Abstract
Purpose of review
Targeting allergens to surface receptors on antigen presenting cells may provide a therapeutic strategy for allergic disease. This article discusses the immunomodulatory capacity of a molecule (H22-Fel d 1) which targets the major cat allergen, Fel d 1, to the high affinity IgG receptor, FcγRI, on human dendritic cells (DCs).
Recent findings
The fusion protein, H22-Fel d 1, induced a semi-mature phenotype in DCs characterized by production of inflammatory cytokines with no change in surface markers, suggesting tolerogenic capacity. At the T-cell level, H22-Fel d 1 stimulated increased proliferation coupled with amplification of T cells expressing IL-5 and IL-10. Further analysis revealed induction of diverse T cell subtypes characteristic of Th0, regulatory Th1 and regulatory Th2 cells. Notably, this effect was restricted to T cells isolated from cat-allergic subjects. Despite the increase in IL-5-expressing T cells, responses induced by H22-Fel d 1 appeared to be regulated by IL-10. Comparison with non-receptor-targeted allergens from cat and house dust mite confirmed that qualitative T cell changes induced by H22-Fel d 1 were unique.
Summary
H22-Fel d 1 induces a novel variation of the Th2 response which incorporates elements of a protective T-cell response. Exploiting FcγRI-mediated pathways for allergen delivery may offer a new approach for treatment.
Keywords: Fel d 1, Fcγ receptors, FcεRI, CD4+ T cells, dendritic cell-based vaccines
INTRODUCTION
The prevalence of allergic disease continues to rise at an alarming rate, both within the U.S. and on a global scale. Allergic diseases pose a huge public health burden with one out of every four Americans now suffering from an allergic disorder. Vaccination strategies which have the potential to offer long-term symptomatic relief would provide a welcome alternative to pharmaceuticals which remain the mainstay of treatment for allergic disease.
Chronic inflammation is a cardinal feature of a variety of allergic diseases including asthma, atopic dermatitis, and allergic rhinitis. Allergen-specific type 2 CD4+ T lymphocytes (Th2 cells) are central to the initiation and maintenance of the allergic inflammatory response through the cytokines they secrete. The Th2 cytokine, IL-4, mediates antibody isotype switching to IgE while IL-5 and IL-13 are pivotal to other pathognomonic features of allergic disease such as eosinophil migration and airway hyperreactivity. Allergen-specific CD4+ T cells recognize allergen-derived peptides (epitopes) presented in the context of MHC class II molecules by antigen presenting cells (APCs). Dendritic cells (DCs) are professional APCs which are key to priming and maintenance of allergen-specific T cell responses. Consequently, allergen-specific vaccines which target DCs could have therapeutic potential based on their ability to modulate DCs and thus, alter T cell responses to allergens.
Here, we highlight the immunomodulatory properties of a novel allergen variant which binds to the high affinity IgG receptor FcγRI (CD64) on DCs. The purpose of this article is to discuss the therapeutic potential of this strategy in the context of existing knowledge regarding the role of receptor-mediated pathways in T cell responses to allergens.
THE RATIONALE FOR TARGETING RECEPTORS ON ANTIGEN PRESENTING CELLS
In some settings, conventional immunotherapy (IT) has proven clinically efficacious for the treatment of allergic disease [1]. There is mounting evidence to suggest that the long-term protective effects of IT are exerted at the T cell level through induction of regulatory T cells (Tregs) which secrete IL-10 and/or Th1 cells which secrete IFN-γ [2–5*]. This approach, which involves administering allergen extracts, poses several disadvantages: (1) There is a risk of IgE-mediated adverse events; (2) Treatment regimens are often prolonged and require considerable patient compliance; (3) Standardization of allergen extracts remains a challenge making it difficult to discern how much of the requisite immunogen is administered with each injection. Consequently, considerable effort has been devoted to the development of allergen-specific DC- or T cell-based vaccines which may confer long-term protection without these drawbacks. As examples, in an attempt to mitigate IgE-mediated side effects, allergen variants and allergen-derived peptides have been generated which lack conformational epitopes but retain the T cell antigenic determinants necessary to activate T cells [6–11]. There is evidence to suggest that these molecules may be useful clinically; however, further studies are needed.
Targeting Allergen to Receptors on Dendritic Cells
Dendritic cells play a pivotal role in the initiation and maintenance of T cell responses to allergens, both in health and in disease. Specifically, the type of T cell response generated can be influenced by the maturational state of DCs. Engagement of a variety of surface receptors on DCs can induce maturation which is characterized by increased expression of an array of surface markers which are co-stimulatory for T cells (CD40, CD80, CD86, OX40L), as well as secretion of pro-inflammatory cytokines. While mature DCs generally induce effector T cell responses (ie. Th1 or Th2), immature or semi-mature DCs are tolerogenic based on their ability to induce Tregs [12,13]. However, the situation is likely more complex as evidenced by recent reports that both mature and immature DCs can exhibit tolerogenic properties [14].
Despite the fact that DCs express many different types of activating surface receptors with immunomodulatory potential, few receptors have been exploited with a view to altering T cell responses to allergens. One important exception has been the toll like receptors (TLRs). These receptors are a key component in the innate immune response to infectious agents by virtue of their ability to recognize pathogen associated molecular patterns. Popularity of the hygiene hypothesis, which posits that increased exposure to Th1-inducing bacteria reduces the risk of developing allergy, spurred studies on how TLR signaling might influence the T cell response to allergen. In a mouse model, engagement of TLR4 by high dose LPS was shown to favor the induction of Th1 responses to inhaled allergen (OVA) [15]. In humans, results from a clinical trial using a TLR9-binding allergen conjugate showed increased induction of Th1-associated cytokines in allergen-stimulated T cell cultures from treated patients [16]. Though there is no doubt that molecules which target TLRs have immunomodulatory potential, their clinical efficacy remains to be determined (Fig. 1).
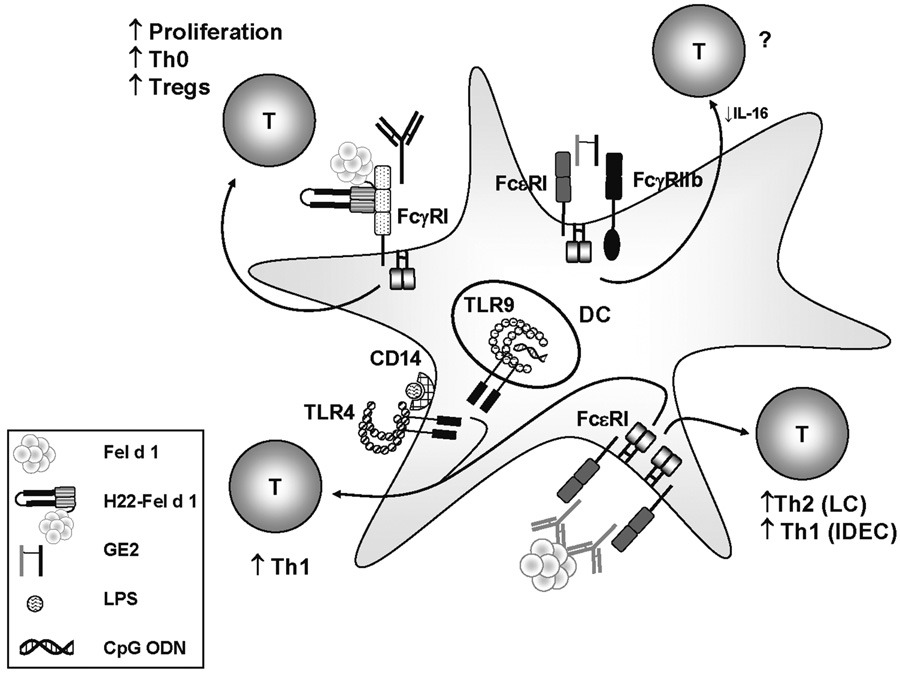
Figure 1. Ligation of Different Receptors on DCs Influences T Cell Responses to Allergens.
Cross-linking of FcεRI on DCs by natural allergen can induce Th1 or Th2 responses depending on the type of DC (IDECs or LCs respectively). However, ligation of FcεRI in conjunction with engagement of TLRs (i.e. TLR4 or TLR9) enhances Th1 responses. Targeting allergen to FcγRI using H22-Fel d 1 enhances T cell proliferation and amplifies Th0 and IL-10-expressing subtypes. Cross-linking FcεRI with the inhibitory receptor FcγRIIb using GE2 (or GFD, not shown) inhibits IL-16 production by DCs; however, its effects on human T cells have not been investigated. CpG ODN: Oligodeoxynucleotides that contain unmethylated cytosine-guanine motifs commonly found in bacteria.
Fc Receptors on Dendritic Cells
The nature of the T cell response generated in response to any antigen is highly dependent on the dose of antigen administered. With this in mind, approaches which increase antigen delivery to the APC have the potential not only to enhance immunogenicity, but also to alter the quality of the T cell response. Dendritic cells express an array of Fc receptors which have the capacity to enhance allergen uptake through internalization of allergen/antibody complexes. The critical role of the high affinity IgE receptor, FcεRI, in triggering mast cell and basophil activation along with the ensuing inflammatory sequelae are well established. In addition to these effects, FcεRI expressed on APCs may influence T cell responses to allergens through facilitated allergen presentation [17,18–20]. On dendritic cells, this receptor exists as a trimer consisting of an α–chain and a γ-chain dimer. The α subunit binds IgE while the γ subunits comprise the signal-transducing element. Each γ-chain contains a conserved immunoreceptor tyrosine-based activation motif (ITAM), which is critical for initiating downstream signaling events. Studies have shown that ligation of FcεRI on human monocyte-derived DCs increases production of proinflammatory cytokines and chemokines [17,21]. Moreover, cross-linking of FcεRI on DCs by allergen induces robust proliferation of allergen-specific T cell clones [17]. Investigation of different DC types present in skin lesions of patients with atopic dermatitis suggests that the nature of FcεRI-mediated T cell responses is dependent upon the type of DC. Specifically, inflammatory dendritic epidermal cells (IDECs) were shown to induce a higher frequency of IFN-γ+ T cells from naïve T cells as compared with Langerhans cells (LCs). Conversely, LCs induced a higher frequency of IL-4+ cells (Fig. 1)[17] .
Dendritic cells express an array of Fc receptors which bind IgG. These include FcγRI, FcγRII and FcγRIII. While FcγRI and FcγRIII constitute activating receptors which contain ITAMs, FcγRII exists in activating (FcγRIIa) and inhibitory (FcγRIIb) forms. The inhibitory properties of FcγRIIb have been manipulated to block the allergic inflammatory cascade. This work is described in detail elsewhere [22**]. Briefly, FcγRIIb contains an immunoreceptor tyrosine-based inhibition motif (ITIM) which induces inhibitory signaling events. This receptor can co-aggregate with FcεRI leading to rapid phosphorylation of the FcγRIIb ITIM which, in turn, triggers a signaling cascade that culminates in inhibition of FcεRI signaling. Saxon and colleagues used this knowledge to develop a fusion protein (GE2) which could non-specifically cross-link FcεRI and FcγRIIb. This molecule consisted of a human IgG Fc fragment fused to a human IgE Fc fragment. Suppression of IgE-mediated signaling by this molecule was evidenced by inhibition of allergen-induced histamine release from human basophils and mast cells in vitro and inhibition of passive cutaneous anaphylaxis in animal models [23, 24]. A fusion protein was developed which had the capacity to coaggregate FcγRIIb and FcεRI via receptor-bound IgE. This molecule (GFD), which contained a human IgG Fc fragment linked to the major cat allergen, Fel d 1, blocked cat-induced allergy in a mouse model [25].
Subsequently, it was shown that GE2 blocked FcεRI-mediated production of IL-16 in human Langerhans-like DCs which expressed FcγRII, but not FcγRI or FcγRIII [26]. However, effects at the T cell level were not examined (Fig. 1). In animal models, there is conflicting data on whether FcγRIIb enhances or inhibits effector T cell responses to antigen complexed with antibody [27, 28]. Thus, further studies are clearly warranted in order to resolve how FcγRIIb-mediated pathways could influence T cell responses to allergen.
TARGETING ALLERGEN TO FcγRI
The high affinity IgG receptor, FcγRI (CD64), is found exclusively on cells of the myeloid lineage. This receptor comprises an IgG binding α-chain complexed with an ITAM-containing γ-chain dimer. The ITAM within the γ-chain is critical to an array of FcγRI-mediated effector functions including phagocytosis, cytokine production and antibody-dependent cell-mediated cytotoxicity. In addition to these functions, FcγRI also facilitates antigen presentation. However, while integrity of the γ-chain ITAM appears to be important for antigen presentation mediated by other Fc activating receptors, this may not be the case for FcγRI. Specifically, FcγRI has been reported to promote antigen presentation independently of a functional γ-chain ITAM [29]. On the other hand, the α-chain appears to be critical not only for antigen uptake, but also for antigen presentation through targeting of receptor-ligand complexes to the appropriate antigen processing compartments within the APC.
In the early 1990’s, the effects of targeting antigen to FcγRI were studied by conjugating antigens to the anti-human FcγRI mAb 22.2. This mAb had been shown to bind to FcγRI via its Fab region outside the Fc binding domain making it feasible to target antigens to the receptor despite occupancy by its normal ligand, IgG. Targeting antigens to human FcγRI using mAb 22 was shown to enhance T cell responses in vitro in human-based systems and augment humoral responses in vivo in a transgenic mouse model [30, 31]. Collectively, these findings pointed to enhanced immunogenicity mediated by FcγRI targeting using mAb 22.
A humanized version of anti-CD64 mAb 22 (H22) was developed which was shown to stimulate receptor internalization, an important first step in antigen processing (Fig. 2A)[32]. This trivalent antibody cross-links FcγRI through binding by its Fc end as well as its Fab ends [33]. Interestingly, targeting antigenic peptides using just the monovalent form of H22 (Fab) enhanced both antigen-specific CD4+ T cell proliferation and cytokine production in vitro [34**]. Using a similar approach to target prostate specific antigen to FcγRI in the human myeloid cell line THP-1 led to enhanced killing of those cells by antigen-specific cytotoxic T lymphocytes [35]. Thus, in addition to facilitating presentation of antigen on MHC class II molecules to CD4+ T cells, exogenous antigens targeted to FcγRI using H22 can also be presented to CD8+ T cells in the context of MHC class I. Moreover, a monovalent form of H22 is sufficient to mediate these effects.
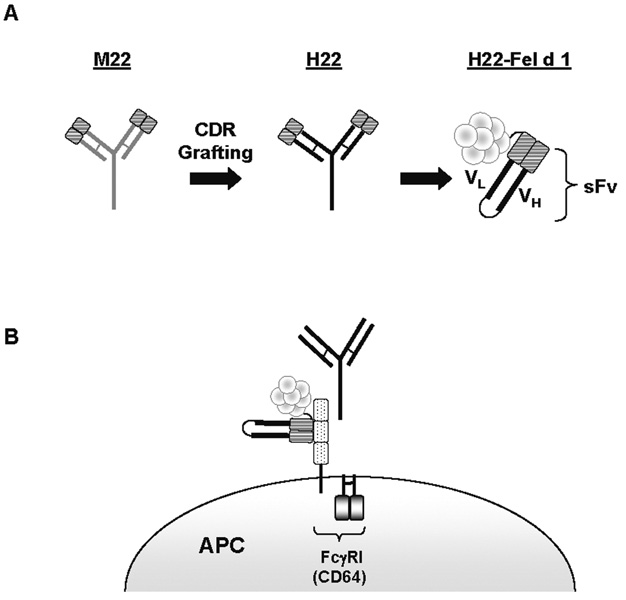
Figure 2. Schematic of H22-Fel d 1.
A) The humanized anti-CD64 monoclonal antibody, H22, was generated by CDR grafting from the mouse mAb M22. The humanized mAb contains 5–10% of the original mouse sequence. The sFv of H22 was linked to Fel d 1 by cloning and H22-Fel d 1 was produced in Baculovirus and Pichia pastoris expression systems [38, 40]. The sFv comprises the variable regions of heavy (VH) and light (VL) chains of H22 produced by joining VH and VL DNAs together with a linker to obtain a single DNA fragment for cloning and expression. B) H22-Fel d 1 binds to FcγRI outside the Fc binding region of the receptor.
The Immunomodulatory Capacity of the Novel Allergen Variant, H22-Fel d 1
In 2001, Platts-Mills and colleagues reported a novel type of immune response (modified Th2 response) in children living with a cat who were exposed to high levels of the major cat allergen, Fel d 1 [36]. This response, which was characterized by the presence of anti-Fel d 1 IgG antibodies in the serum without IgE and without allergic symptoms, was proposed to represent a form of high dose respiratory tolerance. Subsequent T cell studies performed in modified responders suggested a role for enhanced recognition of an immunodominant region within polypeptide chain 2 of Fel d 1 which contained IL-10- and IFN-γ-inducing T cell epitopes [37]. Marked increases in cytokine production to these chain 2 epitopes were also observed in cultures from cat-allergic patients receiving conventional immunotherapy using cat extract. Collectively, these findings supported a role for IL-10 and IFN-γ in protective responses to Fel d 1.
In 2002, Vailes and colleagues developed a recombinant fusion protein which linked Fel d 1 to the single-chain antibody fragment variable regions (sFv) of H22 (H22-Fel d 1)(Fig. 2B)[38**]. The sFv version of H22 (sFv22) binds monovalently and thus, does not cross-link FcγRI, but nevertheless leads to receptor internalization. Interestingly, this effect requires IgG suggesting that occupancy of the ligand binding domain of FcγRI is necessary for receptor uptake mediated by sFv22 [39]. Subsequently, H22-Fel d 1 was shown to bind to FcγRI on monocytes. Moreover, H22-Fel d 1 retained the ability to bind Fel d 1-specific IgE ab, suggesting that fusion of Fel d 1 to sFv22 did not influence allergen folding [38**].
We speculated that targeting Fel d 1 to FcγRI may enhance presentation of major T cell epitopes, including those which induce IL-10 or IFN-γ. Thus, the immunomodulatory properties of H22-Fel d 1 were investigated. In initial studies using PBMC cultures, H22-Fel d 1 stimulated increased T cell proliferation as compared with non-receptor-targeted allergen (rFel d 1)[40**]. This phenomenon was examined further at both the APC and the T cell level. High level expression of CD64 was observed on monocyte-derived dendritic cells (MDCs) confirming that this was an appropriate APC type for analysis. Interestingly, MDCs pulsed with H22-Fel d 1 showed a semi-mature phenotype as judged by production of inflammatory cytokines with no change in surface markers of maturation (HLA-DR, CD40, CD80, CD86). Specifically, H22-Fel d 1 induced increased secretion of Th1-promoting and inflammatory cytokines (IL-1β, IL-12, monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein (MIP)-1α, MIP-1β, RANTES) as compared with rFel d 1. Moreover, levels for selected inflammatory cytokines (MCP-1, MIP-1β) were as high as those induced by the TLR4 ligand, LPS. H22-Fel d 1 also induced increased IL-10, whereas no changes in Th2-attracting chemokines were observed [40**].
Next, we examined the T cell repertoire induced using CD4+ T cells cultured with H22-Fel d 1-pulsed MDCs. Given that semi-mature DCs were previously reported to tolerize T cells, we theorized that changes at the DC level could translate to induction of IL-10-producing T cells [41]. Flow cytometry analysis showed that H22-Fel d 1 amplified both IL-5+ and IL-10+ CD4+ T cells as compared with rFel d 1. This effect was observed for T cells isolated from cat-allergic subjects, but not those from modified responders or serum antibody-negative controls. Since many cytokine-positive T cells induced by H22-Fel d 1 expressed multiple cytokines, we further assessed the nature of these cells by analyzing the following six subtypes: (1) IFN-γ+ only; (2) IL-5+ only; (3) IL-10+ only; (4) IFN-γ+IL-5+; (5) IFN-γ+IL-10+; (6) IL-10+IL-5+. Interestingly, H22-Fel d 1 amplified diverse T cell subtypes which were characteristic of Th0 cells (IFN-γ+IL-5+) and different types of regulatory T cells including T regulatory type 1 (IL-10+ only), regulatory Th1 (IL-10+IFN-γ+) and regulatory Th2 (IL-10+IL-5+) cells. Importantly, the T cell repertoire induced by H22-Fel d 1 did not resemble that induced by rFel d 1 in subjects with a modified Th2 response, showing that it constituted a distinct response. Given that IL-5+ T cells were amplified in response to H22-Fel d 1, we compared the T cell repertoire with that induced by the prototypic dust mite allergen, Der p 1. Notably, despite its Th2 elements, the repertoire induced by H22-Fel d 1 was markedly more diverse as judged by increases in all IL-10-expressing subtypes, as well as Th0 cells [40**]. Thus, targeting Fel d 1 to FcγRI induced a novel variation of the Th2 response in subjects with cat allergy which was characterized by increased T cell diversity (Fig. 1).
WHAT CONSTITUTES A PROTECTIVE T CELL REPERTOIRE?
A key issue which remains is whether our in vitro observations could translate to protective responses in vivo. It is generally accepted that IL-10 suppresses Th2 responses to allergens. However, despite all that we have learned about T cell responses to allergens, including the role of Tregs, what defines a protective T cell response to allergen still remains unclear. For example, how many IL-10-secreting T cells and thus, how much IL-10, is required to confer protection? The frequency of IL-10+ cells induced by H22-Fel d 1 in our system was low (typically less than 5% of total CD4+ T cells) [40**]. To determine whether H22-Fel d 1-induced IL-10 could regulate the response, the effects of IL-10 blockade were tested in our system. Blocking IL-10 was associated with an increase in IL-5+ cells without any change in IFN-γ+ T cells [40**]. Thus, Th2 responses induced by H22-Fel d 1 appear to be “controlled”, at least in part, by IL-10.
In humans, there is considerable confusion regarding the relationship of inducible IL-10-secreting Tregs (so-called adaptive Tregs) to other types of Tregs, including naturally occurring CD25+ Tregs. New data suggests that H22-Fel d 1 can induce IL-10+ T cells within both adaptive and natural Treg populations (unpublished observations), pointing to functional overlap of different Treg types. Regardless, our results highlight the diverse makeup of allergen-induced IL-10-expressing T cells. H22-Fel d 1 will provide a useful molecular tool for analyzing these cells which, until now, have been notoriously difficult to study owing to their low frequency.
FUTURE CONSIDERATIONS
The experimental evidence presented here suggests that targeting allergens to FcγRI may be an appropriate DC-based therapy for allergic disease; however, T cell changes induced by this strategy are complex. It will be interesting to determine whether targeting other allergens via H22 yields similar changes in the T cell repertoire to those induced by H22-Fel d 1. Moving forward, it will also be important to consider whether these responses are influenced by the inflammatory milieu in vivo. Given that H22-Fel d 1 contains intact allergen, the risk for IgE-mediated adverse events will also need to be evaluated. With this in mind, development of H22 conjugates containing allergen-derived T cell epitopes could provide a practical solution to not only eliminate IgE binding, but also stabilize peptides for delivery to APCs.
CONCLUSION
Targeting allergen to the high affinity IgG receptor induces potent immunomodulatory effects. The ability to stimulate a tolerogenic phenotype in DCs coupled with generation of diverse types of IL-10-producing T cells suggests that H22-linked allergens offer promise as a novel therapy for allergic disease.
Acknowledgments
Work supported by: National Institutes of Health R01 grants AI-052196 and AI-020565 and U19 grant AI-070364.
REFERENCES
- 1.Nelson HS. Allergen immunotherapy: where is it now? J Allergy Clin Immunol. 2007;119:769–779. doi: 10.1016/j.jaci.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 2.Nouri-Aria KT, Wachholz PA, Francis JN, et al. Grass pollen immunotherapy induces mucosal and peripheral IL-10 responses and blocking IgG activity. J Immunol. 2004;172:3252–3259. doi: 10.4049/jimmunol.172.5.3252. [DOI] [PubMed] [Google Scholar]
- 3.Jutel M, Akdis M, Blaser K, Akdis CA. Mechanisms of allergen specific immunotherapy--T-cell tolerance and more. Allergy. 2006;61:796–807. doi: 10.1111/j.1398-9995.2006.01175.x. [DOI] [PubMed] [Google Scholar]
- 4.Woodfolk JA. T-cell responses to allergens. J Allergy Clin Immunol. 2007;119:280–294. doi: 10.1016/j.jaci.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 5. Radulovic S, Jacobson MR, Durham SR, Nouri-Aria KT. Grass pollen immunotherapy induces Foxp3-expressing CD4+ CD25+ cells in the nasal mucosa. J Allergy Clin Immunol. 2008;121:1467–1472. doi: 10.1016/j.jaci.2008.03.013. This is one of the most recent articles to support the view that regulatory T cells are induced at inflamed sites by conventional immunotherapy.
- 6.Niederberger V, Horak F, Vrtala S, et al. Vaccination with genetically engineered allergens prevents progression of allergic disease. Proc Nat Acad Sci USA. 2004;101:14677–14682. doi: 10.1073/pnas.0404735101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karamloo F, Schmid-Grendelmeier P, Kussebi F, et al. Prevention of allergy by a recombinant multi-allergen vaccine with reduced IgE binding and preserved T cell epitopes. Eur J Immunol. 2005;35:3268–3276. doi: 10.1002/eji.200425522. [DOI] [PubMed] [Google Scholar]
- 8.Kussebi F, Karamloo F, Rhyner C, et al. A major allergen gene-fusion protein for potential usage in allergen-specific immunotherapy. J Allergy Clin Immunol. 2005;115:323–329. doi: 10.1016/j.jaci.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 9.Pree I, Reisinger J, Focke M, et al. Analysis of epitope-specific immune responses induced by vaccination with structurally folded and unfolded recombinant Bet v 1 allergen derivatives in man. J Immunol. 2007;179:5309–5316. doi: 10.4049/jimmunol.179.8.5309. [DOI] [PubMed] [Google Scholar]
- 10.Tarzi M, Klunker S, Texier C, et al. Induction of interleukin-10 and suppressor of cytokine signalling-3 gene expression following peptide immunotherapy. Clin Exp Allergy. 2006;36:465–474. doi: 10.1111/j.1365-2222.2006.02469.x. [DOI] [PubMed] [Google Scholar]
- 11.Oldfield WL, Larche M, Kay AB. Effect of T-cell peptides derived from Fel d 1 on allergic reactions and cytokine production in patients sensitive to cats: a randomized controlled trial. Lancet. 2002;360:47–53. doi: 10.1016/s0140-6736(02)09332-7. [DOI] [PubMed] [Google Scholar]
- 12.Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–449. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 13.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 14.Rutella S, Danese S, Leone G. Tolerogenic dendritic cells: cytokine modulation comes of age. Blood. 2006;108:1435–1440. doi: 10.1182/blood-2006-03-006403. [DOI] [PubMed] [Google Scholar]
- 15.Eisenbarth SC, Piggott DA, Huleatt JW, et al. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196:1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simons FE, Shikishima Y, Van Nest G, et al. Selective immune redirection in humans with ragweed allergy by injecting Amb a 1 linked to immunostimulatory DNA. J Allergy Clin Immunol. 2004;113:1144–1151. doi: 10.1016/j.jaci.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Novak N, Valenta R, Bohle B, et al. FcεRI engagement of Langerhans cell-like dendritic cells and inflammatory dendritic epidermal cell-like dendritic cells induces chemotactic signals and different T-cell phenotypes in vitro. J Allergy Clin Immunol. 2004;113:949–957. doi: 10.1016/j.jaci.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Schroeder JT, Bieneman AP, Xiao H, et al. TLR9-and FcepsilonRI-mediated responses oppose one another in plasmacytoid dendritic cells by down-regulating receptor expression. J Immunol. 2005;175:5724–5731. doi: 10.4049/jimmunol.175.9.5724. [DOI] [PubMed] [Google Scholar]
- 19.Bajtay Z, Csomor E, Sandor N, Erdei A. Expression and role of Fc- and complement-receptors on human dendritic cells. Immunol Lett. 2006;104:46–52. doi: 10.1016/j.imlet.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 20.Kraft S, Novak N. Fc receptors as determinants of allergic reactions. Trends Immunol. 2006;27:88–95. doi: 10.1016/j.it.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Kraft S, Novak N, Katoh N, et al. Aggregation of the high-affinity IgE receptor Fc(epsilon)RI on human monocytes and dendritic cells induces NF-kappaB activation. J Invest Dermatol. 2002;118:830–837. doi: 10.1046/j.1523-1747.2002.01757.x. [DOI] [PubMed] [Google Scholar]
- 22. Zhang K, Zhu D, Kepley C, et al. Chimeric human fcgamma-allergen fusion proteins in the prevention of allergy. Immunol Allergy Clin North Am. 2007;27:93–103. doi: 10.1016/j.iac.2006.11.002. This review summarizes the immunological properties of a novel human-cat fusion protein, and its potential therapeutic applications.
- 23.Zhu D, Kepley CL, Zhang M, et al. A novel human immunoglobulin Fc gamma Fc epsilon bifunctional fusion protein inhibits Fc epsilon RI-mediated degranulation. Nat Med. 2002;8:518–521. doi: 10.1038/nm0502-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang K, Kepley CL, Terada T, et al. Inhibition of allergen-specific IgE reactivity by a human Ig Fcgamma-Fcepsilon bifunctional fusion protein. J Allergy Clin Immunol. 2004;114:321–327. doi: 10.1016/j.jaci.2004.03.058. [DOI] [PubMed] [Google Scholar]
- 25.Zhu D, Kepley CL, Zhang K, et al. A chimeric human-cat fusion protein blocks cat-induced allergy. Nat Med. 2005;11:446–449. doi: 10.1038/nm1219. [DOI] [PubMed] [Google Scholar]
- 26.Kepley CL, Zhang K, Zhu D, Saxon A. FcεRI-FcγRII coaggregation inhibits IL-16 production from human langerhans-like dendritic cells. Clin Immunol. 2003;108:89–94. doi: 10.1016/s1521-6616(03)00155-4. [DOI] [PubMed] [Google Scholar]
- 27.Yada A, Ebihara S, Matsumura K, et al. Accelerated antigen presentation and elicitation of humoral response in vivo by FcγRIIB- and FcγRI/III-mediated immune complex uptake. Cell Immunol. 2003;225:21–32. doi: 10.1016/j.cellimm.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Desai DD, Harbers SO, Flores M, et al. Fc gamma receptor IIB on dendritic cells enforces peripheral tolerance by inhibiting effector T cell responses. J Immunol. 2007;178:6217–6226. doi: 10.4049/jimmunol.178.10.6217. [DOI] [PubMed] [Google Scholar]
- 29.van Vugt MJ, Kleijmeer MJ, Keler T, et al. The FcγRIa (CD64) ligand binding chain triggers major histocompatibility complex class II antigen presentation independently of its associated FcR γ-chain. Blood. 1999;94:808–817. [PubMed] [Google Scholar]
- 30.Gosselin E, Wardwell K, Gosselin DR, et al. Enhanced antigen presentation using human Fcγ receptor (monocyte/macrophage)-specific immunogens. J Immunol. 1992;149:3477–3481. [PubMed] [Google Scholar]
- 31.Keler T, Guyre PM, Vitale LA, et al. Targeting weak antigens to CD64 elicits potent humoral responses in human CD64 transgenic mice. J Immunol. 2000;165:6738–6742. doi: 10.4049/jimmunol.165.12.6738. [DOI] [PubMed] [Google Scholar]
- 32.Heijnen I, van Vugt MJ, Fanger NA, et al. Antigen targeting to myeloid-specific human FcγRI/CD64 triggers enhanced antibody responses in transgenic mice. J Clin Invest. 1996;97:331–338. doi: 10.1172/JCI118420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guyre CA, Barreda ME, Swink SL, Fanger MW. Colocalization of FcγRI-targeted antigen with class I MHC: implications for antigen processing. J Immunol. 2001;166:2469–2478. doi: 10.4049/jimmunol.166.4.2469. [DOI] [PubMed] [Google Scholar]
- 34. Liu C, Goldstein J, Graziano RF, et al. FcγRI-targeted fusion proteins result in efficient presentation by human monocytes of antigenic and antagonist T cell epitopes. J Clin Invest. 1996;98:2001–2007. doi: 10.1172/JCI119004. This article was the first to report how targeting peptides to CD64 using humanized mAb H22 resulted in increased efficiency of presentation to T cells.
- 35.Wallace PK, Tsang KY, Goldstein J, et al. Exogenous antigen targeted to FcγRI on myeloid cells is presented in association with MHC class I. J Immunol Methods. 2001;248:183–194. doi: 10.1016/s0022-1759(00)00351-3. [DOI] [PubMed] [Google Scholar]
- 36.Platts-Mills T, Vaughan J, Squillace S, et al. Sensitisation, asthma, and a modified Th2 response in children exposed to cat allergen: a population-based cross-sectional study. Lancet. 2001;357:752–756. doi: 10.1016/S0140-6736(00)04168-4. [DOI] [PubMed] [Google Scholar]
- 37.Reefer AJ, Carneiro RM, Custis NJ, et al. A role for IL-10-mediated HLA-DR7-restricted T cell-dependent events in development of the modified Th2 response to cat allergen. J Immunol. 2004;172:2763–2772. doi: 10.4049/jimmunol.172.5.2763. [DOI] [PubMed] [Google Scholar]
- 38. Vailes LD, Sun AW, Ichikawa K, et al. High-level expression of immunoreactive recombinant cat allergen (Fel d 1): targeting to antigen-presenting cells. J Allergy Clin Immunol. 2002;110:757–762. doi: 10.1067/mai.2002.129035. This work described the development of the fusion protein, H22-Fel d 1, which targets the major cat allergen, Fel d 1, to FcγRI on APCs.
- 39.Guyre CA, Keler T, Swink SL, et al. Receptor modulation by FcγRI-specific fusion proteins is dependent on receptor number and modified by IgG. J Immunol. 2001;167:6303–6311. doi: 10.4049/jimmunol.167.11.6303. [DOI] [PubMed] [Google Scholar]
- 40. Hulse KE, Reefer AJ, Engelhard VH, et al. Targeting Fel d 1 to FcγRI induces a novel variation of the Th2 response in subjects with cat allergy. J Allergy Clin Immunol. 2008;121:756–762. doi: 10.1016/j.jaci.2007.10.016. This report describes the immunomodulatory capacity of the fusion protein, H22-Fel d 1, which targets the major cat allergen to FcγRI.
- 41.van Duivenvoorde LM, van Mierlo GJ, Boonman ZF, Toes RE. Dendritic cells: vehicles for tolerance induction and prevention of autoimmune diseases. Immunobiology. 2006;211:627–632. doi: 10.1016/j.imbio.2006.05.014. [DOI] [PubMed] [Google Scholar]