Abstract
Membrane trafficking is well known to regulate receptor-mediated signaling processes, but less is known about whether signaling receptors conversely regulate the membrane trafficking machinery. We investigated this question by focusing on the beta-2 adrenergic receptor (B2AR), a G protein-coupled receptor whose cellular signaling activity is controlled by ligand-induced endocytosis followed by recycling. We used total internal reflection fluorescence microscopy (TIR-FM) and tagging with a pH-sensitive GFP variant to image discrete membrane trafficking events mediating B2AR endo- and exocytosis. Within several minutes after initiating rapid endocytosis of B2ARs by the adrenergic agonist isoproterenol, we observed bright “puffs” of locally increased surface fluorescence intensity representing discrete Rab4-dependent recycling events. These events reached a constant frequency in the continuous presence of isoproterenol, and agonist removal produced a rapid (observed within 1 min) and pronounced (≈twofold) increase in recycling event frequency. This regulation required receptor signaling via the cAMP-dependent protein kinase (PKA) and a specific PKA consensus site located in the carboxyl-terminal cytoplasmic tail of the B2AR itself. B2AR-mediated regulation was not restricted to this membrane cargo, however, as transferrin receptors packaged in the same population of recycling vesicles were similarly affected. In contrast, net recycling measured over a longer time interval (10 to 30 min) was not detectably regulated by B2AR signaling. These results identify rapid regulation of a specific recycling pathway by a signaling receptor cargo.
INTRODUCTION
Membrane trafficking pathways play a fundamental role in shaping cellular responses to receptor-mediated signals. This relationship is clear when one examines endocytic trafficking of transmembrane signaling receptors, such as G protein-coupled receptors (GPCRs), for which the cellular response depends on the number of functional receptors available at the cell surface (Lefkowitz et al., 1998; Moore et al., 2007; von Zastrow and Sorkin, 2007). Removal of surface receptors by endocytosis is one mechanism by which cells can terminate or rapidly attenuate cellular responses to an activating ligand. Subsequent trafficking of internalized receptors to lysosomes promotes receptor proteolysis, typically resulting in a prolonged attenuation of cellular signaling responsiveness (Marchese et al., 2008). Recycling of internalized receptors to the plasma membrane, in contrast, restores the complement of surface receptors and can contribute to rapid recovery of functional signaling (Carman and Benovic, 1998; Ferguson, 2001; Hanyaloglu and von Zastrow, 2008). Despite the established importance of endocytic trafficking in controlling receptor-mediated signaling, relatively little is known about whether or how signaling receptors regulate the endocytic pathway.
The beta-2 adrenergic receptor (B2AR) is a seven-transmembrane G protein-coupled receptor, thus representing the largest known family of signaling receptors. The canonical pathway mediating B2AR signaling after activation by physiological agonists such as epinephrine, as well as the pharmacological agonist isoproterenol, involves G protein-mediated stimulation of adenylyl cyclase and subsequent activation of the cyclic AMP-dependent protein kinase (PKA). Activation of this pathway is thought to occur largely, if not exclusively, at the plasma membrane. Agonist-activated B2ARs undergo extensive phosphorylation and bind arrestins at the plasma membrane, reducing their functional coupling to heterotrimeric G proteins (Hausdorff et al., 1989; Pippig et al., 1993), and are physically removed from the plasma membrane by rapid endocytosis mediated by clathrin-coated pits (von Zastrow and Kobilka, 1992). Subsequent trafficking of receptors via a rapid recycling pathway promotes functional recovery (or resensitization) of cellular signaling responsiveness (Pippig et al., 1995; Lefkowitz et al., 1998; Seachrist et al., 2000; Hanyaloglu et al., 2005).
Endocytosis of the B2AR is exquisitely regulated, both at the level of receptor concentration into clathrin-coated pits (von Zastrow and Kobilka, 1994; Ferguson et al., 1996; Goodman et al., 1996; N′Diaye E et al., 2008) and at the level of endocytic membrane scission (Puthenveedu and von Zastrow, 2006). Subsequent recycling of the B2AR occurs via multiple pathways (Seachrist et al., 2000; Moore et al., 2004; Millman et al., 2008), and involves receptor interaction with specific endocytic sorting machinery (Cao et al., 1999; Hanyaloglu et al., 2005; Millman et al., 2008). Nevertheless, previous studies suggest that recycling of B2ARs occurs with similar kinetics irrespective of ligand binding or agonist-induced activation of signaling (Kurz and Perkins, 1992; von Zastrow and Kobilka, 1992; Moore et al., 1995; Tsao et al., 2001), akin to constitutive recycling of nutrient receptors established previously (see Maxfield and McGraw, 2004 for review).
We recently observed discrete exocytic fusion events mediating recycling of internalized B2ARs in CNS neurons. Surprisingly, a fraction of these events exhibited homeostatic regulation in response to receptor-mediated signaling via PKA (Yudowski et al., 2006). These findings raise fundamental questions regarding the generality, mechanism, and significance of this regulation. First, is regulated recycling of B2ARs restricted to neurons, or can such regulation occur also in less specialized cells? Second, is B2AR-dependent regulation restricted to a specialized pathway of recycling and, if so, what are its distinguishing features? Third, what are the critical PKA substrate(s) that mediate signal-dependent regulation of recycling? The present study addresses these fundamental questions. Our results establish that B2AR-regulated recycling is not restricted to neurons, and they identify a novel mechanism by which GPCR signaling exerts cargo-mediated control over a specific pathway of rapid recycling.
MATERIALS AND METHODS
Expression Constructs, Cell Culture, and Transfection
SpH-B2AR, generated by fusing superecliptic pHluorin (Miesenbock et al., 1998; Sankaranarayanan et al., 2000) preceded by a signal sequence to the amino-terminal ectodomain of the human B2AR, was described previously (Yudowski et al., 2006). FLAG-B2AR, the human B2AR fused to an amino-terminal FLAG epitope tag was also described previously (Cao et al., 1998). The indicated mutations of the PDZ ligand and consensus kinase sites were generated by oligonucleotide site-directed mutagenesis (QuickChange, Stratagene, La Jolla, CA). The SpH-tagged version of the transferrin receptor (SpH-TfR) was generated from a C-terminally EGFP-tagged transferrin receptor construct generously provided by Dr. Gary Banker (Oregon Health & Science University, Portland, OR), and superecliptic pHluorin was amplified by PCR and used to replace the EGFP. Mutant Rab expression constructs were generously provided by Drs. Stephen Ferguson (Robard Institute, London, ON, Canada) and Marino Zerial (Max Planck Institute, Dresden, Germany). DsRed-tagged clathrin light chain was a generous gift of Dr. Wolfhard Almers (Oregon Health & Science University, Portland, OR). Human embryonal kidney (HEK) 293 cells were obtained from ATCC and maintained in Dulbecco's minimal media (UCSF Cell Culture Facility) supplemented 10% fetal bovine serum (Invitrogen, Carlsbad, CA). Transfection was carried out using Effectene (Qiagen, Valencia, CA). Transiently transfected cells were studies 48 to 72 h after transfection. Stably transfected cells were generated by selection in 500 μg/ml G418 (Invitrogen), and a previously described radioligand binding assay (von Zastrow and Kobilka, 1992) was used to select clones expressing recombinant receptors between 0.5 and 2 pmol/mg cell protein. Neuronal cultures were prepared from hippocampi dissected from rats at embryonic day 18 to 19 as described (Yudowski et al., 2006). Neurons were transfected after 5 to 7 d in vitro (DIV) using Lipofectamine 2000 (Invitrogen) and imaged at DIV 17–21. KT-5720 and H-89 were purchased from Sigma (St. Louis, MO).
Total Internal Reflection Fluorescence Microscopy (TIR-FM) and Live Image Analysis
Fluorescence imaging was carried out using a Nikon TE-2000E inverted microscope with a 60 × 1.45 NA TIRF objective, equipped for through-the-objective TIRF illumination using a 488-nm argon laser focused on the periphery of the back focal plane. HEK293 cells were imaged in OptiMEM (UCSF Cell Culture Facility) without added serum. Neurons were imaged in a standard extracellular solution containing (in mM): NaCl (145), KCl (2.4), MgCl2 (2), CaCl2 (4), HEPES (25), glucose (10) adjusted to pH 7.4 with NaOH. Time-lapse sequences were acquired at a continuous rate of either 0.3 or 10 frames per second, as indicated. Live images show raw data with simple background subtraction of the averaged blank field intensity. Dual imaging of SpH-B2AR and TfR was carried out by labeling internalized TfRs with Alexa555-conjugated diferric transferrin (Invitrogen/Molecular Probes, Eugene, OR) and using a beam splitter (Cairn Research, Kent, United Kingdom) mounted in the emission light path. To facilitate unbiased detection and scoring of surface insertion events, image stacks of 600 sequential 100 msec frames were subjected to maximum intensity pixel analysis, using Metamorph software (Molecular Devices, Sunnyvale, CA), revealing individual insertion events as abrupt spikes of increased maximum pixel intensity over background fluctuations as described previously (Yudowski et al., 2006). Event frequencies were compiled across multiple cells and experiments to generate mean determinations shown. In experiments assessing the effects of sequential manipulations on the same cells, event frequencies measured in sequential 1 min imaging episodes were normalized to the starting value, and means were calculated across cells and experiments using the normalized values to reduce spurious effects of cell-to-cell variability. In experiments comparing manipulations or mutations across cells, data were compiled either as total event number or mean events per cell, as indicated. Error bars represent the SE of the mean across individual cells unless indicated otherwise. Statistical significance of differences between groups and treatment conditions was assessed by Student t test. Statistical analyses and curve fitting were carried out using Prism software (GraphPad Software, San Diego, CA).
Flow Cytometric Assay of Net B2AR Recycling
A previously described assay for measuring net recycling of B2ARs was applied, which allows recycling to be measured in the presence of continuing endocytosis (Tsao and von Zastrow, 2000). Briefly, surface FLAG-tagged B2ARs stably expressed in HEK293 cells were labeled with M1 anti-FLAG antibody (1 mg/ml, Sigma) conjugated to Alexa647 isothiocyanate (Invitrogen/Molecular Probes) according to the manufacturer's instructions. Cells were incubated with a saturating concentration (10 μM) of the adrenergic agonist isoproterenol (Sigma) for 30 min at 37°C to drive receptor internalization to steady state (Cao et al., 1998) and cells were rinsed three times with calcium and magnesium -free PBS supplemented with 0.4% EDTA, to dissociate antibody from receptors remaining at the plasma membrane and thereby specifically label internalized receptors. Cells were then incubated at 37°C in EDTA-supplemented PBS, in the presence or absence of 10 μM isoproterenol. At the indicated time points, monolayers were chilled to 4°C, lifted, washed, and analyzed by flow cytometry. M1 antibody dissociates rapidly from receptors upon exposure at the cell surface to calcium-depleted medium, allowing net recycling to be detected by antibody efflux (Tsao and von Zastrow, 2000).
Flow Cytometric Assay of Transferrin Receptor Recycling
A variation of a previously described “pulse-chase” method was used to measure recycling of transferrin receptors by efflux of labeled transferrin bound to the internalized receptor pool (Dunn et al., 1989). The same cell clones of stably transfected HEK293 cells used for FLAG-B2AR recycling assays were preincubated at 37°C in serum-free DMEM for one hour. Transferrin receptors were then surface-labeled with Alexa488-conjugated diferric transferrin (Invitrogen, 1 μg/ml) at 37°C in serum-free DMEM for one hour. Next, cells were washed with PBS and incubated with or without 10 μM isoproterenol in serum-containing DMEM for the indicated time periods. Cells were chilled on ice, lifted, washed, and analyzed by flow cytometry to determine the amount of remaining cell-associated transferrin. The same loading protocol was used to evaluate Tf recycling by wide field fluorescence microscopy, except that cells were labeled with Alexa594-conjugated transferrin and fluorescence intensity was determined by integrated intensity analysis of images captured in the linear detection range after simple background subtraction.
RESULTS
Real-Time Visualization of Discrete Endocytosis and Recycling Events
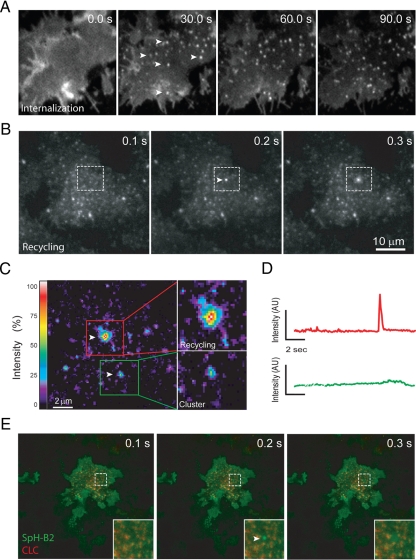
We used TIR-FM (Steyer and Almers, 2001) to image a human B2AR tagged in its ectodomain with the pH-sensitive GFP variant superecliptic pHluorin (SpH-B2AR) (Miesenbock et al., 1998; Yudowski et al., 2006). Stably transfected HEK293 cells were selected for a moderate level of recombinant receptor expression (≈1 pmol/mg), chosen because expression in this range has been shown previously to preserve functional signaling and endocytic trafficking characteristic of endogenous receptors (Yudowski et al., 2006). When imaged at 0.3 frames per second, SpH-B2AR were localized diffusely in the plasma membrane in the absence of ligand and clustered rapidly (within 10 to 30 s) into diffraction-limited clusters after activating receptors with the agonist ligand isoproterenol (Figure 1A, compare first and second panels from left, arrowheads indicate examples of B2AR clusters). Such clusters have been shown previously to represent clathrin-coated pits containing agonist-activated receptors and associated arrestins (Puthenveedu and von Zastrow, 2006).
Figure 1.
Detection of discrete vesicular fusion events mediating surface insertion of SpH-B2AR in HEK293 cells. (A) Representative TIR-FM images of SpH-B2AR fluorescence in the plasma membrane of HEK293 cells incubated the indicated time periods (in sec) after addition of 10 μM isoproterenol, showing rapid agonist-induced clustering of SpH-B2AR. (B) Rapid TIR-FM image series of HEK293 cells expressing SpH-B2AR after more prolonged (10 min) exposure of cells to 10 μM isoproterenol (10 min) to drive receptor internalization to steady state. Boxed area shows a representative SpH-B2AR exocytic insertion event observed under these conditions. (C) Detail view of a representative recycling event and endocytic cluster occurring in the same region of plasma membrane and captured in a single 100-ms frame. Relative fluorescence intensity is scaled in pseudocolor as shown. (D) Fluorescence intensity tracings of the indicated events over time (determined by maximum intensity analysis), using the same time and intensity scales. (E) Dual TIR-FM imaging of SpH-B2AR insertion events relative to clathrin-DsRed, showing that insertion events occur at distinct locations relative to clathrin-coated pits mediating receptor endocytosis.
Within 3 to 5 min after initial application of agonist, and when TIR-FM was carried out at higher temporal resolution (10 frames per second), we observed qualitatively distinct puffs of increased surface receptor fluorescence that appeared within a single 100-msec frame and subsequently dispersed (Figure 1B, boxed region shows an example one such event, and Supplemental Movie 1). These events reached a constant frequency within 10 min in the continued presence of isoproterenol. The absolute frequency of events imaged in individual cells varied somewhat, likely because of differences in cell size and fractional surface area illuminated by the evanescent field, but was typically in the range of 20 to 30 events/cell/min (left bar in Figure 2B). Similar events have been observed previously in hippocampal neurons, and were shown to represent exocytic fusion events mediating recycling of receptors from the endocytic pathway (Yudowski et al., 2006). SpH-B2AR puffs were easily distinguishable from endocytic clusters occurring nearby in the plasma membrane by their much more abrupt appearance and typically higher surface brightness (Figure 1, C and D). SpH-B2AR endocytic clusters colocalized with DsRed-labeled clathrin, as expected, because this construct labels clathrin-coated pits (Merrifield et al., 2002). In contrast, SpH-B2AR exocytic puffs did not colocalize with clathrin (Figure 1E, representative examples are indicated by arrow and arrowhead, respectively).
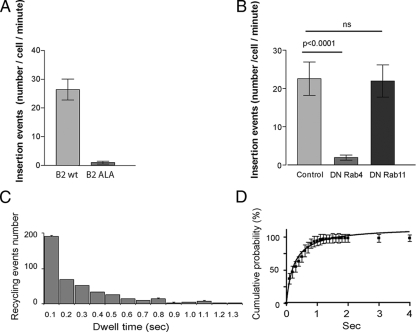
Figure 2.
SpH-B2AR insertion events visible by TIR-FM represent a single kinetic population mediating Rab4-dependent recycling. (A) Mean number of surface insertion events per cell per minute visualized for SpH-B2AR (left) compared with SpH-B2AR ALA (right), as determined from 60-s imaging series of 12 cells expressing each construct at similar level. (B) Mean number of insertion events per cell per minute scored by maximum intensity analysis of SpH-B2AR fluorescence images (15 cells for each condition), comparing the effects of mock (Control), Rab4 S22N, or Rab11 S29N cotransfection. For all conditions, event frequency was normalized to that determined from parallel measurement of the same clone of cells expressing SpH-B2AR and not subsequently transfected with Rab construct. (C) Histogram representation of the duration of locally increased SpH-B2AR fluorescence after exocytic insertion, determined by maximum intensity analysis of sequential 100 msec frames after the initial appearance of the SpH-B2AR puff, and defining dwell time as the time period required for measured fluorescence intensity to return to the baseline value measured in the surrounding plasma membrane (n = 14 cells, 448 insertion events). The majority of events appeared to disperse fully within 1 s after insertion. (D) Dwell times plotted as a cumulative probability curve. The line shows a least-squares best fit of the data to a unimodal population model. The population t1/2 estimated from this analysis is ≈0.4 s.
SpH-B2AR Puffs Represent Discrete Exocytic Fusion Events Mediating Surface Insertion of Receptors from a Rab4-Dependent Recycling Pathway
To determine whether SpH-B2AR puffs originate from the recycling pathway, we constructed a mutant version of the SpH-B2AR construct in which the PDZ domain–interacting sequence required for efficient recycling (Cao et al., 1999) was disrupted by alanine addition (SpH-B2AR ALA). Importantly, this mutation does not prevent biosynthetic delivery of receptors or rapid endocytosis in response to ligand-induced activation (Puthenveedu and von Zastrow, 2006). Fluorescent puffs visualized by rapid TIR-FM were almost completely blocked by this mutation (Figure 2A), suggesting that these events indeed represent PDZ-dependent recycling of B2ARs.
We next examined the effect of dominant negative mutant Rab4 (S22N) and Rab11 (S29N) coexpression on SpH-B2AR puffs. Previous studies indicate that these constructs selectively inhibit distinct pathways contributing to net recycling of B2ARs in HEK293 cells (Seachrist et al., 2000; Moore et al., 2004; Millman et al., 2008). Visible SpH-B2AR puffs were almost completely prevented by coexpression of Rab4 S22N. In contrast, coexpression of Rab11 S29N had no significant effect (Figure 2B), even though independent analysis of transferrin recycling verified the inhibitory activity of this mutant construct under our experimental conditions (Supplemental Figure 1). Together, these results indicate that SpH-B2AR puffs resolved by TIR-FM represent a subset of exocytic events mediating B2AR recycling and originate specifically from a Rab4-dependent recycling pathway.
Recycled SpH-B2ARs Disperse Rapidly after Exocytic Insertion
SpH-B2AR recycling events imaged previously in neurons were found to represent a heterogenous population, with individual events differing greatly in the time required for receptors to disperse laterally from the site of insertion. So-called ‘transient’ events were characterized by rapid dispersion of receptors (occurring within several hundred msec after surface insertion), whereas “persistent” puffs were characterized by apparent retention near the site of exocytic insertion for a prolonged time period (typically >10 s) (Yudowski et al., 2006). In HEK293 cells, the events observed resembled exclusively the transient class of events described in neurons. This was verified by measuring the dwell time during which SpH-B2ARs remained concentrated at the site of exocytic insertion, and doing so in multiple image series (448 events scored in 14 cells, from a total of 10 experiments). SpH-B2ARs dispersed from essentially all exocytic events measured within <1 s (Figure 2C). Cumulative frequency analysis verified the unimodal nature of dwell times in HEK293 cells (Figure 2D), in marked contrast to the bimodal distribution of recycling events evident from similar analysis of hippocampal neurons. The population half-time of lateral dispersion estimated in HEK293 cells was ≈400 msec, further indicating similarity to the transient class of recycling events observed in neurons (Yudowski et al., 2006).
Recycling Events Are Regulated by B2AR-Mediated Signaling via Protein Kinase A
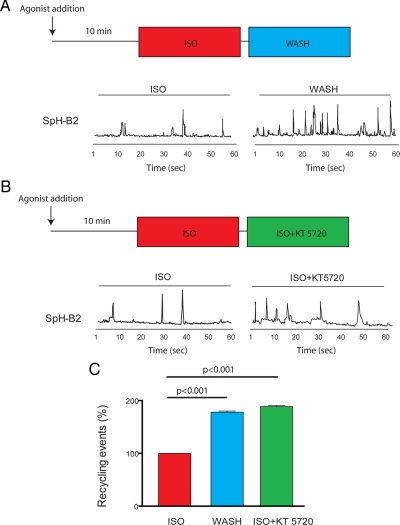
An interesting feature of transient SpH-B2AR recycling events characterized previously in neurons is their homeostatic regulation by downstream signaling (Yudowski et al., 2006). To test whether this was also true in HEK293 cells, we drove SpH-B2AR insertion events to steady state by incubation with isoproterenol and examined the effect of rapid agonist removal on recycling event frequency. To facilitate unbiased analysis, a previously described maximum intensity method (see Materials and Methods for further detail, and the tracing below Supplemental Movie 1 for example) was used to score SpH-B2AR puffs during sequential 1-min imaging periods (diagram in Figure 3A).
Figure 3.
The frequency of SpH-B2AR recycling events is regulated by B2AR activation and PKA. (A) Experimental protocol for analysis of SpH-B2AR recycling event frequency in cells imaged by TIR-FM after driving agonist-induced endocytosis by addition of 10 μM isoproterenol for 10 min, and then incubating cells for sequential 1-min intervals in the continued presence of isoproterenol (ISO) or after acute agonist removal achieved by rapid medium changes (WASH). Representative examples of maximum intensity traces of the plasma membrane area measured over the sequential imaging intervals, showing increased frequency of SpH-B2AR insertion events after agonist washout. (B) Adaptation of the experimental protocol to assess PKA dependence of SpH-B2AR recycling event frequency. Cells were pre-incubated with isoproterenol as in panel A, and sequential 60-s image series were collected in the continued presence of isoproterenol either before (ISO) or after (ISO + KT 5720) addition of the PKA inhibitor KT-5720 (10 μM). (C) Quantification of the frequency of SpH-B2AR insertion events over multiple cells. Mock medium change, maintaining 10 μM isoproterenol in the culture medium, did not detectably change the frequency of SpH-B2AR recycling events (ISO, n = 5 cells, 121 insertion events). SpH-B2AR recycling event frequency was increased ≈twofold either by agonist removal (WASH, n = 6 cells, 254 insertion events) or by addition of the PKA inhibitor KT-5720 (ISO + KT 5720, n = 7 cells, 261 insertion events).
Isoproterenol washout produced a marked increase in the frequency of SpH-B2AR recycling events. This was evident by simple observation of maximum intensity traces (Figure 3A, and Supplemental Movies 2 and 3), whereas a “mock” wash using isoproterenol-containing medium did not change event frequency (not shown). Pharmacological inhibition of PKA, a downstream mediator of B2AR signaling that is required for homeostatic regulation of recycling events in neurons (Yudowski et al., 2006), also increased recycling event frequency in HEK293 cells (Figure 3B shows an example, compare ISO and ISO+KT5720 conditions, respectively). Quantification of these results over multiple cells and experiments verified that PKA inhibition closely mimicked the effect of terminating receptor activation by agonist removal (Figure 3C, see legend for number of cells analyzed for each set of manipulations). Similar results were obtained using H-89, a distinct chemical inhibitor of PKA (Supplemental Figure 2A).
The Essential Substrate for PKA-Dependent Regulation of SpH-B2AR Recycling Events Is the B2AR Itself
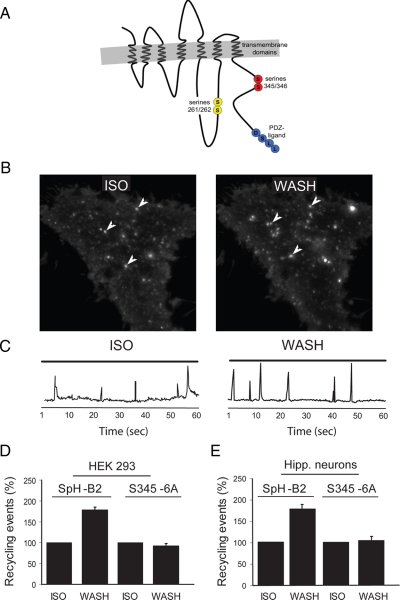
As a next step toward elucidating the mechanistic basis for regulation of SpH-B2AR recycling events, we sought to identify the PKA substrate(s) required. An intriguing candidate was the B2AR itself. This receptor contains two cytoplasmic consensus sites for PKA-mediated phosphorylation, and both are distinct from the PDZ ligand required for sorting of internalized receptors into the rapid recycling pathway (Figure 4A). Phosphorylation of the first PKA site (Ser261/262), located in the third cytoplasmic loop of the receptor (indicated in the schematic by yellow circles), was shown previously to inhibit receptor-G protein coupling without affecting endocytosis (Clark et al., 1989; Hausdorff et al., 1989; Yuan et al., 1994; Lefkowitz et al., 1998). The functional significance of the second PKA site (Ser345/346), present in the carboxyl-terminal cytoplasmic tail of the receptor (indicated by red circles), is less clear (Clark et al., 1989). Importantly, neither PKA site is required for agonist-induced endocytosis of B2ARs (Clark et al., 1985; Bouvier et al., 1989; Hausdorff et al., 1989; Hausdorff et al., 1991; Seibold et al., 2000; Trester-Zedlitz et al., 2005; Iyer et al., 2006).
Figure 4.
The second PKA consensus site in the B2AR is required for signal-dependent regulation of SpH-B2AR recycling events. (A) Schematic representation of B2AR topology indicating the location of the two cytoplasmic PKA sites relative to the PDZ-interacting sequence shown previously to function in B2AR recycling. (B) Maximum intensity projection and (C) kinetic plot of a representative TIR-FM image series (60 s each, 100 msec/frame) derived from a single cell expressing SpH-B2AR with the second PKA site mutated (by alanine substitution of residues corresponding to Ser456 and Ser346 in the wild type receptor), using the experimental protocol diagrammed in Figure 3A. (D) Quantification of insertion event frequency measured in multiple cells expressing SpH-B2AR (left pair of bars) or SpH-B2AR (S345,346A). Insertion events were detected by TIR-FM in multiple cells (n = 5 to 8 cells for each condition) and the frequency of events observed in a 60-s interval after agonist washout (WASH) was normalized to that observed in the presence of 10 μM isoproterenol (ISO). Mutating the second PKA site abrogated agonist-dependent regulation of insertion event frequency. (E) The same analysis conducted in transfected rat hippocampal pyramidal neurons expressing the wild-type SpH-B2AR compared with PKA-mutant SpH-B2AR (ISO, n = 4 cells, WASH, n = 5 cells).
We mutated each candidate PKA site by alanine substitution, and used the sequential incubation protocol to determine the effect of isoproterenol removal on SpH-B2AR recycling event frequency. As expected, PKA site mutations did not prevent agonist-induced endocytic events or the subsequent appearance of discrete recycling events (Figure 4B, examples of discrete events containing Ser345/346 mutant receptors are indicated by arrowheads). Regulation of recycling event frequency by isoproterenol, however, was strongly impaired by mutating the second PKA site (left and right panels in Figure 4B show representative maximum intensity projections, and example maximum intensity traces are shown in Figure 4C). Quantification across multiple cells and experiments (numbers indicated in figure legend) confirmed this observation (Figure 4D). In contrast, mutating the first PKA site (Ser261/262) did not prevent isoproterenol-dependent regulation of recycling event frequency (Supplemental 2B). These findings suggest that B2AR signaling mediates rapid regulation of discrete recycling events, and does so via phosphorylation of the receptor itself, specifically at the second consensus PKA site.
We next asked whether the same phosphorylation site is required for PKA-dependent regulation of recycling events in hippocampal neurons. Although only the transient class of SpH-B2AR recycling events occurring in neurons is regulated by PKA, these events represent the majority (≈90%) visualized by TIR-FM (Yudowski et al., 2006). Hence, it was possible to estimate changes in the frequency of transient SpH-B2AR recycling events in these more complex cells using the simple maximum intensity analysis. These experiments independently verified regulation of wild-type SpH-B2AR recycling events in neurons, and indicated that the second PKA site is also critical for signal-mediated regulation in these cells (Figure 4E).
PKA-Dependent Regulation Affects Copackaged Transferrin Receptors
Control of SpH-B2AR recycling event frequency by the receptor itself is potentially consistent with two distinct mechanistic hypotheses. One possibility is that signaling via PKA controls B2AR sorting into a rapid recycling pathway visible by TIR-FM, without affecting flux of other cargo through this pathway. Alternatively, it is conceivable that B2AR signaling regulates a rapid recycling pathway more generally, thus affecting other copackaged endocytic cargo. To distinguish these hypotheses, we sought another membrane protein that is copackaged with the B2AR in vesicles mediating the observed recycling events. We considered TfR because a significant fraction of internalized TfRs undergo Rab4-dependent recycling (Gruenberg, 2001), and internalized B2ARs colocalize extensively at steady state with TfRs (von Zastrow and Kobilka, 1992; Moore et al., 1995). Further, TfR and B2AR copurify in endocytic vesicles prepared by immunoisolation shortly after driving B2AR endocytosis, but these distinct receptors do not appear to interact directly (Cao et al., 1998).
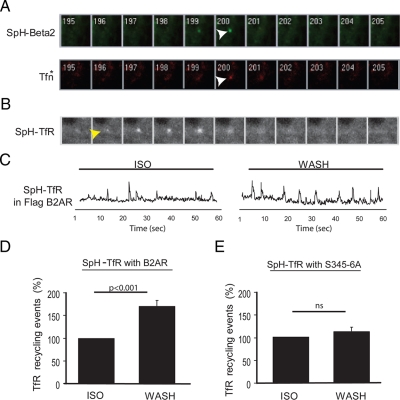
Simultaneous TIR-FM imaging of fluorescent signals representing SpH-B2AR and TfR (labeled with Alexa555-transferrin) using an emission beam splitter revealed that many SpH-B2AR puffs were accompanied by a coincident puff of Alexa555-labeled TfR, confirming that TfRs are coinserted by the rapid recycling events visible by TIR-FM (Figure 5A). We next constructed an SpH-tagged version of TfR (SpH-TfR) to optimize detection of discrete TfR-containing recycling events, and coexpressed this construct in HEK293 cells together with a FLAG epitope-tagged B2AR (to verify efficient coexpression in parallel fixed specimens). As expected from imaging B2AR recycling using the same strategy, SpH-TfR puffs imaged by TIR-FM appeared abruptly (within a single 100-msec frame) and visibly dispersed within <1 s thereafter (Figure 5B and Supplemental Movie 4).
Figure 5.
B2AR-mediated regulation of a distinct endocytic cargo. (A) A region of a two-color TIR-FM image series showing coincident insertion of SpH-B2AR (SpH-B2, top panels in green) and Alexa 555-labeled transferrin (Tfn, bottom panels in red). Sequential 100 msec frames are shown. (B) Sequential 100 msec frames showing a representative insertion event imaged using SpH-TfR. (C) Maximum intensity traces derived from TIR-FM imaging of SpH-TfR insertion in a representative cell coexpressing FLAG-B2AR in sequential 60-s time intervals imaged in the continued presence of 10 μM isoproterenol (ISO) and after agonist removal (WASH). (D and E) Quantification of the effect of agonist washout on insertion event frequency determined by TIR-FM imaging of SpH-TfR in cells coexpressing a FLAG-tagged wild type B2AR (D) or Ser345, 346 Ala mutant FLAG-B2AR (E), showing that this consensus PKA site controls recycling of a distinct endocytic cargo (ISO, n = 4, WASH, n = 6 for D; ISO, n = 4, WASH, n = 6 for E).
SpH-TfR-containing vesicular fusion events were detected with sufficiently high signal-to-noise ratio to allow unbiased scoring of recycling events by maximum intensity analysis (Figure 5C, and trace at bottom of Supplemental Movie 4). Using the same sequential incubation protocol as described above, we were surprised to observe that washout of isoproterenol from the culture medium visibly increased the frequency of discrete recycling events containing SpH-TfR (compare left and right records in the figure). Quantification of this effect across independent experiments verified isoproterenol-dependent regulation of the frequency of discrete SpH-TfR recycling events (Figure 5D). This analysis revealed a degree of isoproterenol-dependent regulation similar to that observed by scoring surface insertion of the SpH-B2AR itself (compare with Figure 2C). Further, this effect was not observed in the absence of cotransfected B2ARs (Supplemental Figure 2C), consistent with the very low level of endogenous B2AR expression in the HEK293 cells studied in our laboratory (Yudowski et al., 2006). Moreover, in cells coexpressing FLAG-B2ARs, isoproterenol-dependent regulation of SpH-TfR puffs was blocked by mutation of the second PKA site (Figure 5E). Together, these observations indicate that B2AR-dependent regulation is not restricted to the B2AR. Instead they suggest that B2AR signaling, via a PKA-dependent autoregulatory loop, essentially confers cargo-dependent regulation over an entire pathway of rapid recycling.
The ability of SpH-TfRs to independently monitor regulation of recycling events allowed us to extend the sequential incubation protocol to examine the effect of both initiating and terminating B2AR-mediated signaling. Activation of B2ARs by acute agonist addition did not detectably affect the frequency of rapid recycling events, whereas subsequent agonist removal produced ≈two-fold increase in event frequency in the same cells (Supplemental Figure 1D). This suggests that the observed increase in rapid recycling events represents a transient response of cells specifically to reduction or termination of B2AR signaling, consistent with the proposed role of Rab4-dependent recycling in promoting recovery of cellular catecholamine responsiveness rapidly after agonist removal (Seachrist et al., 2000).
PKA-Dependent Regulation Specifically Controls a Rapid Recycling Pathway
Such cargo-mediated regulation of recycling was surprising because previous studies, based on conventional biochemical and pharmacological assays, indicate that both TfRs (Dunn et al., 1989; Maxfield and McGraw, 2004) and B2ARs (Kurz and Perkins, 1992; von Zastrow and Kobilka, 1992; Tsao and von Zastrow, 2000) recycle constitutively, irrespective of ligand binding to receptors or B2AR signaling. Conventional assays based on net redistribution of receptors have been noted, however, to underestimate rapid recycling processes (Hao and Maxfield, 2000). In principle, TIR-FM imaging offers substantially higher temporal resolution than conventional biochemical or immunochemical assays of receptor recycling. This suggested the possibility that cargo-mediated regulation might be specific to a very rapid pathway of receptor recycling, and may not affect other pathway(s) that contribute to net recycling measured over a longer time interval.
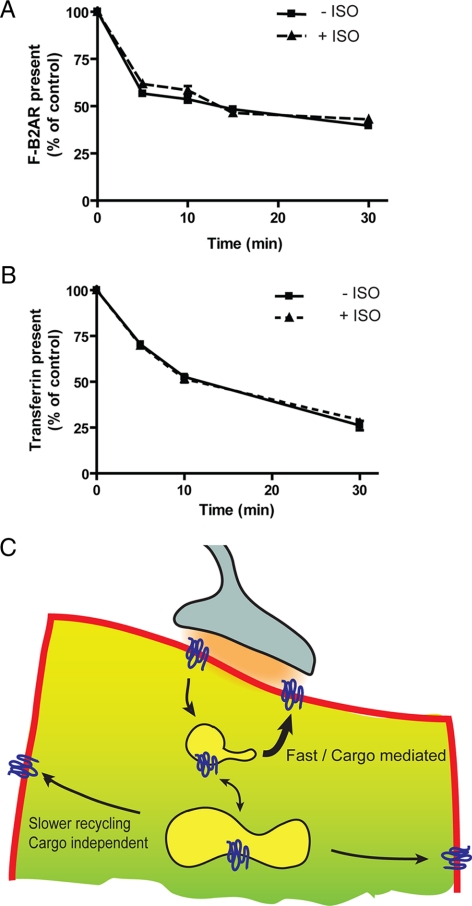
To test this, we applied a previously established flow cytometric method to measure net recycling of FLAG-B2ARs over a 10- to 30-min time course (Tsao and von Zastrow, 2000). In accord with this previous study, antibody efflux curves indicating B2AR recycling over this time period were very similar, irrespective of whether or not isoproterenol was left in the assay medium (Figure 6A). We noticed a slight increase in net recycling of FLAG-B2ARs at the earliest time points examined after isoproterenol removal (compare points representing mean antibody efflux measured in the presence and absence of isoproterenol at 5 and 10 min). This difference was statistically significant (P = 0.029) only at the earliest time point (5 min) examined, however. TfR recycling curves, measured in the same cells and at the same time points by determining efflux of internalized transferrin (Dunn et al., 1989), were essentially superimposable at all time points examined, whether in the presence or absence of isoproterenol (Figure 6B). Thus, in contrast to the pronounced regulation of rapid recycling events resolved in the first minute after isoproterenol removal from the culture medium, net recycling of receptors measured over a longer time interval (>5 min) was not detectably regulated.
Figure 6.
Slower recycling is not detectably regulated by B2AR signaling. (A) Recycling of FLAG-B2ARs measured in stably transfected cells using the antibody efflux assay (as described in Materials and Methods). Internalization of antibody-labeled receptors was carried out by 30-min pre-incubation in the presence of 10 μM isoproterenol. Antibody efflux was assayed at the indicated time points thereafter, in cells incubated in the continuing presence of isoproterenol (+ISO) and in cells subjected to agonist washout (−ISO). Points represent mean determinations calculated from three independent experiments (with triplicate determinations from 10,000 cells in each experiment). Error bars represent the SEM calculated across experiments. (B) Recycling of TfRs estimated by efflux of Alexa488-conjugated transferrin. Measurements were carried out in the same FLAG-B2AR coexpressing cells used in the experiments described in A, and efflux was determined at the indicated time points in the continuous presence (+ISO) or absence (−ISO) of 10 μM isoproterenol. Points represent mean determinations calculated from three independent experiments, and error bars represent the SE of the mean. (C) Proposed model for the role of cargo-mediated regulation of rapid recycling, in context with additional pathway(s) contributing to net recycling measured over longer time periods.
DISCUSSION
Our previous work revealed an unanticipated role of B2AR signaling in regulating the frequency of discrete fusion events mediating receptor recycling to the somatodendritic plasma membrane of neurons. In the present study, we tested whether such regulation occurs also in non-neural cells and further explored its mechanistic basis. We show that B2AR signaling via PKA also regulates discrete recycling events in a heterologous cell model, suggesting a general significance of this mechanism in diverse cell types. Our results indicate that this regulation occurs by a recurrent signaling loop requiring a specific PKA site in the B2AR itself, suggesting a novel form of cargo-specific regulation of a rapid recycling pathway.
PKA-mediated phosphorylation of the B2AR is a well-known biochemical consequence of B2AR signaling via heterotrimeric G proteins, and PKA-mediated phosphorylation of the B2AR contributes to functional desensitization of receptor-mediated signaling (Bouvier et al., 1988; Clark et al., 1988; Hausdorff et al., 1989; Liggett et al., 1989; Tran et al., 2004). Such functional regulation can occur in the absence of endocytosis, however, and we are not aware of previous evidence linking PKA-dependent phosphorylation of the B2AR to regulation of endocytic recycling.
GRK-mediated phosphorylation of multiple Ser/Thr residues in the proximal cytoplasmic tail of the B2AR (Hausdorff et al., 1991; Tran et al., 2004; Trester-Zedlitz et al., 2005) promotes both functional desensitization and receptor endocytosis via clathrin-coated pits, and does so by increasing the affinity of activated receptors for nonvisual (or beta-) arrestins that function as endocytic adaptors (Ferguson et al., 1996; Goodman et al., 1996). A serine residue (Ser411) present in the consensus PDZ domain-binding sequence in the distal tail of the B2AR is another potential substrate for GRKs (but not PKA) (Fredericks et al., 1996), and mutation of this residue prevents PDZ-dependent recycling of receptors (Cao et al., 1999). The distinct PKA site located at Ser 345/346 is not required for PDZ domain-mediated protein interactions measured in vitro, and mutation of this site does not abolish rapid recycling of receptors. Rather, our data suggest that phosphorylation of this site regulates the frequency with which vesicles containing internalized B2AR are reinserted to the plasma membrane. Thus, we anticipate that the presently identified mechanism contributes to dynamic and precise tuning of B2AR number on the cell surface.
B2AR-mediated regulation was not restricted to this receptor, however, as might be expected if PKA simply regulated sorting of the B2AR into a rapid recycling pathway. Rather, B2AR signaling also regulated the frequency of discrete recycling events when scored by imaging copackaged TfRs. Further, mutating the critical PKA site present in the B2AR tail prevented signal-dependent regulation of both this signaling recept or and the TfR. Together these results suggest that B2AR phosphorylation, via G protein-linked signaling activating PKA, exerts cargo-mediated regulation over an entire Rab4-dependent recycling pathway.
The latter observation was a surprise, and raised the question of how such exquisitely regulated recycling could occur for endocytic cargo that are generally thought to recycle in a constitutive manner (Dunn et al., 1989; Kurz and Perkins, 1992; von Zastrow and Kobilka, 1992; Tsao and von Zastrow, 2000; Maxfield and McGraw, 2004). A partial answer to this question came from comparing the results of TIR-FM and conventional flow cytometric analyses when applied to the same cell population. B2AR-mediated regulation of recycling events was clearly evident by TIR-FM within 1 min of terminating receptor activation, the time interval analyzed in TIR-FM imaging series. Recycling measured over a longer time interval by fluorescence flow cytometry, in contrast, was only slightly affected at the earliest time point (5 min) after agonist washout and was indistinguishable thereafter. These observations suggest that the TIR-FM assay selectively measures a very rapid pathway of receptor recycling, which is dynamically regulated in a cargo-mediated manner via PKA. In contrast, slower pathway(s) that dominate cumulative recycling measured over a longer time interval are evidently not regulated by this mechanism.
The TIR-FM imaging method appears, therefore, to be particularly well suited for detecting exocytic fusion events that mediate very rapid recycling. The detection of these events simply by spikes in local surface fluorescence of either B2AR or TfR suggests, further, that the vesicles mediating this rapid recycling contain endocytic cargo at relatively high concentration. In contrast, we speculate that vesicles mediating slower pathway(s) of unregulated recycling contain endocytic cargo at significantly lower concentration, thus making them undetectable by the present imaging method. Further supporting this hypothesis, we note that our TIR-FM data did not detect evidence for biosynthetic insertion of B2ARs, even though this process is thought to occur continuously in transfected cells (Koenig and Edwardson, 1997). It will be interesting in future studies to extend the detection limit of the TIR-FM approach, such as by combining rapid TIR-FM with proteolytic cleavage or photoconversion methods, to reduce the fluorescence signal of existing surface receptors and thereby increase sensitivity for other exocytic pathways.
What might be the physiological significance of the presently described regulatory process? Endocytic recycling of the B2AR is known to mediate rapid regulation of cellular responsiveness to catecholamines in diverse cell types. Rab4-dependent recycling, in particular, has been linked specifically to the process of rapid resensitization in cultured cells (Seachrist et al., 2000) and in sustaining catecholamine responsiveness in the intact heart (Odley et al., 2004). In many physiological settings, including in the nervous system and heart, important B2AR signaling events are thought to occur in limited regions of the plasma membrane near axonal varicosities or boutons, where catecholamines are released in a spatially and temporally controlled manner by regulated exocytosis (Davare et al., 2001; Xiang and Kobilka, 2003). A recycling process capable of mediating rapid regulation of receptor insertion, and delivering receptors at high concentration, might be particularly well suited for mediating dynamic regulation of such localized signaling. Constitutive recycling pathway(s) might, in contrast, play more of a “housekeeping” role in sustaining the total number of surface receptors over a longer time period, as required to support slower and diffusely localized responses to circulating catecholamines (Figure 6C).
It is tempting to speculate that cargo-mediated regulation could have more general significance, particularly in view of previous studies indicating that activated B2ARs present in the plasma membrane locally regulate clathrin-coated pits (Puthenveedu and von Zastrow, 2006). In principle, cargo-mediated regulation of both endocytosis and recycling events offers a simple means for controlling endocytic trafficking of specific signaling receptors with high spatiotemporal precision. Cargo-mediated regulation also offers the potential advantage of being scalable to a large number of spatially segregated surface domains. A neuronal dendrite, for example, may contain hundreds or even thousands of specialized surface domains opposite presynaptic inputs. Regulated endocytic trafficking of diverse signaling receptors is mediated in discrete dendritic domains, however, by an apparently similar mechanism (e.g., Carroll et al., 1999; Haberstock-Debic et al., 2005). Nevertheless, it is thought that trafficking of signaling receptors in dendrites can be controlled in a highly localized manner, and that such localized trafficking is critical for synapse-specific neural plasticity (Malinow and Malenka, 2002; Newpher and Ehlers, 2008). The regulatory mechanism elaborated in the present study indeed functions in neurons, and exocytic events mediating surface delivery of receptors at relatively high local surface concentration have been directly observed in dendrites (Yudowski et al., 2006; Yudowski et al., 2007). Thus we believe that the present study, in addition to revealing a specific additional link between signaling and endocytic membrane traffic, may provide insight to the fundamental problem of how precise spatiotemporal control of signaling receptor insertion is achieved in complex mammalian cells.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank K. Thorn (UCSF/Nikon Imaging Facility) for valuable advice and access to equipment used in some of the experiments. We also thank W. Almers, S. Ferguson, G. Miesenbock (Yale University, New Haven, CT), J. Rothman (Columbia University, New York, NY), and M. Zerial for providing important reagents. These studies were supported by the U.S. National Institutes of Health/National Institute on Drug Abuse to M.v.Z (R01-DA012864), G.A.Y. (K99-DA023444), and M.A.P. (K99-DA024698). A.H. is supported by a predoctoral fellowship from the U.S. National Science Foundation.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E08-08-0892) on April 15, 2009.
REFERENCES
- Bouvier M., Collins S., O'Dowd B. F., Campbell P. T., de Blasi A., Kobilka B. K., MacGregor C., Irons G. P., Caron M. G., Lefkowitz R. J. Two distinct pathways for cAMP-mediated down-regulation of the beta 2-adrenergic receptor. Phosphorylation of the receptor and regulation of its mRNA level. J. Biol. Chem. 1989;264:16786–16792. [PubMed] [Google Scholar]
- Bouvier M., Hausdorff W. P., De Blasi A., O'Dowd B. F., Kobilka B. K., Caron M. G., Lefkowitz R. J. Removal of phosphorylation sites from the beta 2-adrenergic receptor delays onset of agonist-promoted desensitization. Nature. 1988;333:370–373. doi: 10.1038/333370a0. [DOI] [PubMed] [Google Scholar]
- Cao T. C., Mays R. W., von Zastrow M. Regulated endocytosis of G protein-coupled receptors by a biochemically and functionally distinct subpopulation of clathrin-coated pits. J. Biol. Chem. 1998;273:24592–24602. doi: 10.1074/jbc.273.38.24592. [DOI] [PubMed] [Google Scholar]
- Cao T. T., Deacon H. W., Reczek D., Bretscher A., von Zastrow M. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the beta2-adrenergic receptor. Nature. 1999;401:286–290. doi: 10.1038/45816. [DOI] [PubMed] [Google Scholar]
- Carman C. V., Benovic J. L. G-protein-coupled receptors: turn-ons and turn-offs. Curr. Opin. Neurobiol. 1998;8:335–344. doi: 10.1016/s0959-4388(98)80058-5. [DOI] [PubMed] [Google Scholar]
- Carroll R. C., Beattie E. C., Xia H., Lüscher C., Altschuler Y., Nicoll R. A., Malenka R. C., von Zastrow M. Dynamin-dependent endocytosis of ionotropic glutamate receptors. Proc. Natl. Acad. Sci. USA. 1999;96:14112–14117. doi: 10.1073/pnas.96.24.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. B., Friedman J., Dixon R. A., Strader C. D. Identification of a specific site required for rapid heterologous desensitization of the beta-adrenergic receptor by cAMP-dependent protein kinase. Mol. Pharmacol. 1989;36:343–348. [PubMed] [Google Scholar]
- Clark R. B., Friedman J., Prashad N., Ruoho A. E. Epinephrine-induced sequestration of the beta-adrenergic receptor in cultured S49 WT and cyc- lymphoma cells. J. Cyclic Nucleotide Protein Phosphor. Res. 1985;10:97–119. [PubMed] [Google Scholar]
- Clark R. B., Kunkel M. W., Friedman J., Goka T. J., Johnson J. A. Activation of cAMP-dependent protein kinase is required for heterologous desensitization of adenylyl cyclase in S49 wild-type lymphoma cells. Proc. Natl. Acad. Sci. USA. 1988;85:1442–1446. doi: 10.1073/pnas.85.5.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davare M. A., Avdonin V., Hall D. D., Peden E. M., Burette A., Weinberg R. J., Horne M. C., Hoshi T., Hell J. W. A beta2 adrenergic receptor signaling complex assembled with the Ca2+ channel Cav1.2. Science. 2001;293:98–101. doi: 10.1126/science.293.5527.98. [DOI] [PubMed] [Google Scholar]
- Dunn K. W., McGraw T. E., Maxfield F. R. Iterative fractionation of recycling receptors from lysosomally destined ligands in an early sorting endosome. J. Cell Biol. 1989;109:3303–3314. doi: 10.1083/jcb.109.6.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson S. S. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol. Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- Ferguson S. S., Downey W. E., 3rd, Colapietro A. M., Barak L. S., Menard L., Caron M. G. Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science. 1996;271:363–366. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- Fredericks Z. L., Pitcher J. A., Lefkowitz R. J. Identification of the G protein-coupled receptor kinase phosphorylation sites in the human beta2-adrenergic receptor. J. Biol. Chem. 1996;271:13796–13803. doi: 10.1074/jbc.271.23.13796. [DOI] [PubMed] [Google Scholar]
- Goodman O. J., Krupnick J. G., Santini F., Gurevich V. V., Penn R. B., Gagnon A. W., Keen J. H., Benovic J. L. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- Gruenberg J. The endocytic pathway: a mosaic of domains. Nat. Rev. Mol. Cell Biol. 2001;2:721–730. doi: 10.1038/35096054. [DOI] [PubMed] [Google Scholar]
- Haberstock-Debic H., Kim K. A., Yu Y. J., von Zastrow M. Morphine promotes rapid, arrestin-dependent endocytosis of mu-opioid receptors in striatal neurons. J. Neurosci. 2005;25:7847–7857. doi: 10.1523/JNEUROSCI.5045-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanyaloglu A. C., McCullagh E., von Zastrow M. Essential role of Hrs in a recycling mechanism mediating functional resensitization of cell signaling. EMBO J. 2005;24:2265–2283. doi: 10.1038/sj.emboj.7600688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanyaloglu A. C., von Zastrow M. Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu. Rev. Pharmacol. Toxicol. 2008;48:537–568. doi: 10.1146/annurev.pharmtox.48.113006.094830. [DOI] [PubMed] [Google Scholar]
- Hao M., Maxfield F. R. Characterization of rapid membrane internalization and recycling. J. Biol. Chem. 2000;275:15279–15286. doi: 10.1074/jbc.275.20.15279. [DOI] [PubMed] [Google Scholar]
- Hausdorff W. P., Bouvier M., O'Dowd B. F., Irons G. P., Caron M. G., Lefkowitz R. J. Phosphorylation sites on two domains of the beta 2-adrenergic receptor are involved in distinct pathways of receptor desensitization. J. Biol. Chem. 1989;264:12657–12665. [PubMed] [Google Scholar]
- Hausdorff W. P., Campbell P. T., Ostrowski J., Yu S. S., Caron M. G., Lefkowitz R. J. A small region of the beta-adrenergic receptor is selectively involved in its rapid regulation. Proc. Natl. Acad. Sci. USA. 1991;88:2979–2983. doi: 10.1073/pnas.88.8.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer V., Tran T. M., Foster E., Dai W., Clark R. B., Knoll B. J. Differential phosphorylation and dephosphorylation of beta2-adrenoceptor sites Ser262 and Ser355,356. Br. J. Pharmacol. 2006;147:249–259. doi: 10.1038/sj.bjp.0706551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig J. A., Edwardson J. M. Endocytosis and recycling of G protein-coupled receptors. Trends Pharmacol. Sci. 1997;18:276–287. doi: 10.1016/s0165-6147(97)01091-2. [DOI] [PubMed] [Google Scholar]
- Kurz J. B., Perkins J. P. Isoproterenol-initiated beta-adrenergic receptor diacytosis in cultured cells. Mol. Pharmacol. 1992;41:375–381. [PubMed] [Google Scholar]
- Lefkowitz R. J., Pitcher J., Krueger K., Daaka Y. Mechanisms of beta-adrenergic receptor desensitization and resensitization. Adv. Pharmacol. 1998;42:416–420. doi: 10.1016/s1054-3589(08)60777-2. [DOI] [PubMed] [Google Scholar]
- Liggett S. B., Bouvier M., Hausdorff W. P., O'Dowd B., Caron M. G., Lefkowitz R. J. Altered patterns of agonist-stimulated cAMP accumulation in cells expressing mutant beta 2-adrenergic receptors lacking phosphorylation sites. Mol. Pharmacol. 1989;36:641–646. [PubMed] [Google Scholar]
- Malinow R., Malenka R. C. AMPA receptor trafficking and synaptic plasticity. Annu. Rev. Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Marchese A., Paing M. M., Temple B. R., Trejo J. G protein-coupled receptor sorting to endosomes and lysosomes. Annu. Rev. Pharmacol. Toxicol. 2008;48:601–629. doi: 10.1146/annurev.pharmtox.48.113006.094646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield F. R., McGraw T. E. Endocytic recycling. Nat. Rev. Mol. Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- Merrifield C. J., Feldman M. E., Wan L., Almers W. Imaging actin and dynamin recruitment during invagination of single clathrin-coated pits. Nat. Cell Biol. 2002;4:691–698. doi: 10.1038/ncb837. [DOI] [PubMed] [Google Scholar]
- Miesenbock G., De Angelis D. A., Rothman J. E. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- Millman E. E., Zhang H., Godines V., Bean A. J., Knoll B. J., Moore R. H. Rapid recycling of beta-adrenergic receptors is dependent on the actin cytoskeleton and myosin Vb. Traffic. 2008;9:1958–1971. doi: 10.1111/j.1600-0854.2008.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore C. A., Milano S. K., Benovic J. L. Regulation of receptor trafficking by GRKs and arrestins. Annu. Rev. Physiol. 2007;69:451–482. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- Moore R. H., Millman E. E., Alpizar-Foster E., Dai W., Knoll B. J. Rab11 regulates the recycling and lysosome targeting of beta2-adrenergic receptors. J. Cell Sci. 2004;117:3107–3117. doi: 10.1242/jcs.01168. [DOI] [PubMed] [Google Scholar]
- Moore R. H., Sadovnikoff N., Hoffenberg S., Liu S., Woodford P., Angelides K., Trial J. A., Carsrud N. D., Dickey B. F., Knoll B. J. Ligand-stimulated beta 2-adrenergic receptor internalization via the constitutive endocytic pathway into rab5-containing endosomes. J. Cell Sci. 1995:2983–2991. doi: 10.1242/jcs.108.9.2983. [DOI] [PubMed] [Google Scholar]
- N′Diaye E.N., Hanyaloglu A. C., Kajihara K. K., Puthenveedu M. A., Wu P., von Zastrow M., Brown E. J. The ubiquitin-like protein PLIC-2 is a negative regulator of G protein-coupled receptor endocytosis. Mol. Biol. Cell. 2008;19:1252–1260. doi: 10.1091/mbc.E07-08-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newpher T. M., Ehlers M. D. Glutamate receptor dynamics in dendritic microdomains. Neuron. 2008;58:472–497. doi: 10.1016/j.neuron.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odley A., Hahn H. S., Lynch R. A., Marreez Y., Osinska H., Robbins J., Dorn G. W., 2nd Regulation of cardiac contractility by Rab4-modulated beta2-adrenergic receptor recycling. Proc. Natl. Acad. Sci. USA. 2004;101:7082–7087. doi: 10.1073/pnas.0308335101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pippig S., Andexinger S., Daniel K., Puzicha M., Caron M. G., Lefkowitz R. J., Lohse M. J. Overexpression of beta-arrestin and beta-adrenergic receptor kinase augment desensitization of beta 2-adrenergic receptors. J. Biol. Chem. 1993;268:3201–3208. [PubMed] [Google Scholar]
- Pippig S., Andexinger S., Lohse M. J. Sequestration and recycling of beta 2-adrenergic receptors permit receptor resensitization. Mol. Pharmacol. 1995;47:666–676. [PubMed] [Google Scholar]
- Puthenveedu M. A., von Zastrow M. Cargo regulates clathrin-coated pit dynamics. Cell. 2006;127:113–124. doi: 10.1016/j.cell.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan S., De Angelis D., Rothman J. E., Ryan T. A. The use of pHluorins for optical measurements of presynaptic activity. Biophys. J. 2000;79:2199–2208. doi: 10.1016/S0006-3495(00)76468-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seachrist J. L., Anborgh P. H., Ferguson S. S. Beta 2-adrenergic receptor internalization, endosomal sorting, and plasma membrane recycling are regulated by rab GTPases. J. Biol. Chem. 2000;275:27221–27228. doi: 10.1074/jbc.M003657200. [DOI] [PubMed] [Google Scholar]
- Seibold A., Williams B., Huang Z. F., Friedman J., Moore R. H., Knoll B. J., Clark R. B. Localization of the sites mediating desensitization of the beta(2)-adrenergic receptor by the GRK pathway. Mol. Pharmacol. 2000;58:1162–1173. doi: 10.1124/mol.58.5.1162. [DOI] [PubMed] [Google Scholar]
- Steyer J. A., Almers W. A real-time view of life within 100 nm of the plasma membrane. Nat. Rev. Mol. Cell Biol. 2001;2:268–275. doi: 10.1038/35067069. [DOI] [PubMed] [Google Scholar]
- Tran T. M., Friedman J., Qunaibi E., Baameur F., Moore R. H., Clark R. B. Characterization of agonist stimulation of cAMP-dependent protein kinase and G protein-coupled receptor kinase phosphorylation of the beta2-adrenergic receptor using phosphoserine-specific antibodies. Mol. Pharmacol. 2004;65:196–206. doi: 10.1124/mol.65.1.196. [DOI] [PubMed] [Google Scholar]
- Trester-Zedlitz M., Burlingame A., Kobilka B., von Zastrow M. Mass spectrometric analysis of agonist effects on posttranslational modifications of the beta-2 adrenoceptor in mammalian cells. Biochemistry. 2005;44:6133–6143. doi: 10.1021/bi0475469. [DOI] [PubMed] [Google Scholar]
- Tsao P., Cao T., von Zastrow M. Role of endocytosis in mediating downregulation of G-protein-coupled receptors. Trends Pharmacol. Sci. 2001;22:91–96. doi: 10.1016/s0165-6147(00)01620-5. [DOI] [PubMed] [Google Scholar]
- Tsao P. I., von Zastrow M. Type-specific sorting of G protein-coupled receptors after endocytosis. J. Biol. Chem. 2000;275:11130–11140. doi: 10.1074/jbc.275.15.11130. [DOI] [PubMed] [Google Scholar]
- von Zastrow M., Kobilka B. K. Ligand-regulated internalization and recycling of human beta 2-adrenergic receptors between the plasma membrane and endosomes containing transferrin receptors. J. Biol. Chem. 1992;267:3530–3538. [PubMed] [Google Scholar]
- von Zastrow M., Kobilka B. K. Antagonist-dependent and -independent steps in the mechanism of adrenergic receptor internalization. J. Biol. Chem. 1994;269:18448–18452. [PubMed] [Google Scholar]
- von Zastrow M., Sorkin A. Signaling on the endocytic pathway. Curr. Opin. Cell Biol. 2007;19:436–445. doi: 10.1016/j.ceb.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y., Kobilka B. K. Myocyte adrenoceptor signaling pathways. Science. 2003;300:1530–1532. doi: 10.1126/science.1079206. [DOI] [PubMed] [Google Scholar]
- Yuan N., Friedman J., Whaley B. S., Clark R. B. cAMP-dependent protein kinase and protein kinase C consensus site mutations of the beta-adrenergic receptor. Effect on desensitization and stimulation of adenylylcyclase. J. Biol. Chem. 1994;269:23032–23038. [PubMed] [Google Scholar]
- Yudowski G. A., Puthenveedu M. A., Leonoudakis D., Panicker S., Thorn K. S., Beattie E. C., von Zastrow M. Real-time imaging of discrete exocytic events mediating surface delivery of AMPA receptors. J. Neurosci. 2007;27:11112–11121. doi: 10.1523/JNEUROSCI.2465-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudowski G. A., Puthenveedu M. A., von Zastrow M. Distinct modes of regulated receptor insertion to the somatodendritic plasma membrane. Nat. Neurosci. 2006;9:622–627. doi: 10.1038/nn1679. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.