Abstract
Spermatogenesis uses mitotic and meiotic cell cycles coordinated with growth and differentiation programs to generate functional sperm. Our analysis of a Drosophila mutant has revealed that asunder (asun), which encodes a conserved protein, is an essential regulator of spermatogenesis. asun spermatocytes arrest during prophase of meiosis I. Strikingly, arrested spermatocytes contain free centrosomes that fail to stably associate with the nucleus. Spermatocytes that overcome arrest exhibit severe defects in meiotic spindle assembly, chromosome segregation, and cytokinesis. Furthermore, the centriole-derived basal body is detached from the nucleus in asun postmeiotic spermatids, resulting in abnormalities later in spermatogenesis. We find that asun spermatocytes and spermatids exhibit drastic reduction of perinuclear dynein–dynactin, a microtubule motor complex. We propose a model in which asun coordinates spermatogenesis by promoting dynein–dynactin recruitment to the nuclear surface, a poorly understood process required for nucleus–centrosome coupling at M phase entry and fidelity of meiotic divisions.
INTRODUCTION
Spermatogenesis is a dynamic process in which the cell cycle is coordinated with developmental events to produce haploid sperm capable of fertilizing eggs. Drosophila melanogaster has proven to be an excellent model system for the study of spermatogenesis. The relatively large size of meiotic spindles of Drosophila spermatocytes makes them well suited for cytological examination (Cenci et al., 1994). Due to relaxation of checkpoints, meiotic progression occurs even in the face of errors in spindle assembly (Rebollo and Gonzalez, 2000). Immature spermatids, which are abundant in the testis, are highly regular in appearance; deviations in their uniformity are diagnostic of earlier defects in meiotic divisions. These features, combined with genetic tools available in Drosophila, have facilitated mutational analysis of male meiosis (Wakimoto et al., 2004).
The stages of Drosophila spermatogenesis have been clearly defined (Fuller, 1993). Germline stem cells at the apical tip of the testis give rise to spermatogonial cells that proceed through four synchronous rounds of mitosis with incomplete cytokinesis to form 16-cell cysts of primary spermatocytes. After DNA replication, primary spermatocytes enter a prolonged G2 growth phase. Progression through meiosis I leads to formation of 32-cell cysts of secondary spermatocytes, and further division in meiosis II generates 64-cell cysts of spermatids. Immature, round spermatids differentiate into elongated and individualized sperm that move into the seminal vesicles.
Spermatogenesis is one of only a few aspects of Drosophila development that requires centrioles. Although centrioles seem to be largely dispensable for mitosis, acentriolar spermatocytes form highly abnormal meiotic spindles and do not initiate cytokinesis (Bettencourt-Dias et al., 2005; Basto et al., 2006; Rodrigues-Martins et al., 2008). In addition, centrioles are required in postmeiotic spermatids to form the axoneme (Bettencourt-Dias et al., 2005).
During Drosophila male meiosis, unique in that centrosomes undergo dramatic changes in position and a reductive division (Fuller, 1993). After premeiotic S phase, centrosomes separate from the nucleus and move to the cortex. Cortical centrosomes nucleate astral microtubules in late G2 and move to the nucleus in early prophase to initiate spindle formation. Centrosomes divide without duplicating in meiosis II; thus, each postmeiotic spermatid contains one centriole, which converts to a basal body to direct axoneme formation.
Centrosome behavior has been shown in many systems to depend on dynein, a minus-end–directed microtubule motor complex (Hook and Vallee, 2006). There are two general forms of dynein: axonemal dynein, which provides the force for movement of cilia and flagella, and cytoplasmic dynein. Both forms are composed of one to three heavy chain motor subunits, as well as multiple intermediate, light intermediate, and light chains. Another large multimeric complex, dynactin, enhances dynein's motor activity and plays essential roles in regulating its subcellular localization and interactions with other proteins (Schroer, 2004). Cytoplasmic dynein plays key roles in a variety of biological processes such as nucleus–centrosome interactions, spindle assembly, chromosome segregation, nuclear migration, and cell movement. In Drosophila, dynein regulates many aspects of development, including both male and female gametogenesis (Gepner et al., 1996).
We report here that asunder (previously known as Mat89Bb) is required in Drosophila for male fertility. Primary spermatocytes of asunder testes undergo prophase arrest with defects in nucleus–centrosome coupling; cells that overcome arrest exhibit abnormal meiotic spindle assembly, chromosome segregation, and cytokinesis. Additionally, nucleus-basal body associations are disrupted during postmeiotic stages of differentiation. We show that this constellation of defects in germline cells of asunder males is likely due to reduced perinuclear localization of dynein–dynactin.
MATERIALS AND METHODS
Drosophila Stocks
y w was used as the “wild-type” stock. β-tubulin-GFP flies were a gift from H. Oda and Y. Akiyama-Oda (JT Biohistory Research Hall, Osaka, Japan). The Dmn-GFP stock has been previously described previously (Wojcik et al., 2001). piggyBac insertion line f02815 was from the Exelixis Collection (Harvard Medical School, Boston, MA). asp alleles were a gift from C. Gonzalez (Institut de Recerca Biomedica, Barcelona, Spain; Rebollo et al., 2004). The following lines were from Bloomington Stock Center (Indiana University, Bloomington, IN): Df(3R)Exel7329, nanos-Gal4-VP16, piggyBac transposase, twe1, βTub85DDrv1, fwdneo1, Df(3L)7C, Dhc64C4–19, Dhc64C6–10, Df(3L)fz-GF3b, and Gl1.
DNA Clones and Transgenics
cDNA clone LD33046 encoding ASUN was from the Drosophila Gene Collection. A cDNA clone encoding human GCT1 (ID 2989678) was from Open Biosystems (Huntsville, AL). UASp-Myc-ASUN was created by subcloning of polymerase chain reaction (PCR)-amplified coding sequence from LD33046 into a modified version of UASp that confers an N-terminal Myc tag (Rorth, 1998). For male germline expression of fluorescently tagged ASUN, cDNA encoding ASUN with an N-terminal green fluorescent protein (GFP) or Cherry tag was subcloned into testis expression vector tv3 (gift from J. Brill, The Hospital for Sick Children, Toronto, Canada) (Wong et al., 2005). Two independent transgenic lines with male germline expression of GFP-ASUN were used in this study: line 16 (chromosome III) to demonstrate perinuclear localization of GFP-ASUN (Figure 10) and line 11 (chromosome II) for all other experiments. Transgenic lines were generated by P-element–mediated transformation via embryo injection (Rubin and Spradling, 1982).
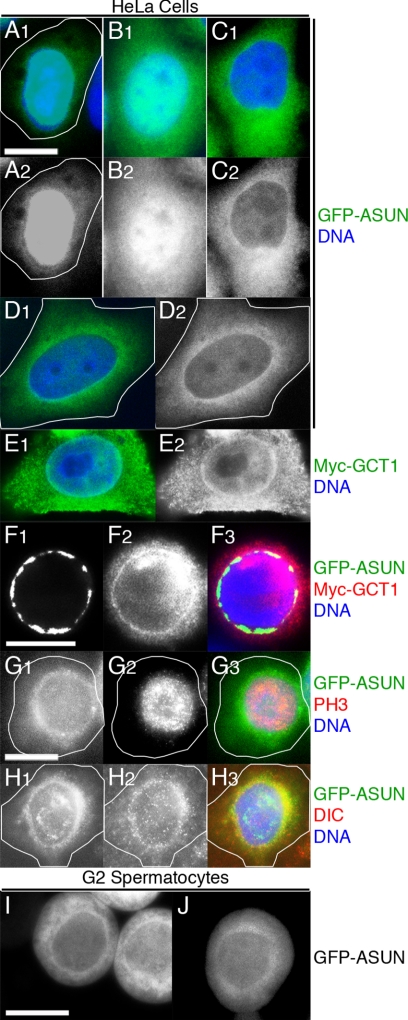
Figure 10.
Perinuclear localization of ASUN. (A–H) Fluorescence microscopy of transfected HeLa cells. DNA is in blue. (A–D) Localization of GFP-ASUN (green in A1–D1; grayscale in A2–D2) ranges from predominantly nuclear (A), distributed between the nucleus and cytoplasm (B), and predominantly cytoplasmic (C) with perinuclear enrichment (D) frequently observed. (E) Perinuclear enrichment of Myc-GCT1, the human homolog of ASUN (green in E1; grayscale in E2). (F) Colocalization of GFP-ASUN (F1; green in merge) and Myc-GCT1 (F2; red in merge) at the nuclear surface (F3; merge). (G and H) Nocodazole-treated cells expressing GFP-ASUN. (G) In prophase cells identified by phospho-histone H3 staining (G2; red in merge), GFP-ASUN (G1; green in merge) displayed a perinuclear localization (G3; merge). (H) Colocalization of GFP-ASUN (H1; green in merge) and dynein IC (H2, red in merge) in cells exhibiting perinuclear dynein localization (H3; merge). (I and J) Perinuclear localization of GFP-ASUN is occasionally observed in late G2 spermatocytes of a Drosophila transgenic line with relatively low expression. Bars, 10 μm.
Cytological Analyses of Live and Fixed Testes
Live testes cells were prepared for examination by phase contrast or fluorescent microscopy as described previously (Kemphues et al., 1980). Methanol fixation (for immunofluorescence and visualization of cells expressing β-tubulin-GFP) was performed as follows: slides of squashed testes were snap-frozen, immersed in methanol for 10 min at −20°C after coverslip removal, and washed twice in phosphate-buffered saline. Formaldehyde fixation (for actin staining and visualization of cells coexpressing Cherry-ASUN and Dynamitin [DMN]-GFP) was performed as described previously (Gunsalus et al., 1995).
Primary antibodies were used as follows: rat anti-α-tubulin (Mca77G, 1:500; Accurate Chemical & Scientific, Westbury, NY), mouse anti-γ-tubulin (GTU-88, 1:250; Sigma-Aldrich, St. Louis, MO), mouse anti-cyclin A (A12, 1:50; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), mouse anti-cyclin B (F2F4, 1:50; Developmental Studies Hybridoma Bank), and mouse anti-dynein heavy chain (P1H4, 1:150) (McGrail and Hays, 1997). The following antibodies were used to assess centrosome integrity: rabbit anti-Centrosomin (CNN) (1:1000), rabbit anti-Spindle defective (SPD)-2 (1:200), rabbit anti-Pericentrin-like protein (D-PLP) (1:250), rabbit anti-SAS-4 (1:100), and rabbit anti-transforming acidic coiled-coil (TACC) protein (1:500) (gifts from J. Raff [The Gurdon Institute, Cambridge, United Kingdom] except for anti-CNN, a gift from W. Theurkauf [University of Massachusetts Medical School, Worcester, MA]). Cy2- and Cy3-conjugated secondary antibodies were used at 1:800. Actin individualization cones were stained with Alexa Fluor 594 phalloidin (1:100; Invitrogen, Carlsbad, CA). Fixed samples were mounted in phosphate-buffered saline with 4,6-diamidino-2-phenylindole (DAPI) (0.2 μg/ml) to visualize DNA.
Fluorescent images were obtained using one of two microscopes: Eclipse 80i (Nikon, Tokyo, Japan) with Plan-Apo 100× and Plan-Fluor 40× objectives or Axioplan (Carl Zeiss, Thornwood, NY) with Neo-Fluor Ph2 40× objective. Bright field images of whole testes were obtained using a Stemi 2000-CS stereoscope (Carl Zeiss). Phase contrast images were captured using one of three microscopes: Eclipse 80i (Nikon) with Plan 20× Ph1 objective, Axiophot (Nikon) with Neo-Fluor Ph2 40× objective, or Axioplan (Carl Zeiss) with Neo-Fluor Ph2 40× objective. The ratio of the intensity of perinuclear to cytoplasmic DMN-GFP signal and of cytoplasmic to nuclear Cherry-ASUN signal in individual G2 spermatocytes was determined using Adobe Photoshop (Adobe Systems, Mountain View, CA).
Mammalian Cell Transfection, Staining, and Microscopy
HeLa cells were maintained in DMEM containing 10% fetal bovine serum. Plasmids for expression of N-terminally tagged versions of Drosophila ASUN and/or human GCT1 (GFP or Myc, respectively) generated by subcloning into pCS2 were transfected into cells by using Lipofectamine 2000 (Invitrogen) according to manufacturer's directions.
Cells were plated on fibronectin-coated coverslips 21 h after transfection and fixed 3 h later. For direct GFP fluorescence alone or in combination with phospho-histone H3 or Myc immunostaining, cells were fixed 20 min with 4% formaldehyde in CBS [10 mM 2-(N-morpholino)ethanesulfonic acid, pH 6.1, 138 mM KCl, 3 mM MgCl2, 2 mM EGTA, and 0.32 M sucrose]. For direct GFP fluorescence in combination with dynein intermediate chain (IC) staining, cells were fixed 5 min at −20°C in methanol. Cells were permeabilized 10 min with 0.5% Triton X-100 in Tris-buffered saline. For nocodazole treatment, cells were exposed to 5 μg/ml nocodazole for 3 h before fixation. Primary antibodies were used as follows: mouse anti-c-Myc (9E10, 1:1000), rabbit anti-phospho-histone H3 (Mitosis Marker, 1:200; Millipore, Billerica, MA), and mouse anti-dynein IC (74.1, 1:100; Santa Cruz Biotechnology, Santa Cruz, CA). Fluorescently conjugated secondary antibodies were used at 1:5000. Slides were mounted in VECTASHIELD with DAPI (Vector Laboratories, Burlingame, CA). Images were acquired using an Eclipse 80i microscope (Nikon) equipped with a CoolSNAP ES camera (Photometrics, Tucson, AZ) and Plan-Apo 60× objective. For experiments involving quantification, at least 400 cells per condition were scored.
Reverse Transcription (RT)-PCR
RNA was extracted from testes of newly eclosed males by using RNA STAT-60 (Tel-Test, Friendswood, TX). RT-PCR was performed using Ready-To-Go RT-PCR Beads (GE Healthcare, Chalfont St. Giles, Buckinghamshire, United Kingdom). 5′ and 3′ regions of asun (products 1 and 2, respectively) were amplified using the following primer sets: 5′-gccgcgcattcccaacaagg-3′ (1F), 5′-gcggcatttccagcaagact-3′ (1R), 5′-actaaatgccaccacaatgc-3′ (2F), and 5′-gcgtcccgagaaatccaatc-3′ (2R). A region of Mst89B, which is expressed specifically in the testes, was amplified as a positive control by using the following primer set: 5′-tgcaacctcaagttcagtcg-3′ (Mst89B-F) and 5′-gcgtcccgagaaatccaatc-3′ (Mst89B-R) (Stebbings et al., 1998).
Immunoblots
Protein extracts were prepared by homogenizing dissected testes from newly eclosed males in SDS sample buffer. The equivalent of two testes pairs was loaded per lane. Proteins were transferred to nitrocellulose for immunoblotting using standard techniques. Primary antibodies were used as follows: mouse anti-cyclin A (A12, 1:50; Developmental Studies Hybridoma Bank), rabbit anti-Cyclin B (Rb271, 1:2000; gift from D. Glover, University of Cambridge, Cambridge, United Kingdom), mouse anti-actin (pan Ab-5, 1:1000; NeoMarkers, Fremont, CA), mouse anti-dynein heavy chain (P1H4, 1:2000) (McGrail and Hays, 1997), mouse anti-dynein IC (74.1, 1:1000; Santa Cruz Biotechnology), mouse anti-Dynamitin (1:250; BD Biosciences, San Jose, CA), and mouse anti-tubulin (DM1α, 1:7000). Horseradish peroxidase-conjugated secondary antibodies and chemiluminescence were used to detect primary antibodies.
RESULTS
asunder Is Required for Male Fertility
Mat89Bb was originally identified by virtue of its rich expression in Drosophila ovaries (Stebbings et al., 1998). In a Drosophila genome-scale biochemical screen, we identified Mat89Bb as a substrate of the kinase encoded by pan gu (png) that is required in the early embryo (Lee et al., 2005). To assess Mat89Bb's developmental role, we obtained a candidate mutant (Figure 1A). f02815 is a piggyBac insertion in the second intron of Mat89Bb predicted to remove the C-terminal 64 residues from the full-length (689-amino acid) protein (Thibault et al., 2004). Homozygous Mat89Bbf02815 adults are viable and appear normal. Unexpectedly, we found that Mat89Bbf02815 males are almost completely sterile (Table 1), whereas mutant females have only slightly decreased fertility (data not shown). We have changed the Mat89Bb gene name to asunder (asun) to better reflect its loss-of-function phenotype as described below.
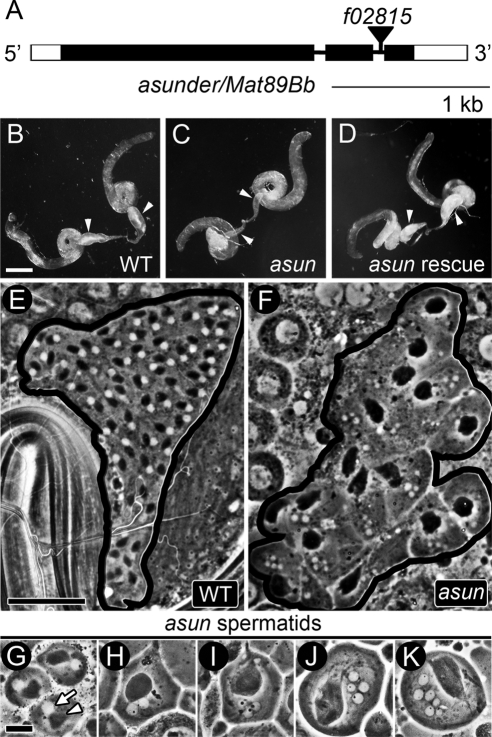
Figure 1.
asun males are sterile. (A) asun gene structure. Coding regions and untranslated regions are represented as filled and unfilled boxes, respectively, introns as thin lines, and piggyBac transposon f02815 as a triangle. (B–D) Testes of males separated from females for 6 d. Arrowheads, seminal vesicles. (B) Wild-type seminal vesicles engorged with mature sperm. (C) Flaccid asun seminal vesicles indicate failed spermatogenesis. (D) Transgenic Myc-ASUN restores sperm to asun seminal vesicles. Bar, 250 μm. (E–K) Phase contrast micrographs of testes. (E and F) Cysts of onion-stage spermatids; black lines mark cyst boundaries. (E) Wild-type cyst with normal complement of 64 cells, each containing a phase-light nucleus and phase-dark Nebenkern of similar size. (F) asun cyst of 16 cells, most with one large Nebenkern and several nuclei of variable size. Bar, 50 μm. (G–K) Range of asun onion-stage spermatid phenotypes. (G) Cell with normal (1:1) ratio of Nebenkern to nuclei. (H–K) Cells with abnormal ratios of Nebenkern to nuclei (1:2 [H], 1:3 [I], 1:4 [J], and 1:6 [K]) and nuclei of variable size. Bar, 10 μm.
Table 1.
Quantification of defects in onion-stage spermatids and sterility of asun males
| Genotypea | nb | Nebenkern-to-nuclei ratio (% cells) |
Macro/micronuclei (% Cells) | Male fertility (% wild type)c | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1:1 | Abnormal |
||||||||
| 1:2 | 1:3 | 1:4 | 1:≥5 | Total | |||||
| Wild type | 2948 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 100 |
| f02815d | 616 | 33 | 16 | 10 | 23 | 18 | 67 | 47 | 8 |
| f02815/Df(3R)Exel7329 | 100 | 9 | 28 | 15 | 33 | 15 | 91 | 56 | 6 |
| f02815 revertant | 2220 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 90 |
| UASp-Myc-asun/Y; f02815 | 2463 | 67 | n.d.e | n.d. | n.d. | n.d. | 33 | n.d. | 22 |
| nanos-Gal4-VP16, f02815 | 1462 | 44 | n.d. | n.d. | n.d. | n.d. | 56 | n.d. | 0 |
| UASp-Myc-asun/Y;f02815/nanos-Gal4-VP16, f02815 | 2297 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 111 |
a All genotypes are w−.
b n is number of onion-stage spermatids scored. Testes from 15 males were scored per genotype.
c Single adult males (2 d old) were placed in vials with five wild-type virgin females (2 d old) and allowed to mate for 5 d. The mean number of adult progeny eclosed per vial is shown as a percentage of wild type (50 males tested per genotype).
d asun allele.
e n.d., not determined.
To elucidate the basis for the male-sterile phenotype of asunf02815 flies (referred to herein simply as asun), we first assessed whether they were capable of producing mature sperm. We dissected whole testes with attached seminal vesicles from males withheld from females to allow accumulation of sperm. Although the overall size and shape of asun testes (Figure 1C) were similar to that of wild type (Figure 1B), suggesting normal proliferation of sperm progenitor cells, their seminal vesicles were largely empty. Mature sperm formed in small amounts in asun testes were immotile or weakly motile (data not shown). Germline expression of Myc-tagged ASUN by using the UAS-Gal4 system fully restored motile sperm production and fertility to asun males (Figure 1D and Table 1) (Brand and Perrimon, 1993; Rorth, 1998). Thus, asun is required for spermatogenesis in Drosophila.
asun Spermatids Display Irregularities in Nuclear Size and Number
To determine which steps of spermatogenesis were impeded in asun males, we used phase contrast microscopy to examine germline cells within live squashed preparations of dissected testes. Intact cysts of asun primary spermatocytes contained exactly 16 cells (50/50 cysts), indicating that the four mitotic spermatogonial divisions occurred; furthermore, asun spermatogonial cells appeared normal (data not shown). When we examined cysts of postmeiotic spermatids in asun testes; however, abnormalities in both cell number and morphology were apparent.
Wild-type spermatids in the “onion” stage (named for their tightly wrapped mitochondrial membranes that resemble onion layers in cross section) have one phase-light nucleus associated with one phase-dark mitochondrial aggregate, the Nebenkern (Figure 1E) (Fuller, 1993). Because nuclear diameter correlates with chromosome content at this stage, errors in chromosome segregation during meiosis yield variably sized nuclei (Gonzalez et al., 1989). Although fusion of interconnected cells within the same cyst commonly occurs during squashing of wild-type testes, resulting in the spurious appearance of multinucleated spermatids, a 1:1 ratio of Nebenkern to nuclei is maintained. Inhibition of meiotic cytokinesis after nuclear division leads to alterations in this ratio because the Nebenkern fuse into a single large mass within multinucleated cells (Liebrich, 1982).
We found that most asun cysts have reduced numbers of onion-stage spermatids, sometimes as few as 16, with a variable number of irregularly sized nuclei and one large Nebenkern (Figure 1, F–K). Such defects are indicative of severe disruptions in chromosome segregation and cytokinesis during the preceding meiotic divisions (Fuller, 1993). These phenotypes were fully rescued by germline expression of Myc-ASUN or reverted to wild type by precise excision of f02815 (Table 1). Thus, asun is required for normal male meiosis.
The frequency of spermatid defects worsened when f02815 was placed in trans to a small chromosomal deficiency that removes asun. Whereas 67% of asunf02815 spermatids were multinucleated, and 47% had variably sized nuclei, the frequency of these phenotypes increased to 91 and 56%, respectively, in hemizygotes, suggesting that asunf02815 may have residual function (Table 1). Consistent with these data, we detected a truncated asun transcript in asunf02815 testes by RT-PCR (Supplemental Figure S1).
Centrosomes Fail to Stably Attach to the Nucleus in asun Primary Spermatocytes
Because the phenotypes that we observed in asun spermatids were diagnostic of earlier disruptions in the meiotic divisions, we shifted our focus to spermatocytes. To aid in this analysis, we crossed into the asun background a transgene that ubiquitously expresses fluorescently labeled β-tubulin (Inoue et al., 2004).
Entry into M phase in primary spermatocytes is marked by centrosome migration, chromosome condensation, and the appearance of specific microtubule structures (Cenci et al., 1994; Rebollo et al., 2004). Paired asters (centrosomes with associated astral microtubules) formed at the cell cortex in late G2 migrate inward from the cortex and stably attach to the nuclear envelope in prophase I (Figure 2, A and E). Asters then separate from each other and move along the nuclear surface to opposite poles to establish the meiotic spindle. In asun spermatocytes, we observed normal cortical positioning in G2 and release of centrosomes upon M phase entry; subsequent nucleus–centrosome coupling, however, was defective (Figure 2, B–D, and F–H; data not shown).
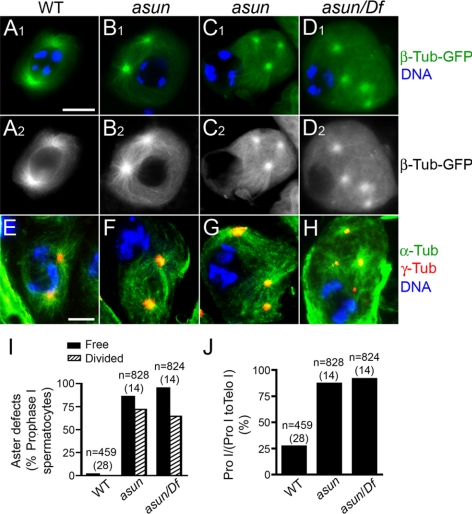
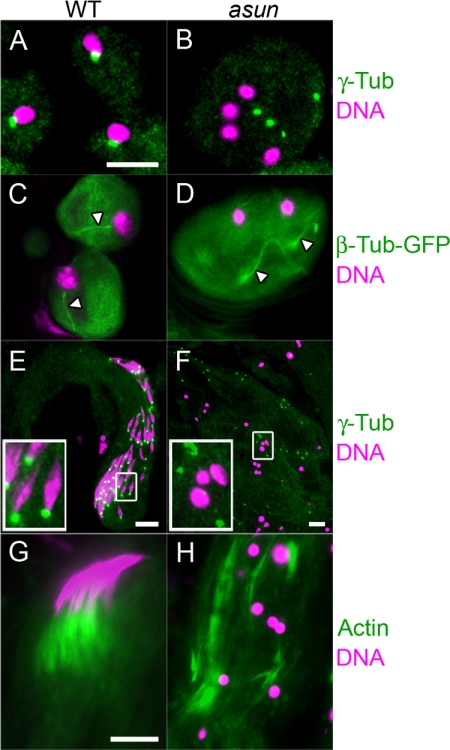
Figure 2.
Loss of nucleus-centrosome attachments in asun spermatocytes. (A–H) Prophase I spermatocytes expressing β-tubulin-GFP (green in A1–D1; grayscale in A2–D2) or stained for microtubules (α-tubulin; green) and centrosomes (γ-tubulin; red) (E–H). DNA is in blue. In wild-type spermatocytes (A and E), centrosomes migrate from cortex and attach to nucleus. Most asun spermatocytes have two (B and F), three (C and G), or four (D and H) unattached centrosomes; spermatocytes in D and H are from hemizygotes. Bar, 10 μm. (I and J) Quantification of aster defects (I) and prophase arrest (J) of asun spermatocytes. Relative to wild type, asun testes contain an increased fraction of prophase I (Pro I) through telophase I (Telo I) spermatocytes that are in prophase I. n, number of prophase I spermatocytes (I) or prophase I through telophase I spermatocytes (J) scored per genotype. Number of males analyzed per genotype is in parentheses. Free, cells with unattached asters. Divided, cells with three or four asters. Df, Df(3R)Exel7329.
We observed a striking loss of association between asters and the nucleus during prophase in asun primary spermatocytes by direct visualization of β-tubulin-GFP (Figure 2, B–D). We confirmed the identity of these free asters by staining for γ-tubulin, a centrosomal marker (Figure 2, F–H). We chose the gene name asunder (set apart in position or space) on the basis of this phenotype. The number of asters per asun prophase spermatocyte ranged from two to four (Figure 2, B–D and F–H), probably due to premature reduction of centrosomes that normally occurs during meiosis. Quantification of these defects revealed that the majority of prophase I spermatocytes from asun homozygous and hemizygous males contain unattached, divided asters (Figure 2I). Furthermore, a disproportionately high number of primary spermatocytes from asun homozygous and hemizygous males were found to be in prophase (Figure 2J), indicative of arrest upon M phase entry. asun spermatocytes seem to normally accumulate cyclin A and cyclin B during prophase I, and asun testes display elevated levels of cyclin A and cyclin B consistent with an increased fraction of cells at prophase I (Supplemental Figure S2).
Meiotic Spindle Assembly and Chromosome Segregation Are Aberrant in asun Spermatocytes
Although asun spermatocytes arrest in prophase I, they can evade arrest and proceed through both meiotic divisions. In wild-type spermatocytes, chromosomes segregate evenly to daughter cells along a bipolar spindle during meiosis I and II (Figure 3, A and B). In contrast, the majority of asun spermatocytes that progress through the meiotic divisions exhibit gross defects in spindle assembly (e.g., tetrapolar spindles) and chromosome segregation (Figure 3, C and D). We infer that these defects in spindle assembly and chromosome segregation occur upon resumption of M phase by asun spermatocytes that underwent premature division of centrioles during a prolonged prophase I. Although Drosophila spermatocytes are capable of forming acentrosomal spindles when nucleus-centrosome coupling is disrupted, we have not observed acentrosomal spindles in asun spermatocytes (Rebollo et al., 2004).
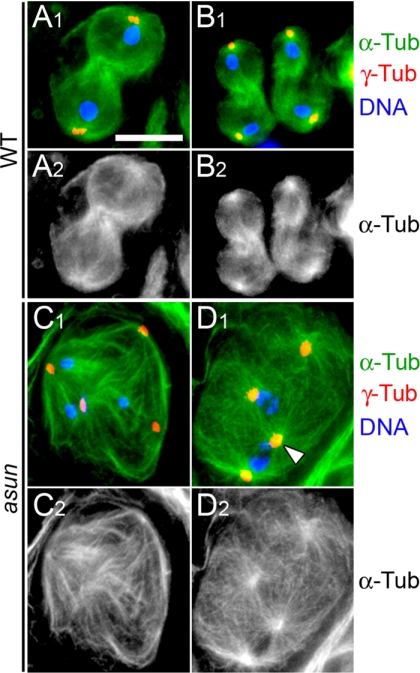
Figure 3.
Aberrant spindle assembly and chromosome segregation in asun spermatocytes. Meiotic cells stained for α-tubulin (green in A1–D1; grayscale in A2–D2), centrosomes (γ-tubulin; red), and DNA (blue). Wild-type primary (A) and secondary (B) spermatocytes in telophase segregate DNA equally to daughter cells. (C and D) Representative asun primary spermatocytes. (C) Unequal segregation of DNA masses to three poles of a tetrapolar spindle. (D) Two spindles sharing a pole (arrowhead). Bar, 10 μm.
Late Events of Spermatogenesis Are Disrupted in asun Males
In Drosophila, key events of spermatid differentiation (e.g., elongation and individualization) can proceed even in the face of severe meiotic defects (Alphey et al., 1992; Castrillon et al., 1993). Indeed, postmeiotic stages of spermatogenesis are present in asun testes despite the disruptions in meiosis that we have reported herein. We examined postmeiotic spermatids in asun testes to determine whether asun regulates late spermatogenesis.
Axoneme assembly is directed by a centriole-derived basal body that embeds in the nuclear envelope immediately after meiosis (Figure 4, A and C). We found that nucleus-basal body coupling is impaired in asun spermatids that have failed to undergo meiotic cytokinesis (Figure 4B). Despite the absence of nucleus-basal body interactions, axonemes nonetheless nucleate from the free basal bodies of asun spermatids (Figure 4D).
Figure 4.
Loss of nucleus-basal body attachment and individualization defects of asun spermatids. (A and B) Onion-stage spermatids stained for basal bodies (γ-tubulin; green) and DNA (magenta). Basal bodies embed in the nuclear envelope after meiosis in wild-type spermatids (A) but fail to attach to nuclei of asun spermatids (B). (C and D) Onion-stage spermatids expressing β-tubulin-GFP (green) stained for DNA (magenta). Sperm axonemes (arrowheads) nucleate from attached basal bodies in wild-type spermatids (C) and from free basal bodies in asun spermatids (D). (E and F) Elongating spermatids stained for basal bodies (γ-tubulin; green) and DNA (magenta). Insets show enlarged views of boxed regions. (E) As wild-type spermatids elongate, basal bodies remain attached to nuclei that acquire a spiculated shape. (F) Disorganized bundles of elongating asun spermatids with dispersed basal bodies and round nuclei. (G and H) Bundles of individualizing spermatids stained for actin (green) and DNA (magenta). (G) In wild-type spermatids, actin cones formed at each nucleus move as a unit along the bundle length. (H) In asun spermatids, disorganized actin cones are found along the bundle length. Bars, 10 μm.
Formation of the sperm axoneme initiates onion-stage spermatid elongation. During elongation of wild-type spermatids, nuclei with their associated basal bodies are positioned at the proximal tips of growing bundles, and the nuclei undergo a change in morphology from round to needle-like (Figure 4E). The nuclei and basal bodies of asun spermatids in elongating bundles, however, are often randomly distributed throughout the length of the bundle, and nuclei remain round (Figure 4F).
Spermatid individualization occurs as actin cones assembled at the nucleus move down the axoneme, pushing out excess cytoplasm and remodeling cell membranes as they travel the bundle length. Wild-type actin cones form synchronously and migrate as a unit (Figure 4G). asun actin cones, however, seem to move down the axoneme independent of one another and are typically dispersed along the entire bundle length (Figure 4H). Thus, asun is required for some, but not all, events of late spermatogenesis.
Dynamic Localization of Dynein–Dynactin throughout Drosophila Spermatogenesis
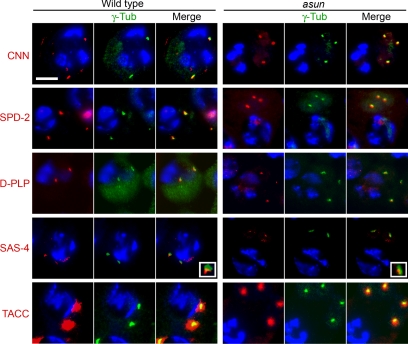
Our analysis of the asun male-sterile phenotype strongly suggested that asun plays a critical role in the formation and/or maintenance of nucleus–centrosome/basal body interactions. Using a panel of antibodies directed against centrosomal proteins, we found that the free asters of asun spermatocytes stained positively for all markers tested (Figure 5) (Gergely et al., 2000; Martinez-Campos et al., 2004; Basto et al., 2006; Dix and Raff, 2007). Thus, loss of nucleus–aster attachments in asun spermatocytes does not seem to be due to a global defect in centrosome composition.
Figure 5.
Normal centrosomes in asun spermatocytes. Wild-type and asun testes were stained for γ-tubulin (green) to mark the position of the centrosomes, DNA (blue), and the indicated centriole/centrosome components (red). asun late primary spermatocytes exhibited staining comparable with that of wild type for all components tested. Insets show enlarged views of SAS-4 localization on centrosomes.
Migration of centrosomes from the cortex to the nucleus in Drosophila primary spermatocytes, a prerequisite step to form nucleus–centrosome attachments, depends upon microtubules, actin, and abnormal spindle (asp) function (Kemphues et al., 1982; Gunsalus et al., 1995; Giansanti et al., 1998; Wakefield et al., 2001; Rebollo et al., 2004). In asun spermatocytes, actin arrays seem grossly normal, and astral microtubule arrays are similar in size to those of wild type (compare Figure 2, B to A and F to E; data not shown). In contrast to asp, centrosomes of asun spermatocytes seem to disassociate from the cell cortex normally upon M-phase entry, suggesting that asun mediates a subsequent event required for nucleus–centrosome coupling (Figure 2, B–D and F–H).
Dynein–dynactin has been shown to play an essential role in anchoring both centrosomes and basal bodies to the nucleus. Dynein–dynactin is recruited to the nuclear periphery in a variety of systems, and this localization has been shown to be critical for centrosome attachment in Caenorhabditis elegans embryos (Gönczy et al., 1999; Salina et al., 2002; Malone et al., 2003). Loss of dynein in Drosophila embryos results in failure of centrosomes to stably attach to nuclei, and nucleus–basal body interactions are lost in Drosophila spermatids null for tctex-1, which encodes a dynein light chain (Robinson et al., 1999; Li et al., 2004). We therefore chose to examine dynein–dynactin localization during spermatogenesis in asun males.
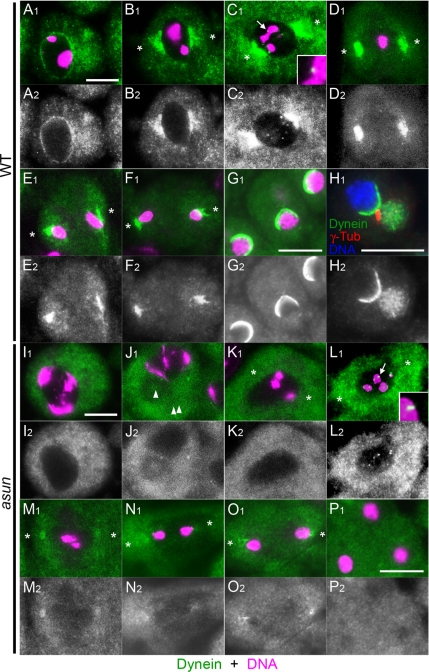
Dynamic localization of dynein at meiotic spindle poles and kinetochores has been observed in grasshopper and crane fly spermatocytes (King et al., 2000; Fabian and Forer, 2005). Localization of dynein–dynactin during Drosophila male meiosis, however, has not been reported to our knowledge. We first established the normal localization pattern of dynein and dynactin in wild-type spermatocytes by examining fixed cells stained for dynein heavy chain and living cells expressing a GFP-tagged version of DMN, the p50 subunit of dynactin (McGrail and Hays, 1997; Wojcik et al., 2001).
Whereas dynein is evenly dispersed in the cytoplasm of early G2 spermatocytes, it accumulates on the nuclear periphery by late G2 (Figure 6A; data not shown). Dynein concentrates near asters attached to the nucleus in prophase I and at spindle poles and kinetochores in prometaphase I (Figure 6, B and C). Kinetochore localization is lost in metaphase I as dynein further concentrates at spindle poles, where it persists during anaphase I and telophase I (Figure 6, D–F). We observed an identical pattern of dynein localization during meiosis II (data not shown). As reported previously, we detected a hemispherical cap of dynein on nuclei of onion-stage spermatids; interestingly, the site of nucleus–basal body attachment lies directly in the middle of this cap (Figure 6, G and H) (Li et al., 2004; Wei et al., 2008). We observed an essentially identical localization pattern throughout spermatogenesis for the DMN subunit of dynactin (Figure 7, A and B; data not shown). We also detected a weak enrichment of dynein–dynactin at the Nebenkern surface that has not been reported previously to our knowledge (Supplemental Figure S3A; data not shown).
Figure 6.
Disrupted dynein localization in asun spermatocytes and spermatids. Wild-type (A–G) and asun (I–P) primary spermatocytes and onion-stage spermatids stained for dynein heavy chain (green in A1–G1, I1–P1; grayscale in A2–G2, I2–P2). DNA is in magenta. α-Tubulin staining (data not shown) was used to determine cell cycle stage and spindle pole positions (asterisks). (A–F) Wild-type spermatocytes. Dynein localizes uniformly to surface of late G2 nuclei (A), near centrosomes attached to the nucleus in prophase (B), and at spindle poles and kinetochores (arrow, enlarged in inset) in prometaphase (C). Kinetochore localization is lost at metaphase (D). Dynein remains at spindle poles in anaphase (E) and telophase (F). (G and H) In wild-type spermatids, dynein localizes to a hemispherical cap on the nucleus. (H) Triple staining for dynein (green in H1; grayscale in H2), basal bodies (γ-tubulin; red), and DNA (blue) reveals proximity of basal body to dynein cap. (I–O) asun spermatocytes. Dynein fails to localize to the nuclear periphery in late G2 (I) and is dispersed in the cytoplasm. asun spermatocytes arrest in prophase I (J) with free asters (arrowheads) and no spindle, although some form bipolar spindles in prophase (K) and proceed through prometaphase (L), metaphase (M), anaphase (N), and telophase (O). In both cases, dynein localization to the nuclear periphery and spindle poles is absent or greatly reduced relative to wild type, whereas localization to prometaphase kinetochores (arrow in L, enlarged in inset) seems normal. (P) Dynein does not localize to a hemispherical nuclear cap in asun spermatids. Bars, 10 μm.
Figure 7.

Disrupted dynactin localization in asun spermatocytes and spermatids. Wild-type (A and B) and asun (C and D) primary spermatocytes and onion-stage spermatids expressing DMN-GFP. Dynactin is at the nuclear periphery (arrows) in wild-type G2 spermatocytes (A) and onion-stage spermatids (B), but not in asun spermatocytes (C) and spermatids (D). Bars, 10 μm.
asun Is Required for Dynein–Dynactin Localization throughout Spermatogenesis
We found dynein-dynactin localization to be severely disrupted in asun spermatocytes and spermatids. Dynein rarely localized to the nuclear periphery in late G2 asun spermatocytes (Figure 6I; 35/320 cells scored) or in arrested asun spermatocytes with free asters (Figure 6J; 0/167 cells scored). Even in asun primary spermatocytes that overcame the prophase arrest and formed spindles, dynein localization to spindle poles was greatly reduced relative to wild type from prophase through telophase (Figure 6, K–O). asun secondary spermatocytes also exhibited decreased dynein at spindle poles (data not shown). Dynein seemed to accumulate normally, however, on kinetochores of asun prometaphase spermatocytes with bipolar spindles (Figure 6L; 64/64 cells). Dynein did not form a hemispherical nuclear cap in multinucleated asun onion-stage spermatids, although very weak perinuclear localization was occasionally seen in asun spermatids with wild-type morphology (Figure 6P; data not shown). A severe reduction in the localization of the DMN subunit of dynactin to the nuclear periphery and to spindle poles throughout spermatogenesis was similarly observed in asun testes (Figure 7, C and D; data not shown). In contrast, localization of dynein–dynactin to the Nebenkern surface seemed to be normal in asun spermatids (Supplemental Figure S3B; data not shown).
Despite the loss of dynein–dynactin localization during meiotic and postmeiotic stages of spermatogenesis in asun males, we found that protein levels of DHC64C and Cdic, core dynein components (heavy and intermediate chains, respectively), are not reduced in asun testes (Supplemental Figure S4A). Likewise, we found that protein levels of DMN, a core dynactin component, are not reduced in asun testes (Supplemental Figure S4B). These results strongly suggest that asun is required for localization, but not stability, of dynein–dynactin during spermatogenesis.
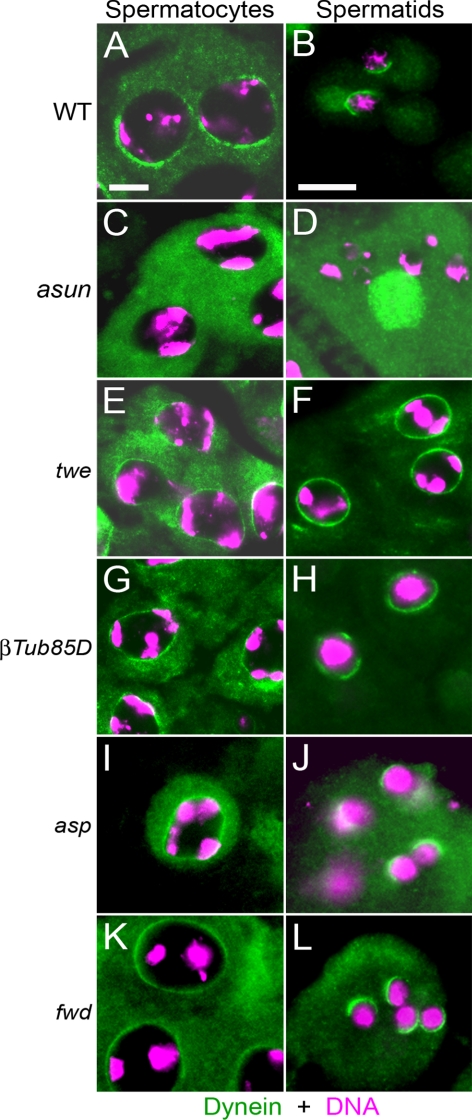
Loss of Perinuclear Dynein Localization Is Not a General Feature of Male-Meiotic Mutants
Loss of dynein–dynactin localization in asun spermatocytes and spermatids could be a general consequence of meiotic failure. To test this possibility, we examined dynein localization to the nuclear surface in four other male-meiotic mutants: twine (twe), βTubulin85D (βTub85D), abnormal spindle (asp), and four wheel drive (fwd). Spermatocytes of twe or βTub85D males arrest at the G2/M phase transition due to loss of Cdk1-cyclin B activity or microtubules, respectively (Kemphues et al., 1982; Alphey et al., 1992). In spermatocytes lacking asp, which encodes a microtubule-associated protein, the two pairs of centrioles fail to migrate from the plasma membrane back to the nucleus during prophase and instead remain located at the plasma membrane throughout meiosis (Wakefield et al., 2001; Rebollo et al., 2004). fwd spermatocytes undergo nuclear divisions, but meiotic cytokinesis fails, resulting in the formation of multinucleated spermatids (Brill et al., 2000). We found that dynein accumulates to normal levels on the nuclear periphery of spermatocytes and spermatids of all four mutants tested (Figure 8), indicating that perinuclear localization of dynein in the male germline occurs via a microtubule-independent mechanism and that loss of dynein localization in asun testes is not likely to be a secondary or indirect consequence of disruption of meiosis.
Figure 8.
Dynein localization in other male-meiotic mutants. Fixed testes from wild-type (A and B), asun (C and D), twe1 (E and F), βTub85DDrv1 (G and H), aspE3/aspL1 (I and J), and fwdneo1/Df(3L)7C (K and L) males were stained for dynein heavy chain (green) and DNA (magenta). In contrast to asun, dynein localizes to the nuclear periphery in spermatocytes and spermatids of other male-meiotic mutants. Perinuclear localization of dynein is expanded in twe and β2tub spermatids relative to wild type.
Genetic Enhancement of asun by Dynein–Dynactin Components
We hypothesized that loss of perinuclear dynein–dynactin is the underlying basis for the defects observed in asun spermatocytes. As a test of this model, we assessed whether reduction of the gene dosage of either of two dynein–dynactin components would enhance the asun phenotype: DHC64C (dynein heavy chain) or Glued (Gl), the p150 subunit of dynactin (Holzbaur et al., 1991). Introduction into the asun background of a single copy of Dhc64C4–19 (null allele), Dhc64C6–10 (hypomorphic allele), or Df(3L)fz-GF3b (genomic deficiency that deletes Gl) significantly increased the incidence of multi-nucleated spermatids (from 64% to 93, 84, and 88%, respectively); dominant enhancement of asun by Gl1 (dominant-negative allele) did not reach statistical significance (Supplemental Figure S5). As control, one copy of any of these alleles in an otherwise wild-type background had no apparent effect on spermatogenesis (data not shown). These genetic data support our model that loss of dynein–dynactin function is responsible for the meiotic defects of asun spermatocytes.
ASUN and Dynactin Undergo Coincident Changes in Localization in Primary Spermatocytes
To gain insight into the mechanism by which ASUN promotes dynein–dynactin localization during spermatogenesis, we examined its localization pattern. We established transgenic lines that express fluorescently tagged versions of ASUN in postmitotic germ cells of the testis under the control of the male-specific βTub85D promoter (Wong et al., 2005). These transgenes could fully rescue the sterility of asun males and did not perturb spermatogenesis in wild-type flies (data not shown). Using a combination of fluorescent and phase contrast microscopy, we assessed ASUN localization throughout male meiosis.
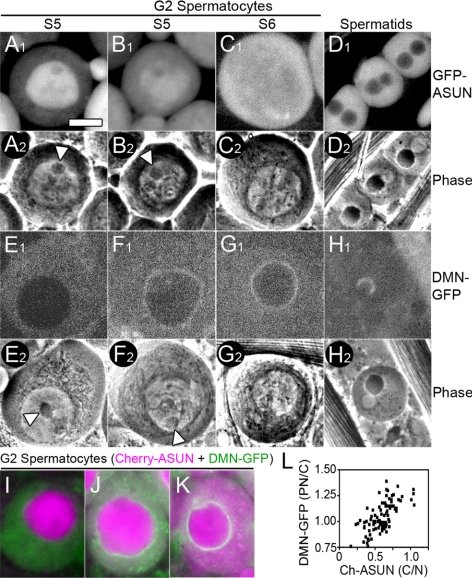
Using this approach, we found that ASUN exhibits stage-specific localization in G2 spermatocytes (Figure 9, A–C). The G2 phase of primary spermatocytes can be divided into six stages (S1–S6) based on the appearance of cytoplasmic and nuclear structures (Cenci et al., 1994). In stage S3–S4 cells, ASUN is largely restricted to the nucleus (253/253 cells). In stage S5 cells, ASUN localization ranges from mostly nuclear to being partitioned between the nucleus and cytoplasm (Figure 9, A and B). By stage S6, which precedes centrosome migration and entry into M phase, ASUN is distributed between the cytoplasm and nucleus (Figure 9C; 236/236 cells). ASUN remains dispersed throughout the cell until the completion of meiosis when it becomes restricted from nuclei of onion-stage spermatids (Figure 9D; data not shown). Based on these observations, we infer that a pool of ASUN is released from the nucleus to the cytoplasm during the S5 stage of G2 spermatocytes.
Figure 9.
Coincident localization changes of ASUN and dynactin in primary spermatocytes. (A–D) Matched fluorescent (A1–D1) and phase contrast (A2–D2) images of representative G2 spermatocytes (A–C) and onion-stage spermatids (D) expressing GFP-ASUN. (A and B) In S5 spermatocytes, GFP-ASUN is largely nuclear (A) or in both nucleus and cytoplasm (B). Arrowheads mark intact nucleoli characteristic of this stage. (C) S6 spermatocyte with uniformly distributed GFP-ASUN. (D) GFP-ASUN is excluded from spermatid nuclei. (E–H) Matched fluorescent (E1–H1) and phase-contrast (E2–H2) images of G2 spermatocytes (E–G) and onion-stage spermatids (H) expressing DMN-GFP. (E and F) S5 spermatocytes with DMN-GFP uniformly distributed in cytoplasm (E) or at nuclear surface (F). Arrowheads mark intact nucleoli characteristic of this stage. (G) S6 spermatocyte in which DMN-GFP has accumulated at the nuclear periphery. (H) DMN-GFP is localized to a hemispherical cap on the nucleus of spermatids. (I–K) Fluorescent images of representative G2 spermatocytes coexpressing Cherry-ASUN (magenta) and DMN-GFP (green). (I) Evenly distributed cytoplasmic DMN-GFP with minimal cytoplasmic Cherry-ASUN. (J) Low levels of perinuclear DMN-GFP with increased cytoplasmic Cherry-ASUN. (K) High levels of perinuclear DMN-GFP with Cherry-ASUN uniformly distributed between nucleus and cytoplasm. Bar, 10 μm. (L) Scatter plot displaying the relationship between release of Cherry-ASUN from the nucleus and accumulation of DMN-GFP at the nuclear periphery in late G2 primary spermatocytes. Each data point represents a single spermatocyte for which the ratio of the intensity of peripheral nuclear to cytoplasmic DMN-GFP signal is plotted against the ratio of the intensity of cytoplasmic to nuclear Cherry-ASUN signal.
Although the localization patterns of ASUN and dynein–dynactin differ during spermatogenesis, we found that the timing of the nuclear-to-cytoplasmic movement of a pool of ASUN in G2 spermatocytes coincides tightly with that of dynein–dynactin recruitment to the nuclear surface. As described above for ASUN, we used a combination of fluorescent and phase contrast microscopy to more precisely define the timing of the perinuclear recruitment of GFP-tagged DMN in living G2 spermatocytes. In stage S3–S4 cells, perinuclear DMN was undetectable (248/248 cells). DMN first accumulates on the nuclear surface during stage S5 (Figure 9, E and F) and uniformly localizes there by stage S6 (Figure 9G; 144/145 cells). When we examined primary spermatocytes expressing both Cherry-ASUN and DMN-GFP, we found a strong temporal correlation between the nuclear-to-cytoplasmic movement of a pool of ASUN and perinuclear accumulation of DMN (Figure 9, I–L). These results are consistent with a model in which release of a pool of ASUN from the nucleus to the cytoplasm in G2 primary spermatocytes stimulates recruitment of dynein–dynactin to the nuclear periphery.
Perinuclear Localization of ASUN
To assess whether the localization of ASUN is conserved, we expressed fluorescently tagged Drosophila ASUN in cultured mammalian (HeLa) cells and examined its subcellular distribution. Similar to our observations in the Drosophila male germline, we found that GFP-ASUN localization in HeLa cells ranged from predominantly nuclear, partitioned between the nucleus and cytoplasm, to predominantly cytoplasmic (Figure 10, A–C). Strikingly, we found that GFP-tagged Drosophila ASUN localized to the perinuclear region in ∼40% of transfected cells (Figure 10D). Essentially identical results were obtained using a Myc-tagged version of GCT1, the candidate human homologue of ASUN, underscoring the possibility that ASUN′s functions are conserved in higher organisms (Figure 10, D–F; data not shown).
Dynein–dynactin has been reported to localize to the nuclear surface during the G2/prophase transition in cultured mammalian cells (Salina et al., 2002; Hebbar et al., 2008). Based on the perinuclear enrichment of both GFP-ASUN and Myc-GCT1 in transfected HeLa cells, we investigated the possibility that ASUN and dynein–dynactin may interact at the nuclear surface. In our hands, we detected only a weak perinuclear localization of dynein in HeLa cells at G2/prophase by immunofluorescence (data not shown). Treatment of cells with nocodazole, which destabilizes microtubules, has been shown to transiently enhance the perinuclear localization of dynein–dynactin in prophase cells (Hebbar et al., 2008). We therefore examined nocodazole-treated HeLa cells expressing GFP-ASUN to assess whether dynein and GFP-ASUN colocalize on the nuclear surface at the G2/prophase transition. We found that GFP-ASUN localized to the nuclear surface in >80% of prophase cells (Figure 10G). Furthermore, we found that dynein tightly colocalized with GFP-ASUN at the nuclear surface in ∼90% of cells displaying perinuclear dynein (Figure 10H). These observations suggest that ASUN and dynein–dynactin may functionally interact at the nuclear surface during the G2/prophase transition.
Given our observations of ASUN/GCT1 localization in HeLa cells, we carefully reassessed the localization pattern of GFP-ASUN in transgenic spermatocytes. Using a second transgenic line with relatively low expression of GFP-ASUN, we occasionally detected perinuclear enrichment of GFP-ASUN in G2 primary spermatocytes (Figure 10, I and J; data not shown). The low frequency at which we detected this perinuclear pool of GFP-ASUN may suggest that it is transient and/or that it is masked by transgenic overexpression. Together with our HeLa cell data, these observations are consistent with a model in which perinuclear ASUN recruits and/or retains dynein–dynactin at the nuclear surface of G2 spermatocytes, thereby mediating nucleus–centrosome coupling at M-phase entry that helps to ensure fidelity of the meiotic divisions.
DISCUSSION
Here, we report that asun is a critical regulator of spermatogenesis in Drosophila. We have shown that primary spermatocytes lacking asun exhibit failure of nucleus–centrosome coupling, which normally occurs upon M phase entry, with subsequent defects in spindle assembly and chromosome segregation. The normal attachment between the nucleus and another centriole-based structure, the basal body, is similarly lost in asun postmeiotic spermatids. Furthermore, we have shown that dynein–dynactin complexes, which mediate nucleus–centrosome/basal body interactions, fail to localize to the nuclear surface in both asun spermatocytes and spermatids (Gönczy et al., 1999; Robinson et al., 1999; Salina et al., 2002; Malone et al., 2003).
We propose the following model to describe the role of ASUN in promoting nucleus–centrosome coupling during male meiosis. In early G2 spermatocytes, ASUN is largely sequestered in the nucleus, and dynein–dynactin localizes diffusely throughout the cytoplasm. In late G2, a pool of ASUN is released from the nucleus into the cytoplasm where it promotes accumulation of dynein–dynactin at the nuclear periphery. Perinuclear dynein–dynactin captures astral microtubules emanating from cortical centrosomes. Due to the minus-end–directed motor activity of dynein, interaction between astral microtubules and anchored dynein–dynactin results in movement of centrosomes toward the nucleus; stable linkage between the nucleus and centrosomes, a prerequisite for fidelity of meiotic divisions, is then established.
Dynein–dynactin-mediated interactions between centrosomes and the nucleus have been shown to be important in a variety of biological processes, including nuclear positioning, nuclear migration, and nuclear envelope breakdown (Reinsch and Gönczy, 1998; Morris, 2000; Salina et al., 2002). Although it is generally assumed that dynein–dynactin on the nuclear envelope interacts with centrosomes via astral microtubules that they nucleate, the mechanism by which dynein–dynactin is anchored to the nucleus remains unclear. The C. elegans Hook protein ZYG-12, which localizes to the nuclear periphery, tethers dynein to this site in embryos; a similar role for Hook proteins in other species, however, has not been reported (Gönczy, 2004; Malone et al., 2003). Nuclear pore complexes have been proposed as likely candidates for sites of dynein–dynactin attachment as they are unique to the nuclear envelope and span both outer and inner nuclear membranes (Salina et al., 2002). In support of this model, dynein–dynactin has been found to physically interact with nuclear pore complex components in bovine zygotes and oocytes (Payne et al., 2003).
Dynein–dynactin accumulation at the nuclear envelope occurs in late G2 in dividing mammalian cells; we have observed a similar pattern of timing during Drosophila male meiosis (Salina et al., 2002). The mechanisms underlying this cell cycle-dependent localization of dynein–dynactin remain to be determined. The coincident timing of ASUN′s release to the cytoplasm and accumulation of dynein–dynactin on the nucleus, however, raises the intriguing possibility that ASUN plays a key role in mediating this event.
We have observed a striking perinuclear localization of fluorescently tagged versions of Drosophila ASUN and its candidate human homologue, GCT1, in transfected HeLa cells at the G2/prophase transition that significantly overlaps with that of dynein IC. Similarly, we have occasionally observed weak perinuclear enrichment of fluorescently tagged ASUN in G2 spermatocytes of transgenic flies. Based on these findings, we propose that this perinuclear pool of ASUN directly promotes dynein–dynactin recruitment and/or retention at the nuclear surface, a prerequisite for nucleus–centrosome coupling. In contrast, dynein–dynactin localization to kinetochores and the Nebenkern seems to be independent of ASUN, suggesting that ASUN mediates localization of a subset of cellular pools of dynein–dynactin. We have been unable to detect physical interactions between ASUN and dynein-dynactin (our unpublished observations). Dynein–dynactin localization and its interactions with molecular cargos depends on several factors, including the presence of adaptors and phosphorylation state of its subunits, thus providing many levels at which it may be regulated by ASUN (Farshori and Holzbaur, 1997; Tai et al., 2001).
In addition to defects in nucleus–centrosome coupling in asun spermatocytes, the centriole-derived basal bodies fail to form stable attachments to the nuclei of asun spermatids. Functional basal bodies, which organize the sperm axoneme, seem to be required for positioning of nuclei within sperm bundles and nuclear shaping during spermatid elongation (Vogt et al., 2006; Texada et al., 2008). Dynein–dynactin localizes to a hemispherical cap on the nuclei of onion-stage spermatids, and the site of nucleus-basal body attachment lies in the middle of this region (Li et al., 2004; this report). Furthermore, spermatids of males lacking tctex-1, which encodes a dynein light chain, exhibit defects in nucleus-basal body coupling similar to what we observe in asun males (Li et al., 2004). We propose that disruption of nucleus–basal body interactions in asun spermatids is due to loss of perinuclear dynein–dynactin.
Expression of asun (previously known as Mat89Bb) in adult flies seems to be limited to the male and female germlines (Stebbings et al., 1998). We previously identified ASUN as an in vitro substrate of PNG kinase (Lee et al., 2005). png expression, which seems to be limited to the female germline with maternal deposition in the egg, is required for cell cycle progression during syncytial embryogenesis (Fenger et al., 2000; Lee et al., 2005). Although we find that asun is essential for gametogenesis in males, we have observed only slightly decreased egg-laying rates of asun females and normal development of their embryos. The lack of defects in asun-derived embryos reported here may be due to the hypomorphic nature of f02815; rigorous assessment of a potential role for asun in early embryogenesis awaits isolation of null alleles. Whereas png-derived embryos have decreased levels of mitotic cyclins, we find elevated levels of cyclin A and cyclin B in asun testes consistent with an increased fraction of cells at prophase I (Fenger et al., 2000; Lee et al., 2001). Furthermore, our demonstration of normal perinuclear localization of dynein in twe spermatocytes suggests that Cdk1-cyclin B does not mediate this process.
Our database searches have revealed putative asun homologues in organisms ranging from C. elegans to humans. The human gene, GCT1, resides in a chromosomal region (12p11-p12) that is both amplified and overexpressed in testicular seminomas (Bourdon et al., 2002; Rodriguez et al., 2003). In contrast to the germline-specific expression of asun in Drosophila, GCT1 is broadly expressed in human tissues, suggesting that it may have functions in somatic cells (Bourdon et al., 2002). We showed previously that down-regulation of GCT1 in HeLa cells resulted in a multinucleated phenotype (Lee et al., 2005). Further experiments will be needed to determine whether human GCT1 coordinates mitosis by regulating dynein–dynactin localization.
Supplementary Material
ACKNOWLEDGMENTS
We thank Julie Brill, David Glover, Cayetano Gonzalez, Hiroki Oda, Y. Akiyama-Oda, Jordan Raff, and Bill Theurkauf for providing vectors, fly stocks, and antibodies. We thank Kate Beckingham and Lee lab members for critical reading of the manuscript. This work was supported by National Institutes of Health grant GM-074044 (to L.A.L.) and National Institutes of Health research training grants T32 HD-007502 and T32 HD-007043 (to M.A.A.). K.G.H. was supported by National Institutes of Health grant R15 GM-080689.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-12-1165) on April 8, 2009.
REFERENCES
- Alphey L., Jimenez J., White-Cooper H., Dawson I., Nurse P., Glover D. M. twine, a cdc25 homolog that functions in the male and female germline of Drosophila. Cell. 1992;69:977–988. doi: 10.1016/0092-8674(92)90616-k. [DOI] [PubMed] [Google Scholar]
- Basto R., Lau J., Vinogradova T., Gardiol A., Woods C. G., Khodjakov A., Raff J. W. Flies without centrioles. Cell. 2006;125:1375–1386. doi: 10.1016/j.cell.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Bettencourt-Dias M., Rodrigues-Martins A., Carpenter L., Riparbelli M., Lehmann L., Gatt M. K., Carmo N., Balloux F., Callaini G., Glover D. M. SAK/PLK4 is required for centriole duplication and flagella development. Curr. Biol. 2005;15:2199–2207. doi: 10.1016/j.cub.2005.11.042. [DOI] [PubMed] [Google Scholar]
- Bourdon V., Naef F., Rao P. H., Reuter V., Mok S. C., Bosl G. J., Koul S., Murty V. V., Kucherlapati R. S., Chaganti R. S. Genomic and expression analysis of the 12p11–p12 amplicon using EST arrays identifies two novel amplified and overexpressed genes. Cancer Res. 2002;62:6218–6223. [PubMed] [Google Scholar]
- Brand A. H., Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Brill J. A., Hime G. R., Scharer-Schuksz M., Fuller M. T. A phospholipid kinase regulates actin organization and intercellular bridge formation during germline cytokinesis. Development. 2000;127:3855–3864. doi: 10.1242/dev.127.17.3855. [DOI] [PubMed] [Google Scholar]
- Castrillon D. H., Gönczy P., Alexander S., Rawson R., Eberhart C. G., Viswanathan S., DiNardo S., Wasserman S. A. Toward a molecular genetic analysis of spermatogenesis in Drosophila melanogaster: characterization of male-sterile mutants generated by single P element mutagenesis. Genetics. 1993;135:489–505. doi: 10.1093/genetics/135.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci G., Bonaccorsi S., Pisano C., Verni F., Gatti M. Chromatin and microtubule organization during premeiotic, meiotic and early postmeiotic stages of Drosophila melanogaster spermatogenesis. J. Cell Sci. 1994;107:3521–3534. doi: 10.1242/jcs.107.12.3521. [DOI] [PubMed] [Google Scholar]
- Dix C. I., Raff J. W. Drosophila Spd-2 recruits PCM to the sperm centriole, but is dispensable for centriole duplication. Curr. Biol. 2007;17:1759–1764. doi: 10.1016/j.cub.2007.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian L., Forer A. Redundant mechanisms for anaphase chromosome movements: crane-fly spermatocyte spindles normally use actin filaments but also can function without them. Protoplasma. 2005;225:169–184. doi: 10.1007/s00709-005-0094-6. [DOI] [PubMed] [Google Scholar]
- Farshori P., Holzbaur E. L. Dynactin phosphorylation is modulated in response to cellular effectors. Biochem. Biophys. Res. Commun. 1997;232:810–816. doi: 10.1006/bbrc.1997.6379. [DOI] [PubMed] [Google Scholar]
- Fenger D. D., Carminati J. L., Burney-Sigman D. L., Kashevsky H., Dines J. L., Elfring L. K., Orr-Weaver T. L. PAN GU: a protein kinase that inhibits S phase and promotes mitosis in early Drosophila development. Development. 2000;127:4763–4774. doi: 10.1242/dev.127.22.4763. [DOI] [PubMed] [Google Scholar]
- Fuller M. T. Spermatogenesis. In: Bate M., Martinez-Arias A., editors. The Development of Drosophila melanogaster. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993. pp. 71–147. [Google Scholar]
- Gepner J., Li M., Ludmann S., Kortas C., Boylan K., Iyadurai S. J., McGrail M., Hays T. S. Cytoplasmic dynein function is essential in Drosophila melanogaster. Genetics. 1996;142:865–878. doi: 10.1093/genetics/142.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergely F., Kidd D., Jeffers K., Wakefield J. G., Raff J. W. D-TACC: a novel centrosomal protein required for normal spindle function in the early Drosophila embryo. EMBO J. 2000;19:241–252. doi: 10.1093/emboj/19.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giansanti M. G., Bonaccorsi S., Williams B., Williams E. V., Santolamazza C., Goldberg M. L., Gatti M. Cooperative interactions between the central spindle and the contractile ring during Drosophila cytokinesis. Genes Dev. 1998;12:396–410. doi: 10.1101/gad.12.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gönczy P. Centrosomes: hooked on the nucleus. Curr. Biol. 2004;14:R268–R270. doi: 10.1016/j.cub.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Gönczy P., Pichler S., Kirkham M., Hyman A. A. Cytoplasmic dynein is required for distinct aspects of MTOC positioning, including centrosome separation, in the one cell stage Caenorhabditis elegans embryo. J. Cell Biol. 1999;147:135–150. doi: 10.1083/jcb.147.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C., Casal J., Ripoll P. Relationship between chromosome content and nuclear diameter in early spermatids of Drosophila melanogaster. Genet. Res. 1989;54:205–212. doi: 10.1017/s0016672300028664. [DOI] [PubMed] [Google Scholar]
- Gunsalus K. C., Bonaccorsi S., Williams E., Verni F., Gatti M., Goldberg M. L. Mutations in twinstar, a Drosophila gene encoding a cofilin/ADF homologue, result in defects in centrosome migration and cytokinesis. J. Cell Biol. 1995;131:1243–1259. doi: 10.1083/jcb.131.5.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebbar S., Mesngon M. T., Guillotte A. M., Desai B., Ayala R., Smith D. S. Lis1 and Ndel1 influence the timing of nuclear envelope breakdown in neural stem cells. J. Cell Biol. 2008;182:1063–1071. doi: 10.1083/jcb.200803071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzbaur E. L., Hammarback J. A., Paschal B. M., Kravit N. G., Pfister K. K., Vallee R. B. Homology of a 150K cytoplasmic dynein-associated polypeptide with the Drosophila gene Glued. Nature. 1991;351:579–583. doi: 10.1038/351579a0. [DOI] [PubMed] [Google Scholar]
- Hook P., Vallee R. B. The dynein family at a glance. J. Cell Sci. 2006;119:4369–4371. doi: 10.1242/jcs.03176. [DOI] [PubMed] [Google Scholar]
- Inoue Y. H., Savoian M. S., Suzuki T., Mathe E., Yamamoto M. T., Glover D. M. Mutations in orbit/mast reveal that the central spindle is comprised of two microtubule populations, those that initiate cleavage and those that propagate furrow ingression. J. Cell Biol. 2004;166:49–60. doi: 10.1083/jcb.200402052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemphues K. J., Kaufman T. C., Raff R. A., Raff E. C. The testis-specific beta-tubulin subunit in Drosophila melanogaster has multiple functions in spermatogenesis. Cell. 1982;31:655–670. doi: 10.1016/0092-8674(82)90321-x. [DOI] [PubMed] [Google Scholar]
- Kemphues K. J., Raff E. C., Raff R. A., Kaufman T. C. Mutation in a testis-specific beta-tubulin in Drosophila: analysis of its effects on meiosis and map location of the gene. Cell. 1980;21:445–451. doi: 10.1016/0092-8674(80)90481-x. [DOI] [PubMed] [Google Scholar]
- King J. M., Hays T. S., Nicklas R. B. Dynein is a transient kinetochore component whose binding is regulated by microtubule attachment, not tension. J. Cell Biol. 2000;151:739–748. doi: 10.1083/jcb.151.4.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L. A., Elfring L. K., Bosco G., Orr-Weaver T. L. A genetic screen for suppressors and enhancers of the Drosophila PAN GU cell cycle kinase identifies cyclin B as a target. Genetics. 2001;158:1545–1556. doi: 10.1093/genetics/158.4.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L. A., Lee E., Anderson M. A., Vardy L., Tahinci E., Ali S. M., Kashevsky H., Benasutti M., Kirschner M. W., Orr-Weaver T. L. Drosophila genome-scale screen for PAN GU kinase substrates identifies Mat89Bb as a cell cycle regulator. Dev. Cell. 2005;8:435–442. doi: 10.1016/j.devcel.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Li M. G., Serr M., Newman E. A., Hays T. S. The Drosophila tctex-1 light chain is dispensable for essential cytoplasmic dynein functions but is required during spermatid differentiation. Mol. Biol. Cell. 2004;15:3005–3014. doi: 10.1091/mbc.E04-01-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebrich W. The effects of cytochalasin B and colchicine on the morphogenesis of mitochondria in Drosophila hydei during meiosis and early spermiogenesis. An in vitro study. Cell Tissue Res. 1982;224:161–168. doi: 10.1007/BF00217275. [DOI] [PubMed] [Google Scholar]
- Malone C. J., Misner L., Le Bot N., Tsai M. C., Campbell J. M., Ahringer J., White J. G. The C. elegans hook protein, ZYG-12, mediates the essential attachment between the centrosome and nucleus. Cell. 2003;115:825–836. doi: 10.1016/s0092-8674(03)00985-1. [DOI] [PubMed] [Google Scholar]
- Martinez-Campos M., Basto R., Baker J., Kernan M., Raff J. W. The Drosophila pericentrin-like protein is essential for cilia/flagella function, but appears to be dispensable for mitosis. J. Cell Biol. 2004;165:673–683. doi: 10.1083/jcb.200402130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrail M., Hays T. S. The microtubule motor cytoplasmic dynein is required for spindle orientation during germline cell divisions and oocyte differentiation in Drosophila. Development. 1997;124:2409–2419. doi: 10.1242/dev.124.12.2409. [DOI] [PubMed] [Google Scholar]
- Morris N. R. Nuclear migration. From fungi to the mammalian brain. J. Cell Biol. 2000;148:1097–1101. doi: 10.1083/jcb.148.6.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne C., Rawe V., Ramalho-Santos J., Simerly C., Schatten G. Preferentially localized dynein and perinuclear dynactin associate with nuclear pore complex proteins to mediate genomic union during mammalian fertilization. J. Cell Sci. 2003;116:4727–4738. doi: 10.1242/jcs.00784. [DOI] [PubMed] [Google Scholar]
- Rebollo E., Gonzalez C. Visualizing the spindle checkpoint in Drosophila spermatocytes. EMBO Rep. 2000;1:65–70. doi: 10.1093/embo-reports/kvd011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebollo E., Llamazares S., Reina J., Gonzalez C. Contribution of noncentrosomal microtubules to spindle assembly in Drosophila spermatocytes. PLoS Biol. 2004;2:E8. doi: 10.1371/journal.pbio.0020008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinsch S., Gönczy P. Mechanisms of nuclear positioning. J. Cell Sci. 1998;111:2283–2295. doi: 10.1242/jcs.111.16.2283. [DOI] [PubMed] [Google Scholar]
- Robinson J. T., Wojcik E. J., Sanders M. A., McGrail M., Hays T. S. Cytoplasmic dynein is required for the nuclear attachment and migration of centrosomes during mitosis in Drosophila. J. Cell Biol. 1999;146:597–608. doi: 10.1083/jcb.146.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues-Martins A., Riparbelli M., Callaini G., Glover D. M., Bettencourt-Dias M. From centriole biogenesis to cellular function: centrioles are essential for cell division at critical developmental stages. Cell Cycle. 2008;7:11–16. doi: 10.4161/cc.7.1.5226. [DOI] [PubMed] [Google Scholar]
- Rodriguez S., et al. Expression profile of genes from 12p in testicular germ cell tumors of adolescents and adults associated with i(12p) and amplification at 12p11.2-p12.1. Oncogene. 2003;22:1880–1891. doi: 10.1038/sj.onc.1206302. [DOI] [PubMed] [Google Scholar]
- Rorth P. Gal4 in the Drosophila female germline. Mech. Dev. 1998;78:113–118. doi: 10.1016/s0925-4773(98)00157-9. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982;218:348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Salina D., Bodoor K., Eckley D. M., Schroer T. A., Rattner J. B., Burke B. Cytoplasmic dynein as a facilitator of nuclear envelope breakdown. Cell. 2002;108:97–107. doi: 10.1016/s0092-8674(01)00628-6. [DOI] [PubMed] [Google Scholar]
- Schroer T. A. Dynactin. Annu. Rev. Cell Dev. Biol. 2004;20:759–779. doi: 10.1146/annurev.cellbio.20.012103.094623. [DOI] [PubMed] [Google Scholar]
- Stebbings L., Grimes B. R., Bownes M. A testis-specifically expressed gene is embedded within a cluster of maternally expressed genes at 89B in Drosophila melanogaster. Dev. Genes Evol. 1998;208:523–530. doi: 10.1007/s004270050211. [DOI] [PubMed] [Google Scholar]
- Tai A. W., Chuang J. Z., Sung C. H. Cytoplasmic dynein regulation by subunit heterogeneity and its role in apical transport. J. Cell Biol. 2001;153:1499–1509. doi: 10.1083/jcb.153.7.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Texada M. J., Simonette R. A., Johnson C. B., Deery W. J., Beckingham K. M. Yuri gagarin is required for actin, tubulin and basal body functions in Drosophila spermatogenesis. J. Cell Sci. 2008;121:1926–1936. doi: 10.1242/jcs.026559. [DOI] [PubMed] [Google Scholar]
- Thibault S. T., et al. A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nat. Genet. 2004;36:283–287. doi: 10.1038/ng1314. [DOI] [PubMed] [Google Scholar]
- Vogt N., Koch I., Schwarz H., Schnorrer F., Nusslein-Volhard C. The gammaTuRC components Grip75 and Grip128 have an essential microtubule-anchoring function in the Drosophila germline. Development. 2006;133:3963–3972. doi: 10.1242/dev.02570. [DOI] [PubMed] [Google Scholar]
- Wakefield J. G., Bonaccorsi S., Gatti M. The Drosophila protein Asp is involved in microtubule organization during spindle formation and cytokinesis. J. Cell Biol. 2001;153:637–648. doi: 10.1083/jcb.153.4.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakimoto B. T., Lindsley D. L., Herrera C. Toward a comprehensive genetic analysis of male fertility in Drosophila melanogaster. Genetics. 2004;167:207–216. doi: 10.1534/genetics.167.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H. C., Rollins J., Fabian L., Hayes M., Polevoy G., Bazinet C., Brill J. A. Depletion of plasma membrane PtdIns(4,5)P2 reveals essential roles for phosphoinositides in flagellar biogenesis. J. Cell Sci. 2008;121:1076–1084. doi: 10.1242/jcs.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik E., Basto R., Serr M., Scaerou F., Karess R., Hays T. Kinetochore dynein: its dynamics and role in the transport of the Rough deal checkpoint protein. Nat. Cell Biol. 2001;3:1001–1007. doi: 10.1038/ncb1101-1001. [DOI] [PubMed] [Google Scholar]
- Wong R., Hadjiyanni I., Wei H. C., Polevoy G., McBride R., Sem K. P., Brill J. A. PIP2 hydrolysis and calcium release are required for cytokinesis in Drosophila spermatocytes. Curr. Biol. 2005;15:1401–1406. doi: 10.1016/j.cub.2005.06.060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.