Abstract
As patients with muscular dystrophy live longer because of improved clinical care, they will become increasingly susceptible to many of the cardiovascular diseases that affect the general population. There is, therefore, a pressing need to better understand both the biology and the mechanics of the arterial wall in these patients. In this paper, we use nonlinear constitutive relations to model, for the first time, the biaxial mechanical behavior of carotid arteries from two common mouse models of muscular dystrophy (dystrophin deficient and sarcoglycan-delta null) and wild-type controls. It is shown that a structurally motivated four-fiber family stress-strain relation describes the passive behavior of all three genotypes better than does a commonly used phenomenological exponential model, and that a Rachev-Hayashi model describes the mechanical contribution of smooth muscle contraction under basal tone. Because structurally motivated constitutive relations can be extended easily to model adaptations to altered hemodynamics, results from this study represent an important step toward the ultimate goal of understanding better the mechanobiology and pathophysiology of arteries in muscular dystrophy.
Keywords: muscular dystrophy, vascular mechanics, constitutive relation, wall stress, compensatory adaptations
Introduction
The dystrophin-glycoprotein complex (DGC) is a transmembrane molecular bridge that links cytoskeletal F-actin with the extracellular matrix protein laminin-2 and localizes many signaling proteins within muscle cells, including vascular smooth muscle. Genetic defects that compromise the DGC have been implicated in muscular dystrophies - progressive muscle disorders characterized by skeletal muscle weakness and wasting that affect approximately 250,000 people in the United States alone. Whereas defects of the DGC are well studied in skeletal muscle, less is known about the role of this complex in vascular smooth muscle. Because the DGC likely plays key roles in mediating mechano-sensitive biological responses, its potential importance in the control of vascular wall biology and mechanics warrants additional study.
Previously, we compared biaxial biomechanical and vasoreactive functional responses of common carotid arteries from wild-type, dystrophin-deficient (mdx), and sarcoglycan-δ null (sgcd−/−) mice (Dye et al., 2007). In this paper, we report constitutive relations and best-fit values of material parameters that describe these previously reported data under both basal smooth muscle tone (basal) and maximally-dilated (passive) conditions. Specifically, using a two-dimensional (2-D) theoretical framework, we evaluated a phenomenological model proposed by Chuong and Fung (1986) and a structurally motivated four-fiber family model proposed by Baek et al. (2007) as possible descriptors of the passive biomechanical responses, and we evaluated the model proposed by Rachev and Hayashi (1999) for active smooth muscle. We found that the four-fiber family model (with 8 parameters) provided a better fit to the passive mechanical data from each genotype than did the Chuong and Fung model (with 7 parameters) and that it also provided quantitative interpretations that were more consistent with the prior experimental findings. In addition, the model of Rachev and Hayashi provided a reasonable description of the mechanical contribution of basal smooth muscle tone for each genotype. Having structurally motivated constitutive relations for the biomechanical behavior of carotid arteries from wild-type and muscular dystrophy mouse models is an important first step toward modeling adaptive processes that are important to clinical care.
Methods
Theoretical Framework
Mouse carotid arteries are long, straight, nearly cylindrical vessels with few or no branches, which facilitates both in vitro experimentation and constitutive formulations. We modeled these arteries using a 2-D framework and thus considered mean values for the intramural stresses. Reasons for this were twofold. First, residual stresses in arteries tend to homogenize the transmural distribution of stress under physiologic conditions, thereby rendering mean values of stress as good estimates of transmural values. Second, murine carotid arteries have a deformed thickness-to-radius ratio of ~0.07 and consist of only two-to-three layers of smooth muscle cells (cf. ~40 layers for human carotid arteries). In a 2-D setting, it is assumed that σrr ≪ σθθ and σrr ≪σzz, where σrr, σθθ, and σzz are mean values of the radial, circumferential, and axial components of Cauchy stress. Mean values for σθθ, and σzz can be calculated directly from experimental data as
| (1) |
where P is the transmural pressure, a and h are the luminal radius and wall thickness in a loaded configuration, and f = fT + πa2 P is the total axial force applied to the vessel; fT is the force measured via an in-line transducer and πa2 P accounts for the end-cap force that arises during in vitro testing. Because of the assumption that σrr ≈0, appropriate nonlinear constitutive relations for mean values of σθθ, and σzz are
| (2) |
where λi are mean in-plane stretch ratios (i = r,θ,z), EAA are mean principal components of the Green strain (A = R,Θ,Z; no sum), which is defined in the reference configuration, W is the strain-energy function for passive behavior, and describes the active contractile response of smooth muscle. Combining equations (1) and (2), we obtain relations suitable for parameter estimation, namely
| (3) |
and
| (4) |
which relate the measurable applied loads on the left-hand side of each equation with geometric changes and the constitutive model on the right hand side.
Constitutive Relations
Consider two candidate strain-energy functions W for the passive behavior: one proposed by Chuong and Fung (1986) and a four-fiber family model proposed by Baek et al. (2007), which is a straightforward extension of the two-fiber family model of Holzapfel et al. (2000). The model of Chuong and Fung is
| (5) |
where and c and c1–6 are material parameters. The four-fiber family model is
| (6) |
where b1, , and are material parameters, with k denoting a fiber family, I1 =tr(C) = CRR + Cθθ + CZZ is the first invariant of the right Cauchy-Green strain tensor C, where principal components of C are related to those of E via CAA = 2EAA − 1, is the stretch of the kth fiber family, is the unit vector along the kth fiber direction in the reference configuration, and is the associated angle between the axial and fiber directions. In general, , but CΘZ = 0 for inflation and extension tests. We considered four fiber families with (axial), (circumferential), and (diagonal), which was left as a variable to be determined along with seven material parameters (with and for the diagonal fibers).
In addition, consider one model for the active (contractile) response of the smooth muscle cells under basal tone. Following Rachev and Hayashi (1999), we let
| (7) |
where λM is the stretch at which the contraction is maximum, λ0 is the stretch at which active force generation ceases, and Tact is a parameter associated with the degree of muscle activation, which may be correlated with the intracellular calcium concentration.
Parameter estimation
Biaxial data came from Dye et al. (2007) for both passive and basal-tone conditions. Briefly, these data resulted from cyclic inflation tests performed at three fixed axial stretches (λz = 1.65, 1.80, and 1.95) and cyclic extension tests performed at three fixed transmural pressures (P = 60, 100, 140 mmHg). P, fT, outer diameter d, and loaded length l were monitored continuously over two to three cycles for each protocol. Also measured directly were the unloaded outer diameter D and unloaded axial length L. In addition, wall thickness was measured at multiple static configurations and a mean value for vessel volume V̄ was used to calculate the luminal radius and thickness h = (b − a) during cyclic testing; here b is the loaded outer radius. Mean circumferential stretch was calculated as λθ = rmid/Rmid, where mid-wall radii rmid = (a+b)/2 and Rmid = (A + B) 2, with A and B the unloaded inner and outer radii, and axial stretch was calculated as λz = l/L.
Best-fit values of material parameters for the passive Chuong and Fung model (c and c1–6) and the four-fiber family model (b1, , and α0, where k = 1, 2, and 3 = 4) were determined via a multi-dimensional nonlinear regression, using the Nelder-Mead direct search method, that minimized the error e between measured values of P and fT on the left-hand side of equations (3) and (4) and calculated values on the right-hand side of equations (3) and (4), given measured values of λθ and λz. Namely, we minimized the error function
| (8) |
using the fminsearch subroutine in MatLab 6.1. Data were taken between 0 and 160 mmHg at increments of 2 mmHg for the P − d tests and from the stretch at zero axial force to λz = 1.95 for the f − l tests; taken together, the three P − d tests and three f − l tests yielded n = 325 to 350 data points. A penalty method was employed to ensure non-negative values of all best-fit parameters.
Data collected at basal smooth muscle tone over these same loading scenarios were used to determine the additional parameters for the active contractile response (λM, λo, and Tact). For these estimations, parameters for the four-fiber family model determined from passive data were used to calculate the passive contribution to stress in equations (3) and (4). The error function (8) was again minimized via nonlinear regression for experimental values during P − d and f − l tests for vessels having a basal smooth muscle tone.
Results
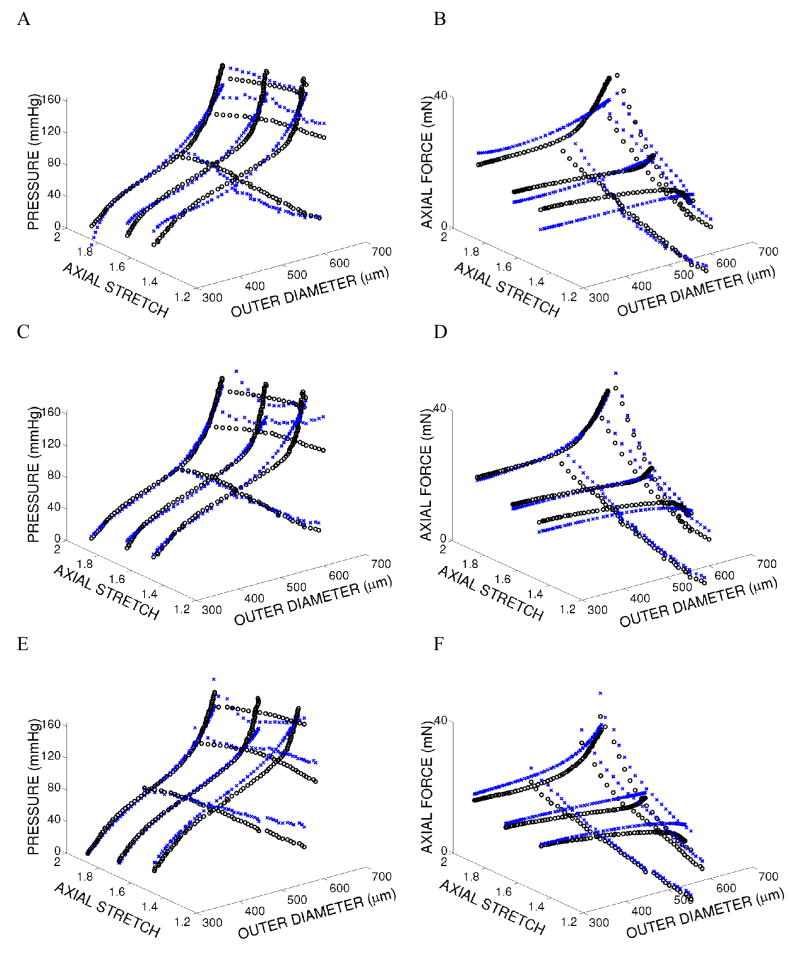
Biomechanical data and modeling results for representative wild-type, mdx, and sgcd−/− carotid arteries illustrate the goodness of fit for the two passive models as well as the active model (Figure 1). Associated best-fit values of the model parameters and minimized error values for each vessel within each genotype (n = 5 or 6) are provided in Tables 1, 2, and 3, respectively. As it can be seen, the four-fiber family model provided a better overall fit to data (i.e., lower value of e) than did the model of Chuong and Fung for each vessel tested. Notice, too, that fiber directions , as determined via nonlinear regression, were significantly different in vessels from the mdx (at p = 0.013) and scgd−/− mice (at p = 0.056) compared to vessels from the wild-type mice (Table 2 and Figure 2).
Figure 1.
Experimental data (open black circles) and model predictions (x’s) for the final cycle of loading for three different fixed-length cyclic inflation and three different fixed-pressure cyclic extension tests for vessel MDX 020105. Panels A and B show passive biomechanical data and the fit of the Chuong and Fung model. Panels C and D show passive biomechanical data and the fit of the four-fiber family model. Panels E and F show biomechanical data under basal tone and the fit of the active model (using the passive four-fiber family model)
Table 1.
Material parameters, error, and strain energy calculated for in vivo loading conditions for the Chuong and Fung (1986) model. Material parameters were determined via a nonlinear least squares regression with mechanical testing data collected from wild-type, mdx, and scgd−/− mouse carotid arteries. Note that the strain energy was evaluated at P = 100 mmHg and the individual in vivo stretches: λz = 1.84, 1.71, and 1.73 for wild-type, mdx, and sgcd−/−, respectively. Superscripts (1) indicates p < 0.05 when comparing mdx and sgcd−/− to wild-type, (2) indicates p < 0.10 when comparing mdx and sgcd−/− to wild-type, (3) indicates p < 0.05 when comparing mdx to sgcd−/−, and (4) indicates p < 0.10 when comparing mdx to sgcd−/−.
| c(kPa) | c1 | c2 | c3 | c4 | c5 | c6 | Error | W(kJ/m3) | |
|---|---|---|---|---|---|---|---|---|---|
| Wild-type | |||||||||
| 011305 | 202.05 | 0.250 | 0.308 | 1.032 | 0.019 | 0.185 | 0.0181 | 0.323 | 97.0 |
| 012705 | 400.32 | 0.113 | 0.234 | 0.474 | 0.044 | 0.149 | 0.0414 | 0.236 | 112.9 |
| 102704 | 171.36 | 0.182 | 0.341 | 0.873 | 0.022 | 0.208 | 0.0000 | 0.287 | 88.8 |
| 110104 | 232.22 | 0.099 | 0.184 | 0.130 | 0.007 | 0.000 | 0.0000 | 0.332 | 79.3 |
| 110304 | 110.12 | 0.282 | 0.384 | 1.339 | 0.071 | 0.284 | 0.1892 | 0.349 | 69.1 |
| 110504 | 302.56 | 0.224 | 0.197 | 0.662 | 0.033 | 0.114 | 0.0743 | 0.306 | 87.4 |
| Average | 236.44 | 0.192 | 0.275 | 0.752 | 0.033 | 0.157 | 0.0538 | 0.305 | 89.1 |
| +/− SD | 102.59 | 0.074 | 0.082 | 0.426 | 0.023 | 0.096 | 0.0721 | 0.040 | 15.0 |
| mdx | |||||||||
| 012505 | 33.15 | 0.551 | 1.038 | 5.381 | 0.216 | 1.233 | 0.4372 | 0.364 | 52.3 |
| 020105 | 48.67 | 0.340 | 0.845 | 3.940 | 0.059 | 0.895 | 0.0000 | 0.339 | 66.1 |
| 112204 | 40.83 | 0.543 | 0.793 | 3.039 | 0.140 | 0.637 | 0.2681 | 0.302 | 49.7 |
| 121404 | 31.30 | 0.594 | 0.939 | 2.111 | 0.137 | 0.387 | 0.0000 | 0.312 | 54.0 |
| 121504 | 67.46 | 0.445 | 0.702 | 1.708 | 0.154 | 0.416 | 0.2408 | 0.277 | 56.1 |
| Average | 44.28(1) | 0.494(1) | 0.864(1) | 3.236(1) | 0.141(1) | 0.714(1) | 0.1892 | 0.319 | 55.6(1) |
| +/− SD | 14.67 | 0.102 | 0.130 | 1.478 | 0.056 | 0.355 | 0.1884 | 0.034 | 6.3 |
| sgcd−/− | |||||||||
| 011405 | 42.26 | 0.253 | 0.994 | 3.638 | 0.088 | 0.993 | 0.0749 | 0.322 | 66.5 |
| 011905 | 66.68 | 0.226 | 0.642 | 1.946 | 0.080 | 0.554 | 0.0000 | 0.307 | 60.2 |
| 012005 | 102.23 | 0.391 | 0.476 | 0.482 | 0.056 | 0.079 | 0.0000 | 0.330 | 65.3 |
| 111704 | 82.32 | 0.401 | 0.623 | 2.211 | 0.128 | 0.511 | 0.2073 | 0.285 | 64.7 |
| 120304 | 96.66 | 0.344 | 0.494 | 1.665 | 0.099 | 0.385 | 0.1156 | 0.275 | 62.1 |
| 122104 | 62.98 | 0.302 | 0.757 | 1.943 | 0.089 | 0.473 | 0.1192 | 0.281 | 66.4 |
| Average | 75.52(1,3) | 0.319(1,3) | 0.664(1,3) | 1.981(1,4) | 0.090(1) | 0.499(1) | 0.0862 | 0.300 | 64.2(1) |
| +/− SD | 22.58 | 0.072 | 0.192 | 1.015 | 0.024 | 0.296 | 0.0795 | 0.023 | 2.5 |
Table 2.
Material parameters, error, and strain energy calculated for in vivo loading conditions for the four-fiber family model. Material parameters were determined via a nonlinear least squares regression with mechanical testing data collected from wild-type, mdx, and scgd−/− mouse carotid arteries. Note that the strain energy was evaluated at P = 100 mmHg and the individual in vivo stretches: λz = 1.84, 1.71, and 1.73 for wild-type, mdx, and sgcd−/−, respectively. Superscripts (1) indicates p < 0.05 when comparing mdx and sgcd−/− to wild-type, (2) indicates p < 0.10 when comparing mdx and sgcd−/− to wild-type, (3) indicates p < 0.05 when comparing mdx to sgcd−/−, and (4) indicates p < 0.10 when comparing mdx to sgcd−/−.
| b1c (kPa) | (kPa) | (kPa) | (Pa) | α0 (deg) | Error | W (kJ/m3) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wild-type | |||||||||||||
| 011305 | 30.11 | 20.65 | 0.067 | 13.93 | 0.202 | 2.43 | 1.811 | 45.1 | 0.254 | 102.6 | |||
| 012705 | 25.93 | 36.35 | 0.019 | 13.32 | 0.104 | 203.87 | 0.834 | 39.0 | 0.180 | 123.1 | |||
| 102704 | 17.11 | 25.84 | 0.040 | 15.06 | 0.080 | 7.55 | 1.239 | 37.8 | 0.225 | 96.5 | |||
| 110104 | 11.60 | 24.63 | 0.011 | 7.61 | 0.061 | 0.91 | 1.248 | 41.2 | 0.264 | 83.7 | |||
| 110304 | 16.35 | 23.38 | 0.000 | 7.61 | 0.193 | 10.42 | 1.330 | 39.2 | 0.248 | 78.4 | |||
| 110504 | 26.39 | 19.32 | 0.038 | 17.48 | 0.183 | 1.14 | 2.171 | 46.3 | 0.226 | 93.1 | |||
| Average | 21.58 | 25.03 | 0.029 | 12.50 | 0.137 | 37.72 | 1.439 | 41.4 | 0.233 | 96.2 | |||
| +/− SD | 7.55 | 6.06 | 0.024 | 4.05 | 0.063 | 81.49 | 0.475 | 3.5 | 0.030 | 15.8 | |||
| mdx | |||||||||||||
| 012505 | 25.95 | 15.42 | 0.112 | 7.30 | 0.262 | 70.70 | 1.394 | 38.8 | 0.287 | 66.9 | |||
| 020105 | 23.11 | 23.11 | 0.052 | 13.78 | 0.131 | 28.40 | 1.184 | 32.3 | 0.258 | 83.7 | |||
| 112204 | 19.41 | 17.98 | 0.063 | 7.50 | 0.351 | 230.98 | 1.033 | 36.5 | 0.219 | 60.7 | |||
| 121404 | 12.75 | 20.28 | 0.076 | 19.76 | 0.176 | 1409.52 | 0.803 | 33.7 | 0.254 | 63.7 | |||
| 121504 | 11.70 | 23.68 | 0.071 | 15.63 | 0.164 | 1479.02 | 0.727 | 35.9 | 0.239 | 63.1 | |||
| Average | 18.59 | 20.09(2) | 0.075(1) | 12.79 | 0.217 | 643.72 | 1.028 | 35.5(1) | 0.251 | 67.6(1) | |||
| +/− SD | 6.26 | 3.48 | 0.022 | 5.38 | 0.089 | 735.10 | 0.273 | 2.5 | 0.025 | 9.2 | |||
| sgcd−/− | |||||||||||||
| 011405 | 10.89 | 31.49 | 0.037 | 10.63 | 0.071 | 130.43 | 0.890 | 30.4 | 0.239 | 82.6 | |||
| 011905 | 13.62 | 25.94 | 0.000 | 11.39 | 0.082 | 243.40 | 0.764 | 31.6 | 0.234 | 73.1 | |||
| 012005 | 21.76 | 30.23 | 0.040 | 12.62 | 0.339 | 106.45 | 1.369 | 40.6 | 0.253 | 77.5 | |||
| 111704 | 23.79 | 23.73 | 0.049 | 14.51 | 0.184 | 272.28 | 0.993 | 36.3 | 0.226 | 77.5 | |||
| 120304 | 21.24 | 20.95 | 0.024 | 14.16 | 0.181 | 568.35 | 0.761 | 36.7 | 0.211 | 72.7 | |||
| 122104 | 12.44 | 28.69 | 0.062 | 9.25 | 0.142 | 705.84 | 0.719 | 33.2 | 0.227 | 76.1 | |||
| Average | 17.29 | 26.84 | 0.035(3) | 12.09 | 0.167 | 337.79(1) | 0.916(1) | 34.8 (2) | 0.232 | 76.6(1) | |||
| +/− SD | 5.58 | 4.04 | 0.021 | 2.06 | 0.097 | 244.27 | 0.244 | 3.8 | 0.014 | 3.6 | |||
Table 3.
Material parameters for the Rachev and Hayashi (1999) model determined via a nonlinear least squares regression with mechanical testing data collected from wild-type, mdx, and scgd−/− mouse carotid arteries under basal tone. Note that the best-fit parameters for the four-fiber family model (Table 2) were used to model the passive mechanical behavior. No significant differences were observed for Tact, λM, and λo across any groups.
| tact (kPa) | λM | λo | Error | |
|---|---|---|---|---|
| Wild-type | ||||
| 011305 | 21.5 | 1.71 | 0.90 | 0.244 |
| 012705 | 12.6 | 1.80 | 0.91 | 0.187 |
| 102704 | 11.7 | 1.81 | 0.91 | 0.646 |
| 110104 | 7.6 | 1.86 | 0.93 | 0.280 |
| 110304 | 14.6 | 1.78 | 0.92 | 0.184 |
| 110504 | 23.8 | 1.74 | 0.89 | 0.190 |
| Average | 15.3 | 1.78 | 0.91 | 0.289 |
| +/− SD | 6.2 | 0.05 | 0.02 | 0.179 |
| mdx | ||||
| 012505 | 17.6 | 1.73 | 0.90 | 0.267 |
| 020105 | 14.8 | 1.80 | 0.92 | 0.358 |
| 112204 | 13.8 | 1.79 | 0.92 | 0.329 |
| 121404 | 13.2 | 1.82 | 0.92 | 0.337 |
| 121504 | 9.6 | 1.84 | 0.92 | 0.244 |
| Average | 13.8 | 1.79 | 0.91 | 0.307 |
| +/− SD | 2.9 | 0.04 | 0.01 | 0.049 |
| sgcd−/− | ||||
| 011405 | 18.7 | 1.70 | 0.89 | 0.280 |
| 011905 | 8.7 | 1.84 | 0.92 | 0.252 |
| 012005 | 23.6 | 1.75 | 0.89 | 0.243 |
| 111704 | 23.3 | 1.77 | 0.87 | 0.265 |
| 120304 | 8.4 | 1.84 | 0.92 | 0.230 |
| 122104 | 13.8 | 1.79 | 0.92 | 0.288 |
| Average | 16.1 | 1.78 | 0.90 | 0.260 |
| +/− SD | 6.8 | 0.06 | 0.02 | 0.022 |
Figure 2.
Mean ± S.D. of best-fit fiber angles ( ) determined via nonlinear regression for wild-type, mdx, and sgcd−/− common carotid arteries. Note: * (p = 0.013) and ** (p = 0.056) indicate the level of statistical significance when compared to wild-type vessels as determined by a paired t-test.
Discussion
By linking the cytoskeleton of muscle cells to adjacent extracellular matrix, the dystrophin-glycoprotein complex (DGC) helps to distribute mechanical stresses on these cells so as to maintain structural integrity during contraction and relaxation (Petrof et al. 1993; Pasternak et al. 1995). Absence of DGC components can thus have important implications for arteries, which consist largely of an abundant extracellular matrix with embedded smooth muscle cells that are subjected to continually changing wall shear and intramural (primarily circumferential and axial) stresses. Indeed, an altered biomechanical function of the vasculature in individuals with muscular dystrophy likely contributes to the progression of skeletal muscle weakness and wasting as well as diverse diseases related to diminished blood flow to end organs (Loufrani et al. 2004; Ito et al. 2006).
Surprisingly few investigators have studied the effects of muscular dystrophies on vascular biology and mechanics. We (Dye et al. 2007) and one group from Paris (Loufrani et al. 2001, 2002, 2004) have shown, however, that passive biomechanical behavior and smooth muscle contractility of carotid arteries are not statistically different under normal hemodynamic conditions in male wild-type (C57BL/6J), mdx barrier AX6 (on a C57BL/10SnJ background), and sgcd−/− (on a C57BL/6J background) mice, noting that mdx mice lack the protein dystrophin. It appears, therefore, that carotid arteries compensate, at least in part, during development for the absence of dystrophin or sarcoglycan-delta such that gross responses to normal hemodynamics are not compromised (e.g., mean arterial pressure and pressure-diameter responses are the same in mdx and wild-type controls). Nevertheless, Loufrani et al. (2001) found that “absence of dystrophin was associated with a defect in signal transduction of [flow-induced wall] shear stress.” That is, overall responses to the exogenous vasodilators acetylcholine (endothelial-dependent) and sodium nitroprusside (endothelial-independent) were normal, but dilation in response to increased shear stress was only 50–60% of normal in mdx mice. Whereas these experiments were performed acutely in isolated perfused vessels, the general importance of shear-regulated remodeling in vivo in response to sustained alterations in flow (e.g., Rudic et al., 2000; Schiffers et al., 2000; Sullivan and Hoying, 2002) suggests that a compromised shear-regulated dilatory function would adversely affect chronic adaptations (i.e., arterial growth and remodeling) to increased flow (as in exercise regimes, which are important but difficult to optimize for muscular dystrophy patients). Indeed, similar compromised adaptations to increased pressure (e.g., as in exercise or hypertension) should be expected, particularly given the important coupling of flow- and pressure-induced growth and remodeling in arteries (Ueno et al., 2000; Dajnowiec and Langille, 2007).
Gleason et al. (2004) and Gleason and Humphrey (2004) recently proposed models of arterial growth and remodeling in special cases of sustained alterations of flow and pressure. Such models promise to increase our understanding of adaptive processes and ultimately to aid in the design of improved clinical interventions (Humphrey and Taylor, 2008). Essential to models of growth and remodeling, however, is knowledge of the constitutive behavior of individual constituents. Although it is not possible, at present, to infer separately the mechanical properties of elastin, fibrillar collagen, and smooth muscle (the primary contributors to wall mechanics) from traditional inflation-extension tests, combining a structurally motivated constitutive model for passive behavior with a model for active smooth muscle enables one to approximate such properties. For example, in contrast to the phenomenological model of Chuong and Fung, the four-fiber family model enables one to estimate contributions due to the elastin-dominated amorphous matrix (isotropic term in equation 6) and contributions due to multiple families of fibrillar collagen (anisotropic terms in equation 6). Indeed, Wicker et al. (2008) show that this four-fiber family model captures mean collagen fiber angles in basilar arteries (measured independently using nonlinear optical microscopy), and comparison of the present best-fit values for b1 (on the order of 101) with those of Wicker and colleagues (on the order of 10−7) showed that the four-fiber family model captured the greater contribution of elastin in the mouse carotid artery (~28% elastin by dry weight) compared to the rabbit basilar artery (~5% elastin). Finally, the best-fit value of Tact (Table 3) was on the order of 10 to 25 kPa, which is reasonable for basal tone (cf. Rachev and Hayashi, 1999). The present results reveal quantitatively (i.e., lower best-fit value of e) and qualitatively (i.e., greater similarity of material parameters across genotypes) that this structurally motivated model fit biaxial data from three genotypes as well as or better than did the phenomenological model, hence providing both a foundation and a motivation for pursuing related growth and remodeling models. Toward this end, it is interesting that the best-fit values of the mean collagen fiber angle were significantly less (at a p ≤ 0.06) for the muscular dystrophy models than for the wild-type controls. Although there is a need to determine directly if such a structural difference exists, this was not possible from the available standard histological data (Dye et al., 2007). In other words, the present theoretical finding provides guidance for future experiments as it should. It is provocative to note, however, that the lower best-fit value of for the two muscular dystrophy models is consistent with the previously observed lower values of in vivo axial stretch (Dye et al., 2007), which in turn was thought to reflect the primary compensatory adaptation by the mdx and sgcd−/− carotids under normal hemodynamics.
In conclusion, much remains to be learned with regard to compensatory adaptations by arteries during development in genetic models of disease as well as how these vessels respond to sustained alterations in hemodynamic loads in maturity. We submit that computational models will play an important role in this endeavor, thus using microstructurally motivated constitutive relations herein to quantify, for the first time, the mechanical behavior of carotid arteries from two common mouse model of muscular dystrophy is an important step toward this ultimate goal.
Acknowledgments
This work was supported, in part, by grants from the Muscular Dystrophy Association (MDA 3681) and the NIH (HL-64372). We also gratefully acknowledge Dr. Kevin P. Campbell, HHMI at the University of Iowa for providing the sgcd−/− breeding pairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baek S, Gleason RL, Rajagopal KR, Humphrey JD. Theory of small on large: Potential utility in computations of fluid-solid interactions in arteries. Comput Methods Appl Mech Engng. 2007;196:3070–3078. [Google Scholar]
- Chuong CJ, Fung YC. On residual stress in arteries. J Biomech Eng. 1986;108:189–192. doi: 10.1115/1.3138600. [DOI] [PubMed] [Google Scholar]
- Dajnowiec D, Langille BL. Arterial adaptations to chronic changes in haemodynamic function: coupling vasomotor tone to structural remodeling. Clin Sci (London) 2007;113:15–23. doi: 10.1042/CS20060337. [DOI] [PubMed] [Google Scholar]
- Dye W, Gleason RL, Wilson E, Humphrey JD. Altered biomechanical properties of carotid arteries in two mouse models of muscular dystrophy. J Appl Physiol. 2007;103:664–672. doi: 10.1152/japplphysiol.00118.2007. [DOI] [PubMed] [Google Scholar]
- Gleason RL, Humphrey JD. A mixture model of arterial growth and remodeling in hypertension: altered muscle tone and tissue turnover. J Vasc Res. 2004;41:352–363. doi: 10.1159/000080699. [DOI] [PubMed] [Google Scholar]
- Gleason RL, Taber LA, Humphrey JD. A 2-D model of flow-induced alterations in the geometry, structure, and properties of carotid arteries. J Biomech Eng. 2004;126:371–381. doi: 10.1115/1.1762899. [DOI] [PubMed] [Google Scholar]
- Holzapfel GA, Gasser TC, Ogden RW. A new constitutive framework for arterial wall mechanics and a comparative study of material models. J Elast. 2000;61:1–48. [Google Scholar]
- Humphrey JD, Taylor CA. Intracranial and abdominal aortic aneurysms: Similarities, differences and need for a new class of computational models. Ann Rev Biomed Eng. 2008 doi: 10.1146/annurev.bioeng.10.061807.160439. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Kimura S, Ozasa S, Matsukura M, Ikezawa M, Yoshioka K, Ueno H, Suzuki M, Araki K, Yamamura K, Miwa T, Dickson G, Thomas G, T M. Smooth muscle-specific dystrophin expression improves aberrant vasoregulation in mdx mice. Human Molc Gen. 2006;15:2266–2275. doi: 10.1093/hmg/ddl151. [DOI] [PubMed] [Google Scholar]
- Loufrani L, Dubroca C, You D, Levy B, Paulin D, Henrion D. Absence of dystrophin in mice reduces NO-dependent vascular function and vascular density: Total recovery after a treatment with the aminogylcoside gentamicin. Arterioscler Thromb Vasc Biol. 2004;20:671–676. doi: 10.1161/01.ATV.0000118683.99628.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loufrani L, Levy B, Henrion D. Defect in microvascular adaptation to chronic changes in blood flow in mice lacking the gene encoding for dystrophin. Circ Res. 2002;91:1183–1189. doi: 10.1161/01.res.0000047505.11002.81. [DOI] [PubMed] [Google Scholar]
- Loufrani L, Matrougui K, Gorny D, Duriez M, Blanc I, Levy B, Henrion D. Flow (shear stress)–induced endothelium-dependent dilation is altered in mice lacking the gene encoding for dystrophin. Circulation. 2001;103:864–870. doi: 10.1161/01.cir.103.6.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak C, Wong S, Elson E. Mechanical function of dystrophin in muscle cells. J Cell Biol. 1995;128:355–361. doi: 10.1083/jcb.128.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrof B, Shrager J, Stedman H, Kelly A, Sweeney H. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci U S A. 1993;90:3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachev A, Hayashi K. Theoretical study of the effects of vascular smooth muscle contraction on strain and stress distributions in arteries. Ann Biomed Eng. 1999;27:459–468. doi: 10.1114/1.191. [DOI] [PubMed] [Google Scholar]
- Rudic RD, Bucci M, Fulton D, Segal SS, Sessa WC. Temporal events underlying arterial remodeling after chronic flow reduction in mice. Correlation of structural changes with a deficit in basal nitric oxide synthesis. Circ Res. 2000;86:1160–1166. doi: 10.1161/01.res.86.11.1160. [DOI] [PubMed] [Google Scholar]
- Schiffers P, Henrion D, Boulanger C, Colucci-Guyon E, Langa-Vuves F, van Essen H, Fazzi G, Levy B, De Mey J. Altered flow-induced arterial remodeling in vimentin-deficient mice. Arterioscler Thromb Vasc Biol. 2000;20:611–616. doi: 10.1161/01.atv.20.3.611. [DOI] [PubMed] [Google Scholar]
- Sullivan CJ, Hoying JB. Flow-dependent remodeling in the carotid artery of fibroblast growth factor-2 knockout mice. Arterioscler Thromb Vasc Biol. 2002;22:1100–1105. doi: 10.1161/01.atv.0000023230.17493.e3. [DOI] [PubMed] [Google Scholar]
- Ueno H, Kanellakis P, Agrotis A, Bobik A. Blood flow regulates the development of vascular hypertrophy, smooth muscle cell proliferation, and endothelial cell nitric oxide synthase in hypertension. Hypertension. 2000;36:89–96. doi: 10.1161/01.hyp.36.1.89. [DOI] [PubMed] [Google Scholar]
- Wicker B, Hutchens H, Wu Q, Yeh A, Humphrey J. Normal basilar artery structure and biaxial mechanical behavior. Comp Meth Biomech Biomed Engng. 2008 doi: 10.1080/10255840801949793. in press. [DOI] [PubMed] [Google Scholar]