Abstract
Background
Studies have been inconsistent in demonstrating that early adversity and specific genotype can be joint risk factors for poor behavioral outcomes. Using a rhesus monkey model, we examined how social context and different forms of early adversity influence whether a specific genotype (polymorphism in the promoter region of monoamine oxidase A) affects display of aggressive, fearful, and anxious behaviors.
Methods
Rhesus monkey infants (n=473) were exposed to brief social challenge at 3–4 months of age. Infants were reared a) with mothers and up to 150 other animals in large cages; b) with mothers in smaller social groups; c) with mother and access to, at most, one other mother-infant pair; and d) without mother, but with access to a same-aged peer in a nursery.
Results
No effects of genotype were found for infants reared by mothers in large social cages, although several genotype by rearing environment interactions were evident. Animals reared in smaller social groups were more likely to display aggression, which was especially true of animals possessing the low-activity MAOA genotype. In addition, animals with low-activity genotypes that had experienced restricted mother-rearing showed more anxious behavior (scratch, yawn).
Conclusions
Among mother-reared animals, broader contextual features, associated with the social environment and experience of the mother, can affect the extent to which genotype contributes to behavioral expression under conditions of challenge. Results also suggest that different forms of early adverse experience can affect the types of responses displayed by animals of different genotypes.
Keywords: MAOA, rearing, aggression, anxiety, fear, rhesus
Introduction
Several studies with humans have demonstrated interactions of genotype and early adversity on later behavior. Particular attention has been paid to a polymorphism in the promoter region of monoamine oxidase A (MAOA), an X-linked gene that codes for an enzyme involved in deamination of monoamine neurotransmitters. In an early report, Caspi et al. (1) found that individuals possessing the genotype associated with lower transcriptional activity of MAOA showed increased risk for antisocial behavior, but only if they had experienced an adverse early environment. Other studies have found similar interactions for behaviors reflecting conduct disorder (2), antisocial behavior and other mental health problems (3), antisocial alcoholism (4), and physical aggression (5). Not all studies have found gene-environment interactions, however; Huizinga et al. (6) described several differences between their work and earlier studies that could account for their differing results, including the age of, and criteria for, early “adversity.” Providing qualified support for the interaction hypothesis, Widom and Brzustowicz (7) found the expected interaction only for white participants; no such interaction was evident for non-whites.
Widom and Brzustowicz’s work (7) suggests the importance of other contextual factors when looking for interactions of genes and experience. Their failure to find a protective effect of genotype among non-whites that suffered abuse suggested that such an effect may be evident only in “the absence of other environmental stressors or adversities (p. 688).” The broader context could be influential at the point of the early adverse event (e.g., abuse may be only one aspect of an adverse environment for a young non-white) or at the point of the later outcome (e.g., the development of later violence and antisocial behavior could be attributed to multiple risk factors among non-whites, such as socio-economic status and racism).
A nonhuman primate model is an excellent means to study the role of broader contextual factors. The ability to raise nonhumans under different conditions can permit study of the multiple factors that might contribute to an adverse environment during development. Rhesus macaques also possess a variable nucleotide tandem repeat polymorphism in the promoter region of MAOA, and functional assessments indicate that the 5- and 6-repeat alleles confer high transcriptional activity, whereas the 7-repeat allele produces low activity (8). In the only published behavioral study with nonhuman primates, Newman et al. (8) contrasted 3–5 year-old male monkeys that had been reared either with their mothers in small social groups, or in the nursery with same-aged peers, an environment generally considered adverse. Contrary to expectation, mother-reared monkeys with low-activity genotypes were more aggressive than were low-activity, nursery-raised animals, or any animals with high-activity genotypes. Newman et al. (8) provided several possible explanations, including that nursery-rearing is characterized by a total absence of experience with adults, and provides little experience with models of aggression. Obviously, these components of the early environment stand in marked contrast to the type of adversity experienced by young, abused, children. Thus, while nursery-rearing is often described as an “adverse” environment, the results of Newman et al. (8) suggest that MAOA genotype does not interact with the qualities of this particular environment to affect aggression-related outcomes.
Here we report data from rhesus monkey infants reared under four standardized protocols at the California National Primate Research Center (CNPRC). Three protocols involved rearing infants with mothers, though there were differences in numbers of social companions and amount of physical space available to infants. The fourth protocol involved nursery-rearing. We had two goals. First, we were interested in whether the effects of MAOA genotype were influenced by broader, contextual features of the animals’ experience. This was accomplished by contrasting the three sets of mother-reared animals. Second, we were interested in the role that genotype might play in different adverse early environments. For this goal, we contrasted nursery-reared animals with monkeys from the mother-reared group that was most restrictive. Restrictive mother-reared infants display more agonistic behavior in later infancy and beyond (9), and nursery-rearing produces monkeys that display greater emotionality (10). We predicted that genotypic influences on behavioral expression would differ for animals reared in an environment that predisposes to high emotional reactivity compared to those reared in an environment that predisposes to aggression.
Methods and Materials
Subjects and Living Arrangements
MAOA genotype was determined for 669 infant rhesus monkeys, but only males (which are hemizygous) and homozygous females were included in the behavioral analyses, because we don’t know, for heterozygous females, which alleles are silenced as a result of X inactivation. Mean age for the final sample (n = 473) was 107.9 days (range: 89–130 days) at time of behavioral testing, which roughly corresponds to the age of 12–16 months for human infants (11).
Field-cage-raised (FR; n=375) monkeys were born to mothers living in one of 17 half-acre outdoor enclosures, constructed of pipe and chain-link, and measuring 40 × 64× 9m. Each cage contained multiple perches, climbing structures, smaller shelters for protection against rain and wind, and up to 150 animals of all age and sex classes. Monkeys raised in corncribs (CR; n=26) lived in small outdoor structures (approximately 4m in diameter) in social groups with their mothers, an adult male and typically two to five other adult females and their offspring. Infants that were mother-reared (MR; n=22) resided with their mothers in standard-sized (0.58 × 0.66 × 0.81m), indoor housing cages. In most cases, two mother-infant pairs were allowed to interact with each other by opening a divider between adjacent cages for approximately 8 hr. per day. Nursery-reared (NR; n=50) infants were derived from the field cages by separating them from their mothers on the day of birth and placing them in incubators (each containing a stuffed animal toy and towels to cling to) for 30 days, after which they were moved into individual units of a quad cage (0.46 × 0.61 × 0.69m) constructed of stainless steel. After 1 or 2 days of habituation, barriers between adjacent units were removed so that infants could interact in pairs. In one birth year, infants were formed into pairs for 6 hours per day while in the subsequent two years barriers were removed permanently, resulting in continuous, dyadic, peer-rearing. Mean age of delivery of the mothers for the FR, CR, and NR animals ranged from 7.5 to 8.3 years and were not significantly different; mothers of MR monkeys, however, were significantly older (11.9 years).
Behavioral Assessment
Five hours after infants were separated from their rearing environment and companion(s) (see Supplement 1) a Human Intruder test was conducted to assess responsiveness of the infants under standardized and graded conditions of challenge (12). This test is an abbreviated version of a test that has been described by others (13, 14), although important differences in procedures (age of animals, duration of exposure [and distance] to the intruder, size of cage, and amount of time since separation from mother) and responses exist. Four consecutive trials, each lasting 1 minute, were conducted in the test cage as follows: an unfamiliar human “intruder” sat approximately 1m in front of the cage, presenting the left profile [profile-far]; after 1 minute, the intruder, maintaining the profile orientation, moved to within 0.3m in front of the cage [profile-near]; after one minute, the intruder returned to the 1m location and attempted to maintain direct eye contact with the infant [stare-far] for 1 min, and finally, the human moved again to within 0.3m of the test cage and attempted to maintain eye contact [stare-near] for 1 min. Behavioral responses were videotaped for later transcription. Because of minimal differences in the two profile conditions, we examined data only from the two stare conditions involving far (mild-challenge) and near (high-challenge) distances. Behaviors of interest reflected fear (grimace), aggression (threat), and anxiety (scratch, yawn) (15–17). The ethogram and definitions can be found in Supplement 1. Correlational analyses (n=473) revealed that, within each challenge condition, rates of behavior were generally independent: in the mild-challenge condition, Pearson correlation coefficients among the four variables ranged from −0.02 to 0.09 and were nonsignificant, and in the high-challenge condition, scratch and yawn were correlated (r = 0.20, p<.001) as were threat and grimace (r = −0.10, p<.05). Across challenge conditions, correlations were higher and significant (all p<.0.001), suggesting reasonable reliability of the individual differences: threat (r=0.37); grimace (r=0.55), scratch (r=0.43), yawn (r=0.41).
Genotyping and Sequencing
(see Supplement 1)
Statistical Analysis
The principal objective of the analysis was to determine the association between MAOA genotype and behavioral responses of rhesus monkeys in the context of early rearing conditions. Evaluation of the significance of genotype by environment interactions was accomplished through the likelihood ratio test, in which a difference is computed between the likelihoods from models with and without interaction terms. This difference is distributed as Chisquare, which was evaluated with alpha=.05. Single degree of freedom contrasts (t-tests) were then made, with alpha=.01 to provide some control for the multiple t-tests. All analyses involved a mixed linear model using a derivative-free restricted maximum likelihood approach (MTDFREML) (24) accounting for relatedness, which was based on a pedigree encompassing 2,280 animals. Separate analyses were conducted for data from the mild-challenge (stare-far) and high-challenge (stare-near) conditions. Based on transcription activities of the promoter variants of MAOA (8), the hemi- and homozygous genotypes possessing the 5- and 6-repeat alleles are referred to as high-activity genotypes while genotypes with 7-repeat allele are low-activity genotypes. Details of our analytic model can be found in Supplement 1.
Results
Genotype Frequencies
Table 1 displays the genotype frequencies for all 669 animals. Chi-square analysis showed no sex bias in genotype frequencies for the 473 individuals used in the behavioral analysis (p=0.44). Table 2 shows the distribution of genotype activity across the four rearing conditions. Chi-square analysis revealed that genotype frequencies did not differ among rearing groups (p=0.16). Finally, because sex and rearing were not significantly related (p=0.08), and in order to keep sample sizes as large as possible, behavioral data for males and females were combined.
Table 1.
Number of individuals for MAOA genotypes by sex.
| Genotypes | |||||||
|---|---|---|---|---|---|---|---|
| 5/5 or 5/- | 6/6 or 6/- | 7/7 or 7/- | 6/5 | 7/5 | 7/6 | Total | |
| Females | 43 | 74 | 100 | 58 | 65 | 73 | 413 |
| Males | 60 | 87 | 109 | 256 | |||
| Total | 103 | 161 | 209 | 58 | 65 | 73 | 669 |
Table 2.
Number of individuals for MAOA genotype activity by rearing condition.
| Rearing Condition | |||||
|---|---|---|---|---|---|
| CR | FR | NR | MR | Total | |
| Low-activity | 15 | 156 | 27 | 11 | 209 |
| High-activity | 11 | 219 | 23 | 11 | 264 |
| Total | 26 | 375 | 50 | 22 | 473 |
Heritabilities
Table 3 displays the heritabilities and standard errors, calculated for our four measures across the two conditions.
Table 3.
Heritability estimates and their standard errors
| Mild-challenge Condition | High-challenge Condition | |
|---|---|---|
| Threat | 0.07 (0.15) | 0.76 (0.20) |
| Grimace | 0.00 (0.09) | 0.27 (0.13) |
| Scratch | 0.06 (0.08) | 0.04 (0.11) |
| Yawn | 1.00 (0.21) | 0.32 (0.11) |
Impact of MAOA Genotype
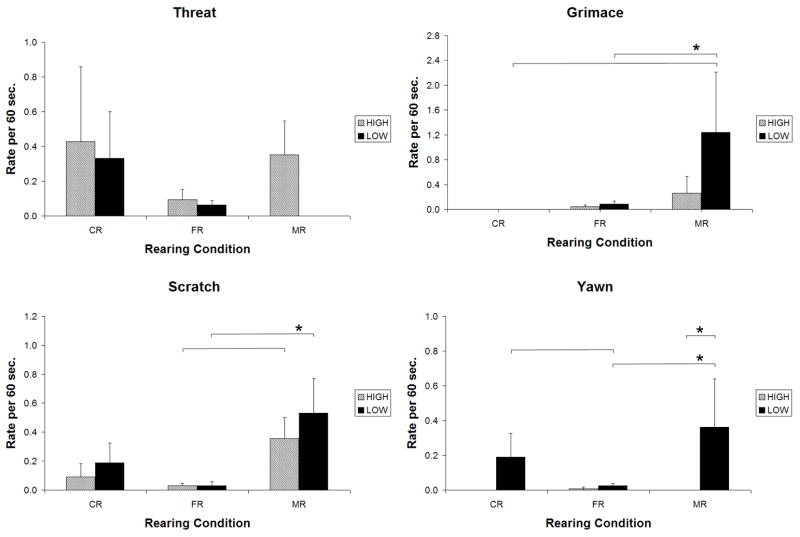
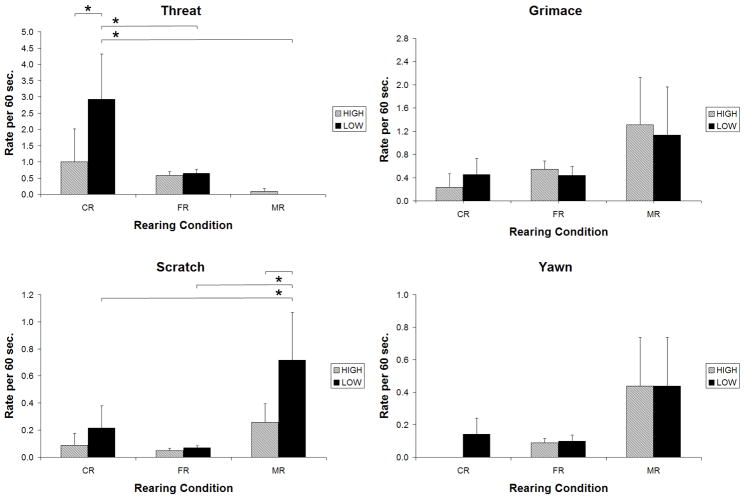
There were no effects of genotype that were consistent across all rearing conditions. Within rearing condition, however, we found several clear effects of genotype. In the mild-challenge condition, MR monkeys with the low-activity genotypes showed significantly more yawns, compared to MR monkeys with the high-activity genotypes (t=4.08, p <0.001) (Fig. 1). In the high-challenge condition, CR monkeys with the low-activity genotypes displayed more threats compared to CR monkeys with the high-activity genotypes (t=3.27, p=0.001) (Fig. 2). Among MR animals, the low-activity genotypes were associated with more scratches than were the high-activity genotypes (t=2.81, p<0.01) (Fig. 2).
Figure 1.
Mild-Challenge Condition: Comparison of animals with low-activity and high-activity MAOA genotypes that were reared in corncribs (CR), field cages (FR), or in indoor cages with mother (MR). Bars indicate effects significant at p<.01; * indicates effects significant at.001 or beyond.
Figure 2.
High-Challenge Condition: Comparison of animals with low-activity and high-activity MAOA genotypes that were reared in corncribs (CR), field cages (FR), or in indoor cages with mother (MR). Bars indicate effects significant at p<.01; * indicates effects significant at.001 or beyond.
Influence of Social Context – CR, FR, and MR Animals (Table 4)
Table 4.
Summary of Rearing Condition Comparisons
CR, FR, MR comparisons
| Mild Challenge Condition (Fig. 1) | High Challenge Condition (Fig. 2) | |||
|---|---|---|---|---|
| Low Activity | High Activity | Low Activity | High Activity | |
| Threat | CR > FR*, MR* | |||
| Grimace | MR > FR*, CR | |||
| Scratch | MR > FR* | MR > FR | MR > CR*, FR* | |
| Yawn | MR*, CR > FR | |||
CR: Corncrib-reared; FR: Fieldcage-reared; MR: Indoor Mother-reared; NR: Nursery-reared all results significant at p <.01, or
p <=.001.
Mild-challenge condition (Fig. 1)
MR animals displayed more fear and anxiety than did members of the other two rearing groups, and this was particularly evident for animals with the low-activity genotypes; differences between CR and FR animals were minimal. These results were indicated by significant genotype by environment interactions for yawn (Chisquare(2)=8.53, p=0.01) and scratch (Chisquare(2)=11.38, p=0.003), and a trend for grimace (Chisquare(2)=4.57, p = 0.10). Follow-up analyses revealed that, among animals with the low-activity genotypes, MR animals displayed more grimaces than did FR or CR monkeys (t=3.29, 2.78; p=0.001, p<0.01, respectively); group differences in rates for animals with the high-activity genotypes were not significant. Similarly, MR monkeys with the low-activity genotypes displayed higher rates of scratch than did FR animals (t=4.56, p<0.001), although for this behavior, MR animals with the high-activity genotypes also had higher rates compared to FR animals (t=2.98, p<0.01). Finally, MR and CR animals with the low-activity genotypes had higher rates of yawn compared to FR monkeys (t=5.25, 3.18; p<0.001, 0.01, respectively), with no group differences (and in fact, virtually no display of this behavior) among monkeys with the high-activity genotypes. No effects were seen for the rate of threats.
High-challenge condition (Fig. 2)
CR animals displayed more aggression and MR animals displayed more anxious behavior, but only if they possessed the low-activity genotypes; these results were indicated by a a significant genotype by environment interaction for threat (Chisquare(2)=7.47, p=0.02), and a trend for scratch (Chisquare(2)=5.16, p=0.08). Follow-up analyses showed that, for monkeys with the low-activity genotype only, CR animals had higher rates of threat than did FR or MR animals (t=5.05, 4.46; p<0.001, 0.001, respectively), and MR animals had higher rates of scratch than did CR or FR animals (t=3.29, 5.47; p=0.001, p<0.001, respectively). No effects were seen for rates of grimace or yawn, or for any contrasts among animals with high-activity genotypes.
Impact of MAOA Genotype in Different Adverse Environments – MR and NR Animals
Mild-challenge condition (Fig. 3)
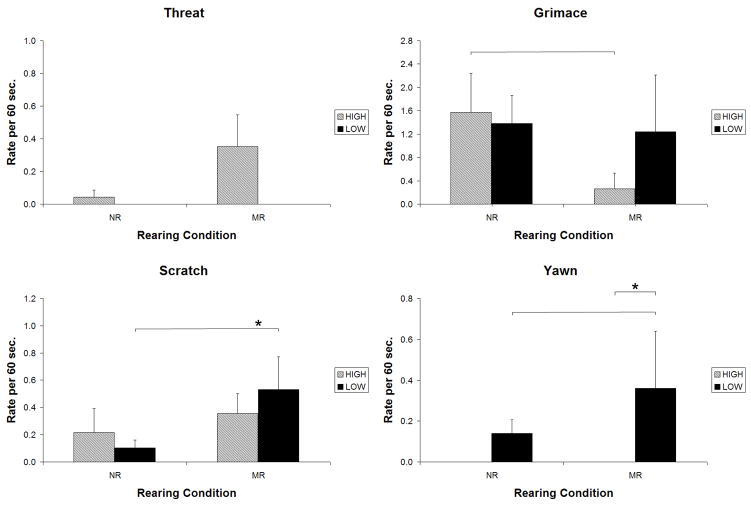
Figure 3.
Mild-Challenge Condition: Comparison of animals with low-activity and high-activity MAOA genotypes that were reared in indoor cages with mother (MR) or in a nursery setting (NR). Bars indicate effects significant at p<.01; * indicates effects significant at.001 or beyond.
Among animals with the low-activity genotypes, MR animals displayed higher rates of scratch than did NR animals, as indicated by a significant genotype by environment interaction (Chisquare=3.76, p=0.05), and a significant follow-up result: t = 3.41; p <.001. Although the pattern for yawn looks identical, the interaction was not significant (p=0.15). There were no effects for grimace or threat.
High-challenge condition (Fig. 4)
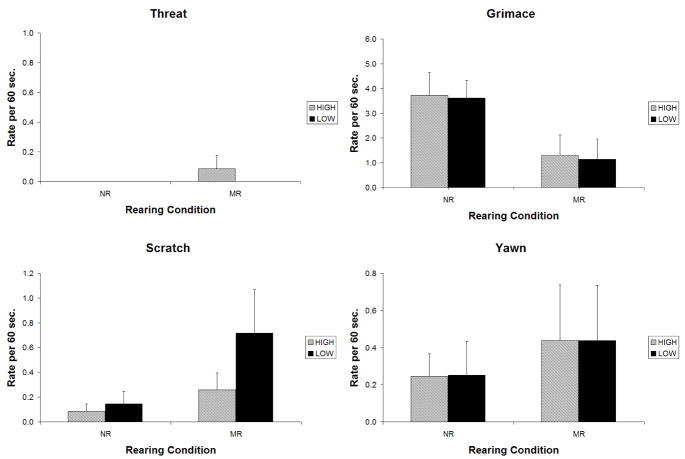
Figure 4.
High-Challenge Condition: Comparison of animals with low-activity and high-activity MAOA genotypes that were reared in indoor cages with mother (MR) or in a nursery setting (NR).
No significant genotype by environment interactions were evident in the high-challenge condition.
Discussion
The present study suggests that genetic variation is an influence on individual differences in behavioral responsiveness; that the broader context associated with early experience is an influential factor in how MAOA genotype influences behavioral expression; and that different forms of adverse rearing affect the genetic contribution to behavioral expression differently. We discuss each of these points below.
Genetic Influences on Behavioral Expression
The heritability analysis indicates that individual differences in behavioral responsiveness are influenced by genetic differences between individuals. This was most apparent in the high-challenge condition where, with the exception of scratch, heritabilities ranged from 0.27 to 0.76. Heritabilities in the mild-challenge condition were lower, and for two behaviors, grimace and yawn, boundary values were obtained (0.0, and 1.0, respectively). While it is possible that these reflect true values, we suspect these values may have arisen owing to problems with distributions of these measures in this condition, or unmeasured contributors to the phenotypic variance. A larger sample may help address this issue. Our finding of negligible heritability for threat in the mild challenge condition is consistent, however, with results from a similar study with juvenile rhesus monkeys (14).
Overall, there were no main effects of MAOA genotype across all rearing conditions, largely as a result of our failure to find any effects for FR animals. This result suggests that rearing in rich, species-typical social groups may buffer individuals from a genotype that, given other circumstances, might be a risk factor for poor behavioral outcomes. Nevertheless, behavioral responsiveness in challenging situations is undoubtedly influenced by many other genes, the identities of which remain to be determined.
Influence of Social Context
One indicator of the importance of social context is evident in a comparison of CR and FR monkeys. While no genotype effects were found for FR animals, CR monkeys with low-activity genotypes showed more threatening in the high-challenge condition when compared to CR monkeys with high-activity genotypes, results that are consistent with those reported by Newman et al. (8; the CR condition in our study is very similar to the mother-rearing condition in the Newman study [Suomi, personal communication to JPC]). Moreover, in a direct comparison of rearing groups, CR animals with low-activity genotypes displayed significantly more yawns (an anxiety-related behavior often associated with conflict: [16]) in the mild-challenge, and significantly more threats in the high-challenge, conditions compared to FR animals with low-activity genotypes. No rearing differences were found for animals with high-activity genotypes, reflecting the significant genotype × environment interactions found for these behaviors.
We believe that the principal differences between field cage and corncrib rearing reflect two important and related factors that we were unable to control: mothers’ parity and group composition. Mothers in corncribs were significantly more likely (p =.004) to be primiparous (15 of 26, or 57.7%) than were FR mothers (115 of 375, or 30.7%). Compared to multiparous mothers, maternal behavior of primipara is usually characterized as more “anxious” (26–28). Moreover, having a primiparous mother means there are no older siblings available for social interaction, and being born into a cage with other primiparous mothers would result in fewer available older peers. Thus it is possible that the greater tendencies of CR infants with low-activity genotypes to display anxious/aggressive behavior in our testing may have reflected greater maternal inexperience and more restricted social networks, compared to the FR animals. With a larger sample of CR animals this hypothesis can be tested in the future.
The importance of social context is also evident in contrasts between the FR and CR animals on the one hand, and MR animals. Overall, the responses of the MR animals could generally be characterized as more fearful and anxious compared to FR and CR monkeys’ responses, and this was particularly true of MR animals with low-activity genotypes: in the mild-challenge condition, low-activity MR animals displayed significantly more grimaces compared to low-activity CR and FR monkeys, significantly more scratches compared to the low-activity FR monkeys, and significantly more yawns compared to FR monkeys. In the high-challenge condition, low-activity MR animals showed significantly fewer threats compared to CR animals, and significantly more scratches compared to FR and CR animals. Three of these results were reflected in significant genotype by environment interactions, and the remaining two reflected trends.
It’s possible that the social restriction and enforced proximity with mother in the MR condition led to the increased fear and anxiety of MR infants: Harlow (9) described the behavior of mothers reared alone with their infants as “overpunishing” when compared to a rearing condition in which infants were able to leave mother behind and interact with multiple peers daily. The infants of such mothers were described as showing less affection and increased fear and aggression beginning at about 4 months of age (9). Our data suggest that this effect is exacerbated in those animals possessing a risky (low activity) genotype. In fact, genotypic differences among MR animals were substantial: two of the three genotypic differences found for the within-rearing-group analyses were exhibited by MR animals for anxious behaviors –compared to high-activity MR animals, low-activity animals showed more yawn in the mild-challenge condition, and more scratch in the high-challenge condition.
Together, these results provide support for the idea that the broader social context (which, in our study, likely includes the numbers and diversity of individuals available for interaction, maternal inexperience, and the possibility that the physical and social environment constrains and influences maternal and infant behavior) can affect the extent to which genotype contributes to behavioral expression. The importance of social context is especially evident in the results for FR animals: this group constituted the largest sub-sample of animals, providing the most statistical power to detect genetic influences. Despite this, no genotypic effects were found in any comparison, suggesting that rearing in a rich social environment can abrogate the effects of possessing a risky MAOA genotype. The results also suggest that the lack of a larger social context, as was evident in the MR condition, itself constitutes an adverse condition, to which animals with a risky genotype are especially susceptible.
Impact of Different Adverse Experiences
To the extent that MR and NR conditions both reflect adverse rearing conditions, the present data suggest that MAOA genotype was not equally responsive to these two conditions. In the mild-challenge condition, MR animals with low-activity genotypes showed more anxious behavior - higher rates of scratch - than did low-activity NR animals, while rates of these behaviors for animals with high-activity genotypes were comparable. The pattern was similar for yawn in the mild-challenge condition, and for scratch in the high-challenge condition, although for these two measures, the genotype by environment interactions were not significant. Notably, the failure to find a genotypic effect for threat for NR animals in either challenge condition replicates Newman et al’s (8) result for similarly-reared animals.
Data from human studies show that a low-activity genotype combined with early adversity tends to be associated with later antisocial behavior. Nursery rearing does not typically result in animals that show antisocial tendencies; rather, NR monkeys are somewhat hyper-social, tending to show high rates of cling and anxious affiliation. In contrast, the primate studies described above suggest that having received punitive mothering tends to promote later antisocial tendencies (9). Thus, restricted mother-rearing (whether in the MR conditions used here, or, possibly, rearing in small groups by primiparous mothers), and not nursery-rearing, may be a better primate model for understanding genetic risk for later anxiety and aggression.
Limitations of the Analysis
We recognize several limitations of the present study. First, we focused on young animals and short-term effects of MAOA genotype and early experience, whereas human studies have generally examined more long-term effects (but see 3). We are unaware of any published research examining specifically how measures such as ours, obtained in infancy, relate to adolescent or adult indicators of anxiety or aggression. Such studies are, however, currently underway in our laboratory. Second, samples sizes for some comparisons were small (Table 2); this is a particular concern inasmuch as several of our effects were evident for MR animals, of which we had only 11 high-activity and 11 low-activity animals. Nevertheless, our results are in good theoretical agreement with human studies that have explored the interaction between genotype and early adversity. In addition, our sample sizes are in the range of the only other nonhuman primate behavioral study of the effects of MAOA and early experience (where n’s ranged from 7 to 15: [8]), suggesting that, when rearing conditions can be carefully controlled in a laboratory setting, the effects of MAOA genotype are so large as to be detectable with such sample sizes. Our ability to continue to accrue cases in rearing conditions such as the MR condition should enable us to replicate the present results with larger sample sizes. Finally, we note that we have no data on the qualities of social interactions that our subjects displayed in their home cages, with either their mothers, peers, or other members of their groups. Do MR infants receive more aggression as early research (9) suggested? What are the social opportunities afforded to an infant of a primiparous mother in a corncrib versus in a fieldcage? Such information would be very useful in understanding how mothers might contribute differently to the behavioral development of their offspring within the broader social context associated with field cages, corncribs, or indoor housing. Such studies are planned for the future.
Summary and Conclusions
Rearing condition was an important contributor to behavioral expression in the human intruder test. As shown in the figures, in general, FR animals showed low responsiveness, MR animals tended to show more anxious responses, NR animals showed the greatest amount of fear, and CR animals generally responded with aggressive behavior. Against this general background, however, there were significant effects of MAOA genotype, with monkeys possessing the low-activity genotype, and having been reared under “adverse” conditions (MR, and to some extent, CR conditions) at greatest risk for negative outcomes. Our data comparing three conditions of mother-rearing indicate that the broader social context can have effects on behavior that differ according to genotype. Furthermore, adverse early rearing that likely involves exposure to more aggression (e.g., restricted mother-rearing) facilitates the impact of genotype on anxiety, while adverse conditions that do not involve such exposure (e.g., nursery-rearing) do not. Further research will be necessary to understand more fully the specific qualities of the social environment that contribute to “adversity” and to consequent poor adaptation by individuals with risky genotypes.
Supplementary Material
Acknowledgments
This study was supported by grants RR000169 to the California National Primate Research Center, RR019970 (JPC), and RR017584 (LAL). The authors thank L. DelRosso, C. Stanko, R. A. Grahn, E. Rettner, M. T. Ruhe, and the animal care and veterinary staffs at CNPRC for technical assistance, and to four anonymous reviewers for helpful comments. All procedures were approved by the University of California, Davis, Institutional Animal Care and Use Committee. UC Davis is an AAALAC accredited institution.
Footnotes
Financial Disclosures
All authors indicate they have no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- 2.Foley DL, Eaves LJ, Wormley B, Silberg JL, Maes HH, Kuhn J, Riley B. Childhood adversity, Monoamine Oxidase A genotype, and risk for conduct disorder. Arch Gen Psychiatry. 2004;61:738–44. doi: 10.1001/archpsyc.61.7.738. [DOI] [PubMed] [Google Scholar]
- 3.Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, Moffitt TE. MAOA, maltreatment, and gene-environment interaction predicting children’s mental health: new evidence and a meta-analysis. Mol Psychiatry. 2006;11:903–913. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- 4.Ducci F, Enoch MA, Hodgkinson C, Xu K, Catena M, Robin RW, Goldman D. Interaction between a functional MAOA locus and childhood sexual abuse predicts alcoholism and antisocial personality disorder in adult women. Mol Psychiatry. 2007 doi: 10.1038/sj.mp.4002034. [DOI] [PubMed] [Google Scholar]
- 5.Frazzetto G, Di Lorenzo G, Carola V, Proietti L, Sokolowska E, Siracusano A, Gross C, Troisi A. Early trauma and increased risk for physical aggression during adulthood: the moderating role of MAOA genotype. PLoS ONE. 2007 May 30;2:e486. doi: 10.1371/journal.pone.0000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huizinga D, Haberstick BC, Smolen A, Menard S, Young SE, Corley RP, Stallings MC, Grotpeter J, Hewitt JK. Childhood maltreatment, subsequent antisocial behavior, and the role of Monoamine Oxidase A genotype. Biol Psychiatry. 2006;60:677–683. doi: 10.1016/j.biopsych.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 7.Widom CS, Brzustowicz LM. MAOA and the “cycle of violence:” childhood abuse and neglect, MAOA genotype, and risk for violent and antisocial behavior. Biol Psychiatry. 2006;60:684–689. doi: 10.1016/j.biopsych.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 8.Newman TK, Syagailo YV, Barr CS, Wendland JR, Champoux M, Graessle M, Suomi SJ, Higley JD, Lesch KP. Monoamine oxidase A gene promoter variation and rearing experience influences aggressive behavior in rhesus monkeys. Biol Psychiatry. 2005;57(2):167–72. doi: 10.1016/j.biopsych.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Harlow HF, Harlow MK. Effects of various mother-infant relationships on rhesus monkey behaviors. In: Foss BM, editor. Determinants of Infant Behaviour IV. NY: Barnes & Noble; 1969. pp. 15–36. [Google Scholar]
- 10.Novak MA, Sackett GP. The effects of rearing experiences: The early years. In: Sackett GP, Ruppenthal GC, Elias K, editors. Nursery rearing of nonhuman primates in the 21stcentury. NY: Springer; 2006. pp. 5–19. [Google Scholar]
- 11.Golub MS, Hogrefe CE, Germann SL, Capitanio JP, Lozoff B. Behavioral consequences of developmental iron deficiency in infant rhesus monkeys. Neurotox Teratol. 2006;28:3–17. doi: 10.1016/j.ntt.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Capitanio JP, Mason WA, Mendoza SP, DelRosso L, Roberts JA. Nursery rearing and biobehavioral organization. In: Sackett GP, Ruppenthal GC, Elias K, editors. Nursery rearing of nonhuman primates in the 21stcentury. NY: Springer; 2006. pp. 191–214. [Google Scholar]
- 13.Kalin NH, Shelton SE. Defensive behaviors in infant rhesus monkeys: environmental cues and neurochemical regulation. Science. 1989;243:1718–1721. doi: 10.1126/science.2564702. [DOI] [PubMed] [Google Scholar]
- 14.Rogers J, Shelton SE, Shelledy W, Garcia R, Kalin NH. Genetic influences on behavioral inhibition and anxiety in juvenile rhesus macaques. Genes, Brain and Behav. 2008 doi: 10.1111/j.1601–183X.2007.00381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Redican WK. Facial expression in nonhuman primates. In: Rosenblum LA, editor. Primate Behavior, Volume 4: Developments in Field and Laboratory Research. NY: Academic; 1975. pp. 103–194. [Google Scholar]
- 16.Hinde RA, Rowell TE. Communication by postures and facial expressions in the rhesus monkey (Macaca mulatta) Proc Zool Soc London. 1962;138:1–21. [Google Scholar]
- 17.Troisi A, Schino G, D’Antoni M, Pandolfi N, Aureli F, D’Amato FR. Scratching as a behavioral index of anxiety in macaque mothers. Behav Neural Biol. 1991;56:307–313. doi: 10.1016/0163-1047(91)90469-7. [DOI] [PubMed] [Google Scholar]
- 18.Capitanio JP, Mendoza SP, Mason WA, Maninger N. Rearing environment and hypothalamic-pituitary-adrenal regulation in young rhesus monkeys (Macaca mulatta) Dev Psychobio. 2005;46:318–330. doi: 10.1002/dev.20067. [DOI] [PubMed] [Google Scholar]
- 19.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16(3):1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toonen RJ, Hughes S. Increased throughput for fragment analysis on an ABI PRISM 377 automated sequencer using a membrane comb and STRand software. Biotechniques. 2001;31(6):1320–4. [PubMed] [Google Scholar]
- 21.Lyons LA, Biller DS, Erdman CA, Lipinski MJ, Young AE, Roe BA, Qin B, Grahn RA. Feline polycystic kidney disease mutation identified in PKD1. J Am Soc Nephrol. 2004;15:2548–2555. doi: 10.1097/01.ASN.0000141776.38527.BB. [DOI] [PubMed] [Google Scholar]
- 22.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 23.Benson DA, Boguski MS, Lipman DJ, Ostell J, Ouellette BF, Rapp BA, Wheeler DL. GenBank. Nucleic Acids Res. 1999;27:12–7. doi: 10.1093/nar/27.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boldman KG, Kriese LA, Van Vleck LD, Van Tassell CP, Kachman SD. A Manual for Use of MTDFREML. A Set of Programs to Obtain Estimates of Variance and Covariances. Lincoln, NE: USDA-ARS; 1995. [Google Scholar]
- 25.McCulloch CE, Searle SR. Generalized, Linear and Mixed Models. NY: Wiley; 2001. [Google Scholar]
- 26.Hooley JM, Simpson MJA. A comparison of primiparous and multiparous mother-infant dyads in Macaca mulatta. Primates. 1981;22:379–392. [Google Scholar]
- 27.Tanaka I. Variability in the development of mother-infant relationships among free-ranging Japanese macaques. Primates. 1989;30:477–491. [Google Scholar]
- 28.Mitchell G, Stevens CW. Primiparous and multiparous monkey mothers in a mildly stressful social situation: First three months. Dev Psychobio. 1968;1:280–286. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.