Abstract
Background
Dextran sodium sulfate (DSS) is a sulfated polysaccharide that has been very widely used to induce inflammation in experimental models of inflammatory bowel disease in which the effects of pharmacologic and biologic therapies are tested. However, the precise mechanisms by which DSS induces inflammation have not been elucidated.
Methods
DSS-induced increases in phospho-IκBα, nuclear NFκB (p65), and IL-8 secretion in human colonic epithelial cells in tissue culture are attributable to a reactive oxygen species (ROS)-induced pathway of inflammation, and do not require TLR4, MyD88, or Bcl10, which are associated with the innate immune pathway of NFκB-IL-8 activation.
Results
DSS-induced increases were inhibited by the ROS scavengers Tempol and Tiron, were associated with decreased phosphorylation of MAPK12 (p38γ), MAPK 13 (p38δ), and Hsp27, and required the IκB kinase (IKK) signalosome component IKKβ. In ex vivo colonic tissue from TLR4-deficient mice, or following knockdown of MyD88 or Bcl10 or exposure to an IRAK 1/4 inhibitor, DSS effects were not suppressed. Data demonstrated that DSS activates IκBα, NFκB, and IL-8 through an ROS-Hsp27-IKKβ-mediated pathway, and not through an innate immune cascade.
Conclusions
These results suggest that DSS models of inflammation may not be optimal for evaluation of interventions that involve mechanisms of innate immunity.
Keywords: inflammation, reactive oxygen species (ROS), sulfated polysaccharide, IKK signalosome, Bcl10, innate immunity, Hsp27
Dextran sodium sulfate (DSS) has been used for decades to induce inflammatory responses in experimental models of inflammatory bowel disease (IBD), yet the precise mechanisms whereby it induces inflammation have not been clarified. Often, DSS has been used to induce inflammation in the intestine, as well as in pleura, peritoneum, bursa, rat hind paw, or other anatomic sites in order to study the effectiveness of potential treatments for inflammation or to determine the mediators of inflammation. An inflammatory process resembling IBD, with ulcerations, leukocytic infiltrate, hemorrhage, and necrosis, follows the oral exposure of animals to DSS and is frequently used to test the effectiveness of potential therapies for IBD.1–10
To clarify the mediators involved in DSS-induced signaling events, the effects of Toll-like receptor 4 (TLR4)-deficiency, myeloid differentiation primary response protein (MyD88), and B-cell CLL/lymphoma 10 (Bcl10) knockdown by RNAi, and chemical inhibition of interleukin receptor-associated kinase (IRAK1/4) on the DSS-induced changes in phospho-Hsp27, phospho-IκBα, NFκB, and IL-8 were determined. Bcl10 is of special interest, since it is emerging as an important mediator of innate immunity in both immune and nonimmune cells. Recent reports have shown that Bcl10 mediates NFκB activation by angiotensin II in HepG2 cells, by lysophosphatidic acid in murine embryonic fibroblasts, by G-protein-coupled receptors in human embryonic kidney 293T cells, and by carrageenan and lipopolysaccharide in colonic epithelial cells.11–15 Previously, Bcl10 was recognized as an important adaptor protein in immune cells, and translocations involving Bcl10 were associated with the constitutive activation of NFκB and the development of MALT (mucosa-associated lymphoid tumor) lymphomas.16–18 To elucidate the cascade of activation of the inflammatory response initiated by exposure to DSS in human colonic epithelial cells, we studied the mechanisms by which DSS induced IκBα, NFκB, and IL-8 activation in NCM460 cells and primary human colonocytes in tissue culture. The effects of DSS exposure were evaluated in colonic tissue from TLR4-deficient mice and in IKKβ−/− and IKKα−/− mouse embryonic fibroblasts, as well as in human colonic tissue following knockdown of MyD88 and Bcl10, two mediators of the innate immune response. Since an increase in reactive oxygen species (ROS) has been associated with clinical IBD, we determined the effects of DSS exposure on ROS and the effect of ROS inhibitors on the DSS-induced activation of phospho-IκBα, NFκB, and IL-8 in these different models of intestinal inflammation.19–21
Proteome profiling was performed in the colonic epithelial cells following DSS exposure and demonstrated changes in several phospho-proteins, including a decline in phospho-Hsp27. Since the phosphorylation of heat-shock protein 27 (Hsp27) is inversely correlated with activation of the IkappaBα kinase (IKK) signalosome, we further evaluated the changes in phosphorylation of Hsp27 that followed DSS exposure.22–24 Since the chemokine IL-8 induces a leukocyte and macrophage infiltrate like that found in active IBD, the DSS-associated change in IL-8 secretion, or in secretion of the murine homolog KC, was used as a primary endpoint.25,26
In the experiments presented in this report, we present the ROS-Hsp27-IKKβ pathway of DSS-induced inflammation and provide evidence that DSS does not activate a pathway of innate immunity, mediated by TLR4-MyD88-IRAK-Bcl10. These findings provide new insight into the signaling mechanisms of a commonly used experimental model of IBD, and suggest that pharmacologic approaches that reduce DSS-stimulated inflammation may not be applicable to innate immune signaling pathways of inflammation.
MATERIALS AND METHODS
Cell preparations, including human colonic epithelial cells, mouse embryonic fibroblasts, and TLR4-deficient mouse colon
NCM460 is a nontransfected human colonic epithelial cell line, originally derived from the normal colon mucosa of a 68-year-old Hispanic male.27 NCM460 cells were grown in M3:10 media (INCELL, San Antonio, TX) and maintained at 37°C in a humidified, 5% CO2 environment with media renewal at 2-day intervals. Confluent cells in T-25 flasks (Costar, Cambridge, MA) were harvested by trypsinization and subcultured in multiwell tissue culture clusters (Costar). Cells were treated with dextran sodium sulfate (DSS; 1 μg/mL; average molecular weight 500,000; Sigma, St. Louis, MO) for time periods ranging from 1 hour to 24 hours, as indicated. Spent media were collected from control and treated wells at specified times and stored at −80°C. Cells were harvested by scraping. Total cell protein was measured by BCA protein assay kit (Pierce, Rockford, IL), using bovine serum albumin as the standard. Cell viability was tested by Trypan Blue exclusion testing and found to be 95% in both treated and control cells.
De-identified colon specimens were obtained at the time of colectomy through an established protocol approved by the Institutional Review Board of the University of Illinois at Chicago (UIC), as previously described.11,12
Mouse embryonic fibroblasts, including wildtype (WT), IKKα−/−, and IKKβ−/− were a generous gift from the laboratory of Dr. Michael Karin (University of California San Diego). These cell lines were obtained from transgenic mice in which IKKα and IKKβ genes were deleted and homozygous mice were bred.28
C57BL/10ScNJ mice that are TLR4-deficient, due to an inherited deficiency of the TLR4 gene locus, and age-matched controls (C57BL/10ScSnJ) were purchased (Jackson Laboratory, Bar Harbor, ME) and euthanized at 9.5 weeks. Colon was dissected and small fragments (≈2 mm2) were exposed to DSS (1 μg/mL) for 6 hours in DMEM with antibiotics and 10% FBS, and incubated at 37°C and 5% CO2. Spent media and tissue samples were collected and frozen for future analysis.
Measurement of Reactive Oxygen Species (ROS)
The production of reactive oxygen species (ROS) by DSS was measured by the method described by Ndengele et al.29 Cells were harvested from T-75 flasks and grown in a 96-well cell culture plate. After 24 hours the medium was changed and treatment with DSS (1 μg/mL × 1–24 hours) was started. At the end of the treatment period the medium in each well was removed, cells were washed with Hank’s Balanced Salt Solution (HBSS), and cells were covered with 200 μL of HBSS containing 10 μM dihydroethidine (HE, Sigma). Cultures were incubated for 1 hour at 37°C, 5% CO2 then the medium was removed and replaced with fresh HBSS (200 μL/well). The intracellular HE fluorescence was measured using a microplate fluorescence reader (FL600, Bio-Tek Instruments, Winooshi, VT) at 488 nm excitation wavelength with a 610-nm emission filter.
Superoxide Scavengers Tempol and Tiron
The superoxide scavengers Tempol(4-hydroxy-2,2,6,6-tetra-methyolpiperidinyloxy; Axxora, San Diego, CA) and Tiron (4,5-dihydroxy-1,3-benzene disulfonic acid; Acros Organics, NJ) were used to inhibit the effects of free oxygen radicals generated by DSS. Tempol and Tiron concentrations were 100 nM for 1, 4, and 24 hours,30–33 co-administered with DSS (1 μg/mL). This concentration of Tempol and of Tiron was determined from review of the literature and following trials of different doses in our experiments.
ELISAs for Measurement of Secreted IL-8, Cellular Bcl10, Nuclear NFκB (p65), Total and Phospho-Hsp27, Phospho-IκBα, and KC
The secretion of IL-8 in the spent media of control and treated NCM460 cells and primary colonic cells was measured using the DuoSet enzyme-linked immunosorbent assay (ELISA) kit for human IL-8 (R&D Systems, Minneapolis, MN), as previously described.11,12
Expression of Bcl10 protein in control and DSS-treated NCM460 cells was determined by a solid-phase ELISA, previously developed by our laboratory for quantitative determination of Bcl10.34 Control or treated cells were lysed in RIPA buffer (50 mM Tris containing 0.15 M NaCl, 1% NP40, 0.5% deoxycholic acid, and 0.1% SDS, pH 7.4), and the cell extracts were stored at − 80°C until assayed using ELISA, as reported.12
Nuclear extracts were prepared from treated and control NCM460 cells using a nuclear extraction kit (Active Motif, Carlsbad, CA). Activated NFκB in the samples was determined by oligonucleotide-based ELISA (Active Motif) as reported previously.12 The specificity of the binding of NFκB of the samples with the coated nucleotide sequence was determined by comparison to the control binding, determined by adding either free consensus nucleotide (5′-AGTTGAGGGGACTTTCCCAGGC-3′) or mutated nucleotide (5′-AGTTGAGGCCACTTTCCCAGGC-3′) to the reaction buffer. The sample values were normalized with the total cell protein determined by protein assay kit (Pierce).
Phospho-IκBα was determined using an ELISA assay in NCM460 cells and in mouse embryonic fibroblasts, including wildtype, IKKα−/− and IKKβ−/− cells, as described previously.11 IκBα phosphorylated at Ser 32 was detected by the PathScan Sandwich ELISA Kit (#7355, Cell Signaling Technology, Beverly, MA) which is a solid phase sandwich ELISA with a mouse monoclonal antibody against IκBα coated onto the microwells of a 96-well plate.
Total and phospho-Hsp27 in control and treated human cell samples were measured by a FACE-ELISA (fast-activated cell-based ELISA; Active Motif), as previously described.35 Mouse phospho-Hsp27 was also determined by a quantitative ELISA in the mouse embryonic fibroblasts by using an antibody directed at phospho-Hsp27 (Ser86) (R&D).
KC, the mouse homolog of IL-8, was measured by ELISA (R&D) in spent media and results were compared to standards, as described previously.36 The sample values were normalized with the total cell protein concentrations determined by BCA Protein assay kit (Pierce), and KC values are expressed as pg/mg protein.
Silencing of Bcl10 mRNA Expression
Silencing of Bcl10 was performed as previously described.11 Efficacy of transfection was monitored by observing the cells that were transfected with control siRNA-rhodamine under fluorescent microscopy. Effectiveness of Bcl10 silencing in the NCM460 cells was demonstrated previously by Western blot and ELISA.12 Silencing of Bcl10 expression was determined by Western blot of the cell lysate using a mouse monoclonal Bcl10 antibody (Santa Cruz Biotechnology, Santa Cruz, CA).
Transfection with Dominant/Negative MyD88 Plasmid
MyD88 is an adaptor molecule for TLR4-induced activation of an inflammatory cascade in immune cells and in epithelial cells.37,38 To determine if MyD88 were required for the DSS-induced activation of inflammation, a plasmid with short hairpin RNA (shRNA) designed to silence MyD88 (NM_002468) and previously found to reduce the effects of LPS36 was introduced into the NCM460 cells (InvivoGen, San Diego, CA). The shRNA was obtained in a plasmid downstream of the RNA polymerase III promoter (7SK). Lyophilized plasmid was centrifuged to pellet the DNA, and DNA was resuspended and amplified in E. coli GT116 using Fast-Media Zeo (InvivoGen). Vector control psiRNA-LucGL3 was processed in parallel to the psiRNA-hMyD88 plasmid DNA, and NCM460 cells were transfected using Hi-Perfect transfection reagent (Qiagen, Chatsworth, CA), as reported previously.36 Twenty-four hours posttransfection, cells were treated with DSS (1 μg/mL × 24 hours), and measurements of IL-8 in the spent media were performed by ELISA.
Measurement of Phospho-proteins by Proteome Array
The NCM460 cells in culture were treated with DSS (1 μg/mL) for 24 hours and cell lysates from control and treated cells were prepared in lysis buffer provided with the phospho-proteome profiler array (R&D), as described previously.35 The proteome profiler detects 21 phosphorylated serine-threonine kinases or related signaling mediators in duplicate simultaneously. Samples were incubated with the nitrocellulose membrane array overnight at 4°C and washed. The arrays were incubated with a cocktail of phospho-site specific biotinylated antibodies for 2 hours at room temperature, and then with Streptavidin-HRP for 30 minutes. The signals were developed with chemiluminescent reagents, and recorded on x-ray film. The densities of individual spots corresponding to a phosphorylated kinase were measured by Image J software (NIH, Bethesda, MD) and compared between treated and control samples.
Inhibition of Interleukin 1b Receptor-associated Kinase (IRAK) by Selective Inhibitor
IRAK 1 and 4 were inhibited by exposure of NCM460 cells to the selective inhibitor N-(2-morpholinylethyl)-2-(3-nitro-benzoyl-amido)-benzimidazole (EMD Bioscience, San Diego, CA) at 50 μM for 2 hours.12,39 IL-8 in the spent media and Bcl10 in the cell lysate were measured following pre-treatment with this inhibitor and exposure to DSS 1 μg/mL for 24 hours at 37°C and 5%CO2. Effectiveness of the IRAK inhibitor was demonstrated previously in the NCM460 cells following exposure to LPS.12
Statistical Analysis
Data presented are the mean ± standard deviation (SD). Unless stated otherwise in the text or figure legend, statistical significance was determined by 1-way analysis of variance (ANOVA), followed by a post-hoc Tukey–Kramer test for multiple comparisons using Instat software. Measurements are the mean of biological triplicates and technical replicates ± SD. A P-value < 0.05 is considered statistically significant. In the figures, 1 asterisk indicates statistical significance at the 0.01 < P ≤0.05 level, 2 asterisks 0.001 < P ≤ 0.01, and three asterisks, P ≤ 0.001.
Ethical Considerations
All procedures involving acquisition of human tissue were approved by the Institutional Review Board of the University of Illinois at Chicago.
RESULTS
Inhibition of DSS-induced Production of ROS by Free Radical Scavenger
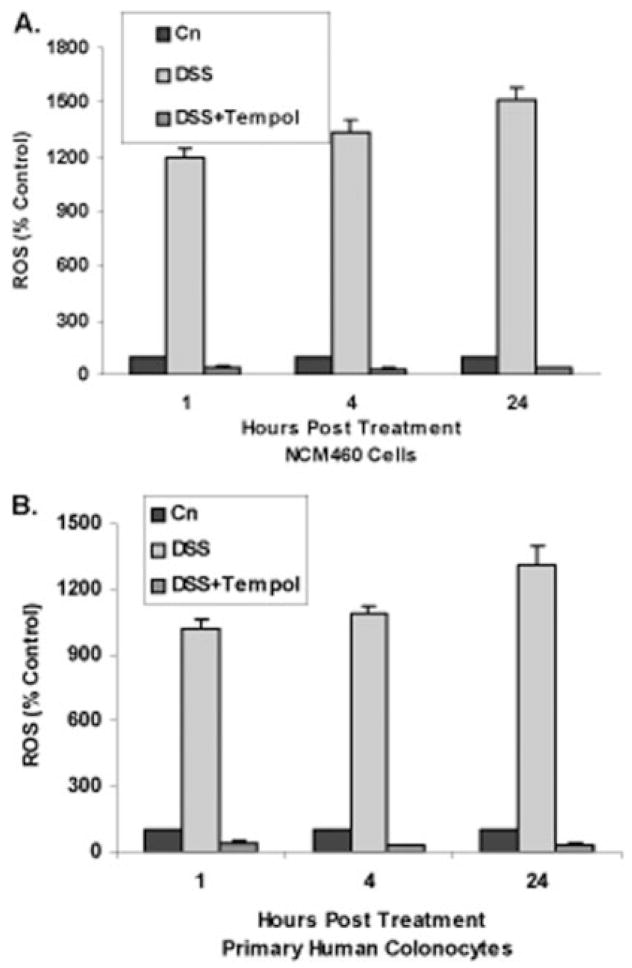
ROS increased in the NCM460 cells (Fig. 1A) and in the normal primary colonic epithelial cells (Fig. 1B) following exposure to DSS (1 μg/mL × 1, 4, and 24 hours). The ROS increased to 12 times the baseline in 1 hour and to 15 times the baseline in 24 hours in the NCM460 cells, and to 10 times and 13 times the baseline in 1 and 24 hours in the primary colonic epithelial cells. The results are statistically significant at all timepoints (P < 0.001).
FIGURE 1.
Reactive oxygen species (ROS) increase following DSS and are inhibited by free radical scavengers. A: In NCM460 cells, ROS increased significantly following exposure to DSS (1 μg/mL) for 1, 4, and 24 hours. These increases were inhibited at all timepoints by combined exposure to Tempol 100 nM. Differences in ROS between control and DSS-treated samples and in ROS following exposure to Tempol are statistically significant at all timepoints (P <0.001, 1-way ANOVA with Tukey–Kramer post-test). Cn, control; DSS, dextran sodium sulfate. B: Similarly, in normal primary human colonocytes, DSS exposure produced marked increase in ROS at 1, 4, and 24 hours and Tempol inhibited these increases (P < 0.001, 1-way ANOVA with Tukey–Kramer post-test; n = 3 independent observations).
Following combined exposure with the free radical scavenger Tempol (100 nM) (Fig. 1A,B) with DSS (1 μg/mL × 1, 4, and 24 hours), ROS activation was completely inhibited in the NCM460 cells and the primary colonocytes (P < 0.001).
Inhibition of DSS-induced Increase in IL-8 by Free Radical Scavengers
DSS (1 μg/mL) stimulated IL-8 secretion from the NCM460 cells by 1 hour, and IL-8 secretion was further increased at 4 and 24 hours (Fig. 2A). At 24 hours, IL-8 secretion had increased from a baseline value at 1 hour of 30 (±2) pg/mg protein to 1516 (±50) pg/mg protein following DSS. In the primary normal colonic epithelial cells, DSS also stimulated IL-8 secretion, but the peak IL-8 value (1121 ± 43 pg/mg protein) was somewhat less than in the NCM460 cells (Fig. 2B). Increases in IL-8 are statistically significant (P < 0.001).
FIGURE 2.
DSS exposure increases IL-8 in colonic epithelial cells and IL-8 increase is inhibited by free radical scavengers. A: In the NCM460 cells, exposure to DSS (1 μg/mL) induced increases in IL-8 from control value of 636 (±26) pg/mg protein to 1516 (±50) pg/mg protein at 24 hours. This increase was inhibited by coadministration of Tempol 100 nM, with IL-8 declining to 433 (±3) pg/mg protein, a decline of 32% from the control value at 24 hours. Differences are statistically significant (P < 0.001, 1-way ANOVA with Tukey–Kramer post-test; n = 3 independent observations). B: Similarly, in the primary colonocytes, exposure to DSS also induced increases in IL-8, but the peak was less. IL-8 increased from 440 (±11) pg/mg protein to 1121 (±43) pg/mg protein, following DSS exposure and declined to 305 (±12) pg/mg protein at 24 hours, a decline of 31% from the baseline, following coadministration of Tempol. Differences are statistically significant (P < 0.001, 1-way ANOVA with Tukey–Kramer post-test; n = 3 independent observations). C: When NCM460 cells were also treated with the free radical scavenger Tiron, the IL-8 value at 24 hours declined significantly from 1498 (±56) pg/mg protein to 414 (±39) pg/mg protein (P < 0.001,1-way ANOVA with Tukey–Kramer post-test; n = 3 independent observations).
Cotreatment with Tempol (100 nM × 24 hours) completely inhibited the DSS-induced increase in IL-8, in the NCM460 (Fig. 2A) and primary colonic epithelial cells (Fig. 2B). In the NCM460 cells, IL-8 declined to 433 (±3) pg/mg protein at 24 hours, a decline of 32% from the control value at 24 hours. In the primary colonocytes, Tempol alone produced a decline of 28% from the baseline IL-8 value, from 440 (±11) pg/mg protein to 317 (±16) pg/mg protein at 24 hours. Similarly, cotreatment with the free radical scavenger Tiron (100 nM × 24 hours) also eliminated the DSS-induced increase in IL-8 secretion in the NCM460 cells (Fig. 2C), and reduced the unstimulated IL-8 secretion to below the control level (from 621 ± 11 pg/mg protein to 424 ± 25 pg/mg protein) in the NCM460 cells (Fig. 2C). The observed differences are statistically significant (P < 0.001).
DSS-induced Increase in Activation of NFκB Inhibited by Tempol
Oligonucleotide assay for nuclear NFκB indicated an increase to 2.24 times the baseline following exposure to DSS. This increase was inhibited by coincident exposure with Tempol (100 nM × 24 hours) (Fig. 3); differences are statistically significant (P < 0.001).
FIGURE 3.
DSS-induced increase in nuclear NFκB (p65) is inhibited by Tempol. Nuclear oligonucleotide assay for NFκB was performed as described in Materials and Methods, and demonstrated a significant increase following exposure to DSS (1 μg/mL × 24 hours) and decline to baseline with Tempol (100 nM × 24 hours) (P < 0.001, 1-way ANOVA with Tukey–Kramer post-test; n = 3 independent observations).
DSS-induced Declines in Phosphorylated MAPK12, MAPK13, and Hsp27
NCM460 cells were treated with DSS (1 μg/mL × 24 hours), and control and treated cell lysates were evaluated by the Phospho-Proteome Profiler Array (Fig. 4A,B). Densitometric analysis (Fig. 4C) confirmed significant declines between the control and the DSS-treated arrays for phosphorylated MAPK12 (p38γ), MAPK13 (p38δ), and Hsp27 (P < 0.001). The phosphorylation of Hsp27 is inversely associated with the phosphorylation of IKKβ, so the DSS-induced declines may provoke increased phosphorylation of the IKK signalosome and thereby of IκBα, affecting the activation of NFκB, as observed in Figure 4.
FIGURE 4.
Proteome profiling demonstrates decreases in phosphorylated MAPK12, MAP13, and phospho-Hsp27 following exposure to DSS. NCM460 cells in culture were treated with DSS for 24 hours, and the cell content of 21 phospho-proteins was determined as indicated in Materials and Methods. A: Representative control array demonstrates paired replicate dots of each of 21 phosphorylated proteins, as well as controls. B: Representative phospho-protein array demonstrates several changes in NCM460 cells that were exposed to DSS (1 μg/mL × 24 hours). Phosphorylated MAPK12 (p38γ), MAPK13 (p38δ), and Hsp27 all demonstrated declines in intensity. C: Differences in optical density between control and DSS-treated arrays were measured by densitometry, and statistical analysis of the differences was determined. Differences in intensity were statistically significant (P < 0.001, 1-way ANOVA with Tukey–Kramer post-test; n = 2 independent observations).
DSS-induced Decline in Phospho-Hsp27 Inhibited by Tempol
To confirm the findings of the phospho-protein profiling, total Hsp27 and phospho-Hsp27 were measured by ELISA in control and DSS-treated NCM460 cells. Total Hsp27 did not change following exposure to DSS, in contrast to a significant decline in phospho-Hsp27 (Fig. 5) (P < 0.001). Combined exposure to DSS and Tempol yielded no change in the total Hsp27, but reversed the DSS-induced decline in phospho-Hsp27. DSS alone reduced the phospho-Hsp27 39 ± 2%, and Tempol alone increased the baseline phospho-Hsp27 24 ± 5%. Together, DSS and Tempol produced an increase of 24 ± 6%. These changes are statistically significant (P < 0.001). Since the DSS-induced decline in phospho-Hsp27 was reversed by Tempol, these data implicate the DSS-induced increase in ROS production as responsible for the decline in phospho-Hsp27.
FIGURE 5.
DSS reduces phospho-Hsp27 in the NCM460 cells. Following exposure to DSS and Tempol, total and phospho-Hsp27 in the NCM460 cells were measured by ELISA. In the cultured NCM460 cells, DSS and Tempol did not affect the total Hsp27. However, in contrast, DSS reduced the phospho-Hsp27 by 61%, and this reduction was reversed by coadministration of Tempol (100 nM). Tempol alone increased the phospho-Hsp27 by 24%. Results are consistent with the role of activated phosphorylated Hsp27 in reduction of the cellular stress response and are statistically significant (P < 0.001, 1-way ANOVA with Tukey–Kramer post-test; n = 3 independent observations). DSS, dextran sodium sulfate; Temp, Tempol.
DSS-induced Increase in Phospho-IκBα Inhibited by Tempol
When NCM460 cells were exposed to DSS (1 μg/mL × 24 hours), phospho-IκBα (Ser32) increased to 233 ± 12% of baseline (P < 0.001) (Fig. 6). This increase was completely inhibited when cells were also exposed to Tempol (reduced to 86 ± 5% of baseline), indicating that the increase was ROS-mediated. The DSS-induced changes in phosphorylation of Hsp27 and IκBα were inverse, and both effects of DSS were reversed by exposure to Tempol.
FIGURE 6.
DSS increases phospho-IκBα in NCM460 cells. Following exposure to DSS and Tempol, phospho-IκBα (Ser32) increased by 133% over baseline in the NCM460 cells. (P <0.001). The increase was inhibited by combined exposure with Tempol 100 nM.
Effects of DSS in IKKα−/− and IKKβ−/− Cells
Since DSS effects on NFκB activation are not mediated through the TLR4-Bcl10 pathway of innate immunity and the associated regulatory domain of the IKK signalosome (IKKγ), we sought to clarify the effects of DSS in relation to IKKα and IKKβ, the catalytic domains of the IKK signalosome. In mouse embryonic fibroblasts (MEF) deficient in either IKKα or IKKβ, the effects of DSS exposure (1 μg/mL × 24 hours) on phospho-Hsp27, phospho-IκBα, and KC were measured. DSS-induced decline in phospho-Hsp27 (Fig. 7) and increases in phospho-IκBα (Fig. 8) and KC (Fig. 9) were dependent on the presence of IKKβ, with no changes in the IKKβ−/− cells. Determinations of phospho-Hsp27, phospho-IκBα, and KC were made in the wildtype (WT), IKKα−/−, and IKKβ −/− cells following DSS, Tempol, and DSS with Tempol. For these determinations, the changes were similar in the WT and the IKKα−/−cells, and no differences were seen following DSS in the IKKβ−/− cells, indicating dependence on the presence of IKKβ for the DSS-induced changes to occur.
FIGURE 7.
No decline in phospho-Hsp27 following DSS in the IKKβ−/− mouse cells. In the wildtype (WT) and IKKα−/− mouse embryonic fibroblasts (MEF), DSS exposure produced decline in phospho-Hsp27 (P < 0.001) that was reversed in the presence of Tempol. In the IKKβ−/− MEF, no change in phospho-Hsp27 occurred with DSS or Tempol, demonstrating that the effects required the presence of IKKβ.
FIGURE 8.
DSS-induced increase in phospho-IκBα requires IKKβ, but not IKKα. In WT and IKKα−/− MEF, DSS exposure produced increased phospho-IκBα (P < 0.001), which was inhibited by Tempol. In the IKKβ−/− MEF, DSS exposure produced no change in phospho-IκBα, demonstrating that the DSS effect required the presence of IKKβ.
FIGURE 9.
DSS-induced increase in KC requires IKKβ, but not IKKα. In WT and IKKα−/− MEF, DSS exposure produced increased KC (P <0.001), which was inhibited by Tempol. In the IKKβ−/− MEF, DSS exposure produced no change in KC, demonstrating that the DSS effect required the presence of IKKβ.
When cells were coincubated with DSS and Tempol, the DSS-induced effects were inhibited in the WT and the IKKα−/− cells. Neither DSS nor Tempol produced changes in phospho-Hsp27, phospho-IκBα, and KC in the IKKβ−/− cells, revealing the requirement for IKKβ, but not IKKα for the activation of NFκB.
Effects of DSS on IL-8, NFκB, or Phospho-Hsp27 Are Not Mediated by Bcl10
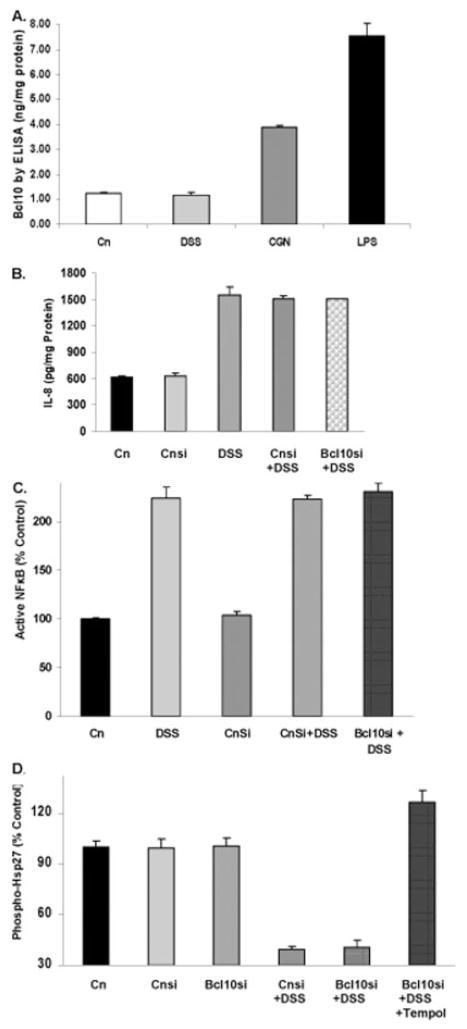
Cellular Bcl10 protein was measured by a solid-phase ELISA in the DSS-treated NCM460 cells, since previous work demonstrated that Bcl10 mediated increases in NFκB and IL-8 following exposures to carrageenan (CGN) and to lipopolysaccharide (LPS). In contrast, no change in Bcl10 occurred following DSS exposure (Fig. 10A). Consistent with this finding, silencing Bcl10 by siRNA had no effect on the increase in IL-8 secretion that followed exposure to DSS (Fig. 10B), or on the activation of NFκB (Fig. 10C), or on the increase in phospho-Hsp27 (Fig. 10D). Hence, Bcl10 does not mediate the effects of DSS.
FIGURE 10.
DSS effects on IL-8 and NFκB are not mediated by Bcl10. A: Bcl10 was measured by ELISA and found to be increased following exposure to lipopolysaccharide (10 ng/mL × 6 hours) and carrageenan (1 μg/mL × 24 hours), but not following exposure to DSS (1 μg/mL × 24 hours). Differences are statistically significant (P = 0.001, 1-way ANOVA with Tukey–Kramer post-test; n = 3 independent observations). B: NCM460 cells were grown in culture and cells were transfected with either control siRNA (con si) or Bcl10 siRNA, and treated with DSS, with or without Tempol for 24 hours. In contrast to the inhibitory effects of Tempol or Tiron, Bcl10 silencing by siRNA had no impact on the IL-8 secretion. C: Similarly, silencing of Bcl10 in the NCM460 cells had no effect on activation of NFκB, in contrast to the pronounced effect of Tempol on reduction of NFκB activation. Cnsi, control siRNA; Bcl10 si, Bcl10 siRNA. D: Bcl10 silencing had no effect on phospho-Hsp27, in contrast to DSS which reduced phospho-Hsp27 and Tempol which increased it.
DSS-induced Increases in IL-8 Are Not Inhibited by Silencing of MyD88 or by Treatment with IRAK Inhibitor
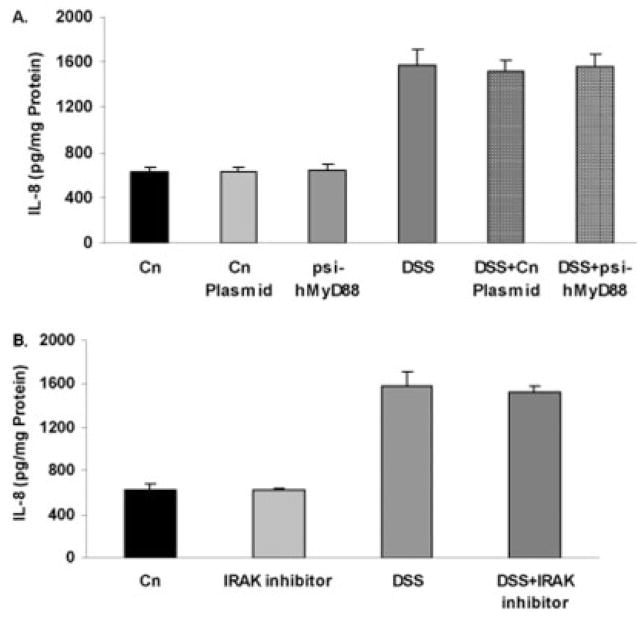
When NCM460 cells were treated with a plasmid that contained RNAi to silence MyD88 expression, the DSS-induced increase in IL-8 was unaffected, rising from 625 ± 51 to 1572 ± 145 pg/mg protein with DSS and remaining at 1559 ± 119 pg/mg protein with DSS and psi-hMyD88 (Fig. 11A). Similarly, exposure to the IRAK 1 and 4 inhibitor N-(2-morpholinylethyl)-2-(3-nitrobenzoyl-amido)-benzimidazole prior to DSS exposure had no effect on the IL-8 stimulation, remaining at 1528 ± 52 pg/mg protein (Fig. 11B). These results are consistent with DSS mediation of increases in IL-8 secretion proceeding through a mechanism that does not involve MyD88 or IRAK.
FIGURE 11.
DSS-induced increase in IL-8 is not reduced by psi-hMyD88 or by IRAK 1/4 inhibitor. A: When NCM460 cells were treated with plasmid designed to inhibit MyD88, an adaptor of TLR4 signaling, there was no impact on the DSS-induced increase in IL-8. B: Similarly, when NCM460 cells were pretreated with a specific inhibitor of IRAK 1 and 4 prior to exposure to DSS, there was no effect on the DSS-induced increase in IL-8 in the NCM460 cells.
DSS-induced Increase in KC Does Not Decline in the TLR4-deficient Mouse
Ex vivo colonic tissue from TLR4-deficient mice was exposed to DSS (1 μg/mL) for 6 hours. KC was measured and the values were compared to untreated tissue samples, as well as to DSS-treated and untreated samples from age-matched, normal control mice. The results showed no difference between the KC determinations in the DSS-exposed TLR4-deficient and control mice (Fig. 12). These findings demonstrated that the DSS pathway of KC activation was not mediated by TLR4 in the mouse colon, and are consistent with the lack of impact of Bcl10 silencing on the DSS-induced inflammatory cascade in the human colonic epithelial cells. By inference, DSS is not likely to affect IKKγ, the regulatory component of the IKK signalosome, which interacts with Bcl10.
FIGURE 12.
DSS-induced increase in KC is not reduced in the TLR4-deficient mouse. When ex vivo colonic tissue from C57BL/10ScNJ mice that have a deletion of TLR4 and from normal control mice were exposed to DSS (1 μg/mL × 6 hours), no difference in the KC response was found (n = 6). These findings are consistent with the lack of impact of Bcl10 silencing on the inflammatory response to DSS.
DISCUSSION
The sulfated polysaccharide DSS, as well as other bioreactive sulfated polysaccharides, are frequently used to induce inflammation in experimental models. The data presented in this report provide new insight into the mechanism by which DSS exposure provokes an inflammatory response. DSS activates IκBα, NFκB, and IL-8 by ROS, Hsp27, and IKKβ, and does not require TLR4, MyD88, IRAK, or Bcl10 (Fig. 13). In contrast, we found that LPS- and CGN-induced activation of IκBα, NFκB, and IL-8 were mediated in large part through the TLR4-MyD88-IRAK-Bcl10 pathway.11,12 The DSS-induced increase in KC, the mouse homolog of IL-8, was not reduced in the TLR4-deficient mouse model, compared to the normal control, providing further evidence that DSS does not induce inflammation through the TLR4-Bcl10 mediated pathway. DSS-induced effects appear to be mediated through the catalytic domain (IKKβ) of the IKK signalosome, rather than the regulatory domain (IKKγ).
FIGURE 13.
Schematic representation of DSS-induced activation of phospho-IκBα, NFκB, and IL-8 via ROS-Hsp27-IKKβ mediated pathway. In contrast, the TLR4-MyD88-IRAK-Bcl10-IKKγ is not activated by DSS exposure. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
ROS are generated as part of normal oxidative metabolism, yet cell damage is induced by their excess. Hsp27 is a stress-responsive protein that is associated with reduced stress response. Both ROS and reactive nitrogen species (RNOS) have been recognized as involved in the initiation and progression of inflammation, and different roles and responses have been associated with superoxide and nitric oxide in inflammation.40–44
In addition to the well-known pathways of free radical generation by oxygen species, redox-active sulfur species have been characterized as part of a sulfate assimilation pathway in protists, yeast, and plants.45–48 Sulfate reactions also involve the metabolism of sulfinic and sulfonic acids that are oxidized sulfur molecules. These sulfur-containing molecules are able to modify cysteine residues and participate in redox reactions that are associated with depletion of glutathi-one.49–52 Since DSS is so highly sulfated, we hypothesize that it may impose a sulfate load on cells and that this is associated with a marked increase in the measurable ROS, leading to activation of an inflammatory cascade. These effects of sulfate are consistent with clinical study of intestinal inflammation that has demonstrated an association between higher dietary sulfate content and increased risk of relapse of ulcerative colitis.53
In the experiments presented in this report, DSS modified the phosphorylation of Hsp27 and increased the phosphorylation of IκBα in cells in which IKKβ was present. This indicated a requirement for the IKKβcomponent of the IKK signalosome in the DSS-induced pathway of NFκB activation. The phosphorylation of IκBα enables the nuclear translocation of NFκB and the enhanced secretion of IL-8 or KC by the effect of NFκB on the IL-8 promoter. The ROS scavenger Tempol antagonized the effect of DSS on phospho-Hsp27, affirming that DSS induced an ROS-mediated process. The phosphorylations of the p38 MAPK kinases and Hsp27 and of IKKβ involve PP2A (protein phosphatase 2A).54–58 Inhibition of PP2A by okadaic acid resulted in reduced IL-8 secretion in the NCM460 cells (data not shown), consistent with an effect on the phosphorylation of the IKK complex and inverse phosphorylation between Hsp27 and IKKβ.22–24 The phosphorylation of Hsp27 has been reported to reduce IKK activity, and decrease in phosphorylation of the IKK complex reduces the phosphorylation of IκBα, thereby leading to reduced nuclear translocation of NFκB.
The effects demonstrated in our experiments are consistent with the effect of increased phospho-Hsp27 as an inhibitor of the cellular stress response, since DSS exposure produced decline in phospho-Hsp27 and increase in the stress response.56,57 The ROS-mediated pathway, which is associated with a decline in phospho-Hsp27, impacts the phosphorylation of the IKK signalosome, thereby affecting the nuclear translocation of NFκB (p65). The association of these cellular responses with the highly sulfated DSS suggests that there may be activation of a sulfate assimilation pathway in the human intestinal cells following exposure to DSS.
The study findings indicate that DSS exposure significantly modifies vital biochemical mechanisms and demonstrate the applicability, as well as the limitations, of DSS as a model for inflammation, including IBD. Study findings demonstrated that DSS exposure in colonic epithelial cells directly induced increases in NFκB nuclear translocation and IL-8 secretion through a distinct pathway activated by ROS, not involving the TLR4-MyD88-IRAK-Bcl10 pathway of innate immunity. In vivo, DSS exposure is expected to lead to activation of secondary immune responses in the macrophages, neutrophils, and lymphocytes of the inflammatory infiltrate that responds to the DSS-induced epithelial cell stress. The lack of DSS-induced signaling via the innate immune pathway has implications for interpretation of the effects of various pharmacological interventions and for evaluation of the impact of the DSS model of inflammation.
Acknowledgments
The authors thank Dr. James Artwohl, clinical veterinarian.
Funded in part by the Department of Veterans Affairs and by NIDDK (DK54016 and DK68324) to P.K.D.
References
- 1.Onderdonk AB. The carrageenan model for experimental ulcerative colitis. Prog Clin Biol Res. 1985;186:237–245. [PubMed] [Google Scholar]
- 2.Kitano A, Matsumoto T, Oshitani N, et al. Distribution and anti-inflammatory effect of mesalazine on carrageenan-induced colitis in the rabbit. Clin Exp Pharmacol Physiol. 1996;23:305–309. doi: 10.1111/j.1440-1681.1996.tb02828.x. [DOI] [PubMed] [Google Scholar]
- 3.Murano M, Maemura K, Hirata I, et al. Therapeutic effect of intracolonically administered nuclear factor kappa B (p65) antisense oligonucleotide on mouse dextran sulphate sodium (DSS)-induced colitis. Clin Exp Immunol. 2000;120:51–58. doi: 10.1046/j.1365-2249.2000.01183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herfarth H, Brand K, Rath HC, et al. Nuclear factor-kappa B activity and intestinal inflammation in dextran sulphate sodium (DSS)-induced colitis in mice is suppressed by gliotoxin. Clin Exp Immunol. 2000;120:59–65. doi: 10.1046/j.1365-2249.2000.01184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venkatraman A, Ramakrishna B, Shaji RV, et al. Amelioration of dextran sulfate colitis by butyrate: role of heat shock protein 70 and NF-κB. Am J Physiol Gastrointest Liver Physiol. 2003;285:G177–G184. doi: 10.1152/ajpgi.00307.2002. [DOI] [PubMed] [Google Scholar]
- 6.Jaysekhar P, Rao SB, Santhakumari G. Effect of 5-substituted benxylideneaminosalicylic acid on carrageenan-induced ulcerative colitis. Boll Chim Farm. 2004;143:309–313. [PubMed] [Google Scholar]
- 7.Oh PS, Lim KT. Plant originated glycoprotein has anti-oxidative and anti-inflammatory effects on dextran sulfate sodium-induced colitis in mouse. J Biomed Sci. 2006;13:549–560. doi: 10.1007/s11373-006-9083-9. [DOI] [PubMed] [Google Scholar]
- 8.Oz HS, Chen TS, McClain CJ, et al. Antioxidants as novel therapy in a murine model of colitis. J Nutr Biochem. 2005;16:297–304. doi: 10.1016/j.jnutbio.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Egger B, Bajaj-Elliott M, MacDonald TT, et al. Characterisation of acute murine dextran sodium sulphate colitis: cytokine profile and dose dependency. Digestion. 2000;62:240–248. doi: 10.1159/000007822. [DOI] [PubMed] [Google Scholar]
- 10.Camuesco D, Gálvez J, Nieto A, et al. Dietary olive oil supplemented with fish oil, rich in EPA and DHA (n-3) polyunsaturated fatty acids, attenuates colonic inflammation in rats with DSS-induced colitis. J Nutr. 2005;135:687–694. doi: 10.1093/jn/135.4.687. [DOI] [PubMed] [Google Scholar]
- 11.Borthakur A, Bhattacharyya S, Dudeja PK, et al. Carrageenan induces Interleukin-8 production through distinct Bcl10 pathway in normal human colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G829–838. doi: 10.1152/ajpgi.00380.2006. [DOI] [PubMed] [Google Scholar]
- 12.Bhattacharyya S, Borthakur A, Pant N, et al. Bcl10 mediates lipopolysaccharide (LPS)-induced activation of NFκB and IL-8 in human intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2007;293:G429–437. doi: 10.1152/ajpgi.00149.2007. [DOI] [PubMed] [Google Scholar]
- 13.McAllister-Lucas LM, Ruland J, Siu K, et al. CARMA3/Bcl10/MALT1-dependent NFkappaB activation mediates angiotensin II-responsive inflammatory signaling in nonimmune cells. Proc Natl Acad Sci U S A. 2007;104:139–144. doi: 10.1073/pnas.0601947103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klemm S, Zimmermann S, Peschel C, et al. Bcl10 and Malt1 control lysophosphatidic acid-induced NF-kappa B activation and cytokine production. Proc Natl Acad Sci U S A. 2007;104:134–138. doi: 10.1073/pnas.0608388103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang D, You Y, Lin PC, et al. Bcl10 plays a critical role in NF-κB activation induced by G protein-coupled receptors. Proc Natl Acad Sci U S A. 2007;104:145–150. doi: 10.1073/pnas.0601894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Q, Siebert R, Yan M, et al. Inactivating mutations and overexpression of BCL10, a caspase recruitment domain-containing gene, in MALT lymphoma with t(1;14)(p22;q32) Nat Genet. 1999;22:63–68. doi: 10.1038/8767. [DOI] [PubMed] [Google Scholar]
- 17.Isaacson PG, Du MQ. MALT lymphoma: from morphology to molecules. Nat Rev Cancer. 2004;4:644–653. doi: 10.1038/nrc1409. [DOI] [PubMed] [Google Scholar]
- 18.Willis TG, Jadayel DM, Du MQ, et al. Bcl10 is involved in t(1;14)(p22; q32) of MALT B cell lymphoma and mutated in multiple tumor types. Cell. 1999;96:335–345. doi: 10.1016/s0092-8674(00)80957-5. [DOI] [PubMed] [Google Scholar]
- 19.Keshavarzian A, Fusunyan RD, Jacyno M, et al. Increased interleukin-8 (IL-8) in rectal dialysate from patients with ulcerative colitis: evidence for a biological role for IL-8 in inflammation of the colon. Am J Gastroenterol. 1999;94:704–712. doi: 10.1111/j.1572-0241.1999.00940.x. [DOI] [PubMed] [Google Scholar]
- 20.Kankuri E, Hämäläinen M, Hukkanen M, et al. Suppression of pro-inflammatory cytokine release by selective inhibition of inducible nitric oxide synthase in mucosal explants from patients with ulcerative colitis. Scand J Gastroenterol. 2003;38:186–192. doi: 10.1080/00365520310000681. [DOI] [PubMed] [Google Scholar]
- 21.Levine JJ, Pettei MJ, Valderrama E, et al. Nitric oxide and inflammatory bowel disease: evidence for local intestinal production in children with active colonic disease. J Pediatr Gastroenterol Nutr. 1998;26:34–38. doi: 10.1097/00005176-199801000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Park KJ, Gaynor RB, Kwak YT. Heat shock protein 27 association with the I kappa B kinase complex regulates tumor necrosis factor alpha-induced NF-kappa B activation. J Biol Chem. 2003;278:35272–35278. doi: 10.1074/jbc.M305095200. [DOI] [PubMed] [Google Scholar]
- 23.Kammanadiminti SJ, Chadee K. Suppression of NF-kappa B activation by Entamoeba histolytica in intestinal epithelial cells is mediated by heat shock protein 27. J Biol Chem. 2006;281:26112–26120. doi: 10.1074/jbc.M601988200. [DOI] [PubMed] [Google Scholar]
- 24.Sur R, Lyte PA, Southall MD. Hsp27 regulates pro-inflammatory mediator release in keratinocytes by modulating NF-kappaB signaling. J Invest Dermatol. 2008;128:1116–1122. doi: 10.1038/sj.jid.5701157. [DOI] [PubMed] [Google Scholar]
- 25.Jobin C, Sartor RB. The I kappa B/NF-kappa B system: a key determinant of mucosal inflammation and protection. Am J Physiol Cell Physiol. 2000;278:C451–C462. doi: 10.1152/ajpcell.2000.278.3.C451. [DOI] [PubMed] [Google Scholar]
- 26.Jung HC, Eckmann L, Yang SK, et al. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moyer MP, Manzano LA, Merriman RL, et al. NCM460, a normal human colon mucosal epithelial cell line. In Vitro Cell Dev Biol Anim. 1996;32:315–317. doi: 10.1007/BF02722955. [DOI] [PubMed] [Google Scholar]
- 28.Li ZW, Chu W, Hu Y, et al. The IKKbeta subunit of IkappaB kinase (IKK) is essential for nuclear factor kappaB activation and prevention of apoptosis. J Exp Med. 1999;189:1839–1845. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ndengele MM, Muscoli C, Wang ZQ, et al. Superoxide potentiates NF-kappaB activation and modulates endotoxin-induced cytokine production in alveolar macrophages. Shock. 2005;23:186–193. doi: 10.1097/01.shk.0000144130.36771.d6. [DOI] [PubMed] [Google Scholar]
- 30.Araki Y, Sugihara H, Hattori T. The free radical scavengers edaravone and tempol suppress experimental dextran sulfate sodium-induced colitis in mice. Int J Mol Med. 2006;17:331–334. [PubMed] [Google Scholar]
- 31.Lejeune D, Hasanuzzaman M, Pitcock A, et al. The superoxide scavenger TEMPOL induces urokinase receptor (uPAR) expression in human prostate cancer cells. Mol Cancer. 2006;5:21. doi: 10.1186/1476-4598-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cuzzocrea S, McDonald MC, Mazzon E, et al. Tempol, a membrane-permeable radical scavenger, reduces dinitrobenezene sulfonic acid-induced colitis. Eur J Pharmacol. 2000;406:127–137. doi: 10.1016/s0014-2999(00)00623-3. [DOI] [PubMed] [Google Scholar]
- 33.Yang J, Su Y, Richmond A. Antioxidants tiron and N-acetyl-L-cysteine differentially mediate apoptosis in melanoma cells via a reactive oxygen species-independent NF-kappaB pathway. Free Radic Biol Med. 2007;42:1369–1380. doi: 10.1016/j.freeradbiomed.2007.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhattacharyya S, Pant N, Dudeja PK, et al. Development, evaluation, and application of a highly sensitive microtiter plate ELISA for human Bcl10 protein. J Immunoassay Immunochem. 2007;28:173–188. doi: 10.1080/15321810701454573. [DOI] [PubMed] [Google Scholar]
- 35.Bhattacharyya S, Dudeja PK, Tobacman JK. Carrageenan-induced NFkappaB activation depends on distinct pathways mediated by reactive oxygen species and Hsp27 or by Bcl10. Biochim Biophys Acta. 2008;1780:973–982. doi: 10.1016/j.bbagen.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhattacharyya S, Gill R, Chen M-L, et al. Toll-like receptor 4 mediates induction of Bcl10-NFκB-IL-8 inflammatory pathway by carrageenan in human intestinal epithelial cells. J Biol Chem. 2008;283:10550–10558. doi: 10.1074/jbc.M708833200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zughaier SM, Zimmer SM, Datta A, et al. Differential induction of the toll-like receptor 4-MyD88-dependent and -independent signaling pathways by endotoxins. Infect Immun. 2005;73:2940–2950. doi: 10.1128/IAI.73.5.2940-2950.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guillot L, Medjane S, Le-Barillec K, et al. Response of human pulmonary epithelial cells to lipopolysaccharide involves Toll-like receptor 4 (TLR4)-dependent signaling pathways. J Biol Chem. 2004;279:2712–2718. doi: 10.1074/jbc.M305790200. [DOI] [PubMed] [Google Scholar]
- 39.Powers JP, Li S, Jaen JC, et al. Discovery and initial SAR of inhibitors of interleukin-1 receptor-associated kinase-4. Bioorg Med Chem Lett. 2006;16:2842–2845. doi: 10.1016/j.bmcl.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 40.Krieglstein CF, Cerwinka WH, Laroux FS, et al. Regulation of murine intestinal inflammation by reactive metabolites of oxygen and nitrogen: divergent roles of superoxide and nitric oxide. J Exp Med. 2001;94:1207–1218. doi: 10.1084/jem.194.9.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kolios G, Rooney N, Murphy CT, et al. Expression of inducible nitric oxide synthase activity in human colon epithelial cells: modulation by T lymphocyte derived cytokines. Gut. 1998;43:56–63. doi: 10.1136/gut.43.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberts PJ, Riley GP, Morgan K, et al. The physiological expression of inducible nitric oxide synthase (iNOS) in the human colon. J Clin Pathol. 2001;54:293–297. doi: 10.1136/jcp.54.4.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menconi MJ, Unno N, Smith M, et al. Nitric oxide donor-induced hyperpermeability of cultured intestinal epithelial monolayers: role of superoxide radical, hydroxyl radical, and peroxynitrite. Biochim Biophys Acta. 1998;1425:189–203. doi: 10.1016/s0304-4165(98)00072-5. [DOI] [PubMed] [Google Scholar]
- 44.Kruidenier L, van Meeteren ME, Kuiper I, et al. Attenuated mild colonic inflammation and improved survival from severe DSS-colitis of transgenic Cu/Zn-SOD mice. Free Radic Biol Med. 2003;34:753–765. doi: 10.1016/s0891-5849(02)01426-0. [DOI] [PubMed] [Google Scholar]
- 45.Kopriva S. Regulation of sulfate assimilation in Arabidopsis and beyond. Ann Bot (Lond) 2006;97:479–495. doi: 10.1093/aob/mcl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Höfgen R, Kreft O, Willmitzer L, et al. Manipulation of thiol contents in plants. Amino Acids. 2001;20:291–299. doi: 10.1007/s007260170045. [DOI] [PubMed] [Google Scholar]
- 47.Meng Z. Oxidative damage of sulfur dioxide on various organs of mice: sulfur dioxide is a systemic oxidative damage agent. Inhal Toxicol. 2003;15:181–195. doi: 10.1080/08958370304476. [DOI] [PubMed] [Google Scholar]
- 48.Dhia Thabet OB, Fardeau ML, Suarez-Nuñez C, et al. Desulfovibrio marinus sp. nov., a moderately halophilic sulfate-reducing bacterium isolated from marine sediments in Tunisia. Int J Syst Evol Microbiol. 2007;57:2167–2170. doi: 10.1099/ijs.0.64790-0. [DOI] [PubMed] [Google Scholar]
- 49.Jacob C, Holme AL, Fry FH. The sulfinic acid switch in proteins. Org Biomol Chem. 2004;2:1953–1956. doi: 10.1039/b406180b. [DOI] [PubMed] [Google Scholar]
- 50.Chang TS, Jeong W, Woo HA, et al. Characterization of mammalian sulfiredoxin and its reactivation of hyperoxidized peroxiredoxin through reduction of cysteine sulfinic acid in the active site to cysteine. J Biol Chem. 2004;279:50994–51001. doi: 10.1074/jbc.M409482200. [DOI] [PubMed] [Google Scholar]
- 51.Woods JS, Kavanagh TJ, Corral J, et al. The role of glutathione in chronic adaptation to oxidative stress: studies in a normal rat kidney epithelial (NRK52E) cell model of sustained upregulation of glutathione biosynthesis. Toxicol Appl Pharmacol. 1999;160:207–216. doi: 10.1006/taap.1999.8774. [DOI] [PubMed] [Google Scholar]
- 52.Dalton TP, Chen Y, Schneider SN, et al. Genetically altered mice to evaluate glutathione homeostasis in health and disease. Free Radic Biol Med. 2004;37:1511–1526. doi: 10.1016/j.freeradbiomed.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 53.Jowett SL, Seal CJ, Pearce MS, et al. Influence of dietary factors on the clinical course of ulcerative colitis: a prospective cohort study. Gut. 2004;53:1479–1484. doi: 10.1136/gut.2003.024828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cairns J, Qin S, Philp R, et al. Dephosphorylation of the small heat shock protein Hsp27 in vivo by protein phosphatase 2A. J Biol Chem. 1994;69:9176–9183. [PubMed] [Google Scholar]
- 55.Kray AE, Carter RS, Pennington KN, et al. Positive regulation of IkappaB kinase signaling by protein serine/threonine phosphatase 2A. J Biol Chem. 2005;280:35974–35982. doi: 10.1074/jbc.M506093200. [DOI] [PubMed] [Google Scholar]
- 56.Tar K, Csortos C, Czikora I, et al. Role of protein phosphatase 2A in the regulation of endothelial cell cytoskeleton structure. J Cell Biochem. 2006;98:931–953. doi: 10.1002/jcb.20829. [DOI] [PubMed] [Google Scholar]
- 57.Arrigo AP. The cellular “networking” of mammalian Hsp27 and its functions in the control of protein folding, redox state and apoptosis. Adv Exp Med Biol. 2007;594:14–26. doi: 10.1007/978-0-387-39975-1_2. [DOI] [PubMed] [Google Scholar]
- 58.Landry J, Huot J. Modulation of actin dynamics during stress and physiological stimulation by a signaling pathway involving p38 MAP kinase and heat-shock protein 27. Biochem Cell Biol. 1995;73:703–707. doi: 10.1139/o95-078. [DOI] [PubMed] [Google Scholar]