Hematologists have long been fascinated by thrombopoiesis. While it has long been accepted that platelets are derived from megakaryocytes,1 the process by which this occurs and its regulation are still incompletely understood. As they mature, megakaryocytes acquire competency for platelet formation through up-modulation of a vast array of cytoskeletal, membrane and granule regulatory proteins,2,3 differentiating into cells that are large, polyploid, and have a cytoplasm filled with a complex system of interconnected cytoplasmic membranes (dermarcation membrane system or DMS), as well as stores of ribosomes, alpha granules and dense granules.4,5 Although the growth and differentiation of megakaryocytes requires thrombopoietin, the role of this cytokine in the subsequent steps of platelet formation is less well established.6
Models of proplatelet formation
At least two models have been put forward to explain platelet formation. Based on examination of microscopic images, James Homer Wright proposed that platelets are released from pseudopodial processes (later termed proplatelets) that extend from megakaryocytes into blood vessels.1 Alternatively, Sharnoff and colleagues suggested that megakaryocytes travel through the circulation to the lungs where they are physically fragmented into platelets within pulmonary capillaries.7 In the former model, it is hypothesized that the role of the DMS is as a store of membrane to support proplatelet formation, whereas in the latter the DMS defines pre-formed platelet territories.
Although still controversial, recent data favor the proplatelet model of thrombopoiesis. Using live cell microscopy, Italiano and colleagues captured cultured megakaryocytes elaborating branching proplatelet processes.8 Microtubule bundles within the processes were seen to form discrete loops at the end of the processes, correlating to the microtubule marginal band that defines the outline of the discoid platelet (Figure 1). Importantly, the fragmentation model does not account for this essential platelet structure. Subsequent work by Junt and colleagues confirmed that proplatelet formation was not only an in vitro phenomenon, but that YFP-labeled megakaryocytes could be seen to release proplatelet-like bodies into the marrow sinusoids in living mice.9 These studies also suggested that shear forces in the marrow sinusoid may play a role in stimulating platelet release. Despite these remarkable insights from live cell imaging, a number of questions remain regarding the factors that initiate and regulate proplatelet formation.
Figure 1.
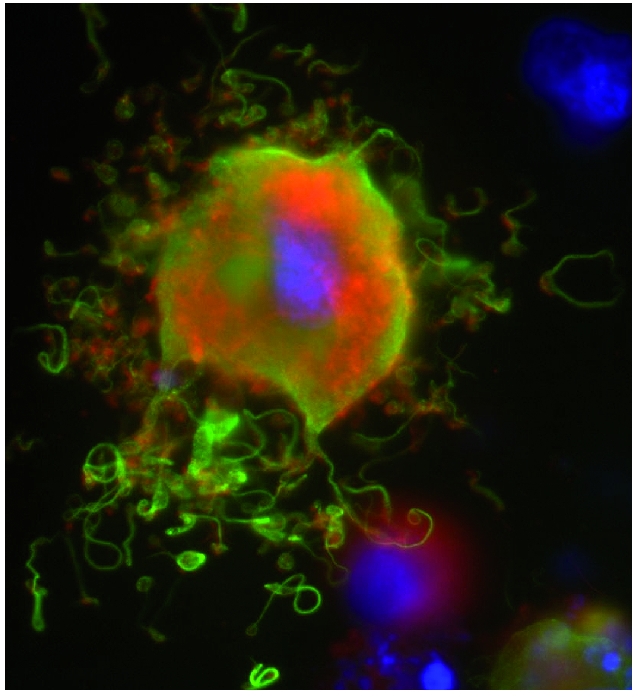
Murine megakaryocyte demonstrating in vitro proplatelet formation. Green: tubulin, Red: actin, Blue: DNA. Note formation of microtubule loops at the end of the proplatelet processes.
The role of apoptosis
Proplatelet formation is a terminal process, and once it has been completed the residual megakaryocyte nucleus is engulfed by macrophages within the bone marrow.8 However, recent studies indicate that localized apoptosis actively contributes to proplatelet formation by an as yet poorly understood mechanism.10,11 For example, using a mouse model in which megakaryocytes over-express Bcl-xL, Kaluhnzy and colleagues found that inhibition of apoptosis results in abnormal DMS formation, impaired in vitro proplatelet formation and a blunted recovery from thrombocytopenia.12 In addition, Morison and colleagues characterized a large family with autosomal dominant thrombocytopenia due to a novel mutation at a conserved residue cytochrome C that led to enhanced apoptosis.13 Surprisingly, platelet lifespan was normal in affected family members; however, the observation of proplatelets forming within the marrow space suggested that premature and ineffective proplatelet formation is the mechanism of peripheral thrombocytopenia in this disorder. Further study of the function of the apoptosis machinery in megakaryocytes is needed to understand its contribution to thrombopoiesis.
The cytoskeleton in proplatelet formation
Reorganization of the megakaryocyte cytoskeleton, including tubulin, actin and myosin, precedes and promotes thrombopoiesis. Dynamic reorganization of tubulin is essential for proplatelet formation, as shown using chemical inhibitors of tubulin polymerization and depolymerization.8 Subsequent work demonstrated that proplatelet elongation requires the sliding of microtubules past one another and can be inhibited by blocking the function of the microtubule-associated motor protein dynein.14 The predominant β-tubulin isoform in platelets is β1-tubulin, which is unique to the megakaryocyte lineage. Mice lacking β1-tubulin are thrombocytopenic and exhibit impaired proplatelet formation compared to controls, although they are still capable of generating platelets.15 Due to disruption of the marginal band, β1-tubulin-null platelets are spherical rather than discoid in appearance.15 Recently, the importance of tubulin in platelet production has been correlated clinically, as familial mutations of β1- tubulin have been discovered in humans and in dogs in association with autosomal dominant macrothrombocytopenia.16,17
Actin and myosin also play important roles in proplatelet formation, likely promoting the branching and amplification of proplatelet tips.8 Additionally, myosin IIA and its upstream regulator RhoA restrain the onset of platelet formation. Consistent with this model, mutations that reduce myosin II activity, such as those occurring in MYH9-related disease, are associated with the premature initiation of proplatelets within the marrow space.18,19 Mutations in the actin-regulating Wiskott Aldrich syndrome protein (WASP) are also associated with ineffective thrombopoiesis,20 which may combine with peripheral platelet destruction to cause thrombocytopenia in patients with Wiskott Aldrich syndrome.
Integrin receptors provide a potential mechanism to link these cytoskeletal changes in the megakaryocyte to the extracellular marrow environment. For example, type I, but not type III or IV, collagen inhibits proplatelet formation,21 whereas fibrinogen promotes it.22 Correspondingly, the integrin α2bβ3 is essential for proplatelet formation in response to fibrinogen22 and mutations resulting in constitutive activation of α2bβ3 further increase thrombopoiesis.23 One interpretation of this constellation of findings is that the differential response to extracellular factors provides a mechanism to prevent platelet release until the megakaryocyte reaches the vascular niche.24
The von Willebrand factor receptor GPIb is another important regulator of proplatelet formation. Antibodies to GPIb have been found experimentally to inhibit megakaryopoiesis and platelet production in vitro,25,26 a finding which may have clinical relevance to immune thrombocytopenia syndromes in which antibodies to GPIb are common. In addition, autosomal recessive inheritance of mutations involving GPIb causes macrothrombocytopenia in humans (Bernard-Soulier syndrome, BSS). Hypothetically, the interaction between GPIb and von Willebrand factor would be one way for the megakaryocyte to sense shear in the sinusoid. However, Kanaji and colleagues expressed a hybrid protein consisting of the intracellular domain of GPIbα and the extracellular domain of the interleukin-4 receptor in GPIbα-null mice and found that platelet production was partially rescued, suggesting that in the absence of interaction with von Willebrand factor the association of GPIbα with cytoskeletal components such as filamin and 14-3-3ζ is still able to contribute to thrombopoiesis.27
Questions of platelet size
Any model of proplatelet formation must account for the variation in platelet size that occurs under conditions of increased platelet destruction and in certain heritable diseases. A population of large platelets is characteristically seen during recovery from acute thrombocytopenia such as occurs in immune-mediated platelet destruction. This has been attributed to the premature release of young platelets and precedes measurable changes in megakaryocyte ploidy.28,29 Curiously, large platelets are not prominent in reactive thrombocytosis and, therefore, the kinetics of platelet production is probably an important factor in this phenomenon. Abnormally large platelets are also characteristic of several of the inherited platelet disorders including the above mentioned BSS and MYH9-related disease, as well as gray platelet syndrome and thrombocytopenia due to mutations involving GATA1.
The marked macrothrombocytopenia of BSS has drawn platelet biologists to study this disease in order to better understand the role of GPIb in thrombopoiesis. Paulus and colleagues observed that the platelet territories defined by the DMS in megakaryocytes from patients with BSS are larger than in those from controls.30 More recently, Balduini and colleagues studied patients with BSS due to a heterozygous mutation of GPIbα and found that their megakaryocytes produce 50% fewer proplatelets than control megakaryocytes, and those proplatelets have abnormally large tips.31 Because further clarification of the mechanisms leading to macrothrombocytopenia is technically difficult in patients, Strassel et al., in a study published in this issue of Haematologica,32 turned to a mouse model to address these questions.
Thrombopoiesis in mouse models of Bernard-Soulier syndrome
Murine models of BSS, including mice with targeted disruption of either GPIbα33 or GPIbβ34 recapitulate the phenotype of the human disease with macrothrombocytopenia and platelet dysfunction. Megakaryocytes from GPIbα-null mice have a poorly developed DMS and a reduced internal membrane pool.35 In this issue of Haematologica Strassel et al. characterize megakaryopoiesis and proplatelet formation in the GPIbβ-null mouse in an attempt to further elucidate the mechanisms of macrothrombocytopenia in BSS.32 The strengths of their elegant work are the similarities between the phenotype of megakaryocytes from the GPIbβ-null mice and those from patients with BSS, the stepwise evaluation of thrombopoiesis in the mice from megakaryocyte commitment to platelet survival, and quantitative analysis of their observations. The results provide clear evidence that macrothrombocytopenia in GPIbβ-null mice is not due to decreased platelet survival or impaired megakaryocyte maturation, but to abnormal DMS formation and impaired proplatelet formation. Curiously, the micro-tubule bundles at the core of the proplatelet and within the marginal band are measurably thicker than those in wild type platelets, suggesting that GPIbβ regulates microtubule organization in an as yet undefined way. Nevertheless, in this model the GPIbβ-null platelets retain their discoid shape, a finding that is in contrast to prior observations that platelets from BSS are spherical.36 Taken together, this work and others demonstrate that there is a growing interest in and understanding of the mechanisms of proplatelet formation, which will provide insight into both acquired and inherited thrombocytopenia syndromes.
Footnotes
Dr. Geddis is an Assistant Professor of pediatrics at the University of California, San Diego, in the division of Pediatric Hematology Oncology.
Dr. Geddis receives funding from NIH DK049855. The author would like to thank Kenneth Kaushansky for critical reading of the manuscript.
References
- 1.Wright JH. The histogenesis of the blood platelets. J Morphol. 1910;21:263–77. [Google Scholar]
- 2.Chen Z, Hu M, Shivdasani RA. Expression analysis of primary mouse megakaryocyte differentiation and its application in identifying stage-specific molecular markers and a novel transcriptional target of NF-E2. Blood. 2007;109:1451–9. doi: 10.1182/blood-2006-08-038901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shim MH, Hoover A, Blake N, Drachman JG, Reems JA. Gene expression profile of primary human CD34+CD38lo cells differentiating along the megakaryocyte lineage. Exp Hematol. 2004;32:638–48. doi: 10.1016/j.exphem.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Behnke O. An electron microscope study of the megacaryocyte of the rat bone marrow. I. The development of the demarcation membrane system and the platelet surface coat. J Ultrastruct Res. 1968;24:412–33. doi: 10.1016/s0022-5320(68)80046-2. [DOI] [PubMed] [Google Scholar]
- 5.Cramer EM, Norol F, Guichard J, Breton-Gorius J, Vainchenker W, Masse JM, et al. Ultrastructure of platelet formation by human megakaryocytes cultured with the Mpl ligand. Blood. 1997;89:2336–46. [PubMed] [Google Scholar]
- 6.Choi ES, Hokom MM, Chen JL, Skrine J, Faust J, Nichol J, et al. The role of megakaryocyte growth and development factor in terminal stages of thrombopoiesis. Br J Haematol. 1996;95:227–33. doi: 10.1046/j.1365-2141.1996.d01-1920.x. [DOI] [PubMed] [Google Scholar]
- 7.Sharnoff JG, Scardino V. Platelet-count differences in blood of the rabbit right and left heart ventricles. Nature. 1960;187:334–5. doi: 10.1038/187334a0. [DOI] [PubMed] [Google Scholar]
- 8.Italiano JE, Jr, Lecine P, Shivdasani RA, Hartwig JH. Blood platelets are assembled principally at the ends of proplatelet processes produced by differentiated megakaryocytes. J Cell Biol. 1999;147:1299–312. doi: 10.1083/jcb.147.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Junt T, Schulze H, Chen Z, Massberg S, Goerge T, Krueger A, et al. Dynamic visualization of thrombopoiesis within bone marrow. Science. 2007;317:1767–70. doi: 10.1126/science.1146304. [DOI] [PubMed] [Google Scholar]
- 10.De Botton S, Sabri S, Daugas E, Zermati Y, Guidotti JE, Hermine O, et al. Platelet formation is the consequence of caspase activation within megakaryocytes. Blood. 2002;100:1310–7. doi: 10.1182/blood-2002-03-0686. [DOI] [PubMed] [Google Scholar]
- 11.Clarke MC, Savill J, Jones DB, Noble BS, Brown SB. Compartmentalized megakaryocyte death generates functional platelets committed to caspase-independent death. J Cell Biol. 2003;160:577–87. doi: 10.1083/jcb.200210111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaluzhny Y, Yu G, Sun S, Toselli PA, Nieswandt B, Jackson CW, et al. BclxL overexpression in megakaryocytes leads to impaired platelet fragmentation. Blood. 2002;100:1670–8. doi: 10.1182/blood-2001-12-0263. [DOI] [PubMed] [Google Scholar]
- 13.Morison IM, Cramer Borde EM, Cheesman EJ, Cheong PL, Holyoake AJ, Fichelson S, et al. A mutation of human cytochrome c enhances the intrinsic apoptotic pathway but causes only thrombocytopenia. Nat Genet. 2008;40:387–9. doi: 10.1038/ng.103. [DOI] [PubMed] [Google Scholar]
- 14.Patel SR, Richardson JL, Schulze H, Kahle E, Galjart N, Drabek K, et al. Differential roles of microtubule assembly and sliding in proplatelet formation by megakaryocytes. Blood. 2005;106:4076–85. doi: 10.1182/blood-2005-06-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Italiano JE, Jr, Bergmeier W, Tiwari S, Falet H, Hartwig JH, Hoffmeister KM, et al. Mechanisms and implications of platelet discoid shape. Blood. 2003;101:4789–96. doi: 10.1182/blood-2002-11-3491. [DOI] [PubMed] [Google Scholar]
- 16.Davis B, Toivio-Kinnucan M, Schuller S, Boudreaux MK. Mutation in beta1- tubulin correlates with macrothrombocytopenia in Cavalier King Charles Spaniels. J Vet Intern Med. 2008;22:540–5. doi: 10.1111/j.1939-1676.2008.0085.x. [DOI] [PubMed] [Google Scholar]
- 17.Kunishima S, Kobayashi R, Itoh TJ, Hamaguchi M, Saito H. Mutation of the beta1-tubulin gene associated with congenital macrothrombocytopenia affecting microtubule assembly. Blood. 2009;113:458–61. doi: 10.1182/blood-2008-06-162610. [DOI] [PubMed] [Google Scholar]
- 18.Chang Y, Aurade F, Larbret F, Zhang Y, Le Couedic JP, Momeux L, et al. Proplatelet formation is regulated by the Rho/ROCK pathway. Blood. 2007;109:4229–36. doi: 10.1182/blood-2006-04-020024. [DOI] [PubMed] [Google Scholar]
- 19.Chen Z, Naveiras O, Balduini A, Mammoto A, Conti MA, Adelstein RS, et al. The May-Hegglin anomaly gene MYH9 is a negative regulator of platelet biogenesis modulated by the Rho-ROCK pathway. Blood. 2007;110:171–9. doi: 10.1182/blood-2007-02-071589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sabri S, Foudi A, Boukour S, Franc B, Charrier S, Jandrot-Perrus M, et al. Deficiency in the Wiskott-Aldrich protein induces premature proplatelet formation and platelet production in the bone marrow compartment. Blood. 2006;108:134–40. doi: 10.1182/blood-2005-03-1219. [DOI] [PubMed] [Google Scholar]
- 21.Balduini A, Pallotta I, Malara A, Lova P, Pecci A, Viarengo G, et al. Adhesive receptors, extracellular proteins and myosin IIA orchestrate proplatelet formation by human megakaryocytes. J Thromb Haemost. 2008;6:1900–7. doi: 10.1111/j.1538-7836.2008.03132.x. [DOI] [PubMed] [Google Scholar]
- 22.Larson MK, Watson SP. Regulation of proplatelet formation and platelet release by integrin alpha IIb beta3. Blood. 2006;108:1509–14. doi: 10.1182/blood-2005-11-011957. [DOI] [PubMed] [Google Scholar]
- 23.Ghevaert C, Salsmann A, Watkins NA, Schaffner-Reckinger E, Rankin A, Garner SF, et al. A nonsynonymous SNP in the ITGB3 gene disrupts the conserved membraneproximal cytoplasmic salt bridge in the alphaIIbbeta3 integrin and cosegregates dominantly with abnormal proplatelet formation and macrothrombocytopenia. Blood. 2008;111:3407–14. doi: 10.1182/blood-2007-09-112615. [DOI] [PubMed] [Google Scholar]
- 24.Larson MK, Watson SP. A product of their environment: do megakaryocytes rely on extracellular cues for proplatelet formation? Platelets. 2006;17:435–40. doi: 10.1080/09537100600772637. [DOI] [PubMed] [Google Scholar]
- 25.Alimardani G, Guichard J, Fichelson S, Cramer EM. Pathogenic effects of antiglycoprotein Ib antibodies on megakaryocytes and platelets. Thromb Haemost. 2002;88:1039–46. [PubMed] [Google Scholar]
- 26.Chang M, Nakagawa PA, Williams SA, Schwartz MR, Imfeld KL, Buzby JS, et al. Immune thrombocytopenic purpura (ITP) plasma and purified ITP monoclonal autoantibodies inhibit megakaryocytopoiesis in vitro. Blood. 2003;102:887–95. doi: 10.1182/blood-2002-05-1475. [DOI] [PubMed] [Google Scholar]
- 27.Kanaji T, Russell S, Ware J. Amelioration of the macrothrombocytopenia associated with the murine Bernard-Soulier syndrome. Blood. 2002;100:2102–7. doi: 10.1182/blood-2002-03-0997. [DOI] [PubMed] [Google Scholar]
- 28.Corash L, Chen HY, Levin J, Baker G, Lu H, Mok Y. Regulation of thrombopoiesis: effects of the degree of thrombocytopenia on megakaryocyte ploidy and platelet volume. Blood. 1987;70:177–85. [PubMed] [Google Scholar]
- 29.Stenberg PE, Levin J. Ultrastructural analysis of acute immune thrombocytopenia in mice: dissociation between alterations in megakaryocytes and platelets. J Cell Physiol. 1989;141:160–9. doi: 10.1002/jcp.1041410124. [DOI] [PubMed] [Google Scholar]
- 30.Paulus JM, Bury J, Grosdent JC. Control of platelet territory development in megakaryocytes. Blood Cells. 1979;5:59–88. [PubMed] [Google Scholar]
- 31.Balduini A, Malara A, Pecci A, Badalucco S, Bozzi V, Pallotta I, et al. Proplatelet formation in heterozygous Bernard-Soulier syndrome type Bolzano. J Thromb Haemost. 2009;7:478–84. doi: 10.1111/j.1538-7836.2008.03255.x. [DOI] [PubMed] [Google Scholar]
- 32.Strassel C, Eckly A, Léon C, Petitjean C, Freund M, Cazenave J-P, et al. Intrinsic impaired proplatelet formation and microtubule coil assembly of megakaryocytes in a mouse model of Bernard-Soulier syndrome. Haematologica. 2009;94:800–10. doi: 10.3324/haematol.2008.001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ware J, Russell S, Ruggeri ZM. Generation and rescue of a murine model of platelet dysfunction: the Bernard-Soulier syndrome. Proc Natl Acad Sci USA. 2000;97:2803–8. doi: 10.1073/pnas.050582097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kato K, Martinez C, Russell S, Nurden P, Nurden A, Fiering S, et al. Genetic deletion of mouse platelet glycoprotein Ibbeta produces a Bernard-Soulier phenotype with i ncreased alpha-granule size. Blood. 2004;104:2339–44. doi: 10.1182/blood-2004-03-1127. [DOI] [PubMed] [Google Scholar]
- 35.Poujol C, Ware J, Nieswandt B, Nurden AT, Nurden P. Absence of GPIbalpha is responsible for aberrant membrane development during megakaryocyte maturation: ultrastructural study using a transgenic model. Exp Hematol. 2002;30:352–60. doi: 10.1016/s0301-472x(02)00774-9. [DOI] [PubMed] [Google Scholar]
- 36.Nurden P, Nurden AT. Congenital disorders associated with platelet dysfunctions. Thromb Haemost. 2008;99:253–63. doi: 10.1160/TH07-09-0568. [DOI] [PubMed] [Google Scholar]


