Abstract
Cytoskeleton microtubules undergo a reversible metamorphosis as cells enter and exit mitosis to build a transient mitotic spindle required for chromosome segregation. Centrosomes play a dominant but dispensable role in microtubule (MT) organization throughout the animal cell cycle, supporting the existence of concurrent mechanisms that remain unclear. Here we investigated MT organization at the entry and exit from mitosis, after perturbation of centriole function in Drosophila S2 cells. We found that several MTs originate from acentriolar microtubule-organizing centers (aMTOCs) that contain γ-tubulin and require Centrosomin (Cnn) for normal architecture and function. During spindle assembly, aMTOCs associated with peripheral MTs are recruited to acentriolar spindle poles by an Ncd/dynein-dependent clustering mechanism to form rudimentary aster-like structures. At anaphase onset, down-regulation of CDK1 triggers massive formation of cytoplasmic MTs de novo, many of which nucleated directly from aMTOCs. CDK1 down-regulation at anaphase coordinates the activity of Msps/XMAP215 and the kinesin-13 KLP10A to favor net MT growth and stability from aMTOCs. Finally, we show that microtubule nucleation from aMTOCs also occurs in cells containing centrosomes. Our data reveal a new form of cell cycle–regulated MTOCs that contribute for MT cytoskeleton remodeling during mitotic spindle assembly/disassembly in animal somatic cells, independently of centrioles.
INTRODUCTION
In animal somatic cells the centrosome is commonly viewed as the major microtubule-organizing center (MTOC) responsible for the architecture of the MT cytoskeleton throughout the cell cycle and plays a central role during spindle assembly as cells enter mitosis. The centrosome is organized as a pair of centrioles surrounded by a pericentriolar material (PCM) where most MTs nucleate. One of the key components of the PCM is the highly conserved protein γ-tubulin, which has been found in association with other proteins that compose the γ-tubulin ring complex (γ-TuRC). During mitosis in humans and Drosophila, this complex is enriched at the centrosome by auxiliary proteins like pericentrin/D-PLP or Cnn and was proposed to play a critical role in MT nucleation (Raynaud-Messina and Merdes, 2007).
Despite the role of the centrosome as MTOC is well established, several noncentrosomal MTs and MTOCs have been found in a wide range of organisms and cell types, including those that normally have a functional centrosome (Spiegelman et al., 1979; Bre et al., 1987; Khodjakov et al., 2000; Sawin and Tran, 2006; Rogers et al., 2008). Early investigations about the origin of these MTs in animal cells revealed that some appear de novo in the cytoplasm (Vorobjev et al., 1997; Yvon and Wadsworth, 1997) and show normal dynamic instability at the plus ends, whereas minus ends are fairly stable. These findings provided the fundamental premises for free MT formation in the cytoplasm and implied the existence of yet poorly characterized “factors” that participate in the assembly process.
The most profound transformation of the MT cytoskeleton during the cell cycle occurs when cells enter mitosis. At this stage, the proportion of MT polymer abruptly decreases, concomitantly with an increase in MT dynamics (Belmont et al., 1990; Zhai et al., 1996). In vitro studies have shown that this correlates with the activation of CDK1 (Verde et al., 1990), which phosphorylates a large number of microtubule-associated proteins (MAPs), thereby favoring the “search-and-capture” of chromosomes by centrosomal MTs and contributing to mitotic spindle morphogenesis (Kirschner and Mitchison, 1986). Higher plants and several female meiotic systems however, build up a microtubular spindle without the contribution of canonical centrioles, supporting the existence of one or more acentriolar spindle assembly pathways (Lloyd and Chan, 2006). One of these pathways has been particularly well characterized using Xenopus oocyte extracts in vitro. In this system, spindles form “inside-out” after initial MT nucleation in the vicinity of chromosomes, in a process involving a RanGTP gradient and several molecular motors (Karsenti and Vernos, 2001). Interestingly, acentriolar spindle assembly appears to be a conserved process in Drosophila and mammalian somatic cells (Debec et al., 1982; Khodjakov et al., 2000) and seems sufficient to drive the development of adult flies from late embryonic stages (Basto et al., 2006). Yet, the underlying molecular and structural requirements behind acentriolar spindle formation in animal somatic cells remain largely unknown.
An even more mysterious and equally extraordinary transformation occurs as mitotic spindles disassemble and the interphase MT cytoskeleton is reestablished when cells exit mitosis. On entering anaphase, CDK1 activity drops as a result of cyclin B degradation by the anaphase-promoting complex (APC) and a balance with the activity of protein phosphatases regulates MT dynamics, favoring their stabilization (Tournebize et al., 1997). In fission yeast this process has been shown to involve nonspindle pole body (the yeast centrosome equivalent) MTOCs, which nucleate MTs from the equatorial site at the end of mitosis and from multiple sites near the cell nucleus during interphase (Sawin and Tran, 2006). More recently, the AAA-ATPase Cdc48/p97 and its adapter Ufd1-Npl4, were implicated in mitotic spindle disassembly in Saccharomyces cerevisiae and Xenopus oocyte extracts and proposed to regulate several spindle assembly factors like XMAP215, TPX2, and Plx1 (Cao et al., 2003). Overall, these data suggest that MT cytoskeleton rearrangements during mitotic exit involve both cell cycle–dependent regulation of MT dynamics and acentriolar MT nucleation, two features that remain to be demonstrated in animal somatic cells.
To understand the molecular and structural requirements involved in MT cytoskeleton remodeling at the entry and exit from mitosis here, we investigated the impact of the absence of centrioles in spindle assembly and disassembly and the respective role of γ-tubulin in MT nucleation in Drosophila somatic cells.
MATERIALS AND METHODS
Transmission Electron Microscopy
Transmission electron microscopy in Drosophila S2 and 1182-4D cells was performed as previously described (Debec et al., 1982; Maiato et al., 2002).
Cell Culture and RNA Interference
Drosophila S2 cells and the derivative clone expressing γ-tubulin-green fluorescent protein (GFP) and mCherry-α-tubulin (gift of G. Goshima, Nagoya University, Japan) were cultured as previously described (Maiato et al., 2002). 1182-4D cells were grown at 25°C, with a 1:1 mixture of D22 (United States Biological, Swampscott, MA) and M3 media (Sigma, St. Louis, MO) with 10% fetal bovine serum (FBS). RNA interference (RNAi) was performed as described previously using published targeting sequences (Maiato et al., 2002; Verollet et al., 2006; Goshima et al., 2007; Rodrigues-Martins et al., 2007). For DSas-4 RNAi, cells were harvested after 6 d (for immunofluorescence analysis) or over two to four transfection cycles with 4-d interval for live imaging (as indicated in the text) also as previously described (Rodrigues-Martins et al., 2007). For codepletion experiments, cells were transfected at the time of the last pulse of DSas-4 RNAi (during 4 d), except in the case of EB1 and Msps where cells were transfected at the time of the last two pulses of DSas-4 RNAi (3 + 3 d). Primary antibodies used for Western blot analysis were mouse anti-γ-tubulin (GTU-88 clone, Sigma), anti-α-tubulin (DM1A, Abcam, Cambridge, MA), anti-dynein heavy chain (gift from T. Hays, University of Minneapolis, MN), rabbit anti-Ncd (gift from J. Scholey, UC Davis, CA), anti-Cenp-C (gift from C. Lehner, University of Zurich, Switzerland), anti-Cnn (gift from T. Megraw, UT Southwestern Medical Center, TX), anti-Dgt5 (gift from G. Goshima, Nagoya University, Japan), anti-KLP10A (gift from D. Sharp, Albert Einstein College of Medicine, NY), anti-Msps (gift from H. Ohkura, University of Edinburgh, UNITED KINGDOM), anti-EB1 (gift from R. Vale, UCSF, CA), and anti-D-CLIP-190 (gift from K. Miller, Washington University, MO). Appropriate secondary antibodies were detected using enhanced chemiluminescence (Pierce, Rockford, IL). Mast and Dgp71WD depletion was assessed by phenotypic analysis (Maiato et al., 2002; Verollet et al., 2006).
Immunofluorescence Microscopy
Cells were placed on 0.5 mg/ml concanavalin A (Sigma)-coated coverslips and fixed after 2 h with 6.4% paraformaldehyde in Cytoskeleton buffer (CB; Maiato et al., 2002) at room temperature or with methanol at −20°C for 10 min, permeabilized with 0.5% SDS in PBS for 3 min (when GTU-88 antibody was used), or extracted with 0.5% Triton X-100 in CB for 10 min and incubated overnight at 4°C with appropriate antibodies. The following primary antibodies were used: rat anti-α-tubulin (YOL1/34, Abcam; or mouse clone B512, Sigma), mouse anti-γ-tubulin (GTU-88, Sigma; or rabbit R62; Debec et al., 1995), rabbit D-PLP and DSas-4 (gifts from J. Raff, Cambridge University, UNITED KINGDOM), Dgp71WD and Dgrip84 (gifts from B. Raynaud-Messina, Institut de Sciences et Technologies du Médicament de Toulouse, France), and guinea-pig Cnn (1:2000; gift from T. Megraw, UT Southwestern Medical Center, TX). Secondary antibodies used were as follows: Alexa 488, 568, and 647 (Invitrogen, Molecular Probes, Eugene, OR) and DAPI (1 μg/ml). Three-dimensional data sets of representative cells were collected with a Zeiss Imager.Z1 microscope (Carl Zeiss, Jena, Germany) equipped with an AxiocamMRm CCD, using a 100× 1.4 NA oil immersion objective. The entire system was driven by Axiovision software. Images were subsequently blind-deconvolved with AutoDeblur X2 software (Media Cybernetics, Silver Spring, MD) and processed for publication using Adobe Photoshop CS3 and Illustrator 8.0 (Adobe Systems, San Jose, CA). For the induction of MT aster formation, cells were incubated with 10 μM taxol (Sigma) for 20 min. For quantification of DSas-4 and γ-tubulin localization in centriolar and acentriolar cells in the absence of MTs, 30 μM colchicine (Sigma) was used for 16 h.
Microtubule Depolymerization/Repolymerization Assay
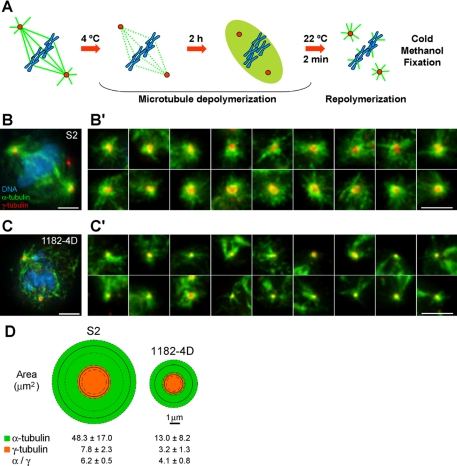
MTs were depolymerized at 4°C for 2 h and allowed to repolymerize at room temperature for 2 min (see Figure 2) or at different time points (Supplementary Figure S4). Cells were then immediately fixed with methanol at −20°C and processed for immunofluorescence as described. In the case of Figure 2, representative image galleries were collected, and the areas of 50 asters (α-tubulin) and respective γ-tubulin foci were measured using the Freehand tool of ImageJ 1.38x software (http://rsb.info.nih.gov/ij/; NIH, Bethesda, MA) and plotted using Windows Visio Software (Microsoft, Redmond, WA). Only α-tubulin staining in the asters was considered.
Figure 2.
Microtubule organization from acentriolar poles containing γ-tubulin. (A) MT depolymerization/repolymerization assay. (B and C) Localization of γ-tubulin (red) in S2 (B) and 1182-4D (C) cells after MT repolymerization and respective image galleries of MT asters obtained from γ-tubulin–positive poles in S2 (B′) and 1182-4D cells (C′). Green, MTs; blue, DNA. Scale bar, (B and C) 5 μm; (B′ and C′) 2 μm. (D) Graphic representation of the areas corresponding to the aster (green) and respective γ-tubulin (orange) recruited in S2 and 1182-4D cells. Solid and dashed lines, respectively, represent the average and SD of the sample (n = 50 asters for each cell line).
Live-Cell Imaging
S2 cells stably expressing γ-tubulin-GFP and mCherry-α-tubulin were plated in 0.25 mg/ml concanavalin A–coated 22 × 22-mm coverslips and mounted in Rose chambers. Four-dimensional data sets were collected with an Andor Revolution Spinning Disk confocal system (Andor Technology, Belfast, United Kingdom)equipped with an Electron Multiplying CCD iXonEM+ camera and a Yokogawa CSU-22 U (Yokogawa Electric, Tokyo, Japan) based on an Olympus IX81 inverted microscope (Tokyo, Japan). Two laser lines (488 and 561 nm) were used for near-simultaneous excitation of GFP and mCherry, and the system was driven by Andor IQ software. Time-lapse image stacks were collected every 20 or 30 s with 0.5-μm z-steps and projected as maximum pixel intensities. For MT stabilization, medium was supplemented with 10 μM taxol (Sigma), respectively.
CDK1 Inhibition Assay
Movies of four cells were analyzed for each RNAi condition. The number of aMTOCs present in each cell was determined before the addition of 50 μM roscovitine (Sigma) and after proteasome inhibition with 20 μM MG132 (Sigma) for 2 h. On addition of roscovitine, MT elongation from aMTOCs and the respective stability was compared with DSas-4 RNAi cells.
RESULTS
Characterization of Acentriolar Spindle Poles in Drosophila 1182-4D Cells
The Drosophila 1182-4D cell line was established from lethal haploid embryos laid by homozygous females for the mh 1182 mutation after spontaneous diploidization under suboptimal culture conditions (Debec et al., 1982). Together with its original haploid line, they remain the only animal somatic cell lines ever obtained that constitutively lack centrioles, a remarkable feature that has been firmly established by two extensive ultrastructural studies, including high-voltage electron microscopy (EM) from thick sections (Debec et al., 1982; Szollosi et al., 1986). The mh 1182 genetic region does not contain any predicted gene encoding for a centriolar protein (Benjamin Loppin, personal communication). Additionally, 1182-4D cells have been kept in culture for several years and express normal levels of SAK/Plk4 (our unpublished observations), a protein required for de novo centriole formation (Rodrigues-Martins et al., 2007), suggesting that this pathway is maintained inactive by a yet unknown mechanism. Although the acentriolar nature of the 1182-4D cell line remains inexplicable, an intriguing property of these cells is their ability to recruit γ-tubulin to some acentriolar spindle poles during mitosis (Debec et al., 1995).
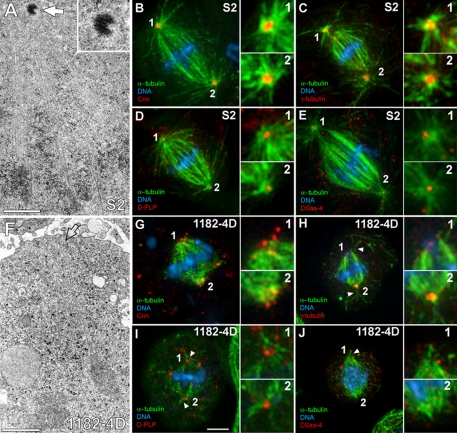
To shed light on the mechanism of spindle MT organization in animal somatic cells lacking centrioles, we investigated the localization of several centrosomal markers at spindle poles of 1182-4D cells. Drosophila S2 cells were used as a control cell line that contains centrioles (Figure 1A). As expected, S2 cells recruited γ-tubulin and Cnn to the PCM and formed robust astral MTs (Figure 1, B and C). Additionally, D-PLP and DSas-4, two bona fide pancentriolar markers (Martinez-Campos et al., 2004; Basto et al., 2006) localized within more restricted areas of the S2 cell mitotic asters that likely represent centrioles (Figure 1, D and E). In 1182-4D cells (Figure 1F) we observed that Cnn and γ-tubulin localization at the poles was disorganized and distinct from S2 cells (Figure 1, G and H). In agreement with previous observations (Debec et al., 1995) we found that approximately one-third of 1182-4D cells showed well-defined γ-tubulin foci in both poles, another third showed focused γ-tubulin in just one pole, whereas the remaining third showed dispersed or undetectable γ-tubulin at both poles (Supplementary Table S1). Noteworthy, a few astral MTs could be detected at the polar regions of 1182-4D cells (Figure 1, H–J). We next evaluated the status of D-PLP and DSas-4 at acentriolar poles of 1182-4D cells. Both proteins were dispersed or eventually formed small aggregates at spindle poles (Figure 1, I and J). Quantification of the polar distribution of DSas-4 in 1182-4D cells indicated that it was undetectable or highly dispersed at the poles in nearly two-thirds of the cells (Supplementary Table S1). Curiously, approximately one-third of 1182-4D cells showed a conspicuous DSas-4 signal in one pole only, whereas a minority of the cells (5%) were able to recruit DSas-4 to both spindle poles, as opposed to ∼90% of S2 cells (Supplementary Table S1). These results may be explained by the fact that a significant rapidly exchanging fraction of DSas-4 and D-PLP is associated with the PCM (Martinez-Campos et al., 2004; Dammermann et al., 2008). Overall, these data indicate that although centrioles enhance the organization of PCM proteins at mitotic spindle poles, they are not absolutely required for this process. Furthermore, they support that acentriolar spindle poles that are able to recruit PCM components retain some MT nucleation capacity.
Figure 1.
Molecular and structural characterization of acentriolar spindle poles in the 1182-4D cell line. (A) EM picture from a serially sectioned S2 cell containing centrioles (arrow). Inset, higher magnification of the centriole. (B–E) Immunofluorescence showing Cnn (B), γ-tubulin (C), D-PLP (D), and DSas-4 (E) localization at spindle poles of S2 cells. (F) EM picture from a serially sectioned 1182-4D cell showing the lack of centrioles (arrow indicates the spindle pole). (G–J) Immunofluorescence showing Cnn (G), γ-tubulin (H), D-PLP (I), and DSas-4 (J) localization at acentriolar spindle poles of 1182-4D cells. DSas-4 was found accumulated at the centrosomal core in S2 cells but was usually dispersed in the cytoplasm of 1182-4D cells (E and J). Note the presence of few astral MTs associated with acentriolar poles (arrowhead in H–J). Numbered insets correspond to enlarged views of the respective spindle poles. Green, MTs; blue, DNA. Scale bar, 1 μm for EM and 5 μm for immunofluorescence pictures.
To quantify the capacity that γ-tubulin–positive spindle poles in 1182-4D cells have to organize MT asters, we performed a MT depolymerization/repolymerization assay and measured the area of the resulting asters (Figure 2A). We have found that the area occupied by the asters in 1182-4D cells was 3–4-fold smaller than in S2 cells (Figure 2, B and C, and B′ and C′), which correlated well with the areas of the respective γ-tubulin foci (Figure 2D). This suggests that centrioles normally determine the amount of γ-tubulin recruited, thereby regulating the size of the asters, as has been shown in other systems (Kirkham et al., 2003). Altogether, these experiments indicate that acentriolar spindle poles in animal somatic cells retain some capacity to recruit γ-tubulin and form rudimentary MT asters.
An Ncd/Dynein-dependent Microtubule Clustering Mechanism Recruits γ-Tubulin to Acentriolar Spindle Poles
1182-4D cells are two- to threefold smaller than S2 cells and assemble a short spindle (typically around 5 μm long), which makes them highly unfavorable for high-resolution microscopy studies. To circumvent this problem and investigate the mechanism of γ-tubulin recruitment to acentriolar spindle poles in living cells, we knocked down DSas-4 by RNAi in S2 cells. DSas-4 is a conserved protein required for centriole duplication, and after several cell cycles with reduced levels of DSas-4, cells are gradually born without centrioles (Basto et al., 2006; Rodrigues-Martins et al., 2007). As so, we performed several rounds of DSas-4 depletion by RNAi in S2 cells. Because DSas-4 is not an abundant protein in Drosophila cells, we were unable to monitor the efficiency of depletion by Western blot (Basto et al., 2006; Rodrigues-Martins et al., 2007). However, the presence or absence of DSas-4 at spindle poles could easily be inferred by immunofluorescence with anti-DSas-4 antibodies. In control S2 cells DSas-4 was always detected as well-defined spots in the center of γ-tubulin–containing asters (Supplementary Figure S1A), whereas after only two rounds of DSas-4 RNAi over 6–7 d, DSas-4 could not be detected at spindle poles in the vast majority of the cells (Supplementary Figure S1, B–E). As in 1182-4D cells, γ-tubulin recruitment to DSas-4–negative poles still occurred to some extent (either in the form of focused or dispersed aggregates) in nearly two-thirds of the cells (Supplementary Figure S1, B–F). Similar results were obtained after SAK or DSas-6 RNAi (our unpublished observations). We concluded that as in the case of 1182-4D cells, S2 cells retain some capacity to recruit γ-tubulin to acentriolar spindle poles.
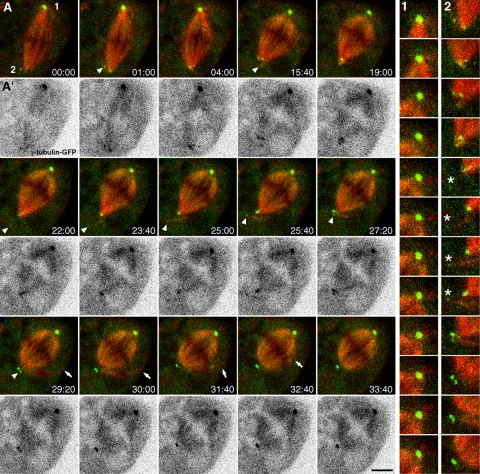
We next used dual-wavelength spinning-disk confocal microscopy to follow mitotic spindle assembly in S2 cells stably expressing mCherry-α-tubulin and γ-tubulin-GFP at near endogenous levels (Figure S2A) after DSas-4 RNAi. We found that, after two rounds of DSas-4 RNAi over 6–7 d, DSas-4 characteristic labeling was occasionally present in only one spindle pole (Supplementary Figure S1G), suggesting that these mitotic spindles were composed of one centriolar and one acentriolar pole. As so, we used this feature to compare the respective behavior of γ-tubulin-GFP at each pole within the same cell (Figure 3 and Supplementary Video S1). Although presumably centriolar poles were found associated with prominent MT asters containing a morphologically unaltered focus of γ-tubulin-GFP over time (Figure 3 A1 and Supplementary Video S1), acentriolar poles showed highly dynamic γ-tubulin-GFP aggregates associated with spindle MT minus ends (Figure 3A2 and Supplementary Video S1). Some γ-tubulin-GFP aggregates in the cytoplasm appear associated with peripheral MTs nucleated de novo, which were subsequently incorporated to the acentriolar pole by an MT-clustering mechanism, thereby contributing to mitotic spindle morphogenesis (Figure 3A2 and Supplementary Video S1). Similar aggregates of endogenous γ-tubulin could be found by immunofluorescence, which colocalize with γ-TuRC components and Cnn, but not with the Golgi apparatus, throughout mitosis in control and DSas-4–depleted cells (Supplementary Figure S3, and our unpublished observations).
Figure 3.
Live-cell imaging of γ-tubulin and spindle microtubules after DSas-4 RNAi in S2 cells. (A) Time-lapse sequence showing mitotic spindle assembly in S2 cells stably expressing mCherry-α-tubulin (red) and γ-tubulin-GFP (green) after DSas-4 RNAi. One centriole-containing pole (A1) and one acentriolar pole (A2) is shown in higher magnification for comparison. Asterisks indicate a small aggregate of γ-tubulin that associates with MTs nucleated de novo, which are subsequently clustered into the spindle pole. Note that γ-tubulin recruited to the acentriolar pole gradually detaches from the MT minus ends. Arrowheads in A highlight small γ-tubulin-GFP aggregates associated with MT minus ends that are clustered into the acentriolar pole. Arrows show the incorporation of peripheral MTs associated with γ-tubulin aggregates into the mitotic spindle. (A′) Same time-lapse sequence depicting γ-tubulin-GFP after contrast inversion. Time is in min:sec; scale bar, 5 μm.
To confirm that the observed cytoplasmic γ-tubulin aggregates are competent to organize MTs, we performed a time-course MT depolymerization/repolymerization assay similar to that described in Figure 2, but over an extended period (Supplementary Figure S4). We observed that endogenous γ-tubulin aggregates in the cytoplasm are resistant to cold-induced MT depolymerization and were present in control or DSas-4–depleted S2 cells (Supplementary Figure S4, A and B). Shortly after rewarming, several cytoplasmic MTs appear to nucleate from γ-tubulin aggregates, regardless of the presence of centrosomes (Supplementary Figure S4, A′ and B′). We named these γ-tubulin aggregates associated with cytoplasmic MTs as aMTOCs. Noteworthy, the relative abundance of MTs associated with aMTOCs decreases over time as spindles assemble after rewarming, suggesting that some of those MTs are either incorporated into the spindle or are unstable (Supplementary Figure S4, A″ and B″).
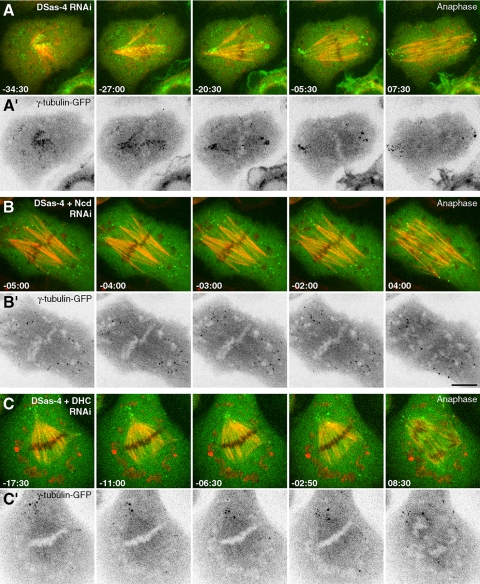
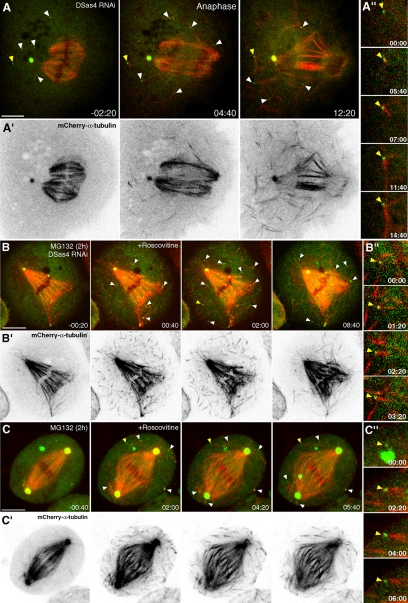
In DSas-4–depleted cells, γ-tubulin-GFP aggregates initially concentrate in a region with high MT density surrounding the chromosomes and some are dispersed in the cytoplasmic periphery (Figure 4, A and A′). As spindles start to form “inside-out,” these aggregates concentrate at acentriolar poles containing focused MT minus ends and remain there in a relatively well-organized manner until anaphase onset when they start to disperse. This behavior is consistent with the involvement of MT minus-end–directed motors with cross-linking activity. Therefore we investigated how codepletion of Ncd or cytoplasmic dynein with DSas-4 impairs the recruitment of γ-tubulin to acentriolar poles (Figure 4, Supplementary Videos S2–S4). As previously shown (Goshima et al., 2005; Morales-Mulia and Scholey, 2005), Ncd is required to maintain MT minus ends clustered, which we found to contribute to focus γ-tubulin-GFP aggregates at acentriolar poles (Figure 4, B and B′). Dynein also enhances this process, but its role appears to be less important than Ncd, as acentriolar poles in 25% of the depleted cells retain MT-clustering capacity with few associated γ-tubulin-GFP aggregates (Figure 4, C and C′).
Figure 4.
Ncd/dynein-dependent microtubule clustering mechanism recruits γ-tubulin to acentriolar spindle poles. (A–C) Time-lapse sequences showing mitotic spindle assembly in S2 cells stably expressing mCherry-α-tubulin (red) and γ-tubulin-GFP (green) after DSas-4 RNAi. (A′–C′) Corresponding time-lapse sequences depicting γ-tubulin-GFP after contrast inversion. (A and A′) Spindle assembly in an S2 cell after DSas-4 RNAi showing γ-tubulin aggregates moving poleward and remaining clustered at acentriolar spindle poles. (B and B′) Spindle assembly in a cell codepleted for DSas-4 and Ncd. (C and C′) Spindle assembly in a cell codepleted for DSas-4 and DHC. Time is in min:sec; scale bar, 5 μm. Time zero was set from the moment of anaphase onset.
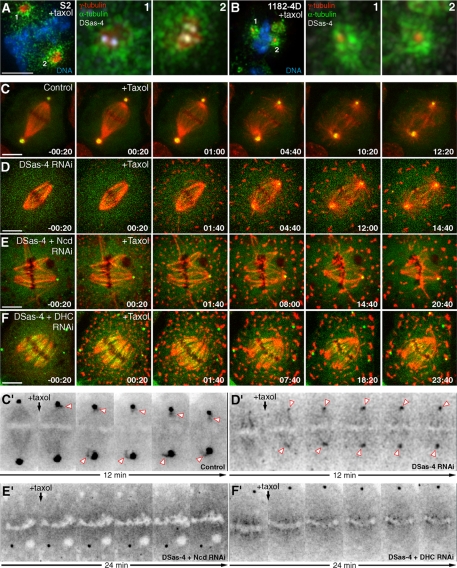
γ-Tubulin Recruitment into Acentriolar Poles after Taxol-induced Aster Formation Requires Ncd and Dynein
Aster formation induced by taxol in acentriolar Xenopus oocyte extracts in vitro rely on the motor activity of cytoplasmic dynein, which is also necessary to recruit γ-tubulin (Verde et al., 1991; Stearns and Kirschner, 1994). The mammalian orthologue of Ncd, HSET, has also been shown to be specifically required for taxol-induced aster formation both in cultured cells and cell-free extracts (Mountain et al., 1999), but whether it plays a role in recruiting γ-tubulin is not known. Treatment of S2 or acentriolar 1182-4D cells with taxol leads to the formation of asters containing γ-tubulin, regardless of the presence of centrioles (Figure 5A, 1 and 2, and B, 1 and 2). Because spindle MTs are recruited to the aster after taxol addition in control cells, spindle-associated γ-tubulin-GFP starts to concentrate at the MT minus ends close to the centrosome (Figure 5, C and C′). In DSas-4–depleted S2 cells treated with taxol the transport of spindle-associated γ-tubulin-GFP to the center of the acentriolar aster (Figure 5, D and D′) was found to rely on the activity of Ncd and dynein (Figure 5, E and E′, and F and F′). These experiments reveal that, in addition to MT clustering of γ-tubulin aggregates in the cytoplasm, MT minus-end–directed transport may also contribute to recruit γ-tubulin from the spindle to acentriolar poles.
Figure 5.
γ-Tubulin recruitment into taxol-induced acentriolar microtubule asters requires Ncd and dynein. (A and B) Immunofluorescence of S2 (A) and 1182-4D cells (B) after taxol treatment showing the distribution of endogenous γ-tubulin (red) and DSas-4 (white). Numbered pictures correspond to enlarged views of the respective spindle poles in A and B. MTs are in green and DNA in blue. (C–F) Time-lapse sequences showing γ-tubulin redistribution upon taxol-induced aster formation in control, DSas-4 RNAi, DSas-4+Ncd RNAi, DSas-4+DHC RNAi, respectively, in S2 cells stably expressing mCherry-α-tubulin (red) and γ-tubulin-GFP (green). (C′–F′) Corresponding contrast inversion views. After codepletion of DSas-4 with Ncd or DHC, aster formation after taxol treatment is strongly compromised and γ-tubulin is no longer recruited to acentriolar spindle poles. Time zero was set from the moment of taxol addition. Time lapse is in min:sec; scale bars, 5 μm.
De Novo Microtubule Nucleation from aMTOCs during Anaphase Is Regulated by CDK1
After spindle formation many aMTOCs are not found associated with MTs (Figure 6A and Supplementary Videos S5 and S6). To test whether MT nucleation from aMTOCs is cell cycle–regulated, we followed MTs and γ-tubulin-GFP in living S2 cells after DSas-4 RNAi at the metaphase–anaphase transition (Figure 6A and Supplementary Videos S5 and S6). Remarkably, upon anaphase onset, several MTs abruptly form de novo, many of which nucleated directly from aMTOCs that were already present during metaphase (Figure 6, A and A″ and Supplementary Videos S5 and S6).
Figure 6.
De novo microtubule nucleation from cytoplasmic aMTOCs after anaphase onset is controlled by CDK1. (A–C) Time-lapse sequences of S2 cells stably expressing mCherry-α-tubulin (red) and γ-tubulin-GFP (green) after DSas-4 RNAi (A and B) or control cells (C). (A′–C′) Corresponding time-lapse sequences depicting mCherry-α-tubulin after contrast inversion. (A) After anaphase onset, cytoplasmic aMTOCs (arrowheads) show a sudden formation of MTs while the cytoskeleton is remodeled during mitotic exit. Time zero was set as the time of anaphase onset. (B and C) DSas-4 RNAi (B and B″) and control (C and C″) cells, blocked in metaphase for 2 h with MG132 upon addition of roscovitine (time zero). On CDK1 inhibition, cytoplasmic aMTOCs (arrowheads) abruptly organize MTs, regardless of the presence of centrosomes. (A″–C″) Enlarged views of cytoplasmic aMTOCs nucleating MTs de novo (yellow arrowheads in A–C, respectively). Time is in min:sec; scale bars, 5 μm.
CDK1 is a key cell cycle regulator whose levels influence MT dynamics and stability in interphase and mitosis in vitro (Verde et al., 1990). As so, we reasoned that MT nucleation from aMTOCs could be regulated by CDK1. To test this hypothesis, we treated control and DSas-4–depleted cells with the selective CDK inhibitor roscovitine (Meijer et al., 1997). To rule out unspecific effects on CDKs other than CDK1, we previously blocked cells in metaphase with the proteasome inhibitor MG132, which prevents sister-chromatid separation in a CDK1-independent manner (Skoufias et al., 2007). Addition of roscovitine to MG132-treated metaphase cells immediately triggers formation of MTs in the cytoplasm, many of which nucleated directly from aMTOCs, regardless of the presence of centrosomes (Figures 6, B and B″, and C and C″, and 7D, and Supplementary Video S7). Because CDK1 is the main CDK controlling mitotic progression, these results indicate that de novo MT nucleation from aMTOCs during anaphase requires down-regulation of CDK1 and occurs in cells that normally contain centrioles.
Figure 7.
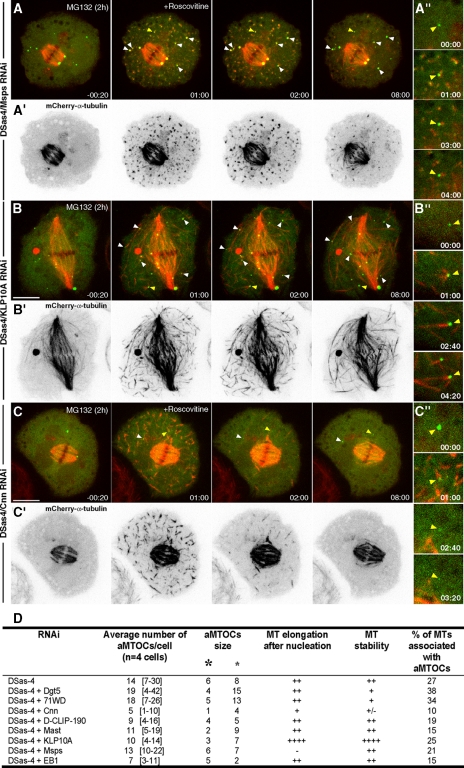
Cnn, Msps and KLP10A regulate the formation and function of aMTOCs. (A–C) Time-lapse sequences of S2 cells stably expressing mCherry-α-tubulin (red) and γ-tubulin-GFP (green) after block in metaphase for 2 h with MG132 and upon addition of roscovitine (time 0). (A′–C′) Corresponding time-lapse sequences depicting mCherry-α-tubulin after contrast inversion. (A and A″) De novo MT formation after codepletion of DSas-4 and Msps. Note that MTs do not elongate (A″). (B and B″) De novo MT formation after codepletion of DSas-4 and KLP10A. Note that MTs are very long and stable (B″). (C and C″) De novo MT formation after codepletion of DSas-4 and Cnn. Only a few aMTOCs can be observed and MTs that formed de novo are unstable, disappearing shortly after (C″). (A″–C″) Enlarged views of cytoplasmic aMTOCs nucleating MTs de novo (yellow arrowheads in A–C, respectively). Time is in min:sec; scale bars, 5 μm. (D) Quantification of the number and size of aMTOCs (large and small asterisks represent big and small, respectively), respective MT elongation/stability potential and frequency of de novo nucleated MTs associated with aMTOCs. MT elongation/stability is expressed as a measure relative to DSas-4 RNAi.
CDK1 Controls the Activity of Microtubule-stabilizing and -destabilizing Proteins Involved in Microtubule Formation from aMTOCs during Anaphase
To identify MAPs that participate in de novo MT formation from aMTOCs during anaphase and are under control of CDK1 activity, we performed the same roscovitine/MG132 assay after codepletion of DSas-4 with candidate MAPs, namely Msps, EB1, D-CLIP-190, Mast/Orbit/CLASP, and the kinesin-13 KLP10A (Figure 7, A and B″, and D, and data not shown). We found that RNAi depletion of Msps significantly affected normal MT elongation from aMTOCs (cf. Figures 7, A and A″, and B and B″, with Figure 6, B and B″), whereas knockdown of KLP10A leads to the formation of longer MTs from aMTOCs after addition of roscovitine. RNAi depletion of EB1, D-CLIP-190 and Mast did not significantly affect de novo MT formation from aMTOCs in our assay (Figure 7D and data not shown). These results support a model where CDK1 inactivation during anaphase coordinates the activity of key MT-stabilizing and -destabilizing proteins, which is essential to mediate MT growth from aMTOCs and regulate MT length.
Cnn Is Required for Normal Architecture and Function of aMTOCs
Acentriolar γ-tubulin aggregates extensively colocalize with Cnn and other γ-TuRC components throughout mitosis, suggesting that these accessory proteins might be required for the normal architecture and/or function of aMTOCs. To directly test whether Cnn and γ-TuRCs are involved in MT formation from aMTOCs, we reproduced our roscovitine/MG132 assay after codepletion of DSas-4 with Cnn or the γ-TuRC component Dgp71WD. This allowed us to circumvent the problem associated with depletion of γ-TuRCs, which severely affects spindle assembly and function (Verollet et al., 2006). We observed that depletion of Cnn reduces the number and size of cytoplasmic γ-tubulin aggregates in metaphase, before roscovitine addition (Figure 7, C, C′, and D). Depletion of Dgp71WD or the Augmin component Dgt5 (Goshima et al., 2008), on the other hand, appears to impair the size of aMTOCs (Figure 7D and data not shown). Importantly, upon addition of roscovitine, only depletion of Cnn significantly impaired the function of aMTOCs and affected the stability of MTs formed de novo (Figure 7, C, C″, and D).
DISCUSSION
Role of aMTOCs in Microtubule Organization during Spindle Assembly
Recruitment and stabilization of PCM proteins in animal somatic cells is thought to be a centriole-dependent function (Kirkham et al., 2003; Martinez-Campos et al., 2004; Basto et al., 2006; Rodrigues-Martins et al., 2007). This view is largely supported by the results presented in our work in the sense that centrioles work as a platform for the organized assembly of PCM. However, here we report that many MTs in Drosophila somatic cells with compromised centriole biogenesis are nucleated de novo from cytoplasmic aMTOCs containing several PCM components and may contribute for the remodeling of the MT cytoskeleton at the entry and exit from mitosis in acentriolar systems. Likewise, higher plants are naturally devoid of centrioles and are still able to organize complex microtubule networks from multiple sites that recruit γ-tubulin and associated proteins (Liu et al., 1993; Murata et al., 2005). Importantly, the aMTOCs described here are also present in centriole-containing cells, and their contribution to MT organization should not be underestimated given that cytosolic γ-tubulin comprises more than 80% of the total cellular pool (Moudjou et al., 1996). In support of this idea, γ-tubulin has been localized in noncentrosomal cytoplasmic patches of polarized mammalian cells (Reilein et al., 2005) and recently shown to mediate microtubule nucleation from the Golgi apparatus in epithelial cells (Efimov et al., 2007). Our results thus uncover an important evolutionary conservation in MT cytoskeleton organization between centriolar and acentriolar systems. It should be noted, however, that the contribution of acentriolar MT organization in animal somatic cells may vary from cell type to cell type (e.g., fibroblasts vs. epithelial cells; Bre et al., 1987), depending on the relative pressure imposed by centrioles in the concerted recruitment of γ-tubulin and PCM components to the centrosome.
Acentriolar spindle assembly in Xenopus oocyte extracts is mediated by a RanGTP gradient concentrated on chromatin and by the action of microtubule motors (Karsenti and Vernos, 2001). However, acentriolar poles of female mouse meiotic spindles can form in the absence of chromatin and independently of RanGTP by self-assembled MT asters that recruit γ-tubulin (Gueth-Hallonet et al., 1993; Palacios et al., 1993; Brunet et al., 1998; Dumont et al., 2007; Schuh and Ellenberg, 2007). In the case of plants, it has been argued that some possess a pole-based mechanism that mediates acentriolar spindle formation during mitosis (Lloyd and Chan, 2006), where γ-tubulin nucleates spindle MTs from polar caps around the nucleus during prophase (Liu et al., 1993; Brown et al., 2004). We envision that an equivalent mechanism may contribute to spindle morphogenesis in animal somatic cells and work in concert with the centrosome- and/or chromatin-mediated pathways. In fact, EB1-GFP tracking in Drosophila S2 cells after centrosome detachment from the spindle revealed new MT nucleation events at acentriolar spindle MT minus ends (Mahoney et al., 2006). The vast majority of EB1 tracks were oriented toward chromosomes but few were found to move away from the pole toward the periphery of the cell. This is consistent with our observation that acentriolar poles that recruit γ-tubulin were able to organize rudimentary astral microtubules. One interesting question arises though—if most cytoplasmic aMTOCs are repressed until anaphase, how would they conceivably contribute to spindle assembly? One possibility would be that additional MT-stabilizing factors allow MT nucleation to take place in the vicinity of the poles or in proximity with unaligned chromosomes. This would also be consistent with the formation of a RanGTP gradient centered on chromosomes that promotes MT nucleation and the existence of factors (e.g., TPX2) involved in this pathway, which are associated with the poles (Wittmann et al., 2000). Another possibility would be that MT nucleation from aMTOCs before anaphase might be regulated by a different balance between the activity of other protein kinases and/or phosphatases, which render MTs to be less stable.
Here we report that an Ncd/dynein-dependent MT clustering mechanism involved in spindle assembly is important to recruit γ-tubulin associated with peripheral MT minus ends to acentriolar spindle poles (Figure 8). Homozygous Ncd mutants in Drosophila are viable but female sterile because of the requirement of Ncd activity for acentriolar spindle assembly in meiotic oocytes (Matthies et al., 1996). It is also known that in mammalian epithelial cells noncentrosome-associated MTs contribute to spindle formation even in the presence of centrosomes, in a dynein-dependent manner (Rusan et al., 2002). Our correlative live-cell imaging of MTs and γ-tubulin supports that γ-tubulin is actively involved in nucleation/stability of MTs from noncentrosomal sites, while participating in spindle formation in animal cells. Whether the contribution of peripheral MTs is essential for spindle formation is unlikely, given the possible redundancy with concurrent centrosome- and/or chromosome-mediated pathways.
Figure 8.
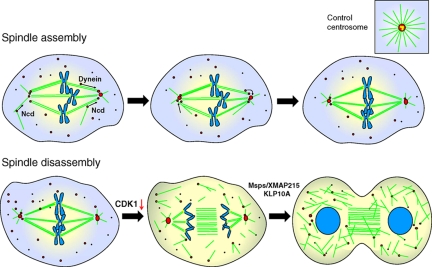
Model for the role of aMTOCs during mitotic spindle assembly and disassembly. Ncd/dynein-dependent mechanism is responsible for recruiting and maintaining γ-tubulin (red circles) in acentriolar poles during mitotic spindle assembly. These poles form rudimentary MT asters when compared with poles containing centrioles, which may work as platforms for the organized recruitment of PCM to the centrosome. De novo MT formation from aMTOCs upon anaphase onset is driven by CDK1 down-regulation, which controls the activity of Msps/XMAP215 and KLP10A to promote net MT growth as cells exit mitosis, thereby contributing to reestablish the interphase MT cytoskeleton.
Finally, γ-tubulin and γ-TuRCs have recently been demonstrated to play a role in acentrosomal spindle assembly in animal somatic cells (Luders et al., 2006; Mahoney et al., 2006). Noteworthy, γ-TuRCs were dispensable for MT nucleation and to target γ-tubulin to centrosomes, but were critical to recruit γ-tubulin to the spindle (Verollet et al., 2006). More recently a new complex composed of at least five proteins, known as Augmin, also targets γ-tubulin to the spindle and is necessary to increase MT density within the spindle (Goshima et al., 2008). Despite γ-TuRC components were recruited to some cytoplasmic γ-tubulin aggregates, depletion of the γ-TuRC component Dgp71WD or the Augmin protein Dgt5 caused little impact in the formation and function of cytoplasmic aMTOCs, suggesting that the mechanisms regulating MT nucleation from aMTOCs and those involving spindle-associated γ-tubulin are different.
Role of aMTOCs in Microtubule Cytoskeleton Remodeling during Mitotic Spindle Disassembly
Similar to what we observe during spindle assembly, several γ-tubulin aggregates dispersed in the cytoplasm were found to nucleate MTs de novo as mitotic spindles disassemble and cells transit into G1 stage (Figure 8). Surprisingly, very little is known about this process because most of the attention has been focused in understanding how spindle MTs are organized. We found that CDK1 down-regulation triggers cytoplasmic MT nucleation after anaphase, supporting that MT cytoskeleton remodeling at the exit from mitosis in animal somatic cells does not exclusively rely on centrosomes and is a cell cycle–regulated process. The mitosis–G1 transition has been shown to be accompanied by a redistribution of tubulin at an essentially constant polymer level (Zhai and Borisy, 1994), which means that, as kinetochore microtubules disassemble during anaphase, new microtubule polymer has to be formed. This is likely to be due to the increased microtubule stability and growth after anaphase onset but is also consistent with de novo microtubule nucleation at this stage. Our data further indicates that microtubule nucleation activity from cytoplasmic aMTOCs is promoted after anaphase onset, strongly suggesting that this process is under the control of the APC. The APC is responsible for the degradation of cyclin B at the metaphase–anaphase transition, thereby negatively regulating the activity of CDK1 and consequently microtubule dynamics (Verde et al., 1990; Wheatley et al., 1997). In agreement, assembly of equatorial MTOCs at the end of anaphase in fission yeast was also shown to require the activity of the APC (Heitz et al., 2001).
Our results further implicate Cnn in the normal architecture and function of aMTOCs, which affects MT stability upon nucleation from noncentrosomal sites after CDK1 down-regulation. The first conclusion from these data are that MT nucleation events can occur from cytoplasmic sites that lack aMTOCs. However, we propose that these are important to sustain MT growth and confer stability to noncentrosomal nucleated MTs. In agreement with our results, de novo MT nucleation at nonspindle pole body MTOCs during mitotic exit in fission yeast was shown to require the Cnn-related protein mod20p, which binds and recruits γ-tubulin to these sites (Sawin and Tran, 2006). In Drosophila, Cnn has been shown to recruit γ-tubulin to centrosomes but also to form “flares” (Megraw et al., 2002) that look similar to the γ-tubulin–containing aMTOCs described in this work. However, despite the coexistence of Cnn flares with centrosomes, they appear to lack γ-tubulin (Megraw et al., 2002). In our hands, Cnn colocalizes with many endogenous γ-tubulin aggregates throughout mitosis and has a role in their formation and function. These results may be reconciled by the fact that detection of cytoplasmic γ-tubulin aggregates in the presence of centrosomes by fluorescence microscopy is difficult and requires oversaturation of the γ-tubulin signal at centrosomes, as well as highly sensitive detection devices.
We further found that Msps/XMAP215 and KLP10A critically affect net MT growth from aMTOCs upon down-regulation of CDK1. Members of the XMAP215 protein family were shown to be phosphorylated by CDK1 in vitro and in vivo and this modification decreases the MT elongation rate and stability (Vasquez et al., 1999; Aoki et al., 2006). Down-regulation of CDK1 during anaphase might alter the balance with protein phosphatases that control the phosphorylation state of XMAP215 (Tournebize et al., 1997) and promote net MT growth from aMTOCs. This must be coordinated with the regulation of proteins that promote MT catastrophes, such as KLP10A, in order to maintain proper MT dynamics as cells exit mitosis. Interestingly, Msps/XMAP215 was recently implicated in acentrosomal MT formation from multiple cytoplasmic sites during interphase in Drosophila S2 cells (Rogers et al., 2008). Importantly, the effect of depleting Msps/XMAP215 in MT regrowth after depolymerization was similar but not additive to that observed after γ-tubulin depletion, suggesting that these proteins work in the same pathway. Finally, CLASPs, which have been recently implicated in MT nucleation from the Golgi apparatus (Efimov et al., 2007), appear to have no role in MT formation from aMTOCs in S2 cells, suggesting the existence of multiple pathways involved in noncentrosomal MT organization in animals.
Supplementary Material
ACKNOWLEDGMENTS
We thank B. Loppin for the communication of results before publication. We are also indebted with G. Goshima, J. Raff, J. Scholey, T. Hays, T. Megraw, K. Miller, R. Vale, C. Lehner, H. Ohkura, D. Sharp, and B. Raynaud-Messina for the generous gift of reagents used in this study. S.M.-P. holds a studentship from Fundação para a Ciência e a Tecnologia (FCT) of Portugal (SFRH/BD/15244/2004) under the GABBA PhD Program. We acknowledge the Portuguese-French cooperation program PESSOA for traveling support during this project. Work in the laboratory of H.M. is supported by Grants PTDC/BIA-BCM/66106/2006 and PTDC/SAU-OBD/66113/2006 from FCT and the Gulbenkian Programmes for Research Stimulation and Frontiers in the Life Sciences.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E09-01-0011) on April 15, 2009.
REFERENCES
- Aoki K., Nakaseko Y., Kinoshita K., Goshima G., Yanagida M. CDC2 phosphorylation of the fission yeast dis1 ensures accurate chromosome segregation. Curr. Biol. 2006;16:1627–1635. doi: 10.1016/j.cub.2006.06.065. [DOI] [PubMed] [Google Scholar]
- Basto R., Lau J., Vinogradova T., Gardiol A., Woods C. G., Khodjakov A., Raff J. W. Flies without centrioles. Cell. 2006;125:1375–1386. doi: 10.1016/j.cell.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Belmont L. D., Hyman A. A., Sawin K. E., Mitchison T. J. Real-time visualization of cell cycle-dependent changes in microtubule dynamics in cytoplasmic extracts. Cell. 1990;62:579–589. doi: 10.1016/0092-8674(90)90022-7. [DOI] [PubMed] [Google Scholar]
- Bre M. H., Kreis T. E., Karsenti E. Control of microtubule nucleation and stability in Madin-Darby canine kidney cells: the occurrence of noncentrosomal, stable detyrosinated microtubules. J. Cell Biol. 1987;105:1283–1296. doi: 10.1083/jcb.105.3.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. C., Lemmon B. E., Horio T. Gamma-tubulin localization changes from discrete polar organizers to anastral spindles and phragmoplasts in mitosis of Marchantia polymorpha L. Protoplasma. 2004;224:187–193. doi: 10.1007/s00709-004-0061-7. [DOI] [PubMed] [Google Scholar]
- Brunet S., Polanski Z., Verlhac M. H., Kubiak J. Z., Maro B. Bipolar meiotic spindle formation without chromatin. Curr. Biol. 1998;8:1231–1234. doi: 10.1016/s0960-9822(07)00516-7. [DOI] [PubMed] [Google Scholar]
- Cao K., Nakajima R., Meyer H. H., Zheng Y. The AAA-ATPase Cdc48/p97 regulates spindle disassembly at the end of mitosis. Cell. 2003;115:355–367. doi: 10.1016/s0092-8674(03)00815-8. [DOI] [PubMed] [Google Scholar]
- Dammermann A., Maddox P. S., Desai A., Oegema K. SAS-4 is recruited to a dynamic structure in newly forming centrioles that is stabilized by the gamma-tubulin-mediated addition of centriolar microtubules. J. Cell Biol. 2008;180:771–785. doi: 10.1083/jcb.200709102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debec A., Detraves C., Montmory C., Geraud G., Wright M. Polar organization of gamma-tubulin in acentriolar mitotic spindles of Drosophila melanogaster cells. J. Cell Sci. 1995;108(Pt 7):2645–2653. doi: 10.1242/jcs.108.7.2645. [DOI] [PubMed] [Google Scholar]
- Debec A., Szollosi A., Szollosi D. A Drosophila melanogaster cell line lacking centriole. Biol. Cell [under the auspices of the European Cell Biology] Organization. 1982;44:133–138. [Google Scholar]
- Dumont J., Petri S., Pellegrin F., Terret M. E., Bohnsack M. T., Rassinier P., Georget V., Kalab P., Gruss O. J., Verlhac M. H. A centriole- and RanGTP-independent spindle assembly pathway in meiosis I of vertebrate oocytes. J. Cell Biol. 2007;176:295–305. doi: 10.1083/jcb.200605199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efimov A., et al. Asymmetric CLASP-dependent nucleation of noncentrosomal microtubules at the trans-Golgi network. Dev. Cell. 2007;12:917–930. doi: 10.1016/j.devcel.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima G., Mayer M., Zhang N., Stuurman N., Vale R. D. Augmin: a protein complex required for centrosome-independent microtubule generation within the spindle. J. Cell Biol. 2008;181:421–429. doi: 10.1083/jcb.200711053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima G., Nedelec F., Vale R. D. Mechanisms for focusing mitotic spindle poles by minus end-directed motor proteins. J. Cell Biol. 2005;171:229–240. doi: 10.1083/jcb.200505107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima G., Wollman R., Goodwin S. S., Zhang N., Scholey J. M., Vale R. D., Stuurman N. Genes required for mitotic spindle assembly in Drosophila S2 cells. Science. 2007;316:417–421. doi: 10.1126/science.1141314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueth-Hallonet C., Antony C., Aghion J., Santa-Maria A., Lajoie-Mazenc I., Wright M., Maro B. γ-Tubulin is present in acentriolar MTOCs during early mouse development. J. Cell Sci. 1993;105(Pt 1):157–166. doi: 10.1242/jcs.105.1.157. [DOI] [PubMed] [Google Scholar]
- Heitz M. J., Petersen J., Valovin S., Hagan I. M. MTOC formation during mitotic exit in fission yeast. J. Cell Sci. 2001;114:4521–4532. doi: 10.1242/jcs.114.24.4521. [DOI] [PubMed] [Google Scholar]
- Karsenti E., Vernos I. The mitotic spindle: a self-made machine. Science. 2001;294:543–547. doi: 10.1126/science.1063488. [DOI] [PubMed] [Google Scholar]
- Khodjakov A., Cole R. W., Oakley B. R., Rieder C. L. Centrosome-independent mitotic spindle formation in vertebrates. Curr. Biol. 2000;10:59–67. doi: 10.1016/s0960-9822(99)00276-6. [DOI] [PubMed] [Google Scholar]
- Kirkham M., Muller-Reichert T., Oegema K., Grill S., Hyman A. A. SAS-4 is a C. elegans centriolar protein that controls centrosome size. Cell. 2003;112:575–587. doi: 10.1016/s0092-8674(03)00117-x. [DOI] [PubMed] [Google Scholar]
- Kirschner M., Mitchison T. Beyond self-assembly: from microtubules to morphogenesis. Cell. 1986;45:329–342. doi: 10.1016/0092-8674(86)90318-1. [DOI] [PubMed] [Google Scholar]
- Liu B., Marc J., Joshi H. C., Palevitz B. A. A gamma-tubulin-related protein associated with the microtubule arrays of higher plants in a cell cycle-dependent manner. J. Cell Sci. 1993;104(Pt 4):1217–1228. doi: 10.1242/jcs.104.4.1217. [DOI] [PubMed] [Google Scholar]
- Lloyd C., Chan J. Not so divided: the common basis of plant and animal cell division. Nat. Rev. 2006;7:147–152. doi: 10.1038/nrm1831. [DOI] [PubMed] [Google Scholar]
- Luders J., Patel U. K., Stearns T. GCP-WD is a gamma-tubulin targeting factor required for centrosomal and chromatin-mediated microtubule nucleation. Nat. Cell biology. 2006;8:137–147. doi: 10.1038/ncb1349. [DOI] [PubMed] [Google Scholar]
- Mahoney N. M., Goshima G., Douglass A. D., Vale R. D. Making microtubules and mitotic spindles in cells without functional centrosomes. Curr. Biol. 2006;16:564–569. doi: 10.1016/j.cub.2006.01.053. [DOI] [PubMed] [Google Scholar]
- Maiato H., Sampaio P., Lemos C. L., Findlay J., Carmena M., Earnshaw W. C., Sunkel C. E. MAST/Orbit has a role in microtubule-kinetochore attachment and is essential for chromosome alignment and maintenance of spindle bipolarity. J. Cell Biol. 2002;157:749–760. doi: 10.1083/jcb.200201101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Campos M., Basto R., Baker J., Kernan M., Raff J. W. The Drosophila pericentrin-like protein is essential for cilia/flagella function, but appears to be dispensable for mitosis. J. Cell Biol. 2004;165:673–683. doi: 10.1083/jcb.200402130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthies H. J., McDonald H. B., Goldstein L. S., Theurkauf W. E. Anastral meiotic spindle morphogenesis: role of the non-claret disjunctional kinesin-like protein. J. Cell Biol. 1996;134:455–464. doi: 10.1083/jcb.134.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megraw T. L., Kilaru S., Turner F. R., Kaufman T. C. The centrosome is a dynamic structure that ejects PCM flares. J. Cell Sci. 2002;115:4707–4718. doi: 10.1242/jcs.00134. [DOI] [PubMed] [Google Scholar]
- Meijer L., Borgne A., Mulner O., Chong J. P., Blow J. J., Inagaki N., Inagaki M., Delcros J. G., Moulinoux J. P. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur. J. Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- Morales-Mulia S., Scholey J. M. Spindle pole organization in Drosophila S2 cells by dynein, abnormal spindle protein (Asp), and KLP10A. Mol. Biol. Cell. 2005;16:3176–3186. doi: 10.1091/mbc.E04-12-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moudjou M., Bordes N., Paintrand M., Bornens M. γ-Tubulin in mammalian cells: the centrosomal and the cytosolic forms. J. Cell Sci. 1996;109(Pt 4):875–887. doi: 10.1242/jcs.109.4.875. [DOI] [PubMed] [Google Scholar]
- Mountain V., Simerly C., Howard L., Ando A., Schatten G., Compton D. A. The kinesin-related protein, HSET, opposes the activity of Eg5 and cross-links microtubules in the mammalian mitotic spindle. J. Cell Biol. 1999;147:351–366. doi: 10.1083/jcb.147.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata T., Sonobe S., Baskin T. I., Hyodo S., Hasezawa S., Nagata T., Horio T., Hasebe M. Microtubule-dependent microtubule nucleation based on recruitment of gamma-tubulin in higher plants. Nat. Cell Biol. 2005;7:961–968. doi: 10.1038/ncb1306. [DOI] [PubMed] [Google Scholar]
- Palacios M. J., Joshi H. C., Simerly C., Schatten G. Gamma-tubulin reorganization during mouse fertilization and early development. J. Cell Sci. 1993;104(Pt 2):383–389. doi: 10.1242/jcs.104.2.383. [DOI] [PubMed] [Google Scholar]
- Raynaud-Messina B., Merdes A. γ-Tubulin complexes and microtubule organization. Curr. Opin. Cell Biol. 2007;19:24–30. doi: 10.1016/j.ceb.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Reilein A., Yamada S., Nelson W. J. Self-organization of an acentrosomal microtubule network at the basal cortex of polarized epithelial cells. J. Cell Biol. 2005;171:845–855. doi: 10.1083/jcb.200505071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues-Martins A., Riparbelli M., Callaini G., Glover D. M., Bettencourt-Dias M. Revisiting the role of the mother centriole in centriole biogenesis. Science. 2007;316:1046–1050. doi: 10.1126/science.1142950. [DOI] [PubMed] [Google Scholar]
- Rogers G. C., Rusan N. M., Peifer M., Rogers S. L. A multicomponent assembly pathway contributes to the formation of acentrosomal microtubule arrays in interphase Drosophila cells. Mol. Biol. Cell. 2008;19:3163–3178. doi: 10.1091/mbc.E07-10-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusan N. M., Tulu U. S., Fagerstrom C., Wadsworth P. Reorganization of the microtubule array in prophase/prometaphase requires cytoplasmic dynein-dependent microtubule transport. J. Cell Biol. 2002;158:997–1003. doi: 10.1083/jcb.200204109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin K. E., Tran P. T. Cytoplasmic microtubule organization in fission yeast. Yeast. 2006;23:1001–1014. doi: 10.1002/yea.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh M., Ellenberg J. Self-organization of MTOCs replaces centrosome function during acentrosomal spindle assembly in live mouse oocytes. Cell. 2007;130:484–498. doi: 10.1016/j.cell.2007.06.025. [DOI] [PubMed] [Google Scholar]
- Skoufias D. A., Indorato R. L., Lacroix F., Panopoulos A., Margolis R. L. Mitosis persists in the absence of Cdk1 activity when proteolysis or protein phosphatase activity is suppressed. J. Cell Biol. 2007;179:671–685. doi: 10.1083/jcb.200704117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman B. M., Lopata M. A., Kirschner M. W. Multiple sites for the initiation of microtubule assembly in mammalian cells. Cell. 1979;16:239–252. doi: 10.1016/0092-8674(79)90002-3. [DOI] [PubMed] [Google Scholar]
- Stearns T., Kirschner M. In vitro reconstitution of centrosome assembly and function: the central role of gamma-tubulin. Cell. 1994;76:623–637. doi: 10.1016/0092-8674(94)90503-7. [DOI] [PubMed] [Google Scholar]
- Szollosi A., Ris H., Szollosi D., Debec A. A centriole-free Drosophila cell line. A high voltage EM study. Eur. J. Cell Biol. 1986;40:100–104. [PubMed] [Google Scholar]
- Tournebize R., Andersen S. S., Verde F., Doree M., Karsenti E., Hyman A. A. Distinct roles of PP1 and PP2A-like phosphatases in control of microtubule dynamics during mitosis. EMBO J. 1997;16:5537–5549. doi: 10.1093/emboj/16.18.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez R. J., Gard D. L., Cassimeris L. Phosphorylation by CDK1 regulates XMAP215 function in vitro. Cell Motil. Cytoskelet. 1999;43:310–321. doi: 10.1002/(SICI)1097-0169(1999)43:4<310::AID-CM4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Verde F., Berrez J. M., Antony C., Karsenti E. Taxol-induced microtubule asters in mitotic extracts of Xenopus eggs: requirement for phosphorylated factors and cytoplasmic dynein. J. Cell Biol. 1991;112:1177–1187. doi: 10.1083/jcb.112.6.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde F., Labbe J. C., Doree M., Karsenti E. Regulation of microtubule dynamics by cdc2 protein kinase in cell-free extracts of Xenopus eggs. Nature. 1990;343:233–238. doi: 10.1038/343233a0. [DOI] [PubMed] [Google Scholar]
- Verollet C., Colombie N., Daubon T., Bourbon H. M., Wright M., Raynaud-Messina B. Drosophila melanogaster gamma-TuRC is dispensable for targeting gamma-tubulin to the centrosome and microtubule nucleation. J. Cell Biol. 2006;172:517–528. doi: 10.1083/jcb.200511071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorobjev I. A., Svitkina T. M., Borisy G. G. Cytoplasmic assembly of microtubules in cultured cells. J. Cell Sci. 1997;110(Pt 21):2635–2645. doi: 10.1242/jcs.110.21.2635. [DOI] [PubMed] [Google Scholar]
- Wheatley S. P., Hinchcliffe E. H., Glotzer M., Hyman A. A., Sluder G., Wang Y. CDK1 inactivation regulates anaphase spindle dynamics and cytokinesis in vivo. J. Cell Biol. 1997;138:385–393. doi: 10.1083/jcb.138.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann T., Wilm M., Karsenti E., Vernos I. TPX2, A novel Xenopus MAP involved in spindle pole organization. J. Cell Biol. 2000;149:1405–1418. doi: 10.1083/jcb.149.7.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yvon A. M., Wadsworth P. Non-centrosomal microtubule formation and measurement of minus end microtubule dynamics in A498 cells. J. Cell Sci. 1997;110(Pt 19):2391–2401. doi: 10.1242/jcs.110.19.2391. [DOI] [PubMed] [Google Scholar]
- Zhai Y., Borisy G. G. Quantitative determination of the proportion of microtubule polymer present during the mitosis-interphase transition. J. Cell Sci. 1994;107(Pt 4):881–890. doi: 10.1242/jcs.107.4.881. [DOI] [PubMed] [Google Scholar]
- Zhai Y., Kronebusch P. J., Simon P. M., Borisy G. G. Microtubule dynamics at the G2/M transition: abrupt breakdown of cytoplasmic microtubules at nuclear envelope breakdown and implications for spindle morphogenesis. J. Cell Biol. 1996;135:201–214. doi: 10.1083/jcb.135.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.