Abstract
Multiple interactive domains are involved in the activity of the stress protein, αB crystallin that protects against the unfolding, aggregation, and toxicity of amyloidogenic proteins. Five peptides corresponding to the interactive sequences 41STSLSPFYLRPPSFLRAP58, 73DRFSVNLDVKHFS85, 101HGKHEERQDE110, 113FISREFHR120, 131LTITSSLSSDGV142, and 156ERTIPITRE164 in human αB crystallin were synthesized and evaluated in Thioflavin T fluorescence assays for their effects on the modulation of fibrillation of four disease-related amyloidogenic proteins: amyloid-β, α-synuclein, transthyretin, and β2-microglobulin. The 73DRFSVNLDVKHFS85 and 101HGKHEERQDE110 peptides in the conserved α crystallin core domain of αB crystallin were the most effective fibril inhibitors. 73DRFSVNLDVKHFS85 completely inhibited α-synuclein fibrillation and reduced the fibrillation of amyloid-β, transthyretin, and β2-microglobulin by >50%. 101HGKHEERQDE110 completely inhibited amyloid-β fibrillation and reduced the fibrillation of α-synuclein, transthyretin, and β2-microglobulin by >50%. The peptides FSVN, NLDV, HGKH, and HEER, which are synthetic fragments of 73DRFSVNLDVKHFS85 and 101HGKHEERQDE110, inhibited fibrillation of all four amyloidogenic proteins by >75%. In contrast, the peptides FISREFHR, ERTIPITRE, DRFS, KHFS, and EERQ were the strongest promoters of fibrillation. Molecular modeling of the interactions between transthyretin and β2-microglobulin and the synthetic bioactive peptides determined that residues Phe-75, Ser-76, Val-77, Asn-78, Leu-79, and Asp-80 in 73DRFSVNLDVKHFS85 and residues His-101, Lys-103, His-104, Glu-105, and Arg-107 in 101HGKHEERQDE110 interact with exposed residues in the β strands, F and D of transthyretin and β2-microglobulin respectively to modulate fibrillation. This is the first characterization of specific bioactive peptides synthesized on the basis of interactive domains in the small heat shock protein, αB crystallin that protect against the fibrillation of amyloidogenic proteins.
Keywords: crystallin, chaperone, fibril, amyloid, synuclein, transthyretin, microglobulin, Alzheimer’s disease, Parkinson’s disease
Introduction
Protein unfolding and aggregation of cytosolic lens crystallins, desmin filaments, microtubules, and amyloidogenic proteins including amyloid-β (Aβ), α-synuclein, transthyretin, and β2-microglobulin are the hallmarks of aggregation diseases that include cataract, desmin-related myopathy (DRM), Alzheimer’s disease (AD), Parkinson’s disease (PD), senile systemic amyloidosis (SSA), hemodialysis-related amyloidosis (HRA), age related macular degeneration (AMD), and familial amyloidotic polyneuropathy (FAP) (Dobson, 2001; Gorevic et al., 1985; J. Hardy & Selkoe, 2002; Selkoe, 2003; Sunde et al., 1997; Vicart et al., 1998). While formation of light scattering protein aggregates in the ocular lens results in cataracts, abnormal assembly of filaments in cardiac tissues results in DRM, and formation of oligomers and structured amyloid-like fibrils results in neurodegenerative diseases including AD and PD (Dobson, 2004). Small heat shock proteins (sHSP) are a family of molecular chaperones (molecular weight < 43kDa) that protect against the harmful effects of protein unfolding, misfolding, and aggregation (Hartl, 1996). The sHSPs, human αB crystallin and sHSP27 are ubiquitously expressed in response to cellular stress and protect against protein unfolding, misfolding, and aggregation in the normal unfolding protein response (UPR) (Haslbeck, Franzmann, Weinfurtner, & Buchner, 2005; Winter & Jakob, 2004). Clinically, sHSPs including αB crystallin have been observed in neuritic plaques and Lewy bodies in brain tissues of AD and PD patients, and in Drusen of patients with age-related macular degeneration (Dabir, Trojanowski, Richter-Landsberg, Lee, & Forman, 2004; De et al., 2007; Johnson, Brown, Pulliam, Anderson, & Johnson, 2005; Nakata, Crabb, & Hollyfield, 2005; Renkawek, Bosman, & de Jong, 1994; Renkawek et al., 1992; Renkawek, Stege, & Bosman, 1999; Wilhelmus, Boelens, Otte-Holler, Kamps, de Waal et al., 2006; Wilhelmus, Boelens, Otte-Holler, Kamps, Kusters et al., 2006). A recent study reported a marked increase in the expression of αB crystallin and sHSP25 in transgenic mouse models of familial amyotrophic lateral sclerosis (ALS), PD, dentato-rubral pallido-luysian atrophy, and Huntington’s disease (HD) (Wang, Martin, Gonzales, Borchelt, & Lee, 2007). Although, human αB crystallin and other sHSPs interact with and inhibit the fibrillation of Aβ1–42, α-synuclein, β2-microglobulin, and insulin in vitro (Goldstein et al., 2003; Hatters, Lindner, Carver, & Howlett, 2001; Lee, Carson, Rice-Ficht, & Good, 2005, 2006; Liang, 2000; Raman et al., 2005; Rekas et al., 2004; Santhoshkumar & Sharma, 2004; Wilhelmus, Boelens, Otte-Holler, Kamps, de Waal et al., 2006), it has been reported that αB crystallin enhances the neurotoxicity of Aβ in cultured neuronal cells (Stege et al., 1999). The consistent observation of the expression and localization of sHSPs in association with abnormal protein aggregates in diseased tissues suggests an important biological function for sHSPs in aggregation diseases. Identification of the bioactive sequences in sHSPs that mediate protein-protein interactions with the amyloidogenic proteins Aβ, α-synuclein, transthyretin, and β2-microglobulin will provide a molecular basis for the mechanism of sHSP activity in protein aggregation diseases including AD and PD, cataract, and AMD.
The goal of the current study was the characterization of molecular interactions between human αB crystallin, the archetype of sHSPs and four amyloidogenic proteins (Aβ1–42, α-synuclein, transthyretin, and β2-microglobulin) that are known to form toxic aggregates and fibrils. Systematic protein pin array analysis, site-directed mutagenesis, and chaperone assays characterized the 41STSLSPFYLRPPSFLRAP58 sequence in the N-terminal domain, the sequences 73DRFSVNLDVKHFS85, 101HGKHEERQDE110, 113FISREFHR120, and 131LTITSSLSSDGV142 in the exposed interface of the α crystallin core domain, and the sequence 156ERTIPITRE164 in the C-terminal domain of human αB crystallin as interactive sequences responsible for the recognition, binding, and solubilization of unfolding/misfolding substrate proteins including lens crystallins, enzymes, filaments, and growth factors (Bhattacharyya, Padmanabha Udupa, Wang, & Sharma, 2006; Ghosh & Clark, 2005; Ghosh, Estrada, & Clark, 2005, 2006; Ghosh, Estrada, Houck, & Clark, 2006; Ghosh, Houck, & Clark, 2007a, 2007b; Ghosh, Shenoy, & Clark, 2006b; Ghosh, Shenoy, & Clark, 2007a). Synthetic peptides corresponding to these sequences suppressed the aggregation of β/γ crystallins, alcohol dehydrogenase, citrate synthase, actin, FGF, and VEGF, and promoted microtubule assembly (Ghosh & Clark, 2005; Ghosh, Estrada, & Clark, 2005, 2006; Ghosh, Estrada, Houck, & Clark, 2006; Ghosh, Houck, & Clark, 2007a, 2007b; Ghosh, Shenoy, & Clark, 2006b; Ghosh, Shenoy, & Clark, 2007a). In this study, we demonstrated the effectiveness of these five αB crystallin sequences in inhibiting the fibrillation of Aβ1–42, α-synuclein, transthyretin, and β2-microglobulin using Thioflavin T fluorescence assays. The synthetic 73DRFSVNLDVKHFS85 and 101HGKHEERQDE110 peptides were the most effective modulators of fibrillation. Systematic truncation of these two bioactive peptides led to the identification of four amino acid long peptides (molecular weight <500Da) that were more effective in modulating the fibrillation of Aβ, α-synuclein, transthyretin, and β2-microglobulin than Tramiprosate, NAP, diflunisal, and mecloflenic acid, which are anti-amyloidogenic molecules currently in clinical development (Aisen, 2005; Gervais et al., 2007; Gozes et al., 2005). The results define specific interactions that are important in the protective activity of the sHSP, human αB crystallin, as a molecular chaperone in protein aggregation diseases.
Materials and Methods
Materials
The αB crystallin peptides DRFSVNLDVKHFS (DR) and HGKHEERQDE (HG) were synthesized by Advanced ChemTech (Louisville, KY). The remaining three αB crystallin peptides, STSLSPFYLRPPSFLRAP (ST), FISREFHR, LTITSSLSSDGV (LT) and ERTIPITRE (ER), were synthesized by Genscript (Piscataway, NJ). The peptides, NAPVSIPQ (NAP) and DRVYIHPFHL (Angiotensin 1, AT1) were purchased from Genscript (Piscataway, NJ). Aβ1–42 (American Peptide, Sunnyvale, CA), transthyretin (Sigma-Aldrich, St. Louis, MO), β2-microglobulin (MP Biomedical, Solon, OH), α-synuclein (rPeptide, Bogart, GA) were also procured from external vendors as listed above. Tramiprosate (3-aminopropanesulfonic acid, APS), diflunisal, flufenamic acid, and meclofenamic acid were purchased from Sigma-Aldrich (St. Louis, MO). The truncated DR and HG peptides were synthesized by Genscript (Piscataway, NJ). All peptides and small molecules obtained from the suppliers were directly dissolved in 0.25% DMSO at a concentration of 2mM without any additional treatment. For all experiments, fresh solutions were prepared immediately prior to use. Assays were performed in 384-well fluorescence plates in duplicates or triplicates and fluorescence was measured using a Perkin Elmer Victor3 V plate reader. Two molecules, Tramiprosate and NAP that are known inhibitors of Aβ1–42 fibrillation and are currently in clinical trials for AD were included in the Aβ1–42 and α-synuclein assays for comparison (Ashur-Fabian et al., 2003; Gervais et al., 2007). Diflunisal, flufenamic acid, and meclofenamic acid which are known inhibitors of transthyretin fibril formation were included in the transthyretin and β2-microglobulin assays. A synthetic neuropeptide Angiotensin 1 (AT1) that has no effect on the fibrillation of Aβ, α-synuclein, transthyretin, and β2-microglobulin was included as a negative control in all assays.
Thioflavin T fluorescence assays
The effect of the αB crystallin peptides on Aβ1–42, α-synuclein, transthyretin, and β2-microglobulin fibrillation was measured using the Thioflavin T (ThT) fluorescence assay (Hurshman, White, Powers, & Kelly, 2004; Ivanova, Sawaya, Gingery, Attinger, & Eisenberg, 2004; Naiki, Higuchi, Hosokawa, & Takeda, 1989; Raman et al., 2005; Walsh et al., 2005; Zhu et al., 2004). Thioflavin T is a fluorescent dye that has a high affinity for amyloid fibrils and becomes fluorescent (excitation λ = 450nm, emission λ = 486nm) when bound to fibrils. Fibril formation was verified by fluorescence and bright-field microscopy (data not shown).
For the Aβ1–42 1:1 assays, 10μL of 90μM test peptides dissolved in 0.25%DMSO were added to 7μL of buffer. Finally, 3μL of 1μM ThT and 10μL of 90μM Aβ1–42 were added to these solutions to start the experiment. The final buffer composition for the assay was 20mM Tris-Cl, 100mM NaCl, 0.08% DMSO, pH 7.4. For the Aβ1–42 5:1 assays, 10μL of 18μM test peptides dissolved in 0.25%DMSO were added to 7μL of buffer. 3μL of 1mM ThT and 10μL of 90μM Aβ1–42 were added to these solutions to start the experiment. The final buffer composition for the assay was 20mM Tris-Cl, 100mM NaCl, 0.08% DMSO, pH 7.4. The fluorescence of all samples was simultaneously measured in a plate reader (excitation λ = 450nm, emission λ = 486nm) immediately after addition of ThT and Aβ1–42, and after 24 and 48hrs of incubation at 37°C. The effect of the peptides and control molecules on fibril formation of Aβ1–42 was determined using the calculation: Aβ fibrillation = ΔFAβ+peptide/ΔFAβ, where ΔFAβ = ThT fluorescence of Aβ at 48hrs - ThT fluorescence of Aβ at 0hrs and ΔFAβ+peptide = ThT fluorescence of Aβ+peptide at 48hrs - ThT fluorescence of Aβ+peptide at 0hrs.
For the α-synuclein 1:1 assays, 5μL of 140μM test peptides dissolved in 0.25%DMSO were added to 17μL of buffer. Finally, 3μL of 1mM ThT and 5μL of 140μM α-synuclein were added to these solutions to start the experiment. The final buffer composition for the assay was 20mM Tris-Cl, 100mM NaCl, 0.04% DMSO, pH 7.4. For the α-synuclein 5:1 assays, 5μL of 28μM test peptides dissolved in 0.25%DMSO were added to 17μL of buffer. Finally, 3μL of 1mM ThT and 5μL of 140μM α-synuclein were added to these solutions to start the experiment. The final buffer composition for the assay was 20mM Tris-Cl, 100mM NaCl, 0.04% DMSO, pH 7.4. The fluorescence of all samples was simultaneously measured in a plate reader (excitation λ = 450nm, emission λ = 486nm) immediately after addition of ThT and α-synuclein and after 24, 48, and 96 hrs of incubation at 37°C. The effect of the peptides and control molecules on α-synuclein fibrillation was determined using the calculation: α-synuclein fibrillation = ΔFα-synuclein+peptide/ΔFα-synuclein, where ΔFα-synuclein = ThT fluorescence of α-synuclein at 96hrs - ThT fluorescence of α-synuclein at 0hrs and ΔFα-synuclein+peptide = ThT fluorescence of α-synuclein+peptide at 96hrs - ThT fluorescence of α-synuclein+peptide at 0hrs.
For the transthyretin 1:1 assays, 5μL of 64μM test peptides dissolved in 0.25%DMSO were added to 10μL of buffer. Finally, 1μL of 1mM ThT and 10μL of 32μM transthyretin were added to these solutions to start the experiment. The final buffer composition for the assay was 5mM PBS, 200mM KCl, 2mM EDTA, 200mM Na acetate, 0.06% DMSO, pH 4.4. For the transthyretin 5:1 assays, 5μL of 6.4μM test peptides dissolved in 0.25%DMSO were added to 10μL of buffer. Finally, 1μL of 1mM ThT and 10μL of 32μM transthyretin were added to these solutions to start the experiment. The final buffer composition for the assay was 5mM PBS, 200mM KCl, 2mM EDTA, 200mM Na acetate, 0.06% DMSO, pH 4.4. The fluorescence of all samples was simultaneously measured in a plate reader (excitation λ = 450nm, emission λ = 486nm) continuously after addition of ThT for 150 minutes at 37°C. The effect of the peptides and control molecules on transthyretin fibrillation was determined using the calculation: Transthyretin fibrillation = ΔFtransthyretin+peptide/ΔFtransthyretin, where ΔFtransthyretin = ThT fluorescence of transthyretin at 2.5hrs - ThT fluorescence of transthyretin at 0hrs and ΔFtransthyretin+peptide = ThT fluorescence of transthyretin +peptide at 2.5hrs - ThT fluorescence of transthyretin+peptide at 0hrs.
For the β2-microglobulin 1:1 assays, 10μL of 86μM test peptides dissolved in 0.25%DMSO were added to 7μL of buffer. Finally, 3μL of 1mM ThT and 10μL of 86μM β2-microglobulin were added to these solutions to start the experiment. The final buffer composition for the assay was 25mM Na acetate, 25mM Na phosphate, 0.08% DMSO, pH 2.4. For the β2-microglobulin 5:1 assays, 10μL of 17.2μM test peptides dissolved in 0.25%DMSO were added to 7μL of buffer. Finally, 3μL of 1mM ThT and 10μL of 86μM β2-microglobulin were added to these solutions to start the experiment. The final buffer composition for the assay was 25mM Na acetate, 25mM Na phosphate, 0.08% DMSO, pH 2.4. The fluorescence of all samples was simultaneously measured in a plate reader (excitation λ = 450nm, emission λ = 486nm) continuously after addition of ThT for 150 minutes at 37°C. The effect of the peptides and control molecules on β2-microglobulin fibrillation was determined using the calculation: β2-microglobulin fibrillation = ΔFβ2-microglobulin +peptide/ΔFβ2-microglobulin, where ΔFβ2-microglobulin = ThT fluorescence of β2-microglobulin at 2.5hrs - ThT fluorescence of β2-microglobulin at 0hrs and ΔFβ2-microglobulin +peptide = ThT fluorescence of β2-microglobulin+peptide at 2.5hrs - ThT fluorescence of β2-microglobulin+peptide at 0hrs
3D molecular modeling of the interactive site for the bioactive peptides
3D co-ordinates for the αB crystallin peptides were extracted from the computed homology model of human αB crystallin described previously using Molecular Operating Environment (Chemical Computing Group, Montreal, Canada) (Ghosh & Clark, 2005; Ghosh, Estrada, & Clark, 2005). 3D co-ordinates for human transthyretin and human β2-microglobulin were obtained from the crystal structures with PDB IDs 1DVQ and 1LDS respectively (Klabunde et al., 2000; Trinh, Smith, Kalverda, Phillips, & Radford, 2002). Co-ordinates for the peptides and the target proteins were provided to the molecular docking program ClusPro (Comeau, Gatchell, Vajda, & Camacho, 2004a, 2004b), which computed docking models for each protein-peptide combination. It is important to note that only the 3D co-ordinates for the αB crystallin peptides and crystal structures of transthyretin and β2-microglobulin were provided to the ClusPro docking program. The active site for docking the αB crystallin peptides was not specified. The docking was performed 3–5 times for each protein-peptide pair and produced the same results. The Cluspro docking program used a fast algorithm for filtering docked conformations with good surface complementarity, and ranked them based on their clustering properties. The free energy filters selected complexes with lowest desolvation and electrostatic energies. Finally, clustering smoothed the local minima and selected the centers of the most populated clusters as predictions of the unknown complex.
Results
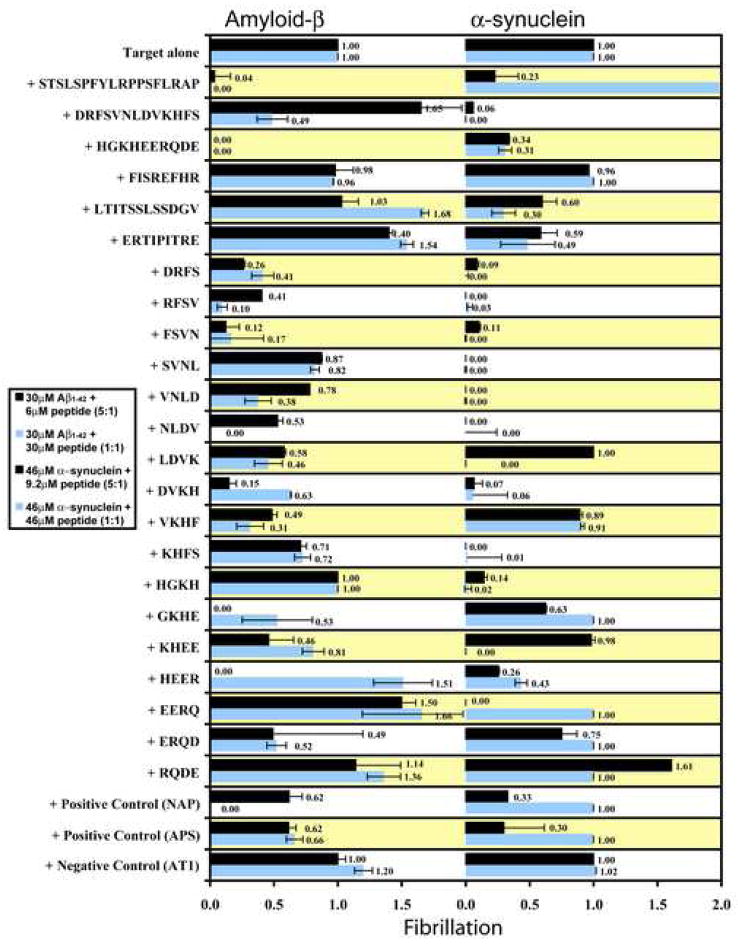
The five full-length human αB crystallin peptides that were synthesized for use in fibril assays corresponded to five previously identified functional sequences in the molecular chaperone, human αB crystallin (Figure 1). The Thioflavin T (ThT) fluorescence assay identified αB crystallin peptides that inhibited or promoted fibril formation of Aβ1–42, α-synuclein, transthyretin, and β2-microglobulin in vitro (Figures 2 and 3). The effects of the αB crystallin peptides on the fibrillation of Aβ1–42 and α-synuclein are summarized in Figure 2 and the effects of the αB crystallin peptides on the fibrillation of transthyretin and β2-microglobulin are summarized in Figure 3. At a concentration of 6μM peptide (Aβ1–42:peptide ratio of 5:1), the full-length ST and HG peptides, the truncated DR peptides DRFS, RFSV, FSVN, NLDV, LDVK, DVKH, VKHF, and KHFS, and the truncated HG peptides GKHE, KHEE, HEER, and ERQD had the strongest inhibitory effect on Aβ1–42 fibrillation and had ThT fluorescence values between 0.00 and 0.71 of the ThT fluorescence of Aβ1–42 alone (Figure 2). At the same concentrations, the full-length DR and ER peptides and the truncated HG peptides, EERQ, and RQDE increased Aβ1–42 fibrillation and had ThT fluorescence values between 1.14 and 1.65 of the ThT fluorescence of Aβ1–42 alone. The full-length FI and LT peptide, the truncated DR peptides SVNL and VNLD, and the truncated HG peptide HGKH had no effect on Aβ1–42 fibrillation and had ThT fluorescence values similar to the control peptide AT1. At a concentration of 30μM peptide (Aβ1–42:peptide ratio of 1:1), the full-length ST, DR, and HG peptides, the truncated DR peptides DRFS, RFSV, FSVN, VNLD, NLDV, LDVK, DVKH, VKHF, and KHFS, and the truncated HG peptides GKHE and ERQD had the strongest inhibitory effect on Aβ1–42 fibrillation and had ThT fluorescence values between 0.00 and 0.72 of the ThT fluorescence of Aβ1–42 alone. At the same concentration, the full-length LT and ER peptides and the truncated HG peptides, HEER, EERQ, and RQDE increased Aβ1–42 fibrillation and had ThT fluorescence values between 1.36 and 1.68 of the ThT fluorescence of Aβ1–42 alone. The full-length FI peptide, the truncated DR peptide SVNL and the truncated HG peptides HGKH and KHEE had no effect on Aβ1–42 fibrillation and had ThT fluorescence values similar to the control peptide AT1.
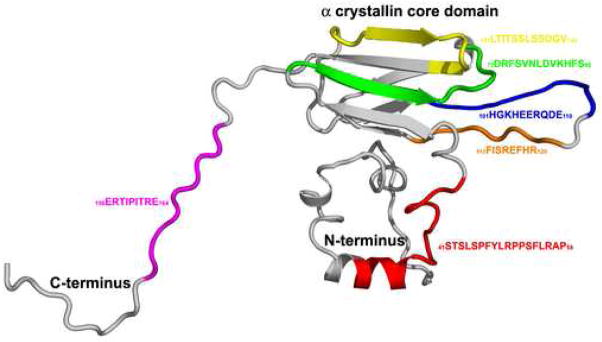
Figure 1.
3D map of the interactive domains in human αB crystallin. The interactive sequences in human αB crystallin were mapped onto the 3D homology model of human αB crystallin (grey). The N-terminal helical sequence 41STSLSPFYLRPPSFLRAP58 (red) is important for substrate recognition and binding (Ghosh, Shenoy, & Clark, 2006). On the exposed surface of the α crystallin core domain, the sequences, 73DRFSVNLDVKHFS85 (green), which forms the β3 strand, 101HGKHEERQDE110 (blue) and 113FISEFHR120 (orange) which form the loop region connecting the β strands 5 and 7, and 131LTITSSLSSDGV142 (yellow) which forms the β8 strand are important for substrate binding (Ghosh, Estrada, & Clark, 2005, 2006; Ghosh, Estrada, Houck, & Clark, 2006). The flexible C-terminal sequence 156ERTIPITRE164 (magenta) is important for solubility of the sHSP-substrate complex (Ghosh, Shenoy, & Clark, 2006). The interactive domains in human αB crystallin have multiple functions and the Thioflavin T fibril assays identified synthetic αB crystallin peptides that inhibit or promote fibril formation of the four amyloidogenic proteins amyloid-β, α-synuclein, transthyretin, and β2-microglobulin.
Figure 2.
Bioactive synthetic αB crystallin peptides promoted or inhibited Aβ1–42 and α-synuclein fibrillation in Thioflavin T fluorescence assays. The STSLSPFYLRPPSFLRAP, HGKHEERQDE, FSVN, and ERQD peptides had the strongest inhibitory effect and the LTITSSLSDGV, ERTIPITRE, HEER, and EERQ peptides had the strongest promoting effect on Aβ1–42 fibrillation. The DRFSVNLDVKHFS, DRFS, RFSV, FSVN, SVNL, VNLD, NLDV, DVKH, KHFS, and HGKH peptides had the strongest inhibitory effect and the STSLSPFYLRPPSFLRAP and RQDE peptides had the strongest promoting effect on α-synuclein fibrillation. The control peptide AT1 had no effect on Aβ1–42 or α-synuclein fibrillation, and the two positive control molecules NAP and APS had modest effects on Aβ1–42 and α-synuclein fibrillation. Each assay was done in triplicate and the error bars indicate the standard deviation. The results demonstrated the effectiveness of individual interactive domains in αB crystallin on modulation of fibril formation in vitro.
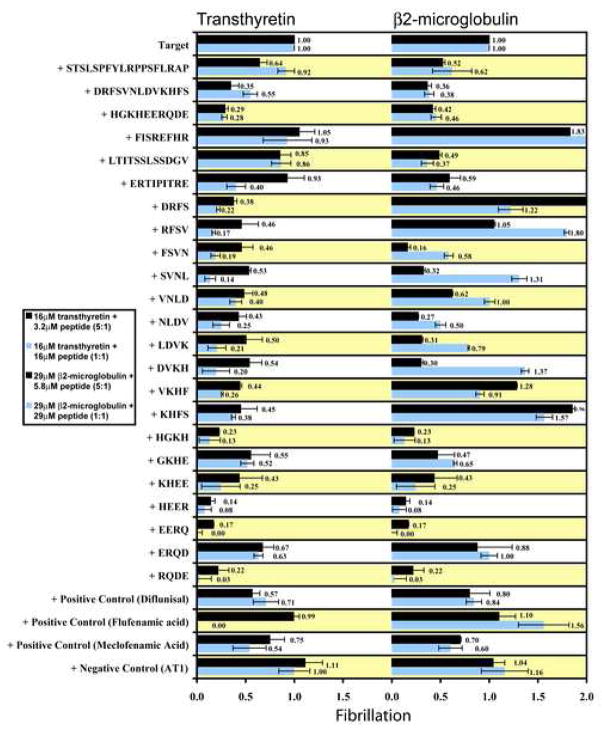
Figure 3.
Bioactive synthetic αB crystallin peptides promoted or inhibited transthyretin and β2-microglobulin fibrillation in Thioflavin T fluorescence assays. The DRFSVNLDVKHFS, HGKHEERQDE, DRFS, HGKH, HEER, EERQ, and RQDE peptides had the strongest inhibitory effect on transthyretin fibrillation. No peptides promoted transthyretin fibrillation. The DRFSVNLDVKHFS, HGKHEERQDE, LTITSSLSDGV, ERTIPITRE, FSVN, HGKH, HEER, EERQ, and RQDE peptides had the strongest inhibitory effect and the FISREFHR, DRFS, RFSV, DVKH, and KHFS peptides had the strongest promoting effect on β2-microglobulin fibrillation. The control peptide AT1 had no effect on transthyretin or β2-microglobulin fibrillation, and the three positive control molecules diflunisal, flufenamic acid, and mecloflenamic acid had modest effects on transthyretin and β2-microglobulin fibrillation. Each assay was done in triplicate and the error bars indicate the standard deviation. The results demonstrated the effectiveness of individual interactive domains in αB crystallin on modulation of fibril formation in vitro.
At a concentration of 9.2μM peptide (α-synuclein:peptide ratio of 5:1), the full-length ST, DR, HG, LT, and ER peptides, the truncated DR peptides DRFS, RFSV, FSVN, SVNL, VNLD, NLDV, DVKH, and KHFS, and the truncated HG peptides, HGKH, GKHE, HEER, EERQ, ERQD, and RQDE had the strongest effect on α-synuclein fibrillation and had ThT fluorescence values between 0.0 and 0.75 of the ThT fluorescence of α-synuclein alone (Figure 2). At the same concentration, the truncated HG peptide increased α-synuclein fibrillation and ThT fluorescence values 1.61 times the ThT fluorescence of α-synuclein alone. The full-length FI peptide, the truncated DR peptides, LDVK and VKHF and the truncated HG peptide KHEE had no effect on α-synuclein fibrillation and had ThT fluorescence values similar to the control peptide AT1. At a concentration of 46μM peptide (α-synuclein:peptide ratio of 1:1), the full-length DR, HG, LT, and ER peptides, the truncated DR peptides DRFS, RFSV, FSVN, SVNL, VNLD, NLDV, LDVK, DVKH, and KHFS, and the truncated HG peptides, HGKH, KHEE, and HEER had the strongest effect on α-synuclein fibrillation and had ThT fluorescence values between 0.00 and 0.49 of the ThT fluorescence of α-synuclein alone. At the same concentration, full-length ST peptide increased α-synuclein fibrillation and a ThT fluorescence value 4.46 times the ThT fluorescence of α-synuclein alone. The full-length FI peptide, the truncated DR peptide VKHF and the truncated HG peptides GKHE, EERQ, ERQD, and RQDE had no effect on α-synuclein fibrillation and had ThT fluorescence values similar to the control peptide AT1.
The full-length ST and HG peptides, the truncated DR peptide FSVN, and the truncated HG peptide ERQD were the strongest inhibitors of Aβ1–42 fibrillation. The full-length DR peptide, the truncated DR peptides DRFS, RFSV, FSVN, SVNL, VNLD, NLDV, DVKH, and KHFS, and the truncated HG peptide HGKH were the strongest inhibitors of α-synuclein fibrillation (Figure 2).
At a concentration of 3.2μM peptide (transthyretin:peptide ratio of 5:1), the full-length ST, DR, and HG peptides, the truncated DR peptides DRFS, RFSV, FSVN, SVNL, VNLD, NLDV, LDVK, DVKH, VKHF, and KHFS, and the truncated HG peptides HGKH, GKHE, KHEE, HEER, EERQ, ERQD, and RQDE had the strongest effect on transthyretin fibrillation and had ThT fluorescence values between 0.14 and 0.67 of the ThT fluorescence of transthyretin alone (Figure 3). The full-length FI, LT and ER peptides had little or no effect on transthyretin fibrillation and had ThT fluorescence values similar to the control peptide AT1. At a concentration of 16μM peptide (transthyretin:peptide ratio of 1:1), the full-length DR, HG, and ER peptides, the truncated DR peptides DRFS, RFSV, FSVN, SVNL, VNLD, NLDV, LDVK, DVKH, VKHF, and KHFS, and the truncated HG peptides HGKH, GKHE, KHEE, HEER, EERQ, ERQD, and RQDE had the strongest effect on transthyretin fibrillation and had ThT fluorescence values between 0.0 and 0.63 of the ThT fluorescence of transthyretin alone. The full-length ST, FI, and LT peptides had little or no effect on transthyretin fibrillation and had ThT fluorescence values similar to the control peptide AT1. No αB crystallin peptides increased transthyretin fibrillation at the measured concentrations.
At a concentration of 5.8μM peptide (β2-microglobulin:peptide ratio of 5:1), the full-length ST, DR, HG, LT, and ER peptides, the truncated DR peptides FSVN, SVNL, VNLD, NLDV, LDVK, and DVKH, and the truncated HG peptides HGKH, GKHE, KHEE, HEER, EERQ, and RQDE had the strongest effect on β2-microglobulin fibrillation and had ThT fluorescence values between 0.16 and 0.62 of the ThT fluorescence of β2-microglobulin alone (Figure 3). The full-length FI peptide, the truncated DR peptides, DRFS, VKHF, and KHFS increased β2-microglobulin fibrillation and had ThT fluorescence values between 1.28 and 2.17 of the ThT fluorescence of β2-microglobulin alone. The truncated DR peptide RFSV and the truncated HG peptide ERQD had no effect on β2-microglobulin fibrillation and had ThT fluorescence values similar to the control peptide AT1. At a concentration of 29μM peptide (β2-microglobulin:peptide ratio of 1:1), the full-length ST, DR, HG, LT, and ER peptides, the truncated DR peptides FSVN and NLDV, and the truncated HG peptides HGKH, GKHE, KHEE, HEER, EERQ, and RQDE had the strongest effect on β2-microglobulin fibrillation and had ThT fluorescence values between 0.0 and 0.65 of the ThT fluorescence of β2-microglobulin alone. The full-length FI peptide, the truncated DR peptides, DRFS, RFSV, SVNL, DVKH, and KHFS increased β2-microglobulin fibrillation and had ThT fluorescence values between 1.22 and 2.29 of the ThT fluorescence of β2-microglobulin alone. The truncated DR peptide VNLD, LDVK, and VKHF, and the truncated HG peptide ERQD had no effect on β2-microglobulin fibrillation and had ThT fluorescence values similar to the control peptide AT1.
The full-length HG peptide and the truncated HG peptides HGKH, EERQ, and RQDE were the strongest inhibitors of transthyretin fibrillation. The full-length DR peptide and the truncated HG peptides HGKH, HEER, and EERQ were the strongest inhibitors of β2-microglobulin fibrillation (Figure 3).
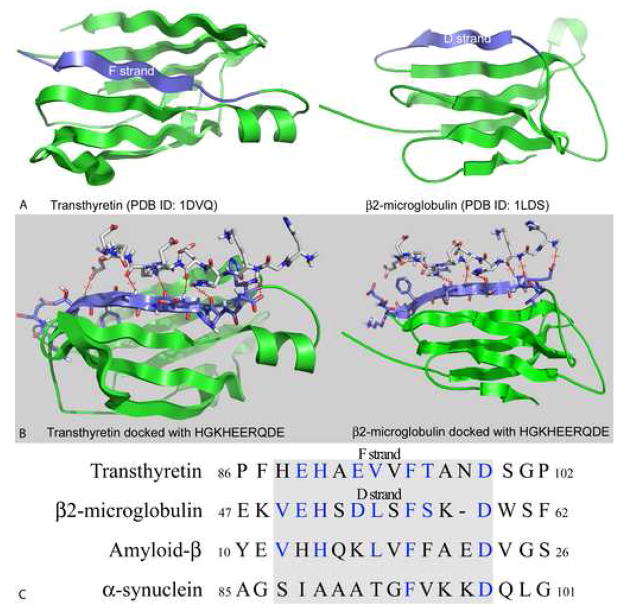
A 3D model of the interactions between the αB crystallin peptides 73DRFSVNLDVKHFS85 which forms the β3 strand and 101HGKHEERQDE110 which forms part of the loop connecting the β5 and β7 strands on the exposed surface of the α crystallin core domain was determined by docking the two peptides with the crystal structures of transthyretin and β2-micrglobulin (Figure 4A). In the transthyretin model, residues Ser-76, Asn-78, and Asp-80 from the 73DRFSVNLDVKHFS85 peptide interacted with residues Glu-83, Val-85, Thr-87, and Asp-90 in transthyretin (Figure 4B). Similarly, residues His-104, Glu-105, and Arg-107 from the 101HGKHEERQDE110 peptide interacted with residues His-79, His-81, Glu-83, and Val-85 in transthyretin (Figure 4B). The sequence 79HEHAEVVFTAND90, which contains these residues forms the amyloidogenic F strand in transthyretin (Figure 4A). In the β2-microglobulin model, residues Phe-75, Val-77, and Leu-79 from the 73DRFSVNLDVKHFS85 peptide interacted with residues Glu-51, Ser-53, Asp-54, Leu-55, and Phe-57 in β2-microglobulin (Figure 4B). Similarly, residues His-101, Lys-103, His-104, Glu-105, and Arg-107 from the 101HGKHEERQDE110 peptide interacted with residues Glu-51, His-52, Ser-53, and Leu-55 in β2-microglobulin. The sequence 50VEHSDLSFSKD60, which contains these residues forms the amyloidogenic D strand in β2-microglobulin (Figure 4A). Direct interactions between residues of the αB crystallin peptides and residues of the amyloidogenic fibril forming β strands of transthyretin and β2-microglobulin can be expected to prevent abnormal self-interactions between transthyretin and β2-microglobulin molecules that result in the formation of β strand rich amyloid-like fibrils. The absence of X-ray crystal or NMR structures for Aβ1–42 and α-synuclein prevented docking studies with these proteins. Alignment of the primary sequences of Aβ1–42 and α-synuclein with the primary sequences of transthyretin and β2-micrglobulin identified the residues 12VHHQKLVFFAED23 and 87SIAAATGFVKKD98 in Aβ1–42 and α-synuclein respectively that were similar in primary sequence to the amyloidogenic F and D β strands of transthyretin and β2-microglobulin (Figure 4C). The inhibitory effect of the αB crystallin peptides on the fibrillation of all four amyloid target proteins and the sequence similarity of the interactive amyloidogenic regions of the four target proteins is consistent with a common mechanism of fibril inhibition by αB crystallin peptides.
Figure 4.
Structural models of transthyretin and β2-microglobulin, protein-peptide docking models and sequence analysis. A) Ribbon diagrams of the monomeric subunits of transthyretin and β2-microglobulin are shown in green. The amyloidogenic β strands, F and D in transthyretin and β2-microgloublin respectively are in blue (McParland et al., 2000; Serag, Altenbach, Gingery, Hubbell, & Yeates, 2002; Thirumalai, Klimov, & Dima, 2003; Trinh, Smith, Kalverda, Phillips, & Radford, 2002). B) The 101HGKHEERQDE110 peptide from the loop region connecting β5 and β7 in the α crystallin core domain of human αB crystallin was docked with the crystal structures of transthyretin (left) and β2-microglobulin (right) using the ClusPro molecular docking program (Comeau, Gatchell, Vajda, & Camacho, 2004a, 2004b). Residues of the 101HGKHEERQDE110 sequence interact with residues of the F and D β strands of transthyretin and β2-microglobulin respectively. Interactions with the same residues in transthyretin and β2-microglobulin were observed when the 73DRFSVNLDVKHFS85 peptide which forms the β3 strand in the α crystallin core domain of αB crystallin was docked with the crystal structures of transthyretin and β2-microglobulin (data not shown). C) Sequence alignment of the amyloidogenic regions of transthyretin and β2-microglobulin with the primary sequences of Aβ1–42 and α-synuclein. The amyloidogenic regions of transthyretin and β2-microglobulin were aligned with the primary sequences of Aβ1–42 and α-synuclein using the DOTTER program (Miake, Mizusawa, Iwatsubo, & Hasegawa, 2002; Serag, Altenbach, Gingery, Hubbell, & Yeates, 2002; Sonnhammer & Durbin, 1995; Thirumalai, Klimov, & Dima, 2003; Trinh, Smith, Kalverda, Phillips, & Radford, 2002). Conserved residues are in blue. The grey box indicates encloses the residues that comprise the F and D β strands in transthyretin and β2-microglobulin. Residues 88–99, 49–59, 12–23, and 87–98 are the amyloidogenic fibril forming regions in transthyretin, β2-microglobulin, Aβ1–42, and α-synuclein respectively (Balbach et al., 2000; McParland et al., 2000; Miake, Mizusawa, Iwatsubo, & Hasegawa, 2002; Morimoto et al., 2004; Serag, Altenbach, Gingery, Hubbell, & Yeates, 2002; Thirumalai, Klimov, & Dima, 2003; Trinh, Smith, Kalverda, Phillips, & Radford, 2002). Structural analysis identified key interactions between amyloidogenic proteins and exposed peptides on the surface of the α crystallin core domain of human αB crystallin that account for modulation of fibril formation observed in the Thioflavin T assembly assays.
Discussion
Five bioactive peptides synthesized on the basis of interactive sequences from the sHSP and molecular chaperone human αB crystallin inhibited or promoted the fibrillation of Aβ1–42, α-synuclein, transthyretin, and β2-microglobulin. The inhibitory effects of the αB crystallin peptides on Aβ1–42, α-synuclein, and β2-microglobulin fibrillation were consistent with previous studies on the effects of full-length αB crystallin and peptide fragments of αA crystallin on Aβ1–42, α-synuclein, and β2-microglobulin fibrillation (Lee, Carson, Rice-Ficht, & Good, 2006; Narayanan, Kamps, Boelens, & Reif, 2006; Raman et al., 2005; Rekas et al., 2004; Santhoshkumar & Sharma, 2004; Stege et al., 1999; Wilhelmus, Boelens, Otte-Holler, Kamps, de Waal et al., 2006). The interactive sequences 73DRFSVNLDVKHFS85 and 101HGKHEERQDE110 in the conserved α crystallin core domain of human αB crystallin reduced the fibrillation of all four target proteins by >50%. The results confirmed previous studies that demonstrated the importance of the 73DRFSVNLDVKHFS85 peptide as a chaperone sequence that inhibited the aggregation of structurally diverse target proteins including lens crystallins, alcohol dehydrogenase, citrate synthase, and FGF (Ghosh, Estrada, & Clark, 2005; Ghosh, Estrada, Houck, & Clark, 2006; Ghosh, Shenoy, & Clark, 2007b). Systematic truncation of the 73DRFSVNLDVKHFS85 and 101HGKHEERQDE110 peptides resulted in the identification of new bioactive peptide inhibitors, FSVN, NLDV, HGKH, and HEER, which reduced fibrillation by >75% as measured by Thioflavin T fluorescence. The identification of these peptides as potent fibril inhibitors was supported by protein-peptide docking studies which identified residues Phe-75, Ser-76, Val-77, Asn-78, Leu-79, and Asp-80 from 73DRFSVNLDVKHFS85 and residues His-101, Lys-103, His-104, Glu-105, and Arg-107 from 101HGKHEERQDE110 as residues that interacted directly with the fibril forming F and D β strands of transthyretin and β2-microglobulin (Figure 4).
The oligomerization and aggregation of amyloidogenic proteins including Aβ proceeds through the formation of various intermediates including dimers, tetramers, soluble oligomers, protofibrils, fibrils, unstructured aggregates, and plaques (Bucciantini et al., 2002; Glabe, 2006; J. Hardy & Selkoe, 2002; J. A. Hardy & Higgins, 1992; Selkoe, 2001). The rate and amount of formation of each intermediate depends on environmental factors including pH, metals, and the presence of stress response proteins like molecular chaperones (Alexandrescu, 2005; Dedmon, Christodoulou, Wilson, & Dobson, 2005; Harper, Wong, Lieber, & Lansbury, 1999). Although, molecular chaperones generally inhibit protein aggregation, abnormal interactions between chaperones and substrate proteins can result in increased aggregation and disease (Liu et al., 2006; Vicart et al., 1998). In the fibril assays reported in this paper, most full-length and truncated αB crystallin peptides inhibited fibrillation. However, the αB crystallin peptides, STSLSPFYLRPPSFLRAP, FISREFHR, LTITSSLSDGV, ERTIPITRE, DRFS, RFSV, DVKH, KHFS, HEER, EERQ, and RQDE increased fibril formation by 14–129%. The fibril promoting effect depended on peptide concentration and the ratio of peptide to target protein used in the assays. The results demonstrated that αB crystallin contains both fibril inhibiting and promoting interactive sequences which may explain the seemingly contradictory effects of αB crystallin on Aβ fibrillation and toxicity reported previously. A previous study demonstrated that the interactive peptides FISREFHR, LTITSSLSDGV, and ERTIPITRE in αB crystallin modulate the assembly of microtubules in vitro (Ghosh, Houck, & Clark, 2007a). The ability of αB crystallin to modulate both Aβ fibrillation and microtubule assembly and disassembly suggests that sHSPs may be a link between the Aβ cascade pathway and the tau-tubulin pathway which results in the formation of neurofibrillary tangles in AD (Ghosh, Houck, & Clark, 2007a).
The results illustrate an important finding that sHSPs like αB crystallin may play a more important and direct role in the pathophysiology of AD than previously thought (Lee, Carson, Rice-Ficht, & Good, 2005, 2006; Liang, 2000; Raman et al., 2005; Santhoshkumar & Sharma, 2004; Stege et al., 1999; Wilhelmus, Boelens, Otte-Holler, Kamps, de Waal et al., 2006). Although, self-interactions of amyloidogenic proteins result in fibril formation, smaller, soluble and more toxic oligomeric intermediates may be the pathological factors for aggregation diseases including AD and PD (McParland et al., 2000; Mornon et al., 1998; Nelson & Eisenberg, 2006; Schormann, Murrell, & Benson, 1998; Serag, Altenbach, Gingery, Hubbell, & Yeates, 2002; Thirumalai, Klimov, & Dima, 2003; Trinh, Smith, Kalverda, Phillips, & Radford, 2002). Our results indicate that αB crystallin peptides modulate the interactions between amyloidogenic proteins to increase or decrease fibrillation. Under conditions in which increasing Aβ fibrillation decreases the levels of more toxic soluble oligomers of Aβ and reduces functional deficits in transgenic AD mice (Cheng et al., 2007; Kirkitadze, Bitan, & Teplow, 2002) peptides of αB crystallin can still have a protective action. Future studies will characterize the interactions between αB crystallin peptides and soluble oligomeric intermediates of amyloidogenic proteins including Aβ1–42, α-synuclein, transthyretin, and β2-microglobulin.
sHSPs and amyloidogenic proteins including transthyretin and β2-microglobulin have similar 3D structures consisting of an immunoglobulin-like fold composed of two anti-parallel β sheets arranged in a compact β sandwich (Mornon et al., 1998; Nelson & Eisenberg, 2006; Sunde et al., 1997). Abnormal interactions of β strands F (79HEHAEVVFTAND90) and D (50VEHSDLSFSKD60) in transthyretin and β2-microglobulin respectively (Figure 4) result in the formation of β strand rich amyloid-like fibrils (McParland et al., 2000; Mornon et al., 1998; Nelson & Eisenberg, 2006; Schormann, Murrell, & Benson, 1998; Serag, Altenbach, Gingery, Hubbell, & Yeates, 2002; Thirumalai, Klimov, & Dima, 2003; Trinh, Smith, Kalverda, Phillips, & Radford, 2002). When the αB crystallin 73DRFSVNLDVKHFS85 and 101HGKHEERQDE110 peptides were docked with the structures of transthyretin and β2-microglobulin, residues from both αB crystallin peptides interacted with residues in the F (79HEHAEVVFTAND90) and D (50VEHSDLSFSKD60) strands of transthyretin and β2-microglobulin (Figure 4). Together with the Thioflavin T assays, these results suggest that surface exposed residues in the 73DRFSVNLDVKHFS85 and 101HGKHEERQDE110 sequences in the α crystallin core domain of human αB crystallin interact with surface exposed residues in the F and D β strands of transthyretin and β2-microglobulin respectively to inhibit fibrillation. The absence of crystal structures for Aβ1–42 and α-synuclein prevented modeling of the interactions between the αB crystallin peptides and Aβ1–42 and α-synuclein. Comparison of the primary sequences of transthyretin and β2-microglobulin with the primary sequences of Aβ1–42 and α-synuclein identified residues 14–23 in Aβ1–42 and 89–98 in α-synuclein as sites of interaction for the αB crystallin peptides (Figure 4C). The identification of these residues in Aβ1–42 and α-synuclein as sites of interaction for the αB crystallin peptide inhibitors was consistent with previous reports that identified these same residues as amyloidogenic residues in Aβ1–42 and α-synuclein (Balbach et al., 2000; Miake, Mizusawa, Iwatsubo, & Hasegawa, 2002; Morimoto et al., 2004). Further support for the inhibitory action of the αB crystallin peptides at these sites comes from a recent NMR study that demonstrated direct interactions between human αB crystallin and residues 17–21 of Aβ1–42 (Narayanan, Kamps, Boelens, & Reif, 2006). Based on the fibril assays, protein-peptide docking results, and sequence analysis, it is hypothesized that the protective effects of the sHSP αB crystallin results from interactions with Aβ1–42, α-synuclein, transthyretin, and β2-microglobulin via residues in the 73DRFSVNLDVKHFS85 and 101HGKHEERQDE110 sequences on the surface interface of the α crystallin core domain.
Development of therapeutics for AD and PD is focused primarily on the amyloid cascade pathway, which proceeds through stages of unfolding, aggregation, oligomerization, and fibrillation of amyloidogenic proteins (Bucciantini et al., 2002; Glabe, 2006; J. Hardy & Selkoe, 2002; J. A. Hardy & Higgins, 1992; Selkoe, 2001). Tramiprosate and NAP, two molecules that disrupt the amyloid cascade pathway (monomer → oligomer → fibril) by preventing the formation of β sheet fibrils have been most successful in clinical trials to date (Gervais et al., 2007; Gozes et al., 2005). The target specificity (Aβ1–42 only) of these two molecules limits their potential use as therapeutics in other non-AD amyloidoses. The results of the current study identified interactive sites in bioactive peptides that use similar mechanisms of action to target the toxic intermediate states of four distinct amyloidogenic proteins and may serve as the basis for a new class of disease modifying therapeutics for a variety of amyloidoses.
In this report, Thioflavin T fluorescence assays, protein-peptide docking, and sequence analysis were conducted with full-length and truncated interactive sequences from the molecular chaperone, human αB crystallin to identify peptides that that interact with and inhibit or promote the fibrillation of the important amyloidogenic proteins: Aβ1–42, α-synuclein, transthyretin, and β2-microglobulin. The αB crystallin peptides identified in this report inhibited or promoted fibrillation at low concentrations indicating excellent potential for the rational design of peptidic and peptidomimetic inhibitors targeting the toxic intermediates of amyloidogenic proteins.
Acknowledgments
This work was supported by grant EY04542 from the National Eye Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aisen PS. The development of anti-amyloid therapy for Alzheimer’s disease: from secretase modulators to polymerisation inhibitors. CNS Drugs. 2005;19(12):989–996. doi: 10.2165/00023210-200519120-00002. [DOI] [PubMed] [Google Scholar]
- Alexandrescu AT. Amyloid accomplices and enforcers. Protein Sci. 2005;14(1):1–12. doi: 10.1110/ps.04887005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashur-Fabian O, Segal-Ruder Y, Skutelsky E, Brenneman DE, Steingart RA, Giladi E, et al. The neuroprotective peptide NAP inhibits the aggregation of the beta-amyloid peptide. Peptides. 2003;24(9):1413–1423. doi: 10.1016/j.peptides.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Balbach JJ, Ishii Y, Antzutkin ON, Leapman RD, Rizzo NW, Dyda F, et al. Amyloid fibril formation by A beta 16–22, a seven-residue fragment of the Alzheimer’s beta-amyloid peptide, and structural characterization by solid state NMR. Biochemistry. 2000;39(45):13748–13759. doi: 10.1021/bi0011330. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya J, Padmanabha Udupa EG, Wang J, Sharma KK. Mini-alphaB-crystallin: a functional element of alphaB-crystallin with chaperone-like activity. Biochemistry. 2006;45(9):3069–3076. doi: 10.1021/bi0518141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucciantini M, Giannoni E, Chiti F, Baroni F, Formigli L, Zurdo J, et al. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature. 2002;416(6880):507–511. doi: 10.1038/416507a. [DOI] [PubMed] [Google Scholar]
- Cheng IH, Scearce-Levie K, Legleiter J, Palop JJ, Gerstein H, Bien-Ly N, et al. Accelerating amyloid-beta fibrillization reduces oligomer levels and functional deficits in Alzheimer disease mouse models. J Biol Chem. 2007 doi: 10.1074/jbc.M701078200. [DOI] [PubMed] [Google Scholar]
- Comeau SR, Gatchell DW, Vajda S, Camacho CJ. ClusPro: a fully automated algorithm for protein-protein docking. Nucleic Acids Res. 2004a;32(Web Server issue):W96–99. doi: 10.1093/nar/gkh354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comeau SR, Gatchell DW, Vajda S, Camacho CJ. ClusPro: an automated docking and discrimination method for the prediction of protein complexes. Bioinformatics. 2004b;20(1):45–50. doi: 10.1093/bioinformatics/btg371. [DOI] [PubMed] [Google Scholar]
- Dabir DV, Trojanowski JQ, Richter-Landsberg C, Lee VM, Forman MS. Expression of the small heat-shock protein alphaB-crystallin in tauopathies with glial pathology. Am J Pathol. 2004;164(1):155–166. doi: 10.1016/s0002-9440(10)63106-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De S, Rabin DM, Salero E, Lederman PL, Temple S, Stern JH. Human retinal pigment epithelium cell changes and expression of alphaB-crystallin: a biomarker for retinal pigment epithelium cell change in age-related macular degeneration. Arch Ophthalmol. 2007;125(5):641–645. doi: 10.1001/archopht.125.5.641. [DOI] [PubMed] [Google Scholar]
- Dedmon MM, Christodoulou J, Wilson MR, Dobson CM. Heat shock protein 70 inhibits alpha-synuclein fibril formation via preferential binding to prefibrillar species. J Biol Chem. 2005;280(15):14733–14740. doi: 10.1074/jbc.M413024200. [DOI] [PubMed] [Google Scholar]
- Dobson CM. The structural basis of protein folding and its links with human disease. Philos Trans R Soc Lond B Biol Sci. 2001;356(1406):133–145. doi: 10.1098/rstb.2000.0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson CM. Principles of protein folding, misfolding and aggregation. Semin Cell Dev Biol. 2004;15(1):3–16. doi: 10.1016/j.semcdb.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Gervais F, Paquette J, Morissette C, Krzywkowski P, Yu M, Azzi M, et al. Targeting soluble Abeta peptide with Tramiprosate for the treatment of brain amyloidosis. Neurobiol Aging. 2007;28(4):537–547. doi: 10.1016/j.neurobiolaging.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Ghosh JG, Clark JI. Insights into the domains required for dimerization and assembly of human alphaB crystallin. Protein Sci. 2005;14(3):684–695. doi: 10.1110/ps.041152805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh JG, Estrada MR, Clark JI. Interactive Domains for Chaperone Activity in the Small Heat Shock Protein, Human alphaB Crystallin. Biochemistry. 2005;44(45):14854–14869. doi: 10.1021/bi0503910. [DOI] [PubMed] [Google Scholar]
- Ghosh JG, Estrada MR, Clark JI. Structure-based analysis of the beta8 interactive sequence of human alphaB crystallin. Biochemistry. 2006;45(32):9878–9886. doi: 10.1021/bi060970k. [DOI] [PubMed] [Google Scholar]
- Ghosh JG, Estrada MR, Houck SA, Clark JI. The function of the beta3 interactive domain in the small heat shock protein and molecular chaperone, human alphaB crystallin. Cell Stress Chaperones. 2006;11(2):187–197. doi: 10.1379/CSC-186.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh JG, Houck SA, Clark JI. Interactive Domains in the Molecular Chaperone Human alphaB Crystallin Modulate Microtubule Assembly and Disassembly. PLoS ONE. 2007a;2:e498. doi: 10.1371/journal.pone.0000498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh JG, Houck SA, Clark JI. Interactive sequences in the stress protein and molecular chaperone human alphaB crystallin recognize and modulate the assembly of filaments. Int J Biochem Cell Biol. 2007b doi: 10.1016/j.biocel.2007.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh JG, Shenoy AK, Jr, Clark JI. N- and C-Terminal Motifs in Human alphaB Crystallin Play an Important Role in the Recognition, Selection, and Solubilization of Substrates. Biochemistry. 2006;45(46):13847–13854. doi: 10.1021/bi061471m. [DOI] [PubMed] [Google Scholar]
- Ghosh JG, Shenoy AK, Jr, Clark JI. Interactions between important regulatory proteins and human alphaB crystallin. Biochemistry. 2007a;46(21):6308–6317. doi: 10.1021/bi700149h. [DOI] [PubMed] [Google Scholar]
- Ghosh JG, Shenoy AK, Jr, Clark JI. Interactions between Important Regulatory Proteins and Human alphaB Crystallin. Biochemistry. 2007b doi: 10.1021/bi700149h. [DOI] [PubMed] [Google Scholar]
- Glabe CG. Common mechanisms of amyloid oligomer pathogenesis in degenerative disease. Neurobiol Aging. 2006;27(4):570–575. doi: 10.1016/j.neurobiolaging.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Goldstein LE, Muffat JA, Cherny RA, Moir RD, Ericsson MH, Huang X, et al. Cytosolic beta-amyloid deposition and supranuclear cataracts in lenses from people with Alzheimer’s disease. Lancet. 2003;361(9365):1258–1265. doi: 10.1016/S0140-6736(03)12981-9. [DOI] [PubMed] [Google Scholar]
- Gorevic PD, Casey TT, Stone WJ, DiRaimondo CR, Prelli FC, Frangione B. Beta-2 microglobulin is an amyloidogenic protein in man. J Clin Invest. 1985;76(6):2425–2429. doi: 10.1172/JCI112257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozes I, Morimoto BH, Tiong J, Fox A, Sutherland K, Dangoor D, et al. NAP: research and development of a peptide derived from activity-dependent neuroprotective protein (ADNP) CNS Drug Rev. 2005;11(4):353–368. doi: 10.1111/j.1527-3458.2005.tb00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Harper JD, Wong SS, Lieber CM, Lansbury PT., Jr Assembly of A beta amyloid protofibrils: an in vitro model for a possible early event in Alzheimer’s disease. Biochemistry. 1999;38(28):8972–8980. doi: 10.1021/bi9904149. [DOI] [PubMed] [Google Scholar]
- Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381(6583):571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- Haslbeck M, Franzmann T, Weinfurtner D, Buchner J. Some like it hot: the structure and function of small heat-shock proteins. Nat Struct Mol Biol. 2005;12(10):842–846. doi: 10.1038/nsmb993. [DOI] [PubMed] [Google Scholar]
- Hatters DM, Lindner RA, Carver JA, Howlett GJ. The molecular chaperone, alpha-crystallin, inhibits amyloid formation by apolipoprotein C-II. J Biol Chem. 2001;276(36):33755–33761. doi: 10.1074/jbc.M105285200. [DOI] [PubMed] [Google Scholar]
- Hurshman AR, White JT, Powers ET, Kelly JW. Transthyretin aggregation under partially denaturing conditions is a downhill polymerization. Biochemistry. 2004;43(23):7365–7381. doi: 10.1021/bi049621l. [DOI] [PubMed] [Google Scholar]
- Ivanova MI, Sawaya MR, Gingery M, Attinger A, Eisenberg D. An amyloid-forming segment of beta2-microglobulin suggests a molecular model for the fibril. Proc Natl Acad Sci U S A. 2004;101(29):10584–10589. doi: 10.1073/pnas.0403756101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PT, Brown MN, Pulliam BC, Anderson DH, Johnson LV. Synaptic pathology, altered gene expression, and degeneration in photoreceptors impacted by drusen. Invest Ophthalmol Vis Sci. 2005;46(12):4788–4795. doi: 10.1167/iovs.05-0767. [DOI] [PubMed] [Google Scholar]
- Kirkitadze MD, Bitan G, Teplow DB. Paradigm shifts in Alzheimer’s disease and other neurodegenerative disorders: the emerging role of oligomeric assemblies. J Neurosci Res. 2002;69(5):567–577. doi: 10.1002/jnr.10328. [DOI] [PubMed] [Google Scholar]
- Klabunde T, Petrassi HM, Oza VB, Raman P, Kelly JW, Sacchettini JC. Rational design of potent human transthyretin amyloid disease inhibitors. Nat Struct Biol. 2000;7(4):312–321. doi: 10.1038/74082. [DOI] [PubMed] [Google Scholar]
- Lee S, Carson K, Rice-Ficht A, Good T. Hsp20, a novel alpha-crystallin, prevents Abeta fibril formation and toxicity. Protein Sci. 2005;14(3):593–601. doi: 10.1110/ps.041020705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Carson K, Rice-Ficht A, Good T. Small heat shock proteins differentially affect Abeta aggregation and toxicity. Biochem Biophys Res Commun. 2006;347(2):527–533. doi: 10.1016/j.bbrc.2006.06.128. [DOI] [PubMed] [Google Scholar]
- Liang JJ. Interaction between beta-amyloid and lens alphaB-crystallin. FEBS Lett. 2000;484(2):98–101. doi: 10.1016/s0014-5793(00)02136-0. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang X, Luo L, Wu M, Zeng R, Cheng G, et al. A novel alphaB-crystallin mutation associated with autosomal dominant congenital lamellar cataract. Invest Ophthalmol Vis Sci. 2006;47(3):1069–1075. doi: 10.1167/iovs.05-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McParland VJ, Kad NM, Kalverda AP, Brown A, Kirwin-Jones P, Hunter MG, et al. Partially unfolded states of beta(2)-microglobulin and amyloid formation in vitro. Biochemistry. 2000;39(30):8735–8746. doi: 10.1021/bi000276j. [DOI] [PubMed] [Google Scholar]
- Miake H, Mizusawa H, Iwatsubo T, Hasegawa M. Biochemical characterization of the core structure of alpha-synuclein filaments. J Biol Chem. 2002;277(21):19213–19219. doi: 10.1074/jbc.M110551200. [DOI] [PubMed] [Google Scholar]
- Morimoto A, Irie K, Murakami K, Masuda Y, Ohigashi H, Nagao M, et al. Analysis of the secondary structure of beta-amyloid (Abeta42) fibrils by systematic proline replacement. J Biol Chem. 2004;279(50):52781–52788. doi: 10.1074/jbc.M406262200. [DOI] [PubMed] [Google Scholar]
- Mornon JP, Halaby D, Malfois M, Durand P, Callebaut I, Tardieu A. alpha-Crystallin C-terminal domain: on the track of an Ig fold. Int J Biol Macromol. 1998;22(3–4):219–227. doi: 10.1016/s0141-8130(98)00019-1. [DOI] [PubMed] [Google Scholar]
- Naiki H, Higuchi K, Hosokawa M, Takeda T. Fluorometric determination of amyloid fibrils in vitro using the fluorescent dye, thioflavin T1. Anal Biochem. 1989;177(2):244–249. doi: 10.1016/0003-2697(89)90046-8. [DOI] [PubMed] [Google Scholar]
- Nakata K, Crabb JW, Hollyfield JG. Crystallin distribution in Bruch’s membrane-choroid complex from AMD and age-matched donor eyes. Exp Eye Res. 2005;80(6):821–826. doi: 10.1016/j.exer.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Narayanan S, Kamps B, Boelens WC, Reif B. alphaB-crystallin competes with Alzheimer’s disease beta-amyloid peptide for peptide-peptide interactions and induces oxidation of Abeta-Met35. FEBS Lett. 2006;580(25):5941–5946. doi: 10.1016/j.febslet.2006.09.063. [DOI] [PubMed] [Google Scholar]
- Nelson R, Eisenberg D. Recent atomic models of amyloid fibril structure. Curr Opin Struct Biol. 2006;16(2):260–265. doi: 10.1016/j.sbi.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Raman B, Ban T, Sakai M, Pasta SY, Ramakrishna T, Naiki H, et al. AlphaB-crystallin, a small heat-shock protein, prevents the amyloid fibril growth of an amyloid beta-peptide and beta2-microglobulin. Biochem J. 2005;392(Pt 3):573–581. doi: 10.1042/BJ20050339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekas A, Adda CG, Andrew Aquilina J, Barnham KJ, Sunde M, Galatis D, et al. Interaction of the molecular chaperone alphaB-crystallin with alpha-synuclein: effects on amyloid fibril formation and chaperone activity. J Mol Biol. 2004;340(5):1167–1183. doi: 10.1016/j.jmb.2004.05.054. [DOI] [PubMed] [Google Scholar]
- Renkawek K, Bosman GJ, de Jong WW. Expression of small heat-shock protein hsp 27 in reactive gliosis in Alzheimer disease and other types of dementia. Acta Neuropathol (Berl) 1994;87(5):511–519. doi: 10.1007/BF00294178. [DOI] [PubMed] [Google Scholar]
- Renkawek K, de Jong WW, Merck KB, Frenken CW, van Workum FP, Bosman GJ. alpha B-crystallin is present in reactive glia in Creutzfeldt-Jakob disease. Acta Neuropathol (Berl) 1992;83(3):324–327. doi: 10.1007/BF00296796. [DOI] [PubMed] [Google Scholar]
- Renkawek K, Stege GJ, Bosman GJ. Dementia, gliosis and expression of the small heat shock proteins hsp27 and alpha B-crystallin in Parkinson’s disease. Neuroreport. 1999;10(11):2273–2276. doi: 10.1097/00001756-199908020-00009. [DOI] [PubMed] [Google Scholar]
- Santhoshkumar P, Sharma KK. Inhibition of amyloid fibrillogenesis and toxicity by a peptide chaperone. Mol Cell Biochem. 2004;267(1–2):147–155. doi: 10.1023/b:mcbi.0000049373.15558.b8. [DOI] [PubMed] [Google Scholar]
- Schormann N, Murrell JR, Benson MD. Tertiary structures of amyloidogenic and non-amyloidogenic transthyretin variants: new model for amyloid fibril formation. Amyloid. 1998;5(3):175–187. doi: 10.3109/13506129809003843. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81(2):741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Folding proteins in fatal ways. Nature. 2003;426(6968):900–904. doi: 10.1038/nature02264. [DOI] [PubMed] [Google Scholar]
- Serag AA, Altenbach C, Gingery M, Hubbell WL, Yeates TO. Arrangement of subunits and ordering of beta-strands in an amyloid sheet. Nat Struct Biol. 2002;9(10):734–739. doi: 10.1038/nsb838. [DOI] [PubMed] [Google Scholar]
- Sonnhammer EL, Durbin R. A dot-matrix program with dynamic threshold control suited for genomic DNA and protein sequence analysis. Gene. 1995;167(1–2):GC1–10. doi: 10.1016/0378-1119(95)00714-8. [DOI] [PubMed] [Google Scholar]
- Stege GJ, Renkawek K, Overkamp PS, Verschuure P, van Rijk AF, Reijnen-Aalbers A, et al. The molecular chaperone alphaB-crystallin enhances amyloid beta neurotoxicity. Biochem Biophys Res Commun. 1999;262(1):152–156. doi: 10.1006/bbrc.1999.1167. [DOI] [PubMed] [Google Scholar]
- Sunde M, Serpell LC, Bartlam M, Fraser PE, Pepys MB, Blake CC. Common core structure of amyloid fibrils by synchrotron X-ray diffraction. J Mol Biol. 1997;273(3):729–739. doi: 10.1006/jmbi.1997.1348. [DOI] [PubMed] [Google Scholar]
- Thirumalai D, Klimov DK, Dima RI. Emerging ideas on the molecular basis of protein and peptide aggregation. Curr Opin Struct Biol. 2003;13(2):146–159. doi: 10.1016/s0959-440x(03)00032-0. [DOI] [PubMed] [Google Scholar]
- Trinh CH, Smith DP, Kalverda AP, Phillips SE, Radford SE. Crystal structure of monomeric human beta-2-microglobulin reveals clues to its amyloidogenic properties. Proc Natl Acad Sci U S A. 2002;99(15):9771–9776. doi: 10.1073/pnas.152337399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicart P, Caron A, Guicheney P, Li Z, Prevost MC, Faure A, et al. A missense mutation in the alphaB-crystallin chaperone gene causes a desmin-related myopathy. Nat Genet. 1998;20(1):92–95. doi: 10.1038/1765. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Townsend M, Podlisny MB, Shankar GM, Fadeeva JV, Agnaf OE, et al. Certain inhibitors of synthetic amyloid beta-peptide (Abeta) fibrillogenesis block oligomerization of natural Abeta and thereby rescue long-term potentiation. J Neurosci. 2005;25(10):2455–2462. doi: 10.1523/JNEUROSCI.4391-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Martin E, Gonzales V, Borchelt DR, Lee MK. Differential regulation of small heat shock proteins in transgenic mouse models of neurodegenerative diseases. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmus MM, Boelens WC, Otte-Holler I, Kamps B, de Waal RM, Verbeek MM. Small heat shock proteins inhibit amyloid-beta protein aggregation and cerebrovascular amyloid-beta protein toxicity. Brain Res. 2006;1089(1):67–78. doi: 10.1016/j.brainres.2006.03.058. [DOI] [PubMed] [Google Scholar]
- Wilhelmus MM, Boelens WC, Otte-Holler I, Kamps B, Kusters B, Maat-Schieman ML, et al. Small heat shock protein HspB8: its distribution in Alzheimer’s disease brains and its inhibition of amyloid-beta protein aggregation and cerebrovascular amyloid-beta toxicity. Acta Neuropathol (Berl) 2006;111(2):139–149. doi: 10.1007/s00401-005-0030-z. [DOI] [PubMed] [Google Scholar]
- Winter J, Jakob U. Beyond transcription--new mechanisms for the regulation of molecular chaperones. Crit Rev Biochem Mol Biol. 2004;39(5–6):297–317. doi: 10.1080/10409230490900658. [DOI] [PubMed] [Google Scholar]
- Zhu M, Rajamani S, Kaylor J, Han S, Zhou F, Fink AL. The flavonoid baicalein inhibits fibrillation of alpha-synuclein and disaggregates existing fibrils. J Biol Chem. 2004;279(26):26846–26857. doi: 10.1074/jbc.M403129200. [DOI] [PubMed] [Google Scholar]