Abstract
It has been shown previously that the binding of oxidized low-density lipoprotein (OxLDL) to resident mouse peritoneal macrophages can be inhibited (up to 70%) by the apoprotein B (apoB) isolated from OxLDL, suggesting that macrophage recognition of OxLDL is primarily dependent on its modified protein moiety. However, recent experiments have demonstrated that the lipids isolated from OxLDL and reconstituted into a microemulsion can also strongly inhibit uptake of OxLDL (up to 80%). The present studies show that lipid microemulsions prepared from OxLDL bind to thioglycollate-elicited macrophages at 4°C in a saturable fashion and inhibit the binding of intact OxLDL and also of the apoB from OxLDL. Reciprocally, the binding of the OxLDL-lipid microemulsions was strongly inhibited by intact OxLDL. A conjugate of synthetic 1-palmitoyl 2(5-oxovaleroyl) phosphatidylcholine (an oxidation product of 1-palmitoyl 2-arachidonoyl phosphatidylcholine) with serum albumin, shown previously to inhibit macrophage binding of intact OxLDL, also inhibited the binding of both the apoprotein and the lipid microemulsions prepared from OxLDL. Finally, a monoclonal antibody against oxidized phospholipids, one that inhibits binding of intact OxLDL to macrophages, also inhibited the binding of both the resolubilized apoB and the lipid microemulsions prepared from OxLDL. These studies support the conclusions that: (i) at least some of the macrophage receptors for oxidized LDL can recognize both the lipid and the protein moieties; and (ii) oxidized phospholipids, in the lipid phase of the lipoprotein and/or covalently linked to the apoB of OxLDL, likely play a role in that recognition.
The generation of foam cells, an early step in atherogenesis, depends in part on the oxidative modification of low-density lipoprotein (LDL) because macrophage scavenger receptors recognize oxidized LDL (OxLDL) but not native LDL (1). It has been generally assumed that macrophage recognition of OxLDL depends on modification of its protein moiety, apoprotein B (apoB). That was implied by the fact that treatment of native LDL with reagents that block the epsilon amino groups of lysine residues (e.g., acetic anhydride or malondialdehyde) convert it to a ligand for one or more scavenger receptors (2, 3). Furthermore, the prototypic lipoprotein receptor, the receptor for native LDL, recognizes a specific segment of native apoB, one that is necessary and sufficient for LDL binding (4). Thus it was reasonable to expect that receptors recognizing OxLDL would likewise exclusively recognize the modified protein moiety. Indeed, Parthasarathy et al. (5) showed some years ago that the apoB recovered from OxLDL after extraction of the lipids, when resolubilized in detergent, could compete with labeled intact OxLDL for binding to mouse peritoneal macrophages. They also showed that the inhibition was reciprocal, i.e., the binding of labeled delipidated apoB was inhibited by intact OxLDL. However, in those studies, the possibility that the lipid moiety of OxLDL might also compete was not directly tested.
Some years later it was found that macrophage binding of oxidized red blood cells and apoptotic cells was inhibited by OxLDL (6, 7). Because the binding of these cells to macrophages had been convincingly shown to correlate with an increase in the level of expression of phosphatidylserine on the outer leaflet of the plasma membrane (8–11), and because the macrophage binding of OxLDL itself was also inhibited by phosphatidylserine-rich liposomes, the possibility that the lipid moiety of OxLDL might play a role in its binding had to be reconsidered. Terpstra et al. (12) showed that the lipids extracted from OxLDL could be reconstituted into a microemulsion that bound to resident macrophages in a saturable fashion and competed for the binding and uptake of intact OxLDL. They also showed that liposomes containing oxidized 1-palmitoyl 2-arachidonoyl phosphatidylcholine (PAPC) inhibited macrophage uptake and degradation of intact OxLDL. The magnitude of the inhibition by the lipids and by the apoprotein (≫50%) required postulating that there must be some receptors that could recognize both the lipid moiety and the protein moiety.
Parallel with these studies, Hörkkö et al. were exploring the nature of the epitopes on OxLDL recognized by monoclonal autoantibodies generated spontaneously in hyperlipidemic apoprotein E (apoE)-deficient mice (13, 14). Several of these autoantibodies were shown to recognize oxidized phospholipids but not unoxidized phospholipids. For example, they bound to oxidized PAPC and to a specific degradation product of oxidized PAPC, namely, 1-palmitoyl 2(5-oxovaleroyl) phosphatidylcholine (POVPC). The latter has been fully characterized as a biologically active component of minimally OxLDL by Watson et al. (15). Each of these autoantibodies studied by Hörkkö et al. recognized both the lipids extracted from OxLDL and the resolubilized apoB. Because these are monoclonal antibodies, the findings indicate that identical or very closely related epitopes are present in both the extracted lipids and the modified apoB. The simplest explanation is that some reactive oxidized phospholipids, such as POVPC, have become covalently attached to apoB during oxidation of the LDL. The presence of lipid covalently bound to the apoB would resolve the apparent paradox that macrophage receptors and monoclonal antibodies recognize both a lipid ligand/epitope and a protein ligand/epitope. Finally, Hörkkö et al. recently demonstrated that several of these antibodies, including one designated E06, are able to strongly inhibit the binding of intact OxLDL to macrophages (14).
In the present studies, we provide evidence that common ligands both present in the extractable lipids and covalently bound to the apoprotein can mediate the binding of OxLDL to macrophages. Specifically, we demonstrate reciprocal competition between the lipids of OxLDL, reconstituted into microemulsions, and both intact OxLDL and the resolubilized modified apoB of OxLDL. We show that a monoclonal antibody against OxLDL (E06) previously shown to inhibit macrophage binding of the lipids from OxLDL (14) also inhibits the binding of the modified apoB. Finally, we show that BSA conjugated with POVPC, previously reported to inhibit binding of intact OxLDL, also inhibits binding of both OxLDL lipid microemulsions and resolubilized apoB from OxLDL.
MATERIALS AND METHODS
Materials.
Polycarbonate membranes were from Poretics. CF50A membrane concentration cones were from Amicon. PAPC was from Avanti Polar Lipids. 3,3′-dihexadecyloxacarbocyanine perchlorate (DiO) was from Molecular Probes. CuSO4 was obtained from Lab Chem (Pittsburgh, PA). Thioglycollate medium was from Difco. N-octyl glucoside was from Boehringer Mannheim. RPMI medium 1640 was from BioWhittaker and was supplemented with Gentamycin from Omega Scientific (Tarzana, CA). Fetal bovine serum was from Gemini Biological Products (Calabasas, CA). Carrier-free Na125I was purchased from ICN. All other reagents were analytical grade.
Lipoproteins.
Human LDL was isolated from normolipemic plasma by density gradient ultracentrifugation (16). The LDL was dialyzed against 6 liters of PBS containing 0.3 mM EDTA, and the protein concentration was measured as described by Lowry et al. (17). For LDL oxidation, the LDL was dialyzed against 4 liters of PBS to remove EDTA, adjusted to 100 μg/ml of LDL protein in PBS, and incubated with 10 μM CuSO4 for 18 h at 37°C. The extent of oxidation was assessed by measuring the thiobarbituric acid-reactive substances (18). To prevent further oxidation, butylated hydroxytoluene and EDTA were then added at 20 μM and 0.1 mM, respectively. The OxLDL was concentrated by centrifugation in CF50A membrane cones to approximately 1 mg/ml.
Lipid Preparation.
Isolation of the lipids from native and oxidized LDL was performed as previously described (12). Briefly, HCl was added to the LDL preparations to a final concentration of 10 mM, and the lipids were extracted with chloroform/methanol (1/1). After centrifugation at 800 × g for 10 min, the chloroform phase was removed, evaporated under nitrogen, and the lipids resuspended in Tris buffer. The lipid suspensions were then extruded through 0.1 μm polycarbonate membranes 8 to 10 times at 37°C to form microemulsions as previously described (12). The final preparations were clear or slightly opalescent and, by light scattering, had an average diameter of 80–120 nm; dried on a grid for transmission electron microscopy, they aggregated to particles sizes of 200–600 nm and showed multilamellar structures, presumably phospholipid, at the surface. Some lipid preparations were fluorescence labeled by adding DiO at a 1% weight ratio to the chloroform phase before resuspension in buffer. Liposomes containing PAPC/cholesterol (2/1 molar ratio) or PAPC that had been oxidized in air for 24 h were also prepared by using this method. The concentration of LDL lipid microemulsions and phospholipid liposomes was determined by measuring the phosphorus as described by Marinetti (19).
Preparation of 125I-Labeled OxLDL and 125I-Labeled apoB from It.
Native LDL was iodinated by the method of Salacinski et al. (20). After removal of free 125I by extensive dialysis against PBS, the LDL was oxidized as described above. The radiolabeled oxidized apoB was isolated through an extraction with ice-cold methanol:chloroform (1:1), followed by water and acetone washes of the protein (5). 125I-apoB from OxLDL was solubilized in octyl glucoside (octyl glucoside:protein ratio, 30:1). Excess detergent was removed by extensive dialysis against PBS. The final specific activity of apoB was approximately 200 cpm/ng protein.
Liposome Binding Assay.
Elicited mouse macrophages were harvested from female Swiss–Webster mice by peritoneal lavage with PBS 3 days after an intraperitoneal injection of 2 ml of thioglycollate medium. The macrophages were plated in 12-well plates at a density of 1.3 × 106 cells per well in RPMI media 1640 containing 5% fetal bovine serum and 0.01 mg/ml Gentamycin. Nonadherent cells were removed by washing the cells 4 h after plating before an overnight incubation at 37°C. Before the binding assay, the cells were placed on ice and washed with PBS. The cells were incubated with DiO-labeled OxLDL-lipid microemulsions with or without competitors for 2 h at 4°C, washed three times, and then scraped into 1 ml of PBS containing 0.1% BSA and 0.01% sodium azide. Binding of the OxLDL lipid microemulsion was measured by flow cytometry counting 30,000 events per sample and quantitated by using cell quest software.
Apoprotein Binding Assay.
Cells were incubated with 125I-apoB prepared from OxLDL for 3 h at 4°C, after which the medium was removed and cells were washed three times with PBS, twice with 1% BSA/PBS, and once with PBS. Monolayers were solubilized in 0.2N NaOH and aliquots were removed for 125I detection in a gamma counter and for protein determination. In most experiments, 125I-apoB from OxLDL and the unlabeled competitor were both added at the beginning of the incubation. However, when competition between 125I-apoB and unlabeled microemulsions or liposomes was investigated, the latter were preincubated with the cells for 1.5 h at 4°C, after which the macrophages were washed to remove unbound lipids, and 125I-apoB was then added for an additional 1.5-h incubation at 4°C. This sequential incubation ensured that the 125I-apoB from OxLDL would not be depleted by adsorption onto the liposomes or microemulsions. The competition between 125I-apoB and OxLDL gave identical results regardless of whether the species were incubated together or sequentially, as just described.
Synthesis of POVPC and Preparation of POVPC-Albumin Conjugates.
POVPC was prepared by bubbling ozone through a methylene chloride solution of PAPC (Avanti Polar Lipids) at −70°C. The ozonide was broken down by the addition of methyl sulfide (Aldrich) (21). The product was purified by silica-based preparative chromatography (Merck) by using chloroform:methanol:water (10:5:1) as the mobile phase. The product was identified by its positive reaction with phosphomolybdate (22) and Schiff base reagent sprays (Aldrich). The Rf of the product was 0.375. Purity was confirmed by mass spectroscopy (m/e = 594), 13C-NMR, 1H-NMR, and reverse-phase HPLC (single peak). Based on the spectroscopic data, the purity of the POVPC was at least 99.5%.
An adduct of POVPC with BSA was made by incubating POVPC (final concentration, 2 mM) with BSA (1 mg/ml) at 37°C for 4 h. Then NaCNBH3 was added (final concentration, 10 mM) and the mixture was incubated overnight at 37°C and exhaustively dialyzed against PBS. From measurements of the number of free epsilon-amino groups masked, it was estimated that 48% of the BSA lysine groups were conjugated to POVPC.
RESULTS
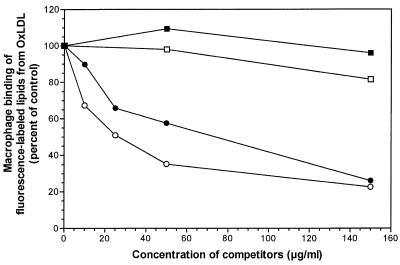
We have reported previously that microemulsions of lipids from OxLDL (but not those of lipids from native LDL) inhibit binding of intact OxLDL to macrophages (12). As shown in Fig. 1, the binding (at 4°C) of DiO-labeled microemulsions prepared from the lipids of OxLDL was inhibited in a concentration-dependent manner both by intact OxLDL and, to a roughly comparable extent, by unlabeled microemulsions prepared from OxLDL lipids. Neither intact native LDL nor microemulsions prepared from the lipids of native LDL had any inhibitory effect. Results similar to these were obtained with resident macrophages (data not shown). Thus, the competition between intact OxLDL and the lipid moiety of OxLDL is reciprocal, compatible with binding to one or more common receptors.
Figure 1.
Binding of DiO-labeled OxLDL lipid microemulsions to mouse macrophages in the presence of unlabeled native LDL lipids (■), OxLDL lipids (●), intact native LDL (□), or OxLDL (○). Elicited mouse macrophages were incubated in the presence of 10 μg/ml DiO-labeled OxLDL lipid microemulsions for 2 h at 4°C with or without competitors added (expressed as μg of phospholipid per ml for lipid microemulsions and μg of protein for intact LDL). The cells were then washed, scraped, and the mean fluorescence intensity of 30,000 events measured by using flow cytometry and cell quest software.
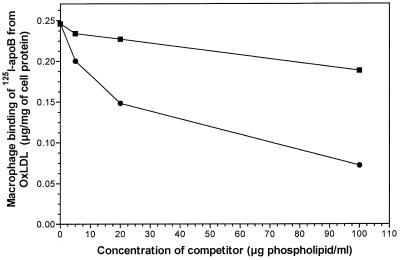
It was shown previously that the delipidated resolubilized apoprotein of 125I-OxLDL binds to macrophages in a saturable fashion and shows reciprocal inhibition either by intact OxLDL or by unlabeled apoB from OxLDL (5). We now show (Fig. 2) that the binding of apoB isolated from OxLDL is also inhibited in a concentration-dependent manner by microemulsions of the lipids derived from OxLDL. The lipids derived from native LDL and reconstituted in the same manner showed little or no inhibition. Reciprocally, the binding of DiO-labeled OxLDL lipid microemulsions was inhibited by the apoB derived from OxLDL—up to 41% (data not shown). Thus, it appears that the lipid and the protein fractions as prepared from intact OxLDL must have some commonality of receptor-binding domains.
Figure 2.
Binding of 125I-apoB from OxLDL to mouse macrophages in the presence of native LDL lipids (■) or OxLDL lipids (●). Elicited mouse macrophages were incubated with native LDL lipid or OxLDL lipid microemulsions for 1.5 h at 4°C. After being washed to remove the unbound lipids, the cells were then incubated with 4 μg/ml of 125I-apoB for an additional 1.5 h at 4°C. Binding was quantitated by measuring the (125I) radioactivity per mg of cell protein as described in Methods.
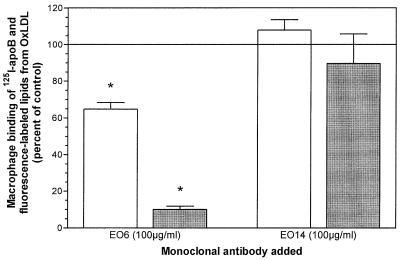
Hörkkö et al. (14) have shown that the binding and uptake of intact OxLDL is inhibited by several monoclonal autoantibodies generated by apoE-deficient hypercholesterolemic mice, including an antibody designated E06, and that these antibodies bind both to the isolated apoprotein of OxLDL and to the isolated OxLDL lipids. To test whether the epitope(s) recognized by E06 also played a role in the binding of the isolated OxLDL lipids and the isolated OxLDL apoB to macrophages, we determined their binding at 4°C in the absence and presence of E06 (Fig. 3). Monoclonal antibody E06 inhibited the binding of both the modified apoB isolated from OxLDL and the lipid microemulsions prepared from OxLDL. The binding of the latter was most strongly inhibited (over 90%). The binding of the isolated apoB was inhibited by only 35%, but the difference was highly significant (P < 0.005). Another monoclonal IgM from the apoE-deficient mice was used as a control (E014) and, as shown in Fig. 3, this IgM showed no inhibitory effect on the binding either of isolated apoB or of the reconstituted lipid microemulsions.
Figure 3.
Inhibition by monoclonal antibody EO6 of the binding of 125I-apoB from OxLDL (open bars) and DiO-labeled OxLDL lipid (closed bars). Binding assays for 125I-apoB (at 4 μg/ml) and DiO-labeled OxLDL lipids (at 10 μg/ml) were performed as described for Figs. 1 and 2, respectively, in the presence of EO6 or EO14 at 100 μg/ml. The values represent the means ± SD (n = 3). ∗, P < 0.005 compared with incubation with EO14, a control IgM.
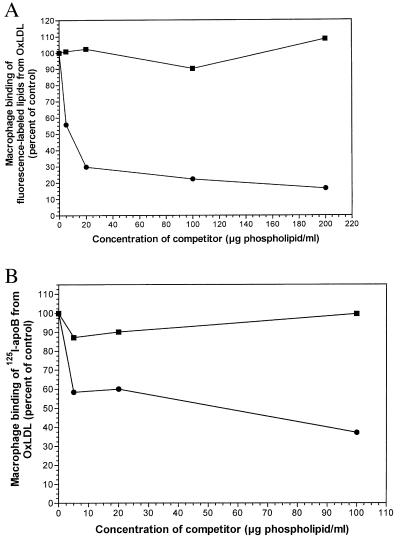
What is the nature of the lipids in OxLDL specifically involved in its binding to macrophage scavenger receptors and in its recognition by monoclonal antibodies? We have shown previously that the uptake and degradation of intact OxLDL by mouse peritoneal macrophages can be strongly inhibited by liposomes containing oxidized PAPC (phospholipid:cholesterol, 2:1) but not by liposomes containing native PAPC (12). Hörkkö et al. have recently shown that antibody EO6 binds to oxidized PAPC but not to native PAPC (14), suggesting that some of the oxidation products are ligands for scavenger receptors. We therefore tested for the ability of oxidized PAPC to inhibit macrophage binding of the separated lipid and apoprotein moieties of OxLDL. As shown in Fig. 4A, the binding of lipid microemulsions prepared from OxLDL was strongly inhibited by liposomes containing oxidized PAPC but not by liposomes containing nonoxidized PAPC. The liposomes containing oxidized PAPC inhibited by more than 70%. As shown in Fig. 4B, the binding of the isolated apoB from OxLDL was also inhibited by liposomes containing oxidized PAPC, although the inhibition was less striking (40% to 60%). Hörkkö et al. have reported that the uptake and degradation of intact OxLDL can be strongly inhibited (more than 80%) by a covalent adduct of POVPC and BSA (14). POVPC is derived from PAPC, the arachidonic acid in the sn-2 position having undergone oxidative fragmentation, leaving a five-carbon aldehydic fragment still attached in ester linkage to the glycerol backbone. This aldehyde function can readily form Schiff bases with the epsilon amino groups of lysine residues, and this is the presumed nature of the complex with BSA used by Hörkkö et al. As shown in Fig. 5, this POVPC–bovine albumin conjugate inhibited in a dose-dependent manner both the binding of the resolubilized 125I apoB (open bars) and that of the reconstituted lipid microemulsions (shaded bars) from OxLDL. Under the conditions used, both were inhibited by about 50% at the highest concentration of bovine albumin-POVPC tested (100 μg/ml).
Figure 4.
(A) Binding of DiO-labeled OxLDL lipid microemulsions to mouse macrophages in the presence of liposomes containing PAPC (■) or oxidized PAPC (●). Elicited mouse macrophages were incubated in the presence of 10 μg/ml DiO-labeled OxLDL lipid microemulsions and PAPC-containing liposomes for 2 h at 4°C. Binding was quantitated as described for Fig. 1. (B) Binding of 125I-apoB from OxLDL to mouse macrophages in the presence of liposomes containing PAPC (■) or oxidized PAPC (●). Elicited mouse macrophages were incubated with PAPC liposomes for 1.5 h at 4°C. After being washed to remove the unbound lipids, the cells were then incubated with 4 μg/ml of 125I-OxApoB for an additional 1.5 h at 4°C. Binding was quantitated as described for Fig. 2.
Figure 5.
Macrophage binding of 125I-apoB (open bars) and of DiO-labeled OxLDL lipids (solid bars) in the presence of unlabeled ligands (15-fold excess) and in the presence of three different concentrations of POVPC-BSA.
DISCUSSION
The data presented here, together with our previous results (6, 7, 12–14), support the conclusion that some macrophage receptors recognize both the modified apoB of OxLDL and the modified lipid moiety of OxLDL. It has been shown that: (i) microemulsions prepared from the lipids of OxLDL bind to macrophages in a saturable fashion and inhibit the binding of intact OxLDL by as much as 70–80%; (ii) this inhibition is reciprocal, i.e., intact OxLDL inhibits the binding of OxLDL-lipid microemulsions to a comparable extent; (iii) the apoB of OxLDL inhibits the binding of intact OxLDL by as much as 70% and this, too, is reciprocal; (iv) microemulsions of OxLDL-lipids inhibit the binding of resolubilized apoB from OxLDL and vice versa; (v) these competitive interactions can occur at 4°C, a temperature at which a nonspecific metabolic basis for inhibition is unlikely; (vi) a monoclonal antibody that binds to intact OxLDL and inhibits its uptake also recognizes both the isolated lipid and protein moieties of OxLDL and inhibits their binding to macrophages; (vii) oxidized PAPC incorporated into liposomes inhibits macrophage binding of intact OxLDL and also of both the apoB and the lipid microemulsions prepared from OxLDL; (viii) finally, POVPC, a specific fully characterized oxidation product of PAPC, when conjugated to BSA, inhibits binding of intact OxLDL and of both the apoB and lipid microemulsions from OxLDL.
One way to account for these observations would be to postulate two different classes of receptors, one binding exclusively the apoprotein moiety of OxLDL and the other binding exclusively one or more lipid moieties of OxLDL. That possibility, however, is not consistent with the quantitative aspects of the data. The cell association and degradation of intact 125I-OxLDL was inhibited by as much as 70% to 80% by lipid microemulsions prepared from OxLDL (12), and the uptake and degradation of intact OxLDL was inhibited by as much as 65% to 70% by the apoB reisolated from OxLDL (5). If there were just two classes of receptors, one recognizing only the lipid moiety and the other recognizing only the protein moiety, and if these shared equally in the binding of OxLDL, one could get up to 50% inhibition (but not more) with either the resolubilized apoB or the reconstituted lipid emulsion from OxLDL. However, if one class (e.g., the class binding lipids) accounts for 70% of total binding, and the other (binding apoB) accounts for 30%, the isolated apoB can inhibit only up to 30%. But what we actually observe is greater than 65% to 80% inhibition by each isolated moiety. Thus, we are driven to conclude that at least some macrophage receptors can recognize and bind to either the lipid moiety of OxLDL or to the “protein” moiety—the latter presumably occurring via lipids covalently bound to it. This conclusion is strongly supported by the monoclonal antibody studies of Hörkkö et al. showing that some monoclonal antibodies that bind specifically to oxidized phospholipids also bind to both the isolated apoprotein from OxLDL and to the microemulsions prepared from OxLDL lipids (14). Moreover, these antibodies inhibited macrophage uptake and degradation of intact 125I-OxLDL by as much as 70% to 90%. Tertov et al. (23) and Karakatsani et al. (24) demonstrated residual phosphate on OxLDL apoB after lipid extraction but did not prove rigorously that it represented phospholipid. Recent studies in this laboratory (K.L.G., J.L.W., and D.S., unpublished results) show that even after exhaustive extraction of OxLDL lipids, there is approximately 70 μmol of phosphorus associated per μmol of recovered protein; after similar treatment of native LDL there was less than 5 μmol of phosphorus per μmol of apoB. This protein-associated phosphorus on OxLDL apparently represents covalently attached phospholipid, because saponification of the delipidated protein released roughly equimolar amounts of phosphorus and of saturated fatty acids (phosphorus/saturated fatty acid molar ratio, 0.84).
At this point, we cannot say which of the receptors that recognize OxLDL bind predominantly to the apoprotein moiety, which to the lipid moiety, and which to both. OxLDL is known to bind to scavenger receptor A (SRA) (25), CD36 (26), macrosialin (CD68) (27), LOX-1 (28), SR-B1/CLA-1 (29), and perhaps still additional receptors. Studies of resident peritoneal macrophages from SRA-knockout mice have shown only a 20–30% decrease in OxLDL binding and uptake, indicating that SRA is not a major receptor for OxLDL (30, 31). CD36, on the other hand, appears to play a major role because OxLDL uptake and degradation by human monocyte-derived macrophages was inhibited by about 50% by a monoclonal antibody against CD36 (32). Nicholson et al. (33) have inferred a role for the lipids of OxLDL in its binding to CD36. They found that the specific binding of intact OxLDL to cells transfected with murine CD36 was 3-fold greater than to wild-type cells, but that the binding of the resolubilized apoB showed no significant difference. The lipid moiety was not directly tested. Little or no information is available with respect to the quantitative roles and the lipid-vs.-protein ligand specificities of other receptors that bind OxLDL.
All of these findings taken together support the conclusion that a large part of the binding of OxLDL to mouse peritoneal macrophages is caused by receptor recognition of partially oxidized phospholipids both present in the lipid phase of the lipoprotein and covalently attached to the apoprotein, as shown in the scheme below. 
The findings do not rule out the participation of other oxidized lipids or modifications of the apoprotein that occur during the process of LDL oxidation. However, the quantitative aspects of the data suggest that the role of oxidized phospholipids is major. If this is correct, low molecular-weight analogs of oxidized phospholipids might prove effective in inhibiting the uptake of OxLDL by some scavenger receptors. Suzuki et al. (34) have shown that knocking out scavenger receptor A, one of the receptors that recognizes OxLDL, reduces the severity of atherosclerosis in apoE-deficient mice. Therefore, antagonists to OxLDL receptors could in theory do the same thing.
These findings also have interesting implications with respect to macrophage scavenger receptor recognition of apoptotic cells. Both intact OxLDL and the lipid microemulsions prepared from it can inhibit the binding of apoptotic thymocytes (by about 50%) (7, 12). Liposomes rich in phosphatidylserine inhibit by almost 90%. The same is true with respect to the binding of oxidatively damaged erythrocytes. In the latter case, the inhibition by OxLDL lipid microemulsions was even greater—closer to 90%. These findings, taken together with the evidence summarized above that oxidized phospholipids play a major role in recognition of OxLDL, suggest the possibility that the recognition of apoptotic cells is also attributable, at least in part, to the presence of oxidized phospholipids generated in the plasma membrane as a part of the apoptotic program. In an accompanying paper (35), Chang et al. in fact show that monoclonal antibody EO6 binds to the surface of apoptotic cells and inhibits their uptake by macrophages. Furthermore, the POVPC-BSA adduct is also an effective competitor. These data suggest that oxidized phospholipids are also ligands on apoptotic cells that mediate macrophage recognition.
Oxidized phospholipids, including POVPC itself, have been shown to have a number of biological effects in cultured cells. These include activation of neutrophils (36, 37), stimulation of smooth muscle growth (37), and induction of endothelial cell adhesion molecules (15, 38, 39). Some or all of these effects are believed to result from interaction with signaling receptors closely related to the platelet-activating factor receptor (36, 37). How do these effects relate, if at all, to functions of POVPC as a ligand mediating the binding and internalization of OxLDL and apoptotic cells? We should note first that our results are thus far all obtained in macrophages, not in the cell types used in the studies mentioned above, so there may be no relationship. Could our results with regard to binding and internalization be indirect results of POVPC stimulation of signaling pathways? That is made unlikely because of our binding studies, which were done at 4°C. It is quite possible that during evolution, two quite different sets of receptors have been selected, both recognizing POVPC and/or other oxidized phospholipids but serving different conservative functions at sites of inflammation.
We have previously suggested that the scavenger receptors that recognize OxLDL have persisted in evolution because of their more general biological function in the recognition of apoptotic cells (6, 7). If oxidized phospholipids and oxidized phospholipid-protein complexes play a major role in the recognition of apoptotic cells, it would be easier to understand why macrophages can recognize a wide variety of apoptotic cells even though those cells differ considerably in their protein makeup. All of them share the predominance of phosphatidylcholine and other phospholipids in their membranes. Oxidative damage, such as has been postulated to occur in the course of inflammation, apoptosis, and/or necrosis would then generate oxidized phospholipid and oxidized phospholipid-protein complexes similar to or even identical with those generated in OxLDL. If this is the case, the autoantibodies that recognize OxLDL may be to some extent generated in response not only to OxLDL itself but to closely related antigens being generated in apoptotic or necrotic cells anywhere in the body. Conversely, antibodies generated in response to OxLDL as the antigen could affect recognition of apoptotic or damaged cells by macrophages and could modify their clearance.
Acknowledgments
This work was supported by National Institutes of Health grants HL56989 (Specialized Center of Research in Molecular Medicine and Atherosclerosis), HL57505 (J.L.W.), Training Grant DK 07044 (K.L.G.), and Training Grant HL07276 (D.A.B.), and by a Postdoctoral Research Fellowship of the American Heart Association (S.H.).
ABBREVIATIONS
- LDL
low-density lipoprotein
- OxLDL
oxidized LDL
- PAPC
1-palmitoyl 2-arachidonoyl phosphatidylcholine
- POVPC
1-palmitoyl 2(5-oxovaleroyl) phosphatidylcholine
- DiO
3,3′-dihexadecyloxacarbocyanine perchlorate
- apoB
apoprotein B
- apoE
apoprotein E
References
- 1.Steinberg D, Parthasarathy S, Carew T E, Khoo J C, Witztum J L. N Engl J Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein J L, Ho Y K, Basu S K, Brown M S. Proc Natl Acad Sci USA. 1979;76:333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fogelman A M, Shechter I, Seager J, Hokom M, Child J S, Edwards P A. Proc Natl Acad Sci USA. 1980;77:2214–2218. doi: 10.1073/pnas.77.4.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein J L, Brown M S. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- 5.Parthasarathy S, Fong L G, Otero D, Steinberg D. Proc Natl Acad Sci USA. 1987;84:537–540. doi: 10.1073/pnas.84.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sambrano G R, Parthasarathy S, Steinberg D. Proc Natl Acad Sci USA. 1994;91:3265–3269. doi: 10.1073/pnas.91.8.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sambrano G R, Steinberg D. Proc Natl Acad Sci USA. 1995;92:1396–1400. doi: 10.1073/pnas.92.5.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schroit A J, Tanaka Y, Madsen J, Fidler I J. Biol Cell. 1984;51:227–238. doi: 10.1111/j.1768-322x.1984.tb00303.x. [DOI] [PubMed] [Google Scholar]
- 9.Schlegel R A, Prendergast T W, Williamson P. J Cell Physiol. 1985;123:215–218. doi: 10.1002/jcp.1041230210. [DOI] [PubMed] [Google Scholar]
- 10.Fadok V A, Voelker D R, Campbell P A, Cohen J J, Bratton D L, Henson P M. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 11.Savill J, Fadok V, Henson P, Haslett C. Immunol Today. 1993;14:131–136. doi: 10.1016/0167-5699(93)90215-7. [DOI] [PubMed] [Google Scholar]
- 12.Terpstra V, Bird D, Steinberg D. Proc Natl Sci USA. 1998;95:1806–1811. doi: 10.1073/pnas.95.4.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hörkkö S, Miller E, Dudl E, Reaven P, Curtiss L K, Zvaifler N J, Terkeltaub R, Pierangeli S S, Branch D W, Palinski W, et al. J Clin Invest. 1996;98:815–825. doi: 10.1172/JCI118854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hörkkö S, Bird D, Miller E, Itabe H, Leitinger N, Subbanagounder G, Berliner J A, Friedman P, Dennis E A, Curtiss L K, et al. J Clin Invest. 1999;103:117–128. doi: 10.1172/JCI4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watson A D, Leitinger N, Navab M, Faull K F, Hörkkö S, Witztum J L, Palinski W, Schwenke D, Salomon R G, Sha W, et al. J Biol Chem. 1997;272:13597–13607. doi: 10.1074/jbc.272.21.13597. [DOI] [PubMed] [Google Scholar]
- 16.Havel R J, Eder H A, Bragdon J H. J Clin Invest. 1955;34:1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowry O H, Rosebrough N J, Farr A L, Randall R J. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 18.Yagi K. Biochem Med. 1976;15:212–216. doi: 10.1016/0006-2944(76)90049-1. [DOI] [PubMed] [Google Scholar]
- 19.Marinetti G. J Lipid Res. 1962;3:1–20. [Google Scholar]
- 20.Salacinski P R P, McLean C, Sykes J E C, Clement-Jones V V, Lowry P J. Anal Biochem. 1981;117:136–146. doi: 10.1016/0003-2697(81)90703-x. [DOI] [PubMed] [Google Scholar]
- 21.Stremler K E, Stafforini D M, Prescott S M, McIntyre T M. J Biol Chem. 1991;266:11095–11103. [PubMed] [Google Scholar]
- 22.Dittmer J C, Lester R L. J Lipid Res. 1964;5:126–127. [PubMed] [Google Scholar]
- 23.Tertov V V, Kaplun V V, Dvoryantsev S N, Orehov A N. Biochem Biophys Res Comm. 1995;214(2):608–613. doi: 10.1006/bbrc.1995.2329. [DOI] [PubMed] [Google Scholar]
- 24.Karakatsani A I, Liapikos T, Troganis A N, Tsoukatos D C. Lipids. 1998;33:1159–1162. doi: 10.1007/s11745-998-0318-3. [DOI] [PubMed] [Google Scholar]
- 25.Krieger M, Acton S, Ashkenas J, Pearson A, Penman M, Resnick D. J Biol Chem. 1993;268:4569–4572. [PubMed] [Google Scholar]
- 26.Endemann G, Stanton L W, Madden K S, Bryant C M, White R T, Protter A A. J Biol Chem. 1993;268:11811–11816. [PubMed] [Google Scholar]
- 27.Ramprasad M, Terpstra V, Kondratenko N, Quehenberger O, Steinberg D. Proc Natl Acad Sci USA. 1996;93:14833–14838. doi: 10.1073/pnas.93.25.14833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sawamura T, Kume N, Aaoyama T, Moriwaki H, Hosokawa H, Aiba Y, Tanakta T, Miwa S, Katsura Y, Kita T, et al. Nature (London) 1997;386:73–77. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- 29.Acton S L, Scherer P E, Lodish H F, Krieger M. J Biol Chem. 1994;269:21003–21009. [PubMed] [Google Scholar]
- 30.Lougheed M, Ming Lum C, Ling W, Suzuki H, Kodama T, Steinbrecher U P. J Biol Chem. 1997;272:12938–12944. doi: 10.1074/jbc.272.20.12938. [DOI] [PubMed] [Google Scholar]
- 31.Terpstra V, Kondratenko N, Steinberg D. Proc Natl Acad Sci USA. 1997;94:8127–8131. doi: 10.1073/pnas.94.15.8127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nozaki S, Kashiwagi H, Yamashita S, Nakagawa T, Kostner B, Tomiyama Y, Nakata A, Ishigami M, Miyagawa J, Kameda-Takemura K, et al. J Clin Invest. 1995;96:1859–1865. doi: 10.1172/JCI118231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicholson A C, Frieda S, Pearce A, Silverstein R L. Arterioscler Thromb Vasc Biol. 1995;15:269–275. doi: 10.1161/01.atv.15.2.269. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki H, Kurihara Y, Takeya M, Kamada N, Kataoka M, Jishage K, Ueda O, Sakaguchi T, Higashi T, Suzuki T, et al. Nature (London) 1997;386:292–296. doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]
- 35.Chang M, Bergmark C, Laurila A, Hörkkö S, Han K, Friedman P, Dennis E A, Witztum J L. Proc Natl Acad Sci USA. 1999;96:6353–6358. doi: 10.1073/pnas.96.11.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smiley P L, Stremler K E, Prescott S M, Zimmerman G A, McIntyre T M. J Biol Chem. 1991;266:11104–11110. [PubMed] [Google Scholar]
- 37.Heery J M, Kozak M, Stafforini D M, Jones D A, Zimmerman G A, McIntyre T M, Prescott S M. J Clin Invest. 1995;96:2322–2330. doi: 10.1172/JCI118288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watson A D, Navab M, Hama S Y, Sevanian A, Prescott S M, Stafforini D M, McIntyre T M, Du B N, Fogelman A M, Berliner J A. J Clin Invest. 1995;95:774–782. doi: 10.1172/JCI117726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leitinger N, Watson A D, Faull K F, Fogelman A M, Berliner J A. Adv Exp Med Biol. 1997;433:379–382. doi: 10.1007/978-1-4899-1810-9_82. [DOI] [PubMed] [Google Scholar]