Summary
Intracellular cargo transport requires microtubule-based motors, kinesin and cytoplasmic dynein, and the actin-based myosin motors to maneuver through the challenges presented by the filamentous meshwork that comprises the cytoskeleton. Recent in vitro single molecule biophysical studies have begun to explore this process by characterizing what occurs as these tiny molecular motors happen upon an intersection between two cytoskeletal filaments. These studies, in combination with in vivo work, define the mechanism by which molecular motors exchange cargo while traveling between filamentous tracks and deliver it to its destination when going from the cell center to the periphery and back again.
Introduction
Cargo transport of organelles, secretory vesicles, and protein complexes by tiny molecular motors is an essential intracellular process. The importance of intracellular transport is highlighted by the fact that mutations to these motors lead to genetic diseases such as amyotrophic lateral sclerosis [1], parapalegia [2], and Griscelli syndrome type 1 [3]. Molecular motors that drive cargo transport along the cytoskeletal highway include myosins traveling along actin filaments and kinesin and cytoplasmic dynein motors traveling on microtubules (Figure 1). These motors share cargo transport duties and face the challenge of maneuvering through a complex cytoskeleton with numerous microtubule and actin filament intersections. How these motors navigate these obstacles and whether they work together to assure that cargo reaches its final destination is still unclear. In this review, we will highlight recent single molecule in vitro experiments that characterize the transport capacity of individual and small ensembles of molecular motors along constructed cytoskeletal networks. We will discuss how these results contribute to our understanding of intracellular cargo transport in vivo.
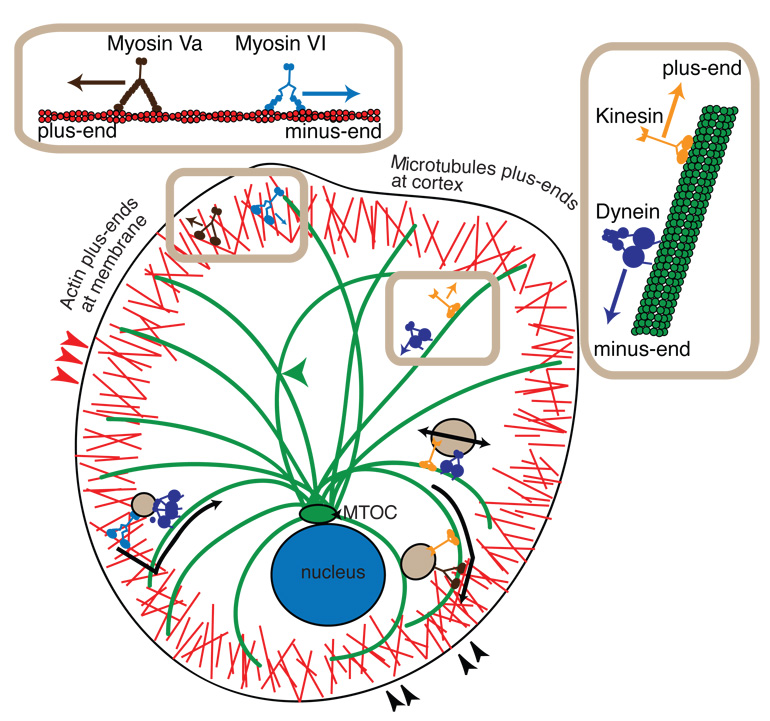
Figure 1.
Cartoon representation of motor proteins and vesicular cargo transport in the cell. Myosin family motors, myosin Va (dark brown) and myosin VI (light blue), walk along actin filaments (red) at the cortex. Myosin Va walks toward the F-actin plus-end, which is oriented towards the membrane. Myosin VI walks towards the minus-end of F-actin, toward the cell interior. Microtubule-based motors include the kinesin family motors (orange) and cytoplasmic dynein (violet). Kinesin motors walk to the plus-ends of microtubules (green), which are oriented toward the actin cortex. Dynein motors walk toward the minus-end of the microtubule, which is located at the microtubule-organizing center (MTOC, green) near the cell nucleus (blue). F-actin and microtubules cross at the cell cortex, as highlighted by black arrowheads (lower right). F-actin can cross itself in the cortex, highlighted by the red arrowheads (left). Microtubules can intersect other microtubules highlighted by the green arrowhead (center). Vesicular cargo (tan) can bind to myosin VI and dynein to switch from actin-based to microtubule-based motion while being transported into the cell interior (lower left). Vesicles can bind kinesin and myosin Va to switch from microtubule-based to actin-based motion in order to be transported to the cell cortex (lower right). Vesicles traveling on microtubules can experience a tug-of-war from kinesin and dynein simultaneously bound (right).
Cargo Transport and Track-Switching
Both the secretory and endocytic pathways require that vesicular cargo be transferred between actin and microtubule tracks. Ideally, microtubules originate from an organizing center near the nucleus and fan out with their plus-ends towards the cell periphery. Cargos (e.g. secretory vesicles) carried by plus-end directed kinesins are translocated along microtubules toward the cortex. Upon reaching the dense cortical actin meshwork the cargo is transferred to myosin Va for delivery to the cell membrane (Figure 1). The overall actin polarity within the cell cortex is directed with barbed or plus-ends towards the cell membrane, and myosin Va walks towards actin plus ends.
In the reverse direction, during endocytosis, vesicles may be transported initially through the actin cortex by the minus-end directed myosin VI. Cargo is then handed-off to minus-end directed cytoplasmic dynein traveling on microtubules (Figure 1). As described, cargo transport involves switching between motor carriers and tracks at actin filament/microtubule intersections as observed in vivo for encapsulated viruses [4], melanosomes and peroxisomes [5••, 6•, 7], vesicles (human epidermal growth factor receptors HER2) [8••], and endocytosed quantum dots [9•].
Unlike the ideal actions outlined above, intracellular transport is plagued with impediments to motion brought about by the intracellular milieu and the motors themselves. For example, multiple motor types are known to be associated with individual cargos (Figure 1). Are their cargo transport activities coordinated or do these motors undergo a constant “tug of war” [6•]? For example, Gross et al. [10] observed that the velocity of melanosomes transported along microtubules increases when the myosin Va is effectively inactivated by a dominant negative construct. This result suggests that kinesin-driven movement may be impeded by myosin Va bound to the same melanosome. Kural et al. [5••, 6•] observed GFP-tagged peroxisomes moving bidirectionally on microtubules, suggesting that both kinesin and dynein are present and that for discrete periods of motion only one of the motor types dominates. Bidirectional excursions are the result of: 1) one of the motor types winning the tug of war, or 2) coordinated dissociation of one motor type so that the opposite type can power motion. Another impediment to motion is the cytoskeleton itself, which presents a crowded sea of physical barriers to forward motion, such as crossing filaments, associated proteins, or other motors with bound cargo all of which may limit the cargo’s forward motion.
Similar questions relate to the specific mechanism by which the hand-off of cargo occurs at cytoskeletal track intersections. Is it a coordinated process or a tug of war, where the stronger or more numerous motor wins? Even more simply, what does a single kinesin or dynein motor do when it encounters a microtubule-microtubule intersection or when a myosin faces an actin-actin intersection? To understand how such motors maneuver through the cytoskeleton, the molecular structure and in vitro function of these molecular motors provide insight into the physical constraints that limit their maneuverability.
Inherent Motor Properties Critical for Cargo Transport
All three motor types (i.e. myosins, kinesins, and cytoplasmic dyneins) have two motor domains that hydrolyze ATP and convert chemical energy into force and motion. These two motor domains are highly coordinated so that the molecule steps processively in a hand-over-hand fashion, taking multiple steps before diffusing away from its track [11•–14]. Although, a single processive motor can, in principal, act as a cargo transporter, it is more likely that several motors are involved, assuring consistent intracellular cargo delivery. For example, Vershinin, et al. showed that multiple kinesin motors working together in vitro can carry cargo far longer than a single motor [15••]. On the other hand, recent in vivo work suggests that only a single motor is actively engaged at any one time since stepwise cargo movements, equaling the stride length of a single motor, were observed inside cells [5, 8, 16]. This follows given that two or more kinesin motors in vitro generate 4 nm or less step sizes when simultaneously interacting with the microtubule [17•]. However, single motor processivity is not an essential property for cargo transport provided there are sufficient numbers so that one motor remains in contact with the track at any point in time, as evidenced by several single-headed or non-processive dimeric motors being able to transport cargo [18–20].
Recent findings suggest that motor processivity can be enhanced by the motor associating via a second binding domain, protein, or protein complex. KIF1A uses a second electrostatic binding domain to power single headed processivity [19]. Melanophilin is an accessory protein that provides a tether for myosin Va motility [21]. Dynactin is a large complex which can dock dynein and kinesin-2 to cargo and enhances processivity by tethering the motors to the microtubule [22•]. These electrostatic tethers effectively prevent the free motor from diffusing away, allowing it to reattach and begin another processive run.
Single molecule optical trapping and total internal reflection fluorescence microscopy studies have determined the step size (more appropriately the stride length) for these motors [11–13, 23, 24, 25•, 26•]. For kinesin and Myosin Va, the step size is fairly constant (8nm, kinesin; 36nm, myosin Va) [11–13, 23], whereas cytoplasmic dynein and myosin VI may have variable step sizes (dynein, up to 32 nm; myosin VI, greater than 30 nm) [14, 24, 25, 27]. These stride lengths reflect both the structure of the motor and the track upon which it steps. For example, myosin Va and dynein dimerize at some distance from their individual motor domains, allowing the motors to skip multiple binding sites on its track before the lead motor domain completes its diffusive search and binds to the track. The dimerization domain for myosin Va is flexible enough so that the unbound leading head can freely explore its diffusional space before attaching to the actin filament [28••, 29•, 30•]. Kinesin, on the other hand, has a short link between the dimerization and motor domains, resulting in a more compact structure and thus less able to skip binding sites on the microtubule. These properties impact these motors’ capacity to maneuver through a cytoskeletal intersection.
Another critical property is the motor’s maximum force generation. Through optical trapping experiments, kinesin is strongest of the motors capable of generating stall forces of 5–8 pN [31, 32], twice that of myosin V [33], and three-times that of cytoplasmic dynein [24••]. These absolute forces may be a factor in cargo transport if tug of wars are common, or when cargo encounter intracellular obstacles that present a load that may impede motion.
In Vitro Maneuvering Through Intersections
A recent series of experiments take a bottom-up approach to build complexity by constructing a simple model of the cytoskeleton on a glass coverslip. By adhering isolated actin filaments and/or microtubules to form filament intersections, one can observe how a single motor, or a small ensemble of motors attached to a bead, navigates an intersection (Figure 2).
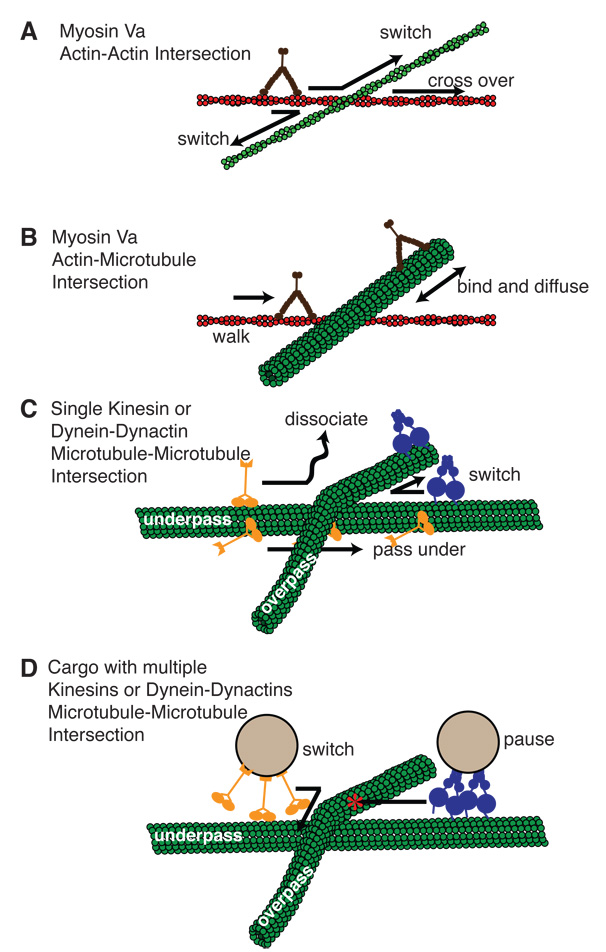
Figure 2.
Schematic diagram of in vitro assays with intersecting cytoskeletal filaments. Intersections have been created between two F-actin filaments, an F-actin and a microtubule, and two microtubules. (A) For actin-actin intersections, recent studies have observed myosin Va switching most frequently to the crossing filament, but also passing over the intersecting F-actin (green). (B) The same study used the microtubule as an obstacle for Myosin Va stepping, but found myosin Va was able to bind and diffuse along microtubules. (C) An interesting feature of microtubule-microtubule intersections is that they have “underpass” and “overpass” tracks. Single kinesin are able to transgress the intersection on the underpass track by going under the overpass bridge. Dynein-dynactin was most likely to switch between microtubules. (D) Two studies of beads decorted with kinesin at microtubule-microtubule intersections have shown that kinesin coated-beads switch frequently at the intersection. Dynein-dynactin coated beads were found to pause at the intersection at high motor density, but pass or switch at low motor density.
Myosin Va at Actin:Actin Intersections
Using this approach, Ali et al., determined that a single myosin Va can deal with an actin-actin intersection (Figure 2A) without hesitation [28••]. In fact, 48% of the time, a myosin Va molecule switched tracks, 37% terminated at the intersection, while 15% crossover [28••]. Given myosin Va’s structure and large 36 nm stride length, one might have expected a higher crossover frequency assuming that myosin Va could easily step over the 7 nm physical barrier presented by the intersecting actin filament. A simple model was developed to explain the result in which myosin Va’s inherent flexibility allows the leading head to undergo a limited three-dimensional, diffusive search [29•, 30•]. It is assumed that all monomers within range are equally attractive. The intersecting filament presents four times as many monomers within reach of the myosin head. Thus, the motor chooses to switch filaments and turn more often than crossing over (Figure 2A), which may account for the 50% probability of melanosomes switching actin tracks in vivo [7]. In fact, the motor’s flexibility is so great that it can take up to a 150° turn at an intersection and thus easily capable of switching filaments at a 70° angle created by the ARP2/3 complex.
Myosin Va at Actin:Microtubule Intersections
In this same study, Ali, et al. also observed how myosin Va deals with an actin-microtubule intersection [28••]. Surprisingly, myosin Va motors can switch from actin to microtubules (Figure 2B). Once associated with the microtubule, the myosin will diffuse along the microtubule due to an electrostatic interaction between the positively-charged surface Loop 2 on myosin Va and the negatively-charged tubulin E-hook. Ali, et al. speculate that this interaction may allow the myosin Va to effectively pace along the microtubule, waiting to link up with cargo being transported by kinesin. A more intriguing possibility is that the myosin Va may enhance kinesin’s processivity through its electrostatic interaction with the microtubule and thus act as a tether preventing the cargo from diffusing away from its track.
Kinesin and Dynein at Microtubule:Microtubule Intersections
Two recent studies have examined how kinesins and dyneins manage at microtubule-microtubule intersections. Using kinesin-coated beads, Vershinin et al. observed that beads with three or more kinesins pause 58% of the time at microtubule intersections with the remaining 42% switching between microtubules after deforming the microtubules [15••]. Interestingly, adding very little tau, a microtubule-associated protein (MAP), increased the switching probability to 91% without any detectable microtubule deformations. This implies that tau, by blocking kinesin binding to the microtubule, reduces the chance that the multiple kinesin motors will engage in a tug of war at the intersection.
A second study by Ross, et al. characterized the behavior of single fluorescently-labeled kinesin or dynein-dynactin molecules at microtubule-microtubule intersections [34••]. Given the 25 nm microtubule diameter, a microtubule-microtubule intersection creates an effective underpass and overpass (Figure 2C). In fact, single kinesins make their way easily through intersections whether traveling on the underpass or overpass microtubule, suggesting that kinesin is small enough to squeeze through the underpass (Figure 2C). However, dynein-dynactin was less likely to pass, but would more easily switch from one microtubule to another at the intersection, reflecting dynein-dynactin’s larger size and flexible nature. These authors then increased complexity of the experiment by decorating beads with dynein-dynactin or kinesin as did Vershinin et al. [15••]. Interestingly, kinesin-decorated beads switch microtubules at intersections much more frequently than single motors: approximately 66% of the time from an underpass and 33% of the time from an overpass. These statistics were quite stable for all decoration densities, in agreement with the results of Vershinin et al. [15••]. However, for dynein-dyanctin decorated beads, as the decoration density increases, the percentage of beads that become moored at the intersection increases (Figure 2D). In fact at the highest dynein-dynactin concentrations, ~100% of beads are stuck at the intersection, no matter if starting from the underpass or overpass microtubule. Since dynein-dynactin may be used to tether organelles in the cell at places of high microtubule density, this in vitro result may support this biological role for dynein-dynactin [34••].
Conclusions
With the advent of single molecule biophysical techniques, the recent flurry of in vivo and in vitro studies have provided significant insight to how molecular motors manage to transport and deliver cargo within the cell. However, many questions still remain that pose experimental challenges. For example, in vitro studies must build further complexity to characterize how cargo with mixed populations of actin and microtubule-based motors navigate through a well-defined three-dimensional array of microtubules and actin filaments. Such studies will help define the exchange and hand-off of cargo that occurs at microtubule-actin filament intersections near the cell cortex. If this is a coordinated process, then understanding the modes by which these motors are regulated will be critical. This is not a trivial matter, since regulation can occur by autoinhibition [35••, 36, 37•, 38], motor-motor interactions [39, 40], and binding partners that exist either on the cargo or the track itself [15, 41]. Therefore, in vitro studies using endogenous, membrane-encapsulated cargos or organelles would be extremely enlightening to determine how motors and their regulating partners on cargo behave at cytoskeletal intersections. For the most part, in vivo studies have relied on the motion of the cargo to infer the motors’ transport properties. The challenge will be to correlate the dynamics of cargo movement with that of the motor or motors themselves in real time. Although the challenges are great, building complexity in vitro and breaking down complexity in vivo will be the key to understanding what is an extremely difficult question to answer, what is involved in getting cargo within the cell from point A to point B?
Acknowledgments
JLR would like to thank the Goldman and Holzbaur laboratories and DMW and MYA the Trybus and Warshaw laboratories for numerous discussions that helped formulate our thoughts around issues presented in this review. DMW and MYA were supported by funds from the National Institutes of Health (HL059408, HL085489).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
• • of outstanding interest
- 1.Chevalier-Larsen E, Holzbaur EL. Axonal transport and neurodegenerative disease. Biochim Biophys Acta. 2006;1762:1094–1108. doi: 10.1016/j.bbadis.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Lo Giudice M, Neri M, Falco M, Sturnio M, Calzolari E, Di Benedetto D, Fichera M. A missense mutation in the coiled-coil domain of the KIF5A gene and late-onset hereditary spastic paraplegia. Arch Neurol. 2006;63:284–287. doi: 10.1001/archneur.63.2.284. [DOI] [PubMed] [Google Scholar]
- 3.Takagishi Y, Murata Y. Myosin Va mutation in rats is an animal model for the human hereditary neurological disease, Griscelli syndrome type 1. Ann N Y Acad Sci. 2006;1086:66–80. doi: 10.1196/annals.1377.006. [DOI] [PubMed] [Google Scholar]
- 4.Lakadamyali M, Rust MJ, Babcock HP, Zhuang X. Visualizing infection of individual influenza viruses. Proc Natl Acad Sci U S A. 2003;100:9280–9285. doi: 10.1073/pnas.0832269100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kural C, Serpinskaya AS, Chou YH, Goldman RD, Gelfand VI, Selvin PR. Tracking melanosomes inside a cell to study molecular motors and their interaction. Proc Natl Acad Sci U S A. 2007;104:5378–5382. doi: 10.1073/pnas.0700145104. Melanosomes are localized with nm resolution inside cells and tracked as they move along microtubules and actin filaments. Single 8 nm steps powered by kinesin or dynein are observed for anterograde or retrograde movements along microtubules, with myosinV-powered 35 nm steps observed along actin filaments. Interestingly, a single melanasome was observed switching between microtubule and actin tracks as inferred from changes in direction that correlate with a change in step size
- 6. Kural C, Kim H, Syed S, Goshima G, Gelfand VI, Selvin PR. Kinesin and dynein move a peroxisome in vivo: a tug-of-war or coordinated movement? Science. 2005;308:1469–1472. doi: 10.1126/science.1108408. GFP-tagged peroxisomes were tracked as they moved bi-directionally on microtubules in S2 cells. The authors proposed that multiple kinesin and dynein may work together to generate cargo speeds 10 times that of speeds measured in vitro
- 7.Snider J, Lin F, Zahedi N, Rodionov V, Yu CC, Gross SP. Intracellular actin-based transport: how far you go depends on how often you switch. Proc Natl Acad Sci U S A. 2004;101:13204–13209. doi: 10.1073/pnas.0403092101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Watanabe TM, Higuchi H. Stepwise movements in vesicle transport of HER2 by motor proteins in living cells. Biophys J. 2007;92:4109–4120. doi: 10.1529/biophysj.106.094649. Anti-HER2 antibody-labeled quantum dots (Qdots) were endocytosed into breast cancer cells. The Qdot-containing vesicles were observed while being transported by myosin VI lying just beneath the cell membrane, then switching to microtubules to be transported by dynein toward the cell nucleus
- 9. Nan X, Sims PA, Chen P, Xie XS. Observation of individual microtubule motor steps in living cells with endocytosed quantum dots. J Phys Chem B. 2005;109:24220–24224. doi: 10.1021/jp056360w. Vesicles with endocytosed Qdots are observed moving birdirectionally towards the cell center and periphery with 8–16 nm steps implying dynein-powered motion
- 10.Gross SP, Tuma MC, Deacon SW, Serpinskaya AS, Reilein AR, Gelfand VI. Interactions and regulation of molecular motors in Xenopus melanophores. J Cell Biol. 2002;156:855–865. doi: 10.1083/jcb.200105055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Warshaw DM, Kennedy GG, Work SS, Krementsova EB, Beck S, Trybus KM. Differential labeling of myosin V heads with quantum dots allows direct visualization of hand-over-hand processivity. Biophys J. 2005;88:L30–L32. doi: 10.1529/biophysj.105.061903. Each myosin V head was labeled with a different color Qdot, demonstrating directly that the heads move in a hand-over-hand manner, with each head taking 72 nm steps
- 12.Yildiz A, Forkey JN, McKinney SA, Ha T, Goldman YE, Selvin PR. Myosin V walks hand-over-hand: single fluorophore imaging with 1.5-nm localization. Science. 2003;300:2061–2065. doi: 10.1126/science.1084398. [DOI] [PubMed] [Google Scholar]
- 13.Yildiz A, Tomishige M, Vale RD, Selvin PR. Kinesin walks hand-over-hand. Science. 2004;303:676–678. doi: 10.1126/science.1093753. [DOI] [PubMed] [Google Scholar]
- 14.Yildiz A, Park H, Safer D, Yang Z, Chen LQ, Selvin PR, Sweeney HL. Myosin VI steps via a hand-over-hand mechanism with its lever arm undergoing fluctuations when attached to actin. J Biol Chem. 2004;279:37223–37226. doi: 10.1074/jbc.C400252200. [DOI] [PubMed] [Google Scholar]
- 15. Vershinin M, Carter BC, Razafsky DS, King SJ, Gross SP. Multiple-motor based transport and its regulation by Tau. Proc Natl Acad Sci U S A. 2007;104:87–92. doi: 10.1073/pnas.0607919104. In vitro study of beads trafficked by one, two, or multiple kinesin motors along tau-coated microtubules. Tau blocks kinesin binding altering the behavior of kinesin-coated beads at microtubule intersections.
- 16.Levi V, Serpinskaya AS, Gratton E, Gelfand V. Organelle transport along microtubules in Xenopus melanophores: evidence for cooperation between multiple motors. Biophys J. 2006;90:318–327. doi: 10.1529/biophysj.105.067843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leduc C, Ruhnow F, Howard J, Diez S. Detection of fractional steps in cargo movement by the collective operation of kinesin-1 motors. Proc Natl Acad Sci U S A. 2007;104:10847–10852. doi: 10.1073/pnas.0701864104. When two surface attached kinesin motors are engaged simultaneously in transporting a microtubule, the step motions of the microtubule were reduced to 4-nm, half of a single motor's step size
- 18.Kon T, Nishiura M, Ohkura R, Toyoshima YY, Sutoh K. Distinct functions of nucleotide-binding/hydrolysis sites in the four AAA modules of cytoplasmic dynein. Biochemistry. 2004;43:11266–11274. doi: 10.1021/bi048985a. [DOI] [PubMed] [Google Scholar]
- 19.Okada Y, Higuchi H, Hirokawa N. Processivity of the single-headed kinesin KIF1A through biased binding to tubulin. Nature. 2003;424:574–577. doi: 10.1038/nature01804. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe T, Hosoya H, Yonemura S. Regulation of myosin II dynamics by phosphorylation and dephosphorylation of its light chain in epithelial cells. Mol Biol Cell. 2007;18:605–616. doi: 10.1091/mbc.E06-07-0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geething NC, Spudich JA. Identification of a minimal myosin Va binding site within an intrinsically unstructured domain of melanophilin. J Biol Chem. 2007;282:21518–21528. doi: 10.1074/jbc.M701932200. [DOI] [PubMed] [Google Scholar]
- 22. Culver-Hanlon TL, Lex SA, Stephens AD, Quintyne NJ, King SJ. A microtubule-binding domain in dynactin increases dynein processivity by skating along microtubules. Nat Cell Biol. 2006;8:264–270. doi: 10.1038/ncb1370. This biophysical study showed that multiple domains on the p150Glued molecule of dynactin complex bind microtubules. The CAP-Gly binding domain, binds statically to microtubules while a second, new domain with basic residues, can tether to and diffuse across the surface of the microtubule
- 23.Block SM. Kinesin motor mechanics: binding, stepping, tracking, gating, and limping. Biophys J. 2007;92:2986–2995. doi: 10.1529/biophysj.106.100677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mallik R, Carter BC, Lex SA, King SJ, Gross SP. Cytoplasmic dynein functions as a gear in response to load. Nature. 2004;427:649–652. doi: 10.1038/nature02293. [DOI] [PubMed] [Google Scholar]
- 25. Reck-Peterson SL, Yildiz A, Carter AP, Gennerich A, Zhang N, Vale RD. Single-molecule analysis of dynein processivity and stepping behavior. Cell. 2006;126:335–348. doi: 10.1016/j.cell.2006.05.046. Single, truncated and artificially dimerized molecules of yeast dynein heavy chain affords great flexibility in labeling and observing velocity and single steps of dynein to characterize its processivity
- 26. Valentine MT, Fordyce PM, Krzysiak TC, Gilbert SP, Block SM. Individual dimers of the mitotic kinesin motor Eg5 step processively and support substantial loads in vitro. Nat Cell Biol. 2006;8:470–476. doi: 10.1038/ncb1394. First characterization of the Eg5 kinesin motor's velocity and stepping under various loading conditions and ATP concentration. A three-state biochemical model is shown to explain Eg5's stepping kinetics
- 27.Sweeney HL, Park H, Zong AB, Yang Z, Selvin PR, Rosenfeld SS. How myosin VI coordinates its heads during processive movement. EMBO J. 2007;26:2682–2692. doi: 10.1038/sj.emboj.7601720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ali MY, Krementsova EB, Kennedy GG, Mahaffy R, Pollard TD, Trybus KM, Warshaw DM. Myosin Va maneuvers through actin intersections and diffuses along microtubules. Proc Natl Acad Sci U S A. 2007;104:4332–4336. doi: 10.1073/pnas.0611471104. Qdot-labeled myosin V maneuvers through actin-actin intersections by switching between filaments or crossing over. Remarkably, myosin V molecules were observed diffusing on microtubules by an electrostatic mechanism which may be an important component associated with intracellular cargo transport
- 29. Dunn AR, Spudich JA. Dynamics of the unbound head during myosin V processive translocation. Nat Struct Mol Biol. 2007;14:246–248. doi: 10.1038/nsmb1206. By labeling single myosin V molecules with gold nanoparticles, the diffusive search of the lead head for its net binding site was characterized during processive stepping
- 30.Shiroguchi K, Kinosita K. Myosin V walks by lever action and Brownian motion. Science. 2007;316:1208–1212. doi: 10.1126/science.1140468. [DOI] [PubMed] [Google Scholar]
- 31.Jaud J, Bathe F, Schliwa M, Rief M, Woehlke G. Flexibility of the neck domain enhances Kinesin-1 motility under load. Biophys J. 2006;91:1407–1412. doi: 10.1529/biophysj.105.076265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Visscher K, Schnitzer MJ, Block SM. Single kinesin molecules studied with a molecular force clamp. Nature. 1999;400:184–189. doi: 10.1038/22146. [DOI] [PubMed] [Google Scholar]
- 33.Mehta AD, Rock RS, Rief M, Spudich JA, Mooseker MS, Cheney RE. Myosin-V is a processive actin-based motor. Nature. 1999;400:590–593. doi: 10.1038/23072. [DOI] [PubMed] [Google Scholar]
- 34. Ross JL, Shuman H, Holzbaur ELF, Goldman YE. Kinesin and Dynein-Dynactin at Intersecting Microtubules: Motor Density Affects Dynein Function. Biophys J. 2007 doi: 10.1529/biophysj.107.120014. Single kinesin and dyneisn motors and beads decorated at systematically increasing motor densities were observed at constructed microtubule-mirotubule intersections. Kinesin moves robustly through intersections, but dynein-dynactin tends to pause at intersections at high decoration densities, properties that should relate to these motors' cellular roles
- 35. Cai D, Hoppe AD, Swanson JA, Verhey KJ. Kinesin-1 structural organization and conformational changes revealed by FRET stoichiometry in live cells. J Cell Biol. 2007;176:51–63. doi: 10.1083/jcb.200605097. Fluorescence resonant energy transfer between the kinesin-1 head, tail, and light chains was used to characterize kinesin-1 regulation inside living cells. Auto-inhibition of the kinesin heavy chain was reversed by the light chain binding to the tail domain, allowing the kinesin can again to bind microtubules
- 36.Coy DL, Hancock WO, Wagenbach M, Howard J. Kinesin's tail domain is an inhibitory regulator of the motor domain. Nat Cell Biol. 1999;1:288–292. doi: 10.1038/13001. [DOI] [PubMed] [Google Scholar]
- 37. Liu J, Taylor DW, Krementsova EB, Trybus KM, Taylor KA. Three-dimensional structure of the myosin V inhibited state by cryoelectron tomography. Nature. 2006;442:208–211. doi: 10.1038/nature04719. Using electron microscopy, the 3D structure of expressed full length myosin V in the inhibited state identifies the cargo-binding domain near the ATP-binding pocket of motor domain. This conformation blocks the motor's ability to bind actin
- 38.Thirumurugan K, Sakamoto T, Hammer JA, Sellers JR, Knight PJ. The cargo-binding domain regulates structure and activity of myosin 5. Nature. 2006;442:212–215. doi: 10.1038/nature04865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hódi Z, Németh AL, Radnai L, Hetényi C, Schlett K, Bodor A, Perczel A, Nyitray L. Alternatively spliced exon B of myosin Va is essential for binding the tail-associated light chain shared by dynein. Biochemistry. 2006;45:12582–12595. doi: 10.1021/bi060991e. [DOI] [PubMed] [Google Scholar]
- 40.Huang JD, Brady ST, Richards BW, Stenolen D, Resau JH, Copeland NG, Jenkins NA. Direct interaction of microtubule- and actin-based transport motors. Nature. 1999;397:267–270. doi: 10.1038/16722. [DOI] [PubMed] [Google Scholar]
- 41.Trinczek B, Ebneth A, Mandelkow EM, Mandelkow E. Tau regulates the attachment/detachment but not the speed of motors in microtubule-dependent transport of single vesicles and organelles. J Cell Sci. 1999;112(Pt 14):2355–2367. doi: 10.1242/jcs.112.14.2355. [DOI] [PubMed] [Google Scholar]