Ginhoux et al., 2007; Poulin et al., 2007).
Molecular control of DC functions
As observed earlier with CD34+-derived in vitro generated DCs (de Saint-Vis et al., 1998), skin LCs and CD14+ DCs produce a dramatically distinct panel of cytokines. In particular CD14+ DCs produce spontaneously and in response to CD40 ligation IL-10 and TGFβ as well as multiple proinflammatory cytokines. In contrast, LCs produce only a limited set of cytokines (IL-6 and IL-8) and most prominently IL-15. Our attempts to identify the molecular mechanisms endowing CD14+ DCs and LCs with their specialized functions have thus far been only partly conclusive. As in Dudziak study (Dudziak et al., 2007), we found that LCs express more genes related to the MHC Class I pathway and CD14+ DCs more genes related to the MHC Class II pathway (not shown), which may explain the superior ability of LCs to crosspresent peptides on Class I molecules to CD8+ T cells. Looking at the cell surface, LCs express more 4-1BB-L an activator of CD8+ T cells (Watts, 2005), but anti-4-1BB-L blocked T cell priming by both subsets (not shown). Furthermore, LCs, but not CD14+ DCs, express IL-15, which is known to enhance CD8+ T cell responses (Oh et al., 2004). However, none of the mAbs we have tried were convincingly able to inhibit LCs-induced CD8+ T cells priming while they were able to inhibit the proliferation of T cells induced by exogenous IL-15 (not shown). We could, however, enhance CD14+ DCs-mediated priming CD8+ T cells by adding external IL-15 to the culture, which could be blocked by anti-IL15 mAb (not shown). CD14+ DCs, on the other hand, produce IL-10 and TGFβ and polarize naïve CD8+ T cells into poor effector cells expressing little Granzyme A/B and Perforin. Addition of IL-10 can induce LCs to polarize CD8+ T cells into cells with low Granzyme A/B and perforin. Indeed, the earlier demonstration that addition of IL-10 of cocultures of monocyte-derived DCs allows the generation of CD4+ T regs is a step in this direction (Roncarolo et al., 2006; Steinbrink et al., 1999). However, blocking IL-10 appears to be insufficient for CD14+ DCs to induce potent effector CD8+ T cells.
Conclusion
The capacity of LCs and CD14+ DCs to respectively preferentially prime cellular immunity and humoral immunity has significant implications, most particularly in the context of novel human vaccines. The effective vaccines developed against a variety of infectious agents, including polio, measles and Hepatitis B, certainly represent major achievements in medicine. Yet these vaccines are all specific for acute infections and their protective capacity arises largely from their induction of humoral immune responses (Pulendran and Ahmed, 2006). Given both the methods by which these vaccines are delivered and the data presented here, it is likely that they principally deliver antigen to and activate CD14+ DCs and possibly CD1a+ DCs but not LCs. In any case, our current study argues that targeting LCs will be important for the design of cutaneous vaccines that aim at eliciting strong cellular immunity. Such vaccines might be particularly useful at preventing, and perhaps even treating, chronic diseases involving viral (HIV, Hepatitis C Virus), bacterial (mycobacteria) and parasitic (malaria) diseases, as well as cancer (Letvin, 2007). The most efficient vaccines might actually be those that will target both CD14+ DCs and LCs, thereby allowing the maximal stimulation of both humoral and cellular immune responses. In this regard it is intriguing to consider that one of the most effective vaccines, smallpox vaccine (Frey et al., 2002; Pulendran and Ahmed, 2006), acts through a combination of strong cellular and humoral immunity and requires scarification of the skin, a procedure that injures both epidermis and dermis and that is likely to mobilize and activate LCs as well as dermal DCs.
Experimental Procedures
Isolation of mDC subsets
LCs and dermal CD1a+ DCs and CD14+ DCs were purified from normal human skin. Specimens were incubated with the bacterial protease dispase type 2 for 18 h at 4°C, and then for 2 h at 37°C. Epidermal and dermal sheets were separated and placed in RPMI 1640 supplemented with 10% fetal bovine serum (FBS). After 2 days, the cells that migrated into the medium were enriched using a Ficoll-diatrizoate gradient. DCs were purified by cell sorting after staining with anti-CD1a FITC and anti-CD14 APC mAbs. LCs, CD1a+ DCs and CD14+ DCs represent 58%, 30% and 12% of the total skin DCs, respectively. Where indicated, sorted DCs were stimulated with either soluble CD40L (200 ng/ml, R&D) or LPS (50 ng/ml, Sigma) for 24 h. Cytokine production was measured in culture supernatants after 24 h using a multiplex bead assay (Luminex). Immunostaining and microscopic analysis of human skin sections as well as Microarray gene analysis are detailed in the supplementary data.
Generation of mDC subsets
DCs were generated in vitro from CD34-HPCs isolated from the blood of healthy volunteers. HPCs were cultured at 5 × 105/ml in Yssel’s medium (Irvine Scientific, CA) supplemented with 5% autologous serum, 50 μM β-mercaptoethanol, 1% L-glutamine, 1% penicillin/streptomycin, GM-CSF (50 ng/ml; Berlex), Flt3L (100 ng/ml; R&D), and (10 ng/ml; R&D) for 9 days. Media and cytokines were refreshed at day 5 of culture. Subsets of DCs, CD1a+CD14-LCs and CD1a-CD14+ DCs were then sorted, yielding a purity of 95−99%. Intracellular IL-15 was analyzed in the cell lysate of purified sorted DC subsets by multiplex bead assay (Luminex).
CD4+ T cell experiments
Naïve CD4+ T cells (CCR7+CD45RA+ CD4+) were sorted from PBMCs following CD8, CD56, CD16 and CD19-magnetic cell depletion (Miltenyi). For assessment of DC allostimulatory capacity, naïve CD4+ T cells (4 × 105/well) were cultured in 96-well plates in Yssel’s medium supplemented with 10% heat-inactivated pooled AB human serum (Yssel’s complete medium), to which allogeneic mDC subsets were added at graded cell numbers. After 5 days, cells were pulsed for 18 h with 1 μCi [3H]-thymidine, then harvested. Alternatively, cell proliferation was assessed by a CFSE dilution.
For cytokine production, naive CD4+ T cells (4 × 105 /well) were stimulated with allogeneic DCs (5 × 104 in vitro DCs per well; 2 × 104 with skin DCs per well) in 96-well plates for 7 days. Proliferating CD4+ T cells were sorted as FSChighCD11c—, and restimulated overnight with anti-CD3 and anti-CD28 coated microbeads. Cytokines were measured with a multiplex bead assay (Luminex), and CXCL13 was measured by ELISA (R&D). For intracytoplasmic cytokine analyses, primed CD4+ T cells were re-stimulated with 50 ng/ml PMA and 1 μg/ml ionomycin (Sigma) for 6 h in the presence of monensin. Fixed and permeabilized cells were stained with anti-IFNγ FITC and anti-IL-4 PE mAbs (BD Biosciences).
To measure functions involving B cell priming/differentiation, sorted FSChighCD11c—CD4+ T cells were co-cultured with autologous naïve B cells. B cells were preincubated with anti-IgM mAb (BD biosciences) and CpG2006 (2.5 μg/ml, InVivoGen) for 2 h. T cells (indicated numbers/well) and B cells (2 × 104 cells/well) were co-cultured in 96-well plates for 14 days. Igs in supernatants were measured by ELISA.
For recall responses, total CD4+ T cells (1 × 105 cells/well) were stimulated in 96-well plates with autologous in vitro mDC subsets (1 × 104 cells/well) preincubated with TT (10 LfU/ml). Cells were harvested at day 7, and re-stimulated with TT-loaded total CD34-DCs for 5 h in the presence of monensin. Intracytoplasmic cytokine secretion was analyzed by flow cytometry.
CD8+ T cell experiments
Naive CD8+ T cells were sorted as CD45RA+CCR7+HLA-DR—CD8+ cells. Allogeneic primed CD8+ T cells were characterized for the expression of cytotoxic effector molecules, Granzyme A (BD Pharmingen), Granzyme B (eBioscience) and perforin (Fitzgerald) after 7 d of coculture with mDC subsets. For primary response, naïve CD8+ T cells (1 ×106 cells/well) were stimulated with autologous LCs or CD14+ DCs (5 × 104 cells/well) that were preincubated for 3 h with the HLA-A201-restricted MART-1 (MART-126-35, ELAGIGILTV), gp100 (gp100209-217, IMDQVPFSV) or a control peptide (3 μM). Cells were cultured for 9 days in 24 well plates with 10 U/ml IL-7 (R&D) and 100 ng/ml CD40L (R&D). IL-2 was added at 10 U/ml at day 3. For cross-presentation experiments, 15 aa MART-121-35 peptide (YTTAEEAAGIGILTV) or Flu-MP protein were incubated with purified CD8+ T cells with 10 U/ml IL-7 (R&D). 100 ng/ml CD40L (R&D) was added after 24 h and IL-2 was added at 10 U/ml at day 3. Expansion of peptide-specific CD8+ T cells was determined by counting the number of cells binding peptide/HLA-A201 tetramers (Beckman Coulter). Cytolytic assays were performed in a standard 51Cr release assay using peptide-loaded T2 cells and an HLA-A201+ melanoma cell line, ME526, as targets, after two rounds of T cell stimulation with the same DC subsets.
Antigen-specific TCR avidity was analyzed by measuring the decay of peptide/HLA-A201 tetramer binding. CD8+ T cells primed by MART-126-35-loaded mDC subsets were incubated with MART-1/HLA-A201 tetramer, and then an excess of unlabeled anti-HLA-A201 Ab (BB7.2; BD Biosciences) was added to prevent rebinding after dissociation. The intensity of tetramer fluorescence, analyzed at various time points, is plotted in the natural logarithm scale of the initial fluorescence (Molldrem et al., 2003). To quantitate pMHC-complexes on DCs, DCs were loaded with HLA-A201-restricted MART-126-35, gp100209-217 or Flu-MP58-66 peptides for 3 h and stained with PE-conjugated tetramerized monoclonal antibodies that recognize each peptide-HLA-A201 complex (Noy et al., 2005). For recall responses, total CD8+ T cells (1 × 106 cells/ml) were stimulated with autologous mDC subsets loaded with HLA-A201-restricted Flu-MP58-66 peptide (GILGFVFTL). The frequency of Flu-MP-specific CD8+ T cells was determined using Flu-MP/HLA-A201 tetramer.
For the production of Flu-MP, the ORF of gi|60458| Flu M1 protein [Influenza A virus (A/WSN/1933(H1N1))] with a V15I substitution was inserted into pET-28b (+) (Novagen). The protein was expressed in T7 Express cells (NEB) and purified by flow through Q Sepharose, then sequential Ni++ chelating and S Sepharose chromatographies.
Supplementary Material
Figure S1. Characterization of human skin epidermal and dermal DC subsets
(A) The relative representation of each DC subset in human skin. Percentage calculated separately according to the mean±SD number of each DC subpopulation isolated from 12 skin specimens processed over 8 months. Epidermal LCs: 58.2±16.4; dermal CD1a:29.6±13.8; dermal CD14:12±10.4.
(B) Immunofluorescent analysis of human skin sections stained with CD1a and CD163 antibodies shows that the majority of the dermal CD1a+ DCs are located in the upper dermis in close proximity of to epidermal LCs. CD163+ cells are located deeper in the dermis. Shown is a representative staining out of 3 independent tissue sections from 3 different donors.
(C)-(F) Differential expression of Langerin and Factor XIIIa by human skin DCs
(C) Gene expression analysis of Langerin by skin DCs isolated from 3 different specimens. RNA was prepared from FACS-sorted migrated skin DC subsets: epidermal LCs, dermal CD1a+ DCs and CD14+ DCs.
(D) Immunofluorescent staining of normal human skin using Langerin and HLA-DR antibodies. Langerin+ cells reside in the epidermis, no Langerin+ cells could be identified in the dermis.
(E) Gene expression analysis of Factor XIIIa by skin DCs isolated from 3 different specimens. RNA was prepared from FACS-sorted migrated skin DC subsets: epidermal LCs, dermal CD1a+ DCs and CD14+ DCs.
(F) Immunofluorescent staining of normal human skin using Factor XIIIa and CD1a antibodies. FXIIIa and CD1a identified 2 discrete populations. Neither CD1aR+ epidermal DCs (LCs) nor dermal CD1a+ DCs express Factor XIIIa.
Figure S2. Distinct cytokine expression pattern by skin and in vitro-generated DC subsets
(A-C) Gene array analysis showing relative amounts of mRNAs expression of TGFβ (A), IL-10 (B) and IL-15 (C) by FACS-sorted skin DC subsets: epidermal LCs, dermal CD1a+ DCs and CD14+ DCs.
(D) Intracellular IL-15 in the cell lysate of in vitro-generated DC subsets as measured by a multiplex bead assay (Luminex). Data of 4 independent experiments for 4 different donors shows higher production level of IL-15 by in vitro LCs compared to CD14+ DCs.
Figure S3. LCs are superior to CD14+ DCs at inducing the proliferation of allogeneic naïve CD4+ T cells
(A) Stimulation of CFSE-labeled naïve CD4+ T cells with in vitro-generated LCs or CD14+ DCs.
(B) LCs yield more activated CD4+ T cells. The ratios of recovered live cells after 6 d of culture with each in vitro-generated DC subset to original cell number. Each point represents an independent experiment.
Figure S4. LCs are more efficient at inducing the differentiation of Type 2 cytokine secreting CD4+ T cells
(A) Both CD40L and LPS stimulation induce maturation of in vitro-generated DC subsets. DC subsets were stimulated for 24 h with either soluble CD40L or LPS. [3H]-thymidine incorporation. One of three experiments.
(B) Each DC subset maintains the ability to skew T cell differentiation irrespective to their maturation stages. Intracytoplasmic cytokine production in CD4+ T cells primed by matured LCs or CD14+ DCs were examined after stimulation with PMA and ionomycin. One of three experiments.
Figure S5. Dermal CD14+ DCs efficiently prime Tfh cells
The levels of secreted immunoglobulins in supernatants of cocultures of naïve B cells and CD4+ T cells pre-activated by each skin DC subset (T cells 20 × 103/well). Results obtained from four independent experiments are shown.
Figure S6. LCs are powerful inducers of naïve CD8+ T cell proliferation
Stimulation of CFSE-labeled naïve CD8+ T cells with in vitro-generated allogeneic LCs or CD14+ DCs.
Figure S7. LCs efficiently prime naïve CD8+ T cells
(A) Frequencies of primed MART-1-specific CD8+ T cells in 15 independent experiments. Paired Student t-test.
(B) Induction of gp100-specific CD8+ T cells as measured by gp100-HLA-A201 tetramer.
(C) Frequencies of primed gp100-specific CD8+ T cells in 10 independent experiments. Paired Student t-test.
(D) In vitro-generated LCs-primed CD8+ T cells express higher level of the effector molecules; granzyme A, Granzyme B and perforin compared to in vitro-generated CD14+ DCs.
Figure S8. In vitro-generated LCs are more efficient than CD14+ DCs at cross-presenting antigens to CD8+ T cells
(A) Frequencies of expanded Flu-MP58-66-specific CD8+ T cells in 12 independent experiments.
(B) Frequencies of primed MART-126-35-specific CD8+ T cells in 10 independent experiments. Paired Student t-test.
Figure S9. In vitro-generated CD14+ DCs are more efficient than LCs at stimulating TT-specific memory CD4+ T cells
The absolute numbers of IFNγ-producing TT-specific CD4+ T cell population in the total CD4+ T cells are shown. Each dot represents each replicate in 4 independent experiments.
Acknowledgments
We thank E. Kowalski, J. Shay, Q. Nguyen, C. Glaser and S.S Clayton for their help. We thank J. Duncan, surgeons and nurses from the Plastic Surgery Department for skin samples. We thank Dr. R. Germain, Dr. I. Mellman and Dr. C. Harrod for critical reading and discussion, Dr. M. Ramsay, Dr. W. Duncan and C. Samuelsen for continuous support. This work was supported by grants from BHCS Foundation, the NIH (RO-1 CA78846, RO-1 CA85540, PO-1 CA84512, U-19 AI- 57234 to JB) and scholarship from Uehara Memorial Foundation (HU). JB holds the W.W. Caruth, Jr. Chair for Transplantation Immunology Research. AKP holds the Ramsay Chair for Cancer Immunology.
JB holds stock options in “Argos therapeutics” a private company which is testing the potential therapeutic applications of Dendritic Cells.
References
- Allan RS, Smith CM, Belz GT, van Lint AL, Wakim LM, Heath WR, Carbone FR. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science. 2003;301:1925–1928. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, Lew AM, Shortman K, Heath WR, Carbone FR. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Alvarez D, Harder G, Fattouh R, Sun J, Goncharova S, Stampfli MR, Coyle AJ, Bramson JL, Jordana M. Cutaneous antigen priming via gene gun leads to skin-selective Th2 immune-inflammatory responses. J Immunol. 2005;174:1664–1674. doi: 10.4049/jimmunol.174.3.1664. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu Y, Pulendran B, Palucka K. Immunobiology of dendritic cells. Ann Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Belz GT, Bedoui S, Kupresanin F, Carbone FR, Heath WR. Minimal activation of memory CD8+ T cell by tissue-derived dendritic cells favors the stimulation of naive CD8+ T cells. Nat Immunol. 2007;8:1060–1066. doi: 10.1038/ni1505. [DOI] [PubMed] [Google Scholar]
- Bennett CL, van Rijn E, Jung S, Inaba K, Steinman RM, Kapsenberg ML, Clausen BE. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J Cell Biol. 2005;169:569–576. doi: 10.1083/jcb.200501071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursch LS, Wang L, Igyarto B, Kissenpfennig A, Malissen B, Kaplan DH, Hogquist KA. Identification of a novel population of Langerin+ dendritic cells. J Exp Med. 2007;204:3147–3156. doi: 10.1084/jem.20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao T, Ueno H, Glaser C, Fay JW, Palucka AK, Banchereau J. Both Langerhans cells and interstitial DC cross-present melanoma antigens and efficiently activate antigen-specific CTL. Eur J Immunol. 2007;37:2657–2667. doi: 10.1002/eji.200636499. [DOI] [PubMed] [Google Scholar]
- Caux C, Massacrier C, Vanbervliet B, Dubois B, Durand I, Cella M, Lanzavecchia A, Banchereau J. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to granulocyte-macrophage colony-stimulating factor plus tumor necrosis factor alpha: II. Functional analysis. Blood. 1997;90:1458–1470. [PubMed] [Google Scholar]
- Celluzzi CM, Falo LD., Jr. Epidermal dendritic cells induce potent antigen-specific CTL-mediated immunity. J Invest Dermatol. 1997;108:716–720. doi: 10.1111/1523-1747.ep12292095. [DOI] [PubMed] [Google Scholar]
- Chomarat P, Dantin C, Bennett L, Banchereau J, Palucka AK. TNF skews monocyte differentiation from macrophages to dendritic cells. J Immunol. 2003;171:2262–2269. doi: 10.4049/jimmunol.171.5.2262. [DOI] [PubMed] [Google Scholar]
- de Saint-Vis B, Fugier-Vivier I, Massacrier C, Gaillard C, Vanbervliet B, Ait-Yahia S, Banchereau J, Liu YJ, Lebecque S, Caux C. The cytokine profile expressed by human dendritic cells is dependent on cell subtype and mode of activation. J Immunol. 1998;160:1666–1676. [PubMed] [Google Scholar]
- Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- Frey SE, Newman FK, Cruz J, Shelton WB, Tennant JM, Polach T, Rothman AL, Kennedy JS, Wolff M, Belshe RB, Ennis FA. Dose-related effects of smallpox vaccine. N Engl J Med. 2002;346:1275–1280. doi: 10.1056/NEJMoa013431. [DOI] [PubMed] [Google Scholar]
- Germain RN. An innately interesting decade of research in immunology. Nat Med. 2004;10:1307–1320. doi: 10.1038/nm1159. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Collin MP, Bogunovic M, Abel M, Leboeuf M, Helft J, Ochando J, Kissenpfennig A, Malissen B, Grisotto M, et al. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J Exp Med. 2007;204:3133–3146. doi: 10.1084/jem.20071733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Zhang J, Donahue C, Falo LD., Jr. Skin-derived dendritic cells induce potent CD8(+) T cell immunity in recombinant lentivector-mediated genetic immunization. Immunity. 2006;24:643–656. doi: 10.1016/j.immuni.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23:611–620. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- King C, Tangye SG, Mackay CR. T Follicular Helper (T(FH)) Cells in Normal and Dysregulated Immune Responses. Annu Rev Immunol. 2008;26:741–766. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- Kissenpfennig A, Henri S, Dubois B, Laplace-Builhe C, Perrin P, Romani N, Tripp CH, Douillard P, Leserman L, Kaiserlian D, et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Letvin Correlates of Immune protection and the Development of Human Immunodeficiency Virus Vaccine. Immunity. 2007;27:366–369. doi: 10.1016/j.immuni.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Maldonado-Lopez R, De Smedt T, Michel P, Godfroid J, Pajak B, Heirman C, Thielemans K, Leo O, Urbain J, Moser M. CD8alpha+ and CD8alpha-subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- Molldrem JJ, Lee PP, Kant S, Wieder E, Jiang W, Lu S, Wang C, Davis MM. Chronic myelogenous leukemia shapes host immunity by selective deletion of high-avidity leukemia-specific T cells. J Clin Invest. 2003;111:639–647. doi: 10.1172/JCI16398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli AE, Rubin JP, Erdos G, Tkacheva OA, Mathers AR, Zahorchak AF, Thomson AW, Falo LD, Jr., Larregina AT. CD4+ T cell responses elicited by different subsets of human skin migratory dendritic cells. J Immunol. 2005;175:7905–7915. doi: 10.4049/jimmunol.175.12.7905. [DOI] [PubMed] [Google Scholar]
- Nestle FO, Filgueira L, Nickoloff BJ, Burg G. Human dermal dendritic cells process and present soluble protein antigens. J Invest Dermatol. 1998;110:762–766. doi: 10.1046/j.1523-1747.1998.00189.x. [DOI] [PubMed] [Google Scholar]
- Nestle FO, Zheng XG, Thompson CB, Turka LA, Nickoloff BJ. Characterization of dermal dendritic cells obtained from normal human skin reveals phenotypic and functionally distinctive subsets. J Immunol. 1993;151:6535–6545. [PubMed] [Google Scholar]
- Noy R, Eppel M, Haus-Cohen M, Klechevsky E, Mekler O, Michaeli Y, Denkberg G, Reiter Y. T-cell receptor-like antibodies: novel reagents for clinical cancer immunology and immunotherapy. Expert Rev Anticancer Ther. 2005;5:523–536. doi: 10.1586/14737140.5.3.523. [DOI] [PubMed] [Google Scholar]
- Oh S, Perera LP, Burke DS, Waldmann TA, Berzofsky JA. IL-15/IL-15Ralpha-mediated avidity maturation of memory CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101:15154–15159. doi: 10.1073/pnas.0406649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin LF, Henri S, de Bovis B, Devilard E, Kissenpfennig A, Malissen B. The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. J Exp Med. 2007;204:3119–3131. doi: 10.1084/jem.20071724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B, Ahmed R. Translating innate immunity into immunological memory: implications for vaccine development. Cell. 2006;124:849–863. doi: 10.1016/j.cell.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Pulendran B, Smith JL, Caspary G, Brasel K, Pettit D, Maraskovsky E, Maliszewski CR. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc Natl Acad Sci U S A. 1999;96:1036–1041. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratzinger G, Baggers J, de Cos MA, Yuan J, Dao T, Reagan JL, Munz C, Heller G, Young JW. Mature human Langerhans cells derived from CD34+ hematopoietic progenitors stimulate greater cytolytic T lymphocyte activity in the absence of bioactive IL-12p70, by either single peptide presentation or cross-priming, than do dermal-interstitial or monocyte-derived dendritic cells. J Immunol. 2004;173:2780–2791. doi: 10.4049/jimmunol.173.4.2780. [DOI] [PubMed] [Google Scholar]
- Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, Shimizu J, Takahashi T, Nomura T. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- Shevach EM. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity. 2006;25:195–201. doi: 10.1016/j.immuni.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nature Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- Silverstein AM. Cellular versus humoral immunology: a century-long dispute. Nat Immunol. 2003;4:425–428. doi: 10.1038/ni0503-425. [DOI] [PubMed] [Google Scholar]
- Soares H, Waechter H, Glaichenhaus N, Mougneau E, Yagita H, Mizenina O, Dudziak D, Nussenzweig MC, Steinman RM. A subset of dendritic cells induces CD4+ T cells to produce IFN-gamma by an IL-12-independent but CD70-dependent mechanism in vivo. J Exp Med. 2007;204:1095–1106. doi: 10.1084/jem.20070176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrink K, Jonuleit H, Muller G, Schuler G, Knop J, Enk AH. Interleukin-10-treated human dendritic cells induce a melanoma-antigen-specific anergy in CD8(+) T cells resulting in a failure to lyse tumor cells. Blood. 1999;93:1634–1642. [PubMed] [Google Scholar]
- Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- Stoitzner P, Tripp CH, Eberhart A, Price KM, Jung JY, Bursch L, Ronchese F, Romani N. Langerhans cells cross-present antigen derived from skin. Proc Natl Acad Sci U S A. 2006;103:7783–7788. doi: 10.1073/pnas.0509307103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valladeau J, Saeland S. Cutaneous dendritic cells. Semin Immunol. 2005;17:273–283. doi: 10.1016/j.smim.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- Zaba LC, Fuentes-Duculan J, Steinman RM, Krueger JG, Lowes MA. Normal human dermis contains distinct populations of CD11c+BDCA-1+ dendritic cells and CD163+FXIIIA+ macrophages. J Clin Invest. 2007;117:2517–2525. doi: 10.1172/JCI32282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Deak E, Soderberg K, Linehan M, Spezzano D, Zhu J, Knipe DM, Iwasaki A. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J Exp Med. 2003;197:153–162. doi: 10.1084/jem.20021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Characterization of human skin epidermal and dermal DC subsets
(A) The relative representation of each DC subset in human skin. Percentage calculated separately according to the mean±SD number of each DC subpopulation isolated from 12 skin specimens processed over 8 months. Epidermal LCs: 58.2±16.4; dermal CD1a:29.6±13.8; dermal CD14:12±10.4.
(B) Immunofluorescent analysis of human skin sections stained with CD1a and CD163 antibodies shows that the majority of the dermal CD1a+ DCs are located in the upper dermis in close proximity of to epidermal LCs. CD163+ cells are located deeper in the dermis. Shown is a representative staining out of 3 independent tissue sections from 3 different donors.
(C)-(F) Differential expression of Langerin and Factor XIIIa by human skin DCs
(C) Gene expression analysis of Langerin by skin DCs isolated from 3 different specimens. RNA was prepared from FACS-sorted migrated skin DC subsets: epidermal LCs, dermal CD1a+ DCs and CD14+ DCs.
(D) Immunofluorescent staining of normal human skin using Langerin and HLA-DR antibodies. Langerin+ cells reside in the epidermis, no Langerin+ cells could be identified in the dermis.
(E) Gene expression analysis of Factor XIIIa by skin DCs isolated from 3 different specimens. RNA was prepared from FACS-sorted migrated skin DC subsets: epidermal LCs, dermal CD1a+ DCs and CD14+ DCs.
(F) Immunofluorescent staining of normal human skin using Factor XIIIa and CD1a antibodies. FXIIIa and CD1a identified 2 discrete populations. Neither CD1aR+ epidermal DCs (LCs) nor dermal CD1a+ DCs express Factor XIIIa.
Figure S2. Distinct cytokine expression pattern by skin and in vitro-generated DC subsets
(A-C) Gene array analysis showing relative amounts of mRNAs expression of TGFβ (A), IL-10 (B) and IL-15 (C) by FACS-sorted skin DC subsets: epidermal LCs, dermal CD1a+ DCs and CD14+ DCs.
(D) Intracellular IL-15 in the cell lysate of in vitro-generated DC subsets as measured by a multiplex bead assay (Luminex). Data of 4 independent experiments for 4 different donors shows higher production level of IL-15 by in vitro LCs compared to CD14+ DCs.
Figure S3. LCs are superior to CD14+ DCs at inducing the proliferation of allogeneic naïve CD4+ T cells
(A) Stimulation of CFSE-labeled naïve CD4+ T cells with in vitro-generated LCs or CD14+ DCs.
(B) LCs yield more activated CD4+ T cells. The ratios of recovered live cells after 6 d of culture with each in vitro-generated DC subset to original cell number. Each point represents an independent experiment.
Figure S4. LCs are more efficient at inducing the differentiation of Type 2 cytokine secreting CD4+ T cells
(A) Both CD40L and LPS stimulation induce maturation of in vitro-generated DC subsets. DC subsets were stimulated for 24 h with either soluble CD40L or LPS. [3H]-thymidine incorporation. One of three experiments.
(B) Each DC subset maintains the ability to skew T cell differentiation irrespective to their maturation stages. Intracytoplasmic cytokine production in CD4+ T cells primed by matured LCs or CD14+ DCs were examined after stimulation with PMA and ionomycin. One of three experiments.
Figure S5. Dermal CD14+ DCs efficiently prime Tfh cells
The levels of secreted immunoglobulins in supernatants of cocultures of naïve B cells and CD4+ T cells pre-activated by each skin DC subset (T cells 20 × 103/well). Results obtained from four independent experiments are shown.
Figure S6. LCs are powerful inducers of naïve CD8+ T cell proliferation
Stimulation of CFSE-labeled naïve CD8+ T cells with in vitro-generated allogeneic LCs or CD14+ DCs.
Figure S7. LCs efficiently prime naïve CD8+ T cells
(A) Frequencies of primed MART-1-specific CD8+ T cells in 15 independent experiments. Paired Student t-test.
(B) Induction of gp100-specific CD8+ T cells as measured by gp100-HLA-A201 tetramer.
(C) Frequencies of primed gp100-specific CD8+ T cells in 10 independent experiments. Paired Student t-test.
(D) In vitro-generated LCs-primed CD8+ T cells express higher level of the effector molecules; granzyme A, Granzyme B and perforin compared to in vitro-generated CD14+ DCs.
Figure S8. In vitro-generated LCs are more efficient than CD14+ DCs at cross-presenting antigens to CD8+ T cells
(A) Frequencies of expanded Flu-MP58-66-specific CD8+ T cells in 12 independent experiments.
(B) Frequencies of primed MART-126-35-specific CD8+ T cells in 10 independent experiments. Paired Student t-test.
Figure S9. In vitro-generated CD14+ DCs are more efficient than LCs at stimulating TT-specific memory CD4+ T cells
The absolute numbers of IFNγ-producing TT-specific CD4+ T cell population in the total CD4+ T cells are shown. Each dot represents each replicate in 4 independent experiments.