Abstract
Context
Both stress-reactive and novelty-seeking individuals are susceptible to alcohol use disorders. Variation in stress reactivity, exploration, and response to novelty have been attributed to differences in corticotropin-releasing hormone (CRH) system function. As such, CRH gene variation may influence risk for alcohol use and dependence.
Objective
To determine whether CRH variation influences relevant intermediate phenotypes, behavior, and alcohol consumption in rhesus macaques.
Design
We sequenced the rhesus macaque CRH locus (rhCRH) and performed cladistic clustering of haplotypes. In silico analysis, gel shift and in vitro reporter assays were performed to identify functional variants. CSF and blood samples were obtained, and levels of CRH and ACTH were measured by RIA. Behavioral data were collected from macaques during infancy. Among adolescent/adult animals, we recorded responses to an unfamiliar conspecific and measured levels of ethanol consumption. Animals were genotyped for a SNP disrupting a glucocorticoid response element, rhCRH -2232 C>G, and the effects of this allele on CSF levels of CRH, plasma levels of ACTH, behavior and ethanol consumption were assessed by ANOVA.
Results
We show that -2232 C>G alters DNA-protein interactions and confers decreased sensitivity of the CRH promoter to glucocorticoids in vitro. Consistent with the known effects of glucocorticoids on CRH expression in brain, carriers of the G allele had lower CSF levels of CRH, but higher levels of ACTH. Infants carrying the G allele were more exploratory and bold, and among adolescent and adult male macaques, the G allele was associated with exploratory/bold responding to an unfamiliar male. Adults with the C/G genotype also exhibited increased alcohol consumption in the social group, a model for high-risk alcohol seeking behavior.
Conclusions
Haplotypes that differ in terms of corticosteroid-sensitivity have been identified in humans. Our data may suggest that functionally similar CRH variants could influence risk for externalizing disorders in human subjects.
Corticotropin-releasing hormone (CRH) is critical to behavioral and neuroendocrine adaptation to stress and is the primary neuropeptide responsible for hypothalamic-pituitary-adrenal (HPA) axis activation. Studies in rodents have shown that various drugs of abuse, including alcohol, can influence CRH release and HPA axis output 1–4, and alcohol preferring strains have been shown to exhibit differences in CRH system function, sensitivity to stress, and HPA axis activity even prior to alcohol exposure 5–8. In humans, perturbations of both the CRH system and the HPA axis are associated with various stress-related neuropsychiatric disorders, and a number of studies have shown that the CRH system and HPA axis are dysregulated in alcohol-dependent subjects 9. However, whether these observed differences antedate or result from prolonged alcohol use is unknown. It is also unknown whether genetic variation at the CRH locus increases the risk for developing stress-related or alcohol use disorders.
Studies utilizing experimental manipulations of CRH system activity suggest that naturally occurring CRH gene variation may mediate individual variability in behavioral and physiological traits that are key to determining an individual’s coping style. One of the most consistent behavioral correlates of CRH system activity is the way an organism approaches novelty and unfamiliar conspecifics 10. Individuals that readily seek out and investigate novel stimuli are considered “exploratory” or “bold”; those more likely to show fear or withdrawal when confronted with new objects or individuals are described as more “inhibited” or “shy.” Individuals show stable tendencies in their behavioral reactions, which are linked–in mice, humans, and nonhuman primates, among others–to biological traits such as heart rate variability 11, frontal brain electrical activity, and cortisol levels 12, 13. As individuals with both anxious/inhibited and impulsive temperaments are more likely to regularly consume alcohol 14, 15, it is possible that those who have either very high or very low levels of CRH system function could be at increased risk for developing alcohol problems. CRH system genes are therefore good candidates for investigating genetic variation as it relates to vulnerability to stress related disorders and alcohol dependence.
Genetic variants that are functionally similar to those that exist in humans have been identified in the rhesus macaque (i.e., HTTLPR, MAOA-LPR, and OPRM1 C77G), offering the opportunity to examine how genetic variation may influence traits linked to alcohol dependence in subjects living in a controlled environment 16–19 . The aim of the present study was to screen the rhesus macaque CRH gene (rhCRH) and its transcriptional control region for variation. Because corticosteroids have a critical role in regulating CRH, and human CRH haplotypes that confer differences in corticosteroid-sensitivity in vitro have been reported 20, we were interested in identifying variants that might alter glucocorticoid receptor (GR)-mediated transcriptional control. We then wanted to determine whether rhCRH variation influenced central CRH levels, HPA axis function, temperament, and behavioral reactions to social challenge. As both dysregulation of the CRH system and certain temperament traits can increase vulnerability to alcohol use disorders, we also tested whether rhCRH genotype influenced voluntary alcohol consumption.
METHODS
IDENTIFICATION OF CRH SEQUENCE VARIANTS
Genomic DNA was extracted from whole blood from rhesus macaques (Macaca mulatta) from the NIH Animal Center (NIHAC), and direct sequencing was performed using samples from 20 unrelated animals (pairwise Identity by Descent, or IBD ≤ 0.0125) that were selected on the basis of variable HPA axis activity. We used primers designed from published human sequence and, subsequently, from rhesus sequence generated in our lab (available upon request). Cycle sequencing was performed using the Big Dye Terminator Version 3.1 reaction in 96-well optical plates (Applied Biosystems, Inc., Foster City, CA). Variants were detected by visualization of electropherograms generated by ABI Sequencing Analysis software.
Haplotype frequencies and agreement with Hardy-Weinberg expectations were predicted using the default settings of PHASE, version 2.1 (based on variation identified from the sequencing of 40 chromosomes). To identify putatively functional variants, we examined regions containing consensus sites for factors known to regulate CRH transcription and also used web-based transcription factor binding site prediction algorithms (TfSitescan, www.ift.org/cgi-bin/ifti/Tfsitescan.pl 21 and Compel Pattern Search 1.0, http://compel.bionet.nsc.ru/FunSite/CompelPatternSearch.html 22).
HAPLOTYPE CLUSTERING AND ANCESTRAL SEQUENCE ESTIMATES
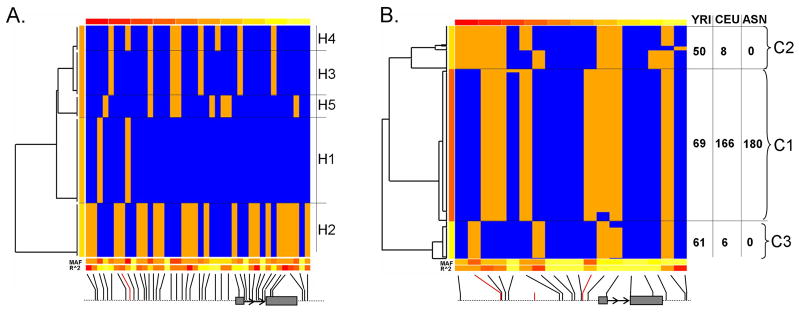
Human haplotypes for Nigeria (YRI) and Utah (CEU) samples in CRH gene region plus 5 Kb upstream and 1 Kb downstream were downloaded from HapMap project Release 21 (http://www.hapmap.org/). The following markers were used in our analyses (Fig 2, in 5′-3′ orientation: rs10098823, rs5030877, rs7839698, rs5030875, rs4501563, rs6472257, rs6999780, rs12721507, rs12721508, rs12721509, rs3176921, rs12721510, rs6158, rs12721511, rs7459924, rs6982394, rs11986876, and rs11997816). Based on Manhattan distances weighted by minor allele frequency and marker average LD, haplotypes for rhesus macaque (five most common) and human were clustered hierarchically using R (http://www.r-project.org). The ancestral sequences at each SNP site were estimated as described by Pollard et al. 23 with multiple alignments of human (March 2006 assembly), chimp (Mar 2006 assembly), and rhesus macaque (January 2006 assembly) from UCSC Genome Browser (http://genome.ucsc.edu/).
Figure 2.
FOCUSED RESEQUENCING
DNA samples from two other rhesus macaque colonies (AlphaGenesis, N = 69; U. of Wisconsin, N = 94 - the kind gift of M. Schneider) were screened for alleles thought to co-segregate with –2232 G, based on our initial sequencing. Focused re-sequencing was also performed on DNA from NIHAC animals of known –2232 C/G genotype (C/C:N=40; C/G: N=60) ) to verify that rhCRH-H2 variants were in allelic identity.
Various other primate samples (New World monkey- Ateles geoffroyi; Old World Monkey – Macaca nemestrina, Macaca fascicularis, Papio hamadryas, Papio anubis; Ape – Pan troglodytes) were sequenced for determination of sequence identity in the region surrounding the -2232 C/G site. DNA samples from 10 wild baboons (Papio hamadryas) from the Awash National Park, Ethiopia were the kind gift of J. Phillips-Conroy and C. J. Jolly. Additional nonhuman primate samples were obtained from the Coriell Institute for Medical Research. Alignments of CRH and 5′ flanking region sequences were performed using ClustalW and/or DNASIS-Mac V.2.0 software. Sequence identity percentages for interspecies comparisons were calculated using the web-based pairwise alignment program EMBOS (http://www.ebi.ac.uk/emboss/align/) using the default parameters.
FUNCTIONAL CHARACTERIZATION OF CRH -2232 C>G
Electrophoretic Mobility Shift Assay
Based on the identification of a variant located within a GRE half-site (-2232C/G), double-stranded DNA oligonucleotides containing the consensus (TTAGCAGTGTGAGAACAGACAAATACA) and non-consensus (TTAGCAGTGTGAGAAGAGACAAATACA) sequences were generated for performance of gel shift assays using nuclear extract generated from an immortalized hypothalamic cell line (IVB cells, the kind gift of Dr. John Kaskow, University of Cincinnati, Cincinnati, OH). Assays were performed using the Gel Shift Assay System (Promega, Madison, WI) per the manufacturer’s instruction. After annealing complementary oligonucleotides (95∞C 5 min, 25∞C 30 min), double-stranded probes were [32P]-ATP labeled using T4 kinase (Promega, Madison, WI) and purified using a Bio-Spin 30 chromatography column (Bio-Rad). Incorporation of radiolabel was > 1 × 105 cpm/ng DNA. Binding assays were performed using the Gel Shift Assay System (Promega, Madison, WI) per the manufacturer’s instructions. Nuclear extract (5 μs incubated for 20 min with 100,000 CPM of each oligonucleotide probe. Competitor oligonucleotides were added at 10X the concentration of the labeled probes. Samples were immediately separated by electrophoresis (250 V for 20 min) on a Novex 6% DNA retardation gel (Invitrogen, Carlsbad, CA), after which gels were dried and bands visualized by autoradiography.
Reporter Assays
Genomic DNA from animals confirmed to be homozygous for the rhCRH-H1 haplotype was extracted for PCR-based cloning. Primers were modified from those previously reported for huCRH reporter constructs 24. The forward primer was 5′ GCG GAA TTC GGC TCA TAA CTC CTT TAT GTG CTT GC 3′ (containing an EcoRI site) and the reverse primer was 5′ AAA GGA TCC GAG GGA CGT CTC CGG GGC 3′ (containing a BamHI site). These primers produced a 783-bp amplification product (from rhCRH −660 to +123) in which none of the rhCRH-H2 co-segregating loci were present. Reaction mixtures (50μl) contained 100 ng DNA, 0.1 mM dNTPs, 0.5 μM of each primer, 2.5 U PfuUltra High Fidelity DNA Polymerase and PfuUltra Buffer (Stratagene, La Jolla, CA). Amplifications were performed using a Perkin Elmer thermocycler (9700) with one cycle at 95∞C, 30 cycles of 95∞C/30 sec, 61.5∞C/30 sec, 72∞C/5 min and a final 10-minute extension at 72∞C. Following cleavage using EcoR1 and BamHI, PCR product was separated by electrophoresis and isolated using a QIAquick gel extraction kit (Qiagen, Valencia, CA). Cleaved products were then ligated into EcoRI/BamHI digested pDsRed-2.1 (BD Biosciences, Mountainview, CA) using standard molecular cloning techniques.
Attempts to test functionality of the -2232 C/G site using rhCRHH1 and H2 reporter constructs (−3458 to +974) generated inconsistent results because of the fact that they exhibited both extremely low levels of transfection efficiency and increased cell mortality, which we attributed to size of these constructs. This was true among experiments performed in a variety of cell lines (COS-7, IV-B, HT22, and JEG). GRE half-sites are known to interact across long distances 25, and there are several GREs and GRE half-sites in the proximal promoter of the CRH gene. Since we specifically wanted to test the function of the -2232 C/G polymorphism, located in a more distal GRE half-site, we designed double-stranded DNA cassettes (-2269 > -2200) containing either the -2232 C or -2232 G allele using oligonucleotides obtained from a commercial vendor (Operon, Huntsville, AL). These cassettes were digested with BglII and HindIII and ligated into the pDsRed MCS in a position 5′ to the rhCRH −660 to +123 insert (which contained the proximal promoter). Fidelity of resultant constructs was verified by sequencing.
We used mouse HT22 hippocampal cells (the kind gift of David Schubert, The Salk Institute, La Jolla, CA, USA). This cell line 26 was selected because of its high concentration of glucocorticoid receptors 27 and because the hippocampus expresses relatively high levels of CRH, especially after stimulation 28. HT22 cells were propagated in DMEM (Gibco) supplemented with 10% fetal bovine serum and maintained at 37∞C in a humidified incubator (5% CO2). Cells were seeded at a density of 5×104/well in a 12-well tissue culture plate. When 60–70% confluent, either the -2232 C or -2232G rhCRH pDsRed reporter constructs (1 μg) was co-transfected with a GFP reporter (0.5μg pGlow-TOPO, Invitrogen, Carlsbad, CA) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA), according to the manufacturer’s instructions. Cells were treated with 30μM Forskolin (Sigma) or 30μM Forskolin + Cortisol (Sigma) 20, 27, 29, 12 h following transfection. We selected a low cortisol dose (1 nM) for this study based on reports that this dose produced Type I GR activation, without Type II GR activation or TypeI:Type II dimerization, in the HT22 cell line 30. This is important because high cortisol doses (sufficient to activate Type II receptors) induce cell death in hippocampus 31, 32. Expression of DsRed was monitored 48h following treatment using an Olympus IX70 microscope (Olympus America, Melville, NY) interfaced with a Hamamatsu ORCA-ER digital camera (Hamamatsu, Tokyo, Japan). Region of interest analysis was performed using OpenLab® software. A CRH promoter expression value was obtained by dividing the DsRed intensity by that for GFP. Experiments were performed four times, with treatments in some instances performed in duplicate. Measurements were made by two observers and averaged. Data were analyzed by ANOVA, with treatment (No Treatment, Forskolin, Forskolin + Cort) and genotype (-2232 C and -2232 G) included as independent variables. Data were analyzed by ANOVA, with treatment (No Treatment, Forskolin, Forskolin + Cort) and genotype (-2232 C and -2232 G) included as independent variables.
GENOTYPING FOR -2232 C>G
Using standard extraction methods, DNA was isolated from whole blood, collected from the femoral vein under ketamine anesthesia (15 mg/kg, IM). To genotype for -2232 C>G, a functional SNP that represented one of the major haplotype clades, a portion of the 5′flanking region (−2730 > −2204) was amplified from 25 ng of genomic DNA with flanking oligonucleotide primers (Forward: 5′-GGT TCT CAT TTA AAC CGA GTG ATC-3′; Reverse: 5′-AAG TGG CTC CAA CTA GGG AGT AAG-3′) in 20 μl reactions using AmpliTaq Gold® and 4 mM MgCl2 according to the manufacturers instructions (Invitrogen, Carlsbad, CA, USA). Amplifications were performed on a PerkinElmer thermocycler (9700) with one cycle at 96oC followed by 30 cycles of 94°C/15 sec, 55°C/15 sec, 72°C/30 sec, and a final 3-minute extension at 72°C. Because results of 5′-Exonuclease assay genotyping for -2232C/G were unreliable, we performed restriction digest by EarI (1.5 μl, New England Biolabs, Beverly, MA, USA) using 10 μl of PCR product in a total volume of 20 μl for 4 h at 37°C. Samples were separated by electrophoresis on 10% polyacrylamide gels, and the C and G alleles were identified by direct visualization following ethidium bromide staining. 307 animals from the NIHAC colony were genotyped using this method.
EXPERIMENTAL ANIMALS: PHYSIOLOGIC AND BEHAVIOR ASSESSMENTS
Animals
Rhesus macaque (Macaca mulatta) infants at NIHAC were randomly selected to be reared with their mothers (MR) or in a nursery by human caregivers (peer-reared, PR). Mother-reared (MR) animals were reared in social groups composed of 8–14 females (about half of whom had same-aged infants) and two adult males. Peer-reared (PR) animals were separated from their mothers at birth and hand-reared in a neonatal nursery for the first 37 days of life. For the first 14 days, they were kept in an incubator and hand-fed. From day 15 until day 37, they were placed alone in a nursery cage and provided a blanket and a terrycloth-covered, rocking surrogate. A bottle from which the infants would feed was fixed to the surrogate. At 37 days of age, they were placed in a cage with three other age-mates with whom they had continuous contact. At approximately 8 months of age, animals (both MR and PR) were placed into age-matched social groups and housed in large indoor-outdoor runs through late adolescence/adulthood (3.5–5 years), at which point the cohorts were divided into sex-limited groups. All procedures described were approved by the NIAAA and NICHD Animal Care and Use Committees.
Sample Collection and Processing
Blood and cerebrospinal fliud (CSF) samples were taken at 3.5–5 years of age (N=72 and N=139, respectively) under ketamine anesthesia (10 mg/kg, IM) for measurement of levels of CRH, ACTH and cortisol. Samples were obtained within five minutes of capture. Cisternal CSF samples were immediately aliquoted in polypropylene tubes, and frozen in liquid nitrogen. Blood samples were placed on wet ice and centrifuged at 4°C for 20 min, after which plasma was aliquoted and immediately frozen in liquid nitrogen. CSF and plasma samples were stored at −70°C until assayed.
CSF was assayed for CRH by radioimmunoassay coupled with C18 Sep-pak extraction using our own antibodies as previously described33. Extracted samples were reconstituted in a buffer at a concentration 3-fold higher than the original samples. The sensitivity of the assays was 15 pg/ml for CRH. Plasma ACTH and cortisol radioimmunoassays utilized commercially available kits (ICN and DPC, Los Angeles, CA, respectively), and were run according to the instructions of the manufacturers. All assays were run in duplicate and the inter- and intra-assay coefficients of variation were all less than 12%.
Behavior Assessments: Early Infant Homecage Behavior and Intruder Challenge Test
Mother-reared rhesus macaque infants (N=95) were scored for 10 min twice a week in the social group for the first 24 weeks of life. Behavior definitions are listed in Table S1. Interobserver reliability was established at greater than r = 0.85 for all behaviors.
Behavioral responses to an unfamiliar intruder were recorded in adolescent/adult rhesus macaques (N = 113). “Intruder” animals were selected based on the age and sex of the test subjects, such that adult animals (7.5 ± 1.6 years of age) were exposed to an unfamiliar adult of the same sex, and subadults (3.3 ± 1 years of age) were exposed to subadult animals of the same sex. Intruders were also selected to match the size of the test subjects as closely as possible. All intruder animals were completely unfamiliar to the test subjects. Prior to the intruder challenge test, the intruder animal was placed into an individual transfer cage, measuring 0.76 m wide × 0.63 m deep × 0.91 m high, for a 30-minute acclimation period. All subjects were tested three at a time in the home-run. For the test, three randomly grouped animals were locked into the outdoor portion of their home run, an enclosure measuring 2.64 m wide × 3 m long × 2.44 m high. After ten minutes, the intruder animal’s cage was placed directly at the front of the enclosure and behavioral scoring of the test subjects was initiated. One observer was assigned to each test subject, which was observed for 30 minutes using focal animal continuous recording. Behaviors recorded are listed in Table S1. Behaviors were generally scored in seconds in duration. Vocalization, aggression, and approach intruder were scored in frequency. Interobserver reliability was established at greater than r = 0.85 for all behaviors.
Alcohol Consumption
Young adult macaques (age 3.5–5, N=74) were allowed to freely consume an aspartame-sweetened 8.4% (v/v) alcohol solution for one hour per day, 5 days a week in their home run. This method consisted of three phases, which have previously been reported 17. (1) Spout Training; (2) Initial Alcohol Exposure; and (3) Experimental Period. During the 6-week experimental phase, alcohol and vehicle were dispensed 5 days a week (Monday-Friday) from 1300 to 1400 while the animals were in their home-cage environment. Animals were fitted with a collar implanted with an identifier chip so that volumes consumed could be recorded for each individual.
STATISTICAL ANALYSES
Using data collected from mother-reared infants in the home cage we performed factor analysis to generate behavioral dimensions from behaviors averaged over the 18th through the 24th weeks of life, a developmental stage during which infants are being weaned by their mothers and begin to spend much of their time interacting with other members of the social group and exploring. Factor analysis was also performed on behaviors collected during the intruder challenge test. In each instance, principal components analysis, followed by standard orthogonal (Varimax) normalized rotation, was performed, and factor scores were generated for each individual. Behavioral dimensions were labeled by investigators with expertise in primate behavior, and were consistent with factors that have been generated by other primatologists studying temperamental differences 34 or responses to an intruder 35. The behavioral dimensions generated were then used as dependent variables in ANOVA, to test the hypothesis that animals carrying the -2232 G allele (carried on the rhCRH H2 haplotype) would show different scores on one or more behavioral dimensions. Because age and sex are known to influence responses to an intruder, age (adult vs. subadult) and sex (male vs. female) were included as independent measures for these analyses.
We analyzed baseline levels of CRH, ACTH and Cortisol using ANOVA. Analyses were performed with rearing condition as a co-independent variable, but since there were no interactions with genotype and because the inclusion of this variable did not reduce the residual variance, it was removed from the analyses. Alcohol consumption data were also analyzed by ANOVA. To control for potential differences in drinking patterns among test cohorts, we also generated z-scores controlling for cohort. Prior reports from this lab have shown that alcohol consumption is higher among peer-reared macaques and that functional genetic variants can interact with early rearing history to influence levels of ethanol self-administration 17, 36. Because of these established differences and the fact that males generally consume more ethanol than females, both sex and rearing condition were included as a co-independent variables in the analysis.
Although this is an outbred colony of macaques 37, to verify that our effects were attributable to CRH variation, and not to general heritability of stress responsivity, we repeated our analyses using a set of six randomly selected bi-allelic genetic markers used for genotyping in our colony. There were no effects of the other markers tested (data not shown), suggesting our results to be attributable to effects of rhCRH variation on our phenotypes of interest. -2232 C/G allele frequencies were in agreement with Hardy-Weinberg. There were two animals with the G/G genotype among these datasets, which were collapsed with G allele carriers for the purpose of this analysis. To account for non-homogeneity of variances, dependent variables were rank-transformed when appropriate. Analyses were performed using Statview 5.01 statistical software. Criterion for significance was set at P ≤ 0.05.
Results
IDENTIFICATION OF VARIANTS AT THE rhCRH LOCUS
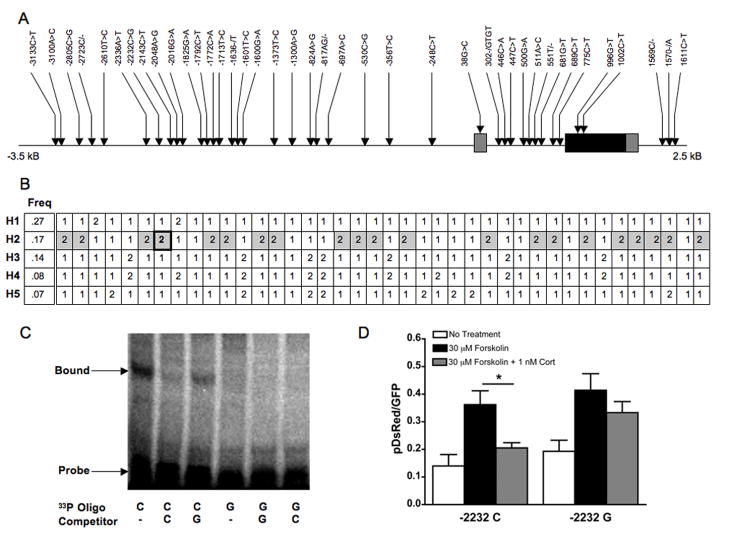
We screened the rhesus macaque CRH gene and 3′ and 5′ flanking regions and identified 40 polymorphic sites (Figure 1A). Variants were assigned positions relative to the rhCRH transcription start site, which was predicted at 68804514 of chromosome 8 (January 2006 assembly-rheMac2, http://genome.ucsc.edu/). The five most common haplotypes are shown in Figure 1B. rhCRH-H1 carried the common allele at almost every site. The second most common (rhCRH-H2) is composed of the minor alleles for a 21-marker haplotype that extends across the 5740-bp region (Figures 1 and 2). Genotyping across populations (NIHAC, University of Wisconsin, Morgan Island) demonstrated this haplotype to be present at similar frequencies (minor allele frequency = 8%, data not shown), and focused re-sequencing showed they were in perfect linkage disequilibrium (LD, D′) (Figure 1B).
Figure 1.
CLADISTIC CLUSTERING OF HAPLOTYPES
Cladistic clustering of the 5 most common rhesus haplotypes demonstrated there to be 2 major haplotype clades (Figure 2, cluster 1= H1, H3-H5 and cluster 2= H2). Using data from European (CEU), African (YRI) and Asian (ASN) human populations for comparison, we also generated cladograms for the human CRH locus (Figure 2). The most common haplotypes (rhCRH-H1 in Rhesus macaque and C1 haplotype cluster in human) share most alleles with the ancestral sequence (data not shown). The African population (YRI) shows more haplotype complexity at the CRH locus than does the Caucasian (CEU) population (Figure 2), whereas all Asians were clustered in C1. The cladograms also demonstrate the existence of alternative (yin-yang) haplotpe clades in both species.
FUNCTIONAL ASSAYS
Glucocorticoids are critical to regulation of the CRH system, and among the SNPs that differed between the 2 major haplotype clades was one that was present in a consensus GRE half-site (-2232 C/G, boxed in Figure 1B) 38, 39. Rhesus exhibited only 92% sequence similarity with humans for the 173bp surrounding this site (-2341>-2168), but among all primate species screened, the GRE half-site within this sequence was 100% conserved (data not shown). In silico analysis indicated that -2232C>G would result in complete disruption of this GRE.
Consistent with the prediction that -2232 C>G would lead to loss of a GRE half-site, gel shift assays performed using hypothalamic nuclear extract (generated from IVB cells, which expresses glucocorticoid receptors and in which corticosteroid effects on CRH expression have been extensively studied) demonstrated an attenuation in protein binding to -2232G probes (Figure 1C). Similar results were obtained using a nuclear extract enriched for GR (data not shown). To test whether corticosteroid-sensitivity of the CRH regulatory region was altered as a consequence of this SNP, reporter assays were performed in HT22 cells transfected with -2232C or G pDsRed constructs. There were main effects of treatment (F(2,27) = 11.8, P = 0.0002) and genotype (F(1,27) = 4.7, P = 0.04). A significant decrease in reporter activity following cortisol treatment was observed in forskolin-stimulated cells expressing the -2232 C allele constructs (Figure 1D, Tukey-Kramer, P < 0.05), but this effect was not observed in those transfected with G allele constructs (Figure 1D, n.s.) (No Treatment: C, N=5, G, N=4; Forskolin: C, N=6, G, N=5; Forskolin + Cort: C, N=6, G, N=7).
No other variants in the H2 haplotype were predicted to disrupt transcription factor binding sites for factors known to be involved in CRH transcriptional control. While another site (-2336 A>T) that was part of this haplotype predicted the creation of a YY1 site, gel shift assays indicated that DNA-protein interactions were not altered as a consequence of this variant (data not shown). There were neither non-synonymous SNPs nor variants predicted to alter exon-intron splicing or mRNA stability.
EFFECTS OF rhCRH -2232 G ALLELE ON INTERMEDIATE PHENOTYPES
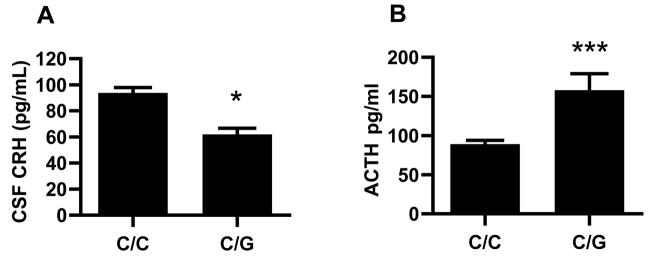
Among adolescent/young adult animals, those with the rhCRH -2232 C/G genotype (H2) had lower CSF levels of CRH (Figure 3A, F(1,70) = 4.7, P < 0.04) and higher plasma levels of ACTH (Figure 3B, F(1,138) = 7.96, P = 0.005), with genotype accounting for 6–7% of the variance. Cortisol levels did not differ according to genotype (n.s., data not shown). These findings were the same after controlling for differences in early stress exposure (i.e., rearing condition).
Figure 3.
BEHAVIORAL EFFECTS OF rhCRH -2232 C>G
Infant Behavioral Dimensions
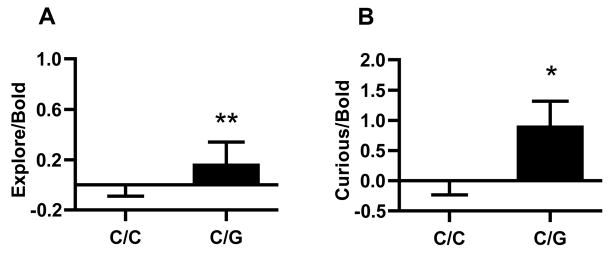
Principal component extraction, followed by standard Varimax normalized rotation, identified three orthogonal factors with Eigenvalues >1.0 which, together, explained 73.0% of the variance (Table 1). These were labeled as “Anxious”, “Exploratory/Bold” and “Attached” 34. Animals with the C/G genotype rated higher on the “Exploratory/Bold” dimension (Figure 4A, F(1, 93)=6.6, P =0.01) than did those homozygous for the C allele, with genotype accounting for 7% of the total variance of this trait. In contrast, the other behavioral dimensions were not influenced by genotype (n.s.).
Table 1.
| FACTOR | Eigen Value | Loading | CRH -2232 C>G | |
|---|---|---|---|---|
| “Anxious” | 4.8 | Locomotion | 0.709 | No effect |
| Passive | 0.650 | |||
| Mutual Ventral | 0.411 | |||
|
| ||||
| “Exploratory/Bold | 1.75 | Explore Environment | 0.639 | P ≤ 0.01 (C/G > C/C) |
| Leave by Infant | 0.603 | |||
| Approach by Infant | 0.560 | |||
| Vocalization | 0.875 | |||
| Aggression | 0.878 | |||
|
| ||||
| “Attached” | 1.37 | Leave by Infant | 0.630 | No effect |
| Approach by Infant | 0.560 | |||
| Social Contact- Mother | 0.597 | |||
| Mutual Break | 0.874 | |||
Figure 4.
Responses to an Unfamiliar Rhesus Macaque
When we performed factor analysis of the behavioral responses to an unfamiliar intruder, five orthogonal factors with Eigenvalues greater than 1 were generated: “Agonistic/Highly Aggressive,” “Harm Avoidant,” “Curious/Bold,” “Threatening,” and “Reactive.” These factors explained 68% of the total variance (Table 2). Prior reports from other laboratories have demonstrated there to be both sex and age differences in responses to an unfamiliar intruder 35. In agreement with this, we found that, during social intrusion, sub-adults were more harm-avoidant than adults (F(1,105) = 12.19, P = 0.007) and that males rated higher on both the Curious/Bold (F(1,105) = 6.7, P = 0.01) and Reactive factors (F(1, 105) = 5.6, P = 0.02) (data not shown).
Table 2.
| FACTOR | Eigen Value | Loading | CRH -2232 C>G | |
|---|---|---|---|---|
| “Agonistic“(High Risk Aggression) | 2.4 | Approach Intruder | 0.61 | No effect |
| Social Contact-INT | 0.89 | |||
| Contact Aggression- INT | 0.89 | |||
| “Harm Avoidant | 1.7 | Social Contact- Other | 0.92 | No effect |
| Passive | −0.68 | |||
| “Curious/Bold” | 1.3 | Locomotion | 0.87 | P ≤ 0.0002 (Males: C/G >C/C) |
| Approach Intruder | 0.48 | |||
| Passive | −0.57 | |||
| “Threatening” (Low Risk Aggression) | 1.1 | Non-Contact Aggression-INT | 0.60 | No effect |
| Explore Environment | 0.59 | |||
| Stereotypy/Stypic | −0.66 | |||
| “Reactive” | 1.05 | Self-Directed Behavior | 0.66 | No effect |
| Vocalization | −0.75 |
While there were no main effects of rhCRH genotype on the Curious/Bold response to an unfamiliar intruder (F(1,105)=1.13, P = 0.29), -2232 C/G genotype strongly interacted with sex (F(1, 105) = 10.4, P = 0.002) to influence this type of response, accounting for 8% of the total variance. Males that were carriers of the G allele exhibited significantly increased levels of Curious/Bold responding when presented with an unfamiliar male, compared to C homozygotes (Figure 4B, Tukey-Kramer, P < 0.05), while no such differences were observed among females (n.s.). This remained true following rank-transformation of the data (F(1,105) = 8.3, P < 0.005). There were no main effects of genotype nor were there interactions among genotype, age and sex on the other reactivity dimensions.
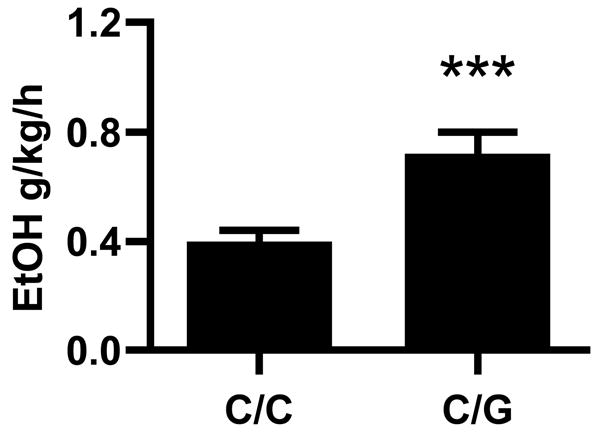
Alcohol Consumption
C/G animals consumed more alcohol in the social group than did those with the C/C genotype (Figure 5, F(1,72) = 8.5, P = 0.005). Genotype accounted for 12% of the total variance in ethanol consumption, and a main effect of genotype remained after controlling for rearing condition (peer-reared vs. mother-reared) and sex (male vs. female) (F(1,66) = 5.04, P ≤ 0.03; C/C vs G, Tukey/Kramer, P < 0.05). It also remained following both statistical correction for testing cohort (F(1,66)=4.5, P ≤ 0.04) and rank transformation of the data (F(1,66) = 7.9, P < 0.007). In each of these analyses, G allele carriers exhibited higher levels of alcohol self-administration (Tukey/Kramer, P < 0.05).
Figure 5.
COMMENT
The neurobiological systems that influence addiction vulnerability may do so by acting on reward pathways, behavioral dyscontrol, and vulnerability to stress and anxiety. The CRH system is one that is known to be dysregulated in alcohol-dependent subjects and in which variation may influence both stress-responding and novelty-seeking. We screened the rhesus macaque CRH gene and regulatory region and identified a SNP that disrupted a GRE half-site in the CRH regulatory region. We demonstrated that this functional variant predicted endocrine and behavioral differences in addition to levels of alcohol self-administration in the rhesus macaque.
Glucocorticoids are known to be critical to CRH transcriptional regulation, via binding to GREs. GREs are hormone inducible enhancer elements 40 that can activate or repress transcription through cooperative action with additional GRE half-sites or other DNA-bound proteins 41, 42. The nucleotide sequence in which we identified variation (-2232 C/G) is a consensus GRE half- site. GREs exert effects in regions distant from the core promoter 43, and half-sites can interact with one another over distances of hundreds of nucleotides 25. It is also known that cooperative binding among receptors bound to GRE half-sites leads to high occupancy at low concentrations and nonlinearity of binding 44. We used GR-containing nuclear extracts, generated from a hypothalamic cell line, to show that -2232 G results in decreased levels of DNA-protein interactions. These results support the notion that the AGAACA (-2232 C) site is functional.
Using an in vitro reporter assay, we also demonstrated there to be attenuated sensitivity of the rhCRH promoter to corticosteroids in cells expressing the G allele construct. Even with the introduction of the G allele, some degree of corticosteroid sensitivity was maintained. This is not surprising, given that other glucocorticoid receptor binding sites are present in the rhCRH promoter. However, it is possible that the GRE half site in which we identified variation could be functionally important through another mechanism. Binding of a hormone-receptor complex to a consensus GRE half-site can aid in recruitment of glucocorticoid receptors to a particular genomic region and facilitate binding of other receptor monomers to surrounding non-consensus sites 45. This site may aid in GR binding to the non-consensus sites in the rhCRH promoter that have been demonstrated to be important to regulation of CRH transcriptional activity, an effect that would be unlikely to be appreciated in a reporter system, which is enriched for the genomic region of interest. Regardless of the exact mechanism, we do indeed find effects of the -2232 G allele on CSF CRH levels and HPA axis output, and these effects are consistent with decreased sensitivity of the CRH promoter to glucocorticoids.
In the paraventricular nucleus (PVN) of the hypothalamus, corticosteroids reduce CRH expression, but in some extrahypothalamic sites, such as the amygdala, they produce opposite effects 29, 46–49. We find that plasma levels of ACTH are increased, whereas, in CSF, levels of CRH are decreased. The lower CSF CRH levels can be accounted for based on the likelihood of a low or nonexistent contribution of PVN-median eminence CRH to the CSF pool relative to other sources, such as the amygdala. 33 Our finding is consistent with what have been previously reported in human and non-human primates, which indicate opposing rhythms of CSF levels of CRH and HPA axis output. 33,43 In rodents, some exploratory/novelty-seeking strains have higher levels of Crh expression in the hypothalamus, but lower levels in the amygdala 50 and others find that bold/aggressive rat strains have higher baseline levels of ACTH 10. We examined behavioral dimensions during infancy and found that carriers of the -2232 G allele were more “Exploratory/bold,” exhibiting relatively high levels of environmental exploration and aggression. We also employed a challenge that has been developed to test behavioral responses to social intrusion in adolescent or adult primates. This test is not only an excellent tool for looking at coping responses during exposures to social stress, but is especially useful for assessment of individual differences in behavioral inhibition, as approaching an unfamiliar animal is considered to be a risky and impulsive behavior 35. Consistent with the fact that G allele carriers rated higher on the exploratory/bold dimension during infancy, we found that male G allele carriers exhibited a more bold and active response to an unfamiliar intruder. An individual that readily approaches novel objects or conspecifics may do well in certain social situations, but may face higher risk of predation or attack than a more cautious, harm-avoidant individual. Such behaviors might, therefore, be predicted to confer selective advantage at particular developmental or life history stages and in certain environmental contexts.
Twenty mutations at the rhesus CRH locus were in linkage disequilibrium (LD, D′) with -2232 C>G, and roughly 15% of individuals in the NIH population were heterozygous at these sites (MAF = 8%). Sequencing of the regions containing each polymorphic site in this haplotype demonstrated that these 21 markers were in perfect LD in multiple macaque populations, in which they also occurred at similar frequencies. Neutral sites in LD with an allele maintained by selection have a better chance of remaining in the population 51, 52. The persistence of the two divergent rhCRH haplotypes over time may suggest that they have been subject to selection such that at least one of the alleles on each background is being selected–possibly in a particular environmental context–while the rest are hitchhiking. The effects of the H2 haplotype on our intermediate phenotypes (i.e., decreased CSF CRH but increased plasma ACTH) are consistent with our in vitro findings, which demonstrated the loss of a GRE half-site and decreased sensitivity to corticosteroids. However, as we did not sequence beyond the boundaries of this haplotype, we do not know whether there is another functional marker in LD with -2232 G, nor do we know about the block size, which might provide further evidence for selection. That being said, several studies in humans 53, 54 have shown there to be evidence for selection at the CRH locus, in which, similar to the rhesus macaque, we have observed alternative, yin-yang haplotype clades (Figure 2). As in the rhesus macaque, the major human CRH haplotypes have been shown to vary in terms of their in vitro promoter activity, and among the observed differences are those pertaining to glucocorticoid-sensitivity20.
Because of differences in their behavioral and physiologic responses to stress, the types of stress-related pathology to which bold, proactive individuals and harm-avoidant, reactive individuals are vulnerable are distinct. Whereas the latter are at risk for internalizing disorders, such as depression and anxiety, the former are more likely to develop externalizing conditions, primarily characterized by impaired impulse control 10. In humans, anxiety is a risk factor for developing alcohol problems, and stress exposures can lead to craving and relapse 14, 15 55. It is also known that impulsivity or behavioral dyscontrol can predispose individuals to early and uncontrolled alcohol intake 14, 15. We find that carriers of the -2232 G allele exhibit higher levels of alcohol consumption when tested in the social group, a model for high-risk, impulsivity-related alcohol consumption. They also exhibit lower levels of the serotonin metabolite, 5-HIAA (data not shown), a neurochemical endophenotype observed both in macaques exposed to early life stress and among individuals with early-onset, Type II alcoholism 37, 56. It may be that, in humans, genetic variation that altered CRH system function could influence multiple behavioral dimensions (i.e., both neuroticism and extraversion) and that variants that placed an individual at the extremes of these spectra (i.e., inhibited and anxious/stress reactive vs. bold/impulsive and novelty seeking) could increase the risk for developing alcohol use disorders.
The present investigation includes some of the largest sample sizes that are currently available for a relatively homogeneous outbred macaque colony. Nevertheless, the numbers of animals represented in the datasets used for this study are quite small relative to those used for performing association studies in human populations. For this reason, we neither have the power to perform refined haplotype analysis nor can we examine complex interactive effects with other functional genetic variants (i.e., HTT-LPR, MAOA-LPR, OPRM1 C77G) that may be contributing to the variance in our phenotypes of interest. We have elected to use an approach that relies on cladistic clustering of haplotypes and functional characterization of variants in order to determine whether genetic variation is associated with our phenotypes of interest, using factor analysis to generate behavioral clusters so that the number of statistical comparisons is minimized. Experimental studies in humans as well as non-human primates 16, 37, 57, 58 have repeatedly demonstrated the gain in power that comes from designs that genotype a relatively small number of subjects for functional genetic variation, measure fine-grained, quantitative phenotypic traits, and apply the power of general linear models to test the a priori hypothesis. In this study, we demonstrate that a functional rhCRH promoter variant is associated with relevant intermediate phenotypes in addition to behavioral traits that were assessed at multiple developmental time points and which have been, in both primate and non-primate species, repeatedly and reliably linked to differences in CRH system activity.
In a social environment and due to niche-specific and frequency-dependent selection, divergent physiologic and behavioral stress responses will be maintained in a population because the benefits and risks of opposing behavioral strategies will balance one another in different contexts. Two basic behavioral phenotypes have been observed and are thought to be maintained by balancing selection in a wide variety of animal species, including humans. While one is characterized as being aggressive and bold, adopting a more proactive coping style, and fight or flight responses to stress, the other is more harm avoidant and exhibits reactive, anxiety-like behaviors following stress. A conceptual framework has been put forth that suggests differences in these behavioral strategies to have implications not only in terms of selection, but in terms of vulnerability to psychopathology 10. The CRH system is proposed to critically contribute to such differences. There is evidence that variation at the CRH locus predicts levels of behavioral inhibition in children 59, and genetic variation at the CRHR1 locus is suggested to be a risk factor for alcohol problems. Functional CRH haplotypes have been associated with various non-psychiatric disorders such as rheumatoid arthritis 60, but few studies examining the role of CRH variation in the vulnerability to psychiatric disorders have been reported. Our data suggest common selective mechanisms in humans and macaques, and may indicate that corticosteroid-insensitive CRH haplotypes could increase risk for externalizing disorders in human subjects.
Supplementary Material
Table S1. Behavior Definitions for Focal Scoring
Acknowledgments
We would like to thank Karen Smith for assistance in the preparation of this manuscript and the research and animal care staff at the NIAAA and NIHAC for their assistance in data collection. This work was funded by NARSAD and the NIAAA and NICHD intramural programs.
Reference List
- 1.Hansson AC, Cippitelli A, Sommer W, Ciccocioppo R, Heilig M. Region-specific down regulation of Crhr1 gene expression in alcohol preferring msP rats following ad lib access to alcohol. Addict Biol. 2007;12:30–4. doi: 10.1111/j.1369-1600.2007.00050.x. [DOI] [PubMed] [Google Scholar]
- 2.Lee S, Schmidt ED, Tilders FJ, Rivier C. Effect of repeated exposure to alcohol on the response of the hypothalamic-pituitary-adrenal axis of the rat: I. Role of changes in hypothalamic neuronal activity. Alcohol Clin Exp Res. 2001;25(1):98–105. [PubMed] [Google Scholar]
- 3.Rasmussen DD, Boldt BM, Bryant CA, Mitton DR, Larsen SA, Wilkinson CW. Chronic daily ethanol and withdrawal: 1. Long-term changes in the hypothalamopituitary-adrenal axis. Alcohol Clin Exp Res. 2000;24(12):1836–49. [PubMed] [Google Scholar]
- 4.Zhou Y, Franck J, Spangler R, Maggos CE, Ho A, Kreek MJ. Reduced hypothalamic POMC and anterior pituitary CRF1 receptor mRNA levels after acute, but not chronic, daily “binge” intragastric alcohol administration. Alcohol Clin Exp Res. 2000;24(10):1575–82. [PubMed] [Google Scholar]
- 5.George SR, Fan T, Roldan L, Naranjo CA. Corticotropin-releasing factor is altered in brains of animals with high preference for ethanol. Alcohol Clin Exp Res. 1990;14(3):425–9. doi: 10.1111/j.1530-0277.1990.tb00499.x. [DOI] [PubMed] [Google Scholar]
- 6.Hansson AC, Cippitelli A, Sommer WH, et al. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc Natl Acad Sci U S A. 2006;103(41):15236–41. doi: 10.1073/pnas.0604419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hwang BH, Stewart R, Zhang JK, Lumeng L, Li TK. Corticotropin-releasing factor gene expression is down-regulated in the central nucleus of the amygdala of alcohol-preferring rats which exhibit high anxiety: a comparison between rat lines selectively bred for high and low alcohol preference. Brain Res. 2004;1026(1):143–50. doi: 10.1016/j.brainres.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 8.Richter RM, Zorrilla EP, Basso AM, Koob GF, Weiss F. Altered amygdalar CRF release and increased anxiety-like behavior in Sardinian alcohol-preferring rats: a microdialysis and behavioral study. Alcohol Clin Exp Res. 2000;24(12):1765–72. [PubMed] [Google Scholar]
- 9.Goldman D, Barr CS. Restoring the addicted brain. N Engl J Med. 2002;347(11):843–5. doi: 10.1056/NEJMcibr021948. [DOI] [PubMed] [Google Scholar]
- 10.Korte SM, Koolhaas JM, Wingfield JC, McEwen BS. The Darwinian concept of stress: benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci Biobehav Rev. 2005;29(1):3–38. doi: 10.1016/j.neubiorev.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Kagan J, Reznick JS, Snidman N. Biological bases of childhood shyness. Science. 1988;240(4849):167–71. doi: 10.1126/science.3353713. [DOI] [PubMed] [Google Scholar]
- 12.Kalin NH. Asymmetric frontal brain activity, cortisol, and behavior associated with fearful temperament in rhesus monkeys. Behav Neurosci. 1998;112(2):286–92. doi: 10.1037//0735-7044.112.2.286. [DOI] [PubMed] [Google Scholar]
- 13.Kalin NH, Shelton SE, Davidson RJ. Cerebrospinal fluid corticotropin-releasing hormone levels are elevated in monkeys with patterns of brain activity associated with fearful temperament. Biol Psychiatry. 2000;47(7):579–85. doi: 10.1016/s0006-3223(99)00256-5. [DOI] [PubMed] [Google Scholar]
- 14.Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6(7):521–32. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- 15.Barr CS, Goldman D. Non-human primate models of inheritance vulnerability to alcohol use disorders. Addict Biol. 2006;11(3–4):374–85. doi: 10.1111/j.1369-1600.2005.00034.x. [DOI] [PubMed] [Google Scholar]
- 16.Barr CS, Newman TK, Lindell S, et al. Interaction between serotonin transporter gene variation and rearing condition in alcohol preference and consumption in female primates. Arch Gen Psychiatry. 2004;61(11):1146–52. doi: 10.1001/archpsyc.61.11.1146. [DOI] [PubMed] [Google Scholar]
- 17.Barr CS, Schwandt M, Lindell SG, et al. Association of a functional polymorphism in the mu-opioid receptor gene with alcohol response and consumption in male rhesus macaques. Arch Gen Psychiatry. 2007;64(3):369–76. doi: 10.1001/archpsyc.64.3.369. [DOI] [PubMed] [Google Scholar]
- 18.Newman TK, Syagailo YV, Barr CS, et al. Monoamine oxidase A gene promoter variation and rearing experience influences aggressive behavior in rhesus monkeys. Biol Psychiatry. 2005;57(2):167–72. doi: 10.1016/j.biopsych.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 19.Miller GM, Bendor J, Tiefenbacher S, Yang H, Novak MA, Madras BK. A mu-opioid receptor single nucleotide polymorphism in rhesus monkey: association with stress response and aggression. Mol Psychiatry. 2004;9(1):99–108. doi: 10.1038/sj.mp.4001378. [DOI] [PubMed] [Google Scholar]
- 20.Wagner U, Wahle M, Moritz F, Wagner U, Hantzschel H, Baerwald CG. Promoter polymorphisms regulating corticotrophin-releasing hormone transcription in vitro. Horm Metab Res. 2006;38(2):69–75. doi: 10.1055/s-2006-925115. [DOI] [PubMed] [Google Scholar]
- 21.Kel-Margoulis OV, Kel AE, Reuter I, Deineko IV, Wingender E. TRANSCompel: a database on composite regulatory elements in eukaryotic genes. Nucleic Acids Res. 2002;30(1):332–4. doi: 10.1093/nar/30.1.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghosh D. Object-oriented transcription factors database (ooTFD) Nucleic Acids Res. 2000;28(1):308–10. doi: 10.1093/nar/28.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pollard KS, Salama SR, Lambert N, et al. An RNA gene expressed during cortical development evolved rapidly in humans. Nature. 2006;443(7108):167–72. doi: 10.1038/nature05113. [DOI] [PubMed] [Google Scholar]
- 24.Budziszewska B, Jaworska-Feil L, Tetich M, et al. Regulation of the human corticotropin-releasing-hormone gene promoter activity by antidepressant drugs in Neuro-2A and AtT-20 cells. Neuropsychopharmacology. 2004;29(4):785–94. doi: 10.1038/sj.npp.1300379. [DOI] [PubMed] [Google Scholar]
- 25.Schuetz JD, Schuetz EG, Thottassery JV, Guzelian PS, Strom S, Sun D. Identification of a novel dexamethasone responsive enhancer in the human CYP3A5 gene and its activation in human and rat liver cells. Mol Pharmacol. 1996;49(1):63–72. [PubMed] [Google Scholar]
- 26.Maher P, Schubert D. Signaling by reactive oxygen species in the nervous system. Cell Mol Life Sci. 2000;57(8–9):1287–305. doi: 10.1007/PL00000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Pongrac JL, DeFranco DB. Glucocorticoid receptors in hippocampal neurons that do not engage proteasomes escape from hormone-dependent down-regulation but maintain transactivation activity. Mol Endocrinol. 2002;16(9):1987–98. doi: 10.1210/me.2001-0287. [DOI] [PubMed] [Google Scholar]
- 28.Hatalski CG, Brunson KL, Tantayanubutr B, Chen Y, Baram TZ. Neuronal activity and stress differentially regulate hippocampal and hypothalamic corticotropin-releasing hormone expression in the immature rat. Neuroscience. 2000;101(3):571–80. doi: 10.1016/s0306-4522(00)00386-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kasckow J, Mulchahey JJ, Aguilera G, et al. Corticotropin-releasing hormone (CRH) expression and protein kinase A mediated CRH receptor signalling in an immortalized hypothalamic cell line. J Neuroendocrinol. 2003;15(5):521–9. doi: 10.1046/j.1365-2826.2003.01026.x. [DOI] [PubMed] [Google Scholar]
- 30.Nishi M, Tanaka M, Matsuda K, Sunaguchi M, Kawata M. Visualization of glucocorticoid receptor and mineralocorticoid receptor interactions in living cells with GFP-based fluorescence resonance energy transfer. J Neurosci. 2004;24(21):4918–27. doi: 10.1523/JNEUROSCI.5495-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Behl C, Lezoualc’h F, Trapp T, Widmann M, Skutella T, Holsboer F. Glucocorticoids enhance oxidative stress-induced cell death in hippocampal neurons in vitro. Endocrinology. 1997;138(1):101–6. doi: 10.1210/endo.138.1.4835. [DOI] [PubMed] [Google Scholar]
- 32.Landfield PW, McEwan BS, Sapolsky RM, Meaney MJ. Hippocampal cell death. Science. 1996;272(5266):1249–51. [PubMed] [Google Scholar]
- 33.Kling MA, DeBellis MD, O’Rourke DK, et al. Diurnal variation of cerebrospinal fluid immunoreactive corticotropin-releasing hormone levels in healthy volunteers. J Clin Endocrinol Metab. 1994;79(1):233–9. doi: 10.1210/jcem.79.1.8027234. [DOI] [PubMed] [Google Scholar]
- 34.Capitanio JP. Personality dimensions in adult male rhesus macaques: prediction of behaviors across time and situation. Am J Primatol. 1999;47(4):299–320. doi: 10.1002/(SICI)1098-2345(1999)47:4<299::AID-AJP3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 35.Fairbanks LA, Newman TK, Bailey JN, et al. Genetic contributions to social impulsivity and aggressiveness in vervet monkeys. Biol Psychiatry. 2004;55(6):642–7. doi: 10.1016/j.biopsych.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Higley JD, Hasert MF, Suomi SJ, Linnoila M. Nonhuman primate model of alcohol abuse: effects of early experience, personality, and stress on alcohol consumption. Proc Natl Acad Sci U S A. 1991;88(16):7261–5. doi: 10.1073/pnas.88.16.7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barr CS, Newman TK, Schwandt M, et al. Sexual dichotomy of an interaction between early adversity and the serotonin transporter gene promoter variant in rhesus macaques. Proc Natl Acad Sci U S A. 2004;101(33):12358–63. doi: 10.1073/pnas.0403763101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nordeen SK, Suh BJ, Kuhnel B, Hutchison CA. Structural determinants of a glucocorticoid receptor recognition element. Mol Endocrinol. 1990;4(12):1866–73. doi: 10.1210/mend-4-12-1866. [DOI] [PubMed] [Google Scholar]
- 39.Wolfl S, Martinez C, Rich A, Majzoub JA. Transcription of the human corticotropin-releasing hormone gene in NPLC cells is correlated with Z-DNA formation. Proc Natl Acad Sci U S A. 1996;93(8):3664–8. doi: 10.1073/pnas.93.8.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Del Monaco M, Covello SP, Kennedy SH, Gilinger G, Litwack G, Uitto J. Identification of novel glucocorticoid-response elements in human elastin promoter and demonstration of nucleotide sequence specificity of the receptor binding. J Invest Dermatol. 1997;108(6):938–42. doi: 10.1111/1523-1747.ep12295241. [DOI] [PubMed] [Google Scholar]
- 41.Morin B, Zhu C, Woodcock GR, et al. The binding site for Xenopus glucocorticoid receptor accessory factor and a single adjacent half-GRE form an independent glucocorticoid response unit. Biochemistry (Mosc) 2000;39(40):12234–42. doi: 10.1021/bi000981s. [DOI] [PubMed] [Google Scholar]
- 42.Peterkofsky B, Gosiewska A, Singh K, Pearlman S, Mahmoodian F. Species differences in cis-elements of the proalpha1(I) procollagen promoter and their binding proteins. J Cell Biochem. 1999;73(3):408–22. [PubMed] [Google Scholar]
- 43.Bailly A, Torres-Padilla ME, Tinel AP, Weiss MC. An enhancer element 6 kb upstream of the mouse HNF4alpha1 promoter is activated by glucocorticoids and liver-enriched transcription factors. Nucleic Acids Res. 2001;29(17):3495–505. doi: 10.1093/nar/29.17.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmid W, Strahle U, Schutz G, Schmitt J, Stunnenberg H. Glucocorticoid receptor binds cooperatively to adjacent recognition sites. EMBO J. 1989;8(8):2257–63. doi: 10.1002/j.1460-2075.1989.tb08350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Freedman LP. Anatomy of the steroid receptor zinc finger region. Endocr Rev. 1992;13(2):129–45. doi: 10.1210/edrv-13-2-129. [DOI] [PubMed] [Google Scholar]
- 46.King BR, Nicholson RC. Advances in understanding corticotrophin-releasing hormone gene expression. Front Biosci. 2007;12:581–90. doi: 10.2741/2084. [DOI] [PubMed] [Google Scholar]
- 47.Makino S, Hashimoto K, Gold PW. Multiple feedback mechanisms activating corticotropin-releasing hormone system in the brain during stress. Pharmacol Biochem Behav. 2002;73(1):147–58. doi: 10.1016/s0091-3057(02)00791-8. [DOI] [PubMed] [Google Scholar]
- 48.Schulkin J. Allostasis: a neural behavioral perspective. Horm Behav. 2003;43(1):21–7. doi: 10.1016/s0018-506x(02)00035-1. [DOI] [PubMed] [Google Scholar]
- 49.Swanson LW, Simmons DM. Differential steroid hormone and neural influences on peptide mRNA levels in CRH cells of the paraventricular nucleus: a hybridization histochemical study in the rat. J Comp Neurol. 1989;285(4):413–35. doi: 10.1002/cne.902850402. [DOI] [PubMed] [Google Scholar]
- 50.Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. J Neurosci. 2000;20(18):6983–8. doi: 10.1523/JNEUROSCI.20-18-06983.2000. 2000 Sep 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bamshad M, Wooding SP. Signatures of natural selection in the human genome. Nat Rev Genet. 2003;4(2):99–111. doi: 10.1038/nrg999. [DOI] [PubMed] [Google Scholar]
- 52.Klein J, Sato A, Nagl S, O’Huigin C. Molecular trans-species polymorphism. 29:1–21. Annu Rev Ecol Syst. 1998;29:1–21. [Google Scholar]
- 53.Baerwald CG, Mok CC, Fife MS, et al. Distribution of corticotropin-releasing hormone promoter polymorphism in different ethnic groups: evidence for natural selection in human populations. Immunogenetics. 1999;49(10):894–9. doi: 10.1007/s002510050570. [DOI] [PubMed] [Google Scholar]
- 54.Shimmin LC. Corticotropin releasing hormone (CRH) gene variation: Comprehensive resequencing for variant and molecular haplotype discovery in monosomic hybrid cell lines. DNA Seq. 2007;18(6):432–42. doi: 10.1080/10425170701388719. [DOI] [PubMed] [Google Scholar]
- 55.Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug & Alcohol Review. 2007;26(1):25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- 56.Higley JD, Suomi SJ, Linnoila M. A nonhuman primate model of type II alcoholism .2. Diminished social competence and excessive aggression correlates with low cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations. Alcoholism Clinical & Experimental Research. 1996;20(4):643–50. doi: 10.1111/j.1530-0277.1996.tb01666.x. [DOI] [PubMed] [Google Scholar]
- 57.Zubieta JK, Heitzeg MM, Smith YR, et al. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299(5610):1240–3. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]
- 58.Hariri AR, Mattay VS, Tessitore A, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297(5580):400–3. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 59.Smoller JW, Rosenbaum JF, Biederman J, et al. Association of a genetic marker at the corticotropin-releasing hormone locus with behavioral inhibition. Biol Psychiatry. 2003;54(12):1376–81. doi: 10.1016/s0006-3223(03)00598-5. [DOI] [PubMed] [Google Scholar]
- 60.Baerwald CG, Mok CC, Tickly M, et al. Corticotropin releasing hormone (CRH) promoter polymorphisms in various ethnic groups of patients with rheumatoid arthritis. Z Rheumatol. 2000;59(1):29–34. doi: 10.1007/s003930050002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Behavior Definitions for Focal Scoring