Abstract
Effects of angiotensin (Ang)-(1–7), an AngII metabolite, on bone marrow-derived hematopoietic cells were studied. We identified Ang-(1–7) to stimulate proliferation of human CD34+ and mononuclear cells in vitro. Under in vivo conditions, we monitored proliferation and differentiation of human cord blood mononuclear cells in NOD/SCID mice. Ang-(1–7) stimulated differentially human cells in bone marrow and accumulated them in the spleen. The number of HLA-I+ and CD34+ cells in the bone marrow was increased 42-fold and 600-fold, respectively. These results indicate a decisive impact of Ang-(1–7) on hematopoiesis and its promising therapeutic potential in diseases requiring progenitor stimulation.
Keywords: angiotensin, CD34, hematopoietic stem cell
Introduction
Angiotensin (Ang) II and other active peptides of the renin-angiotensin system, such as Ang IV [Ang-(3–8)] and Ang-(1–7), are mostly known as regulators in the cardiovascular system.1 However, recent studies have shown that AngII and Ang-(1–7) also have regulatory effect on tissue regeneration, cellular proliferation and growth factor release.2,3 In vivo studies have shown that AngII and Ang-(1–7) increased hematopoietic recovery after myelosuppression and progenitor engraftment.4,5 The increases in cell numbers were most profound and long-lasting in the bone marrow, consistent with the observed effects on early progenitors, and exhibited effects on multiple blood cell lineages. In addition to stimulating bone marrow regeneration in vivo, AngII was also shown to stimulate the proliferation of hematopoietic progenitors in vitro in cells isolated from both mouse bone marrow and human cord blood.5,6 A recent publication showing the presence of a key component of the renin-angiotensin system, angiotensin converting enzyme, in the human hemangioblast, supports the critical nature of this system in hematopoietic development.7 In this report, we expand these observations to show that Ang-(1–7) stimulates the proliferation and differentiation of CD34+ and mononuclear cells isolated from human cord blood.8 Since it is not known how these stimulatory effects of Ang-(1–7) relate to stimulation of specific subspecies of mononuclear and progenitor cells in vivo, we furthermore set out to study the in vivo effects of Ang-(1–7) on human mononuclear cells monitored in NOD/SCID mice.
Design and Methods
Effects of Ang-(1–7) on human mononuclear cells in vitro
Cord blood was obtained from the LAC Obstetrics Service immediately after delivery by vacutainers containing 10 mM of EDTA. The red blood cells were lysed, the nucleated cells were pelleted and resuspended (107 cells/mL). To isolate CD34+ cells, 10 μL of an antibody cocktail, containing antibodies to glycophorin A, CD2, CD3, CD14, CD16, CD19, CD24, CD56, and CD66b (CellSystems, St. Katharinen, Germany), was added per ml of cells and cells were separated with magnetic beads (negative selection).8 After isolation, the CD34+ cells were resuspended (5×104 cells/mL) in serum free StemSpan (CellSystems, St. Katharinen, Germany) containing the following human recombinant factors: 3 IU/mL erythropoietin, 20 ng/mL stem cell factor, 20 ng/mL interleukin 3 and 20 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF). The cells were cultured for six days (medium change all 24 h) at 37°C in atmosphere of 5% CO2 in air in the absence or presence of Ang-(1–7) as indicated in Figure 1A and B. These cells were washed, counted, resuspended (5×105 cells/mL) and cultured in individual cells of a 96-well microtiter plate (5×104 cells/well) in semi-solid medium (0.9% methyl cellulose in Iscove’s modified Dulbecco’s medium, 30% fetal calf serum, 1% bovine serum albumin, 10 μM 2-mercap-toethanol, 2 mM L-glutamine, 10% agar leukocyte conditioned medium with and without 3 IU/mL erythropoietin) without Ang-(1–7). At various time points after initiation of culture, the number of large colonies without evidence of erythroid differentiation as well as the number of erythroid burst-forming units- (BFU-E) per well were counted.
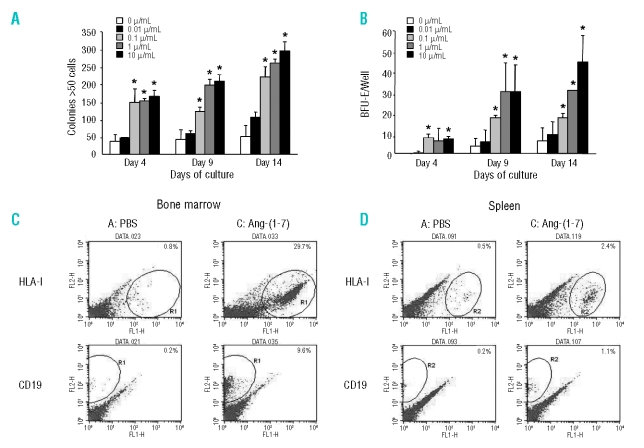
Figure 1.
In vitro effect of Ang-(1–7) on cultured human cord blood derived CD34+ cells. The CD34+ cells were exposed to different concentrations of Ang-(1–7) in suspension culture, and then 5×104 cells/well were transferred to semi-solid medium. The number of large colonies without evidence of erythroid differentiation (A) and BFU-E formed (B) where assessed at various times after initiation of culture in semi-solid medium. *p<0.05 vs. control [0 mg/mL Ang-(1–7)]. In vivo effect of Ang-(1–7) on bone marrow and spleen cells in NOD/SCID mice. Representative flow cytometry density plots showing the effect of PBS and 10.8 μg Ang-(1–7)/mouse on isolated bone marrow cells (C) and spleen cells (D). The percentage of cells positive for human HLA-I and CD19 is indicated in the top right corner of each diagram.
Effects of Ang-(1–7) on human mononuclear cells in NOD/SCID mice
Human cord blood
Human cord blood cells were harvested at the time of delivery at the Charité, Campus Benjamin Franklin, Department of Obstetrics and HELIOS Klinikum Berlin-Buch by gravity into tubes containing 5,000 IU heparin.
Mononuclear cells (MNC) were harvested from the cord blood by density gradient centrifugation using Lymphocytes separation medium (PAA Laboratories, Cölbe, Germany). Cells were centrifuged at 400× g for 30 min at room temperature. The MNCs at the interface were washed and resuspended (5×107 cells/mL).
Mice
NOD/LtSz-SCID/SCID mice were used in the experiments. The animals were maintained under standardized conditions with an artificial 12 h dark-light cycle, with free access to food and water in the animal facility of the Max Delbrück Center (Berlin-Buch, Germany). All animal studies were performed according to national guidelines and approved by the institutional animal care committees. Mice were irradiated sublethally at 6–8 weeks of age with a dose of 1.6 Gy of a 137Cs-γ source before cell transplantation.
Treatment procedure
After isolation of human cord blood MNCs, 1×107 cells were injected intravenously into each mouse. Starting two days after injection, the mice received daily subcutaneous (s.c.) injections of PBS (Group A), 1.08 μg/d Ang-(1–7) (Group B) or 10.8 μg/d Ang-(1–7) (Group C). At day 30, the mice were euthanized and the spleen and bone marrow harvested to assess the engraftment of human progenitors and mature cells by flow cytometry. The average amount of harvested MNCs was 2×107 from bone marrow and 1.5×108 from spleen without differences between the treatment groups.
Flow cytometry
Two-color immunofluorescence cytometry was used to quantify the expression of cell surface molecules on suspended bone marrow and spleen cells. Cell viability was tested by staining with trypanblue. The cells were stained with phycoerythrin (PE) or fluorescein isothiocyanate (FITC) labeled monoclonal antibodies against human anti-CD34 (clone 581), anti-CD11a (clone G43-25B), anti-CD19 (clone HI B19), anti-CD 15 (clone H 198), anti-HLA-DR (clone G46-6), anti-HLA-I (clone G46-2.6) from Becton Dickinson (Becton Dickinson, Heidelberg, Germany). FITC and PE conjugated iso-type-matched mouse IgGs (clone X40) from Becton Dickinson were used to determine background staining. After 25 min of incubation in the dark, cells were washed in PBS and analyzed immediately in a dual-laser FACSCalibur (Becton Dickinson) flow cytometer with Cell-Quest-Software. Each measurement contained 10,000 events and cell debris was excluded by threshold.
Statistical analysis
Data was expressed by mean±SEM. Statistical analysis was performed by the Student’s t test. Statistical significance was assumed when p ≥ 0.05.
Results and Discussion
In vitro Ang-(1–7) effects on human mononuclear cells
CD34+ cells isolated from human cord blood were expanded in vitro in the presence of Ang-(1–7). After the expansion phase, the cells were transferred to semi-solid medium for assessment of colony formation. This experiment showed that in vitro Ang-(1–7) increased the number of large (> 50 cells per colony) colonies without evidence of erythroid differentiation (Figure 1A) and BFU-E (Figure 1B) in a dose-dependent manner.
In vivo Ang-(1–7) effects on human mononuclear cells in mice
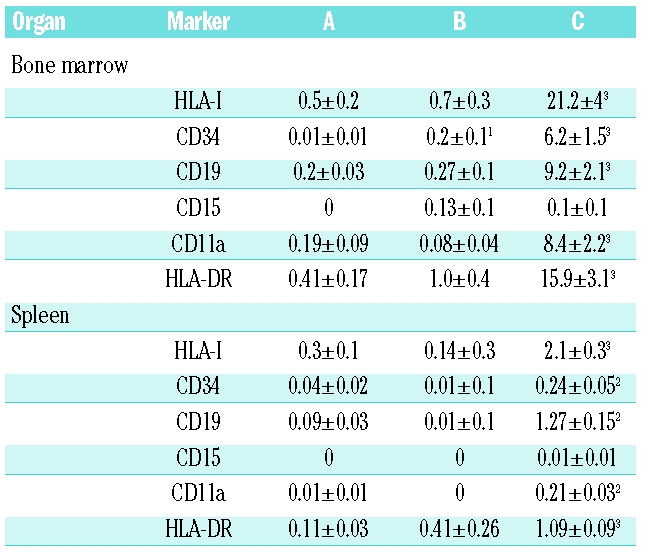
To investigate whether these effects of Ang-(1–7) could also be observed under in vivo conditions and to identify the cell populations that are stimulated, a study was designed to evaluate human cord blood progenitor engraftment into NOD/SCID mice after application of two different concentrations of Ang-(1–7) by daily s.c. injection. Administration of PBS alone resulted in approximately 0.5% of the bone marrow cells (Figure 1C and Table 1) and 0.3% of the splenocytes (Figure 1D and Table 1) being of human origin. Administration of the lower dose of Ang-(1–7) did not have significant impact on the stimulation and recruitment of human cells in bone marrow and spleen, respectively, whereas administration of the high dose of peptide increased the number of human cells (HLA-I+ cells) in the bone marrow approximately 42-fold (Figure 1C and Table 1) and in the spleen approximately 7-fold (Figure 1D and Table 1). CD34+ cells were also identified in the bone marrow and spleen of placebo-treated mice. Both doses of Ang-(1–7) increased CD34+ cells in the bone marrow in a dose-dependent manner (20- and 600-fold for low and high dose, respectively) (Table 1). At the high dose of Ang-(1–7), the percentage of splenocytes that expressed human CD34 was increased 6-fold over placebo (Table 1). Differentiated cells of human origin [myelomonocytic (CD15, CD11a, HLA DR) and B cell (CD19, HLA DR) lineage] were found at very low levels in the bone marrow (0.19–0.41%) and spleen (0–0.11%) of vehicle-treated mice (Figure 1C and D, and Table 1). While administration of low-dose Ang-(1–7) did not affect the number of differentiated cells of the hematopoietic human lineage, administration of the higher dose of the heptapeptide highly significantly increased the number of CD19+, CD15+, CD11a+, and HLA DR+ cells in the bone marrow and spleen of treated animals at day 30 (Figure 1C and D, and Table 1).
Table 1.
Percentage of human cells and subpopulations in bone marrow and spleen of NOD/SCID mice, treated for 30 days post injection of mononuclear cells with A: daily injection of PBS s.c.; B: daily injection of 1.08 μg/mouse Ang-(1–7) s.c.; C: daily injection of 10.8 μg/mouse Ang-(1–7) s.c. 1p≤0.05, 2p≤0.01, 3p≤0.001 vs. A (Student’s t test).
Ang-(1–7) has been described as having a growing number of beneficial effects in cardiovascular diseases. Our data points to the conclusion that a broad spectrum of such beneficial activities observed with Ang-(1–7) may be due to actions on early progenitor cells. Most of the Ang-(1–7) effects are NO-mediated. NO has been shown to have regulatory effects on e.g. endothelial progenitors,9 and first data indicates a potential role in the proliferation and mobilization of hematopoietic stem cells.10 Thus, further sets of experiments have to clarify whether the here newly identified effects of Ang-(1–7) are also NO-mediated.
Since stem cells derived from adults may be highly versatile or plastic, transplantation of bone marrow or activation of endogenous bone marrow cells could result in donor/activated cells possessing the added benefit of mediating the healing of injuries to the CNS, muscle, liver, and heart. Therefore, an agent that stimulates the proliferation and differentiation of stem cells has the potential for similar versatility. Given our present results, defining Ang-(1–7)’s versatility with respect to hematopoietic progenitor cell stimulation, it seems worthwhile to explore this heptapeptide as a regenerative agent beyond the scope of bone marrow repopulation and cardiovascular repair.
Footnotes
Authorship and Disclosures
SHW, SMS, AWG, FG, IF: data collection; KE, LU, HPS: data analysis; KR, TW: study design, data analysis, and writing the manuscript.
The authors reported no potential conflicts of interest.
Funding: this work was supported by a grant from ‘National Institute of Health’ (NIH; 5R01HL082722-02) and from ‘Federal Ministry of Education and Research (BMBF; 01GN0528).
References
- 1.Ferrario CM. Angiotensin I, angiotensin II and their biologically active peptides. J Hypertens. 2002;20:805–7. doi: 10.1097/00004872-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Takeda H, Katagata Y, Hozumi Y, Kondo S. Effects of angiotensin II receptor signaling during skin wound healing. Am J Pathol. 2004;165:1653–62. doi: 10.1016/S0002-9440(10)63422-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Unger T, Chung O, Csikos T, Culman J, Gallinat S, Gohlke P, et al. Angiotensin receptors. J Hypertens Suppl. 1996;14:S95–103. [PubMed] [Google Scholar]
- 4.Ellefson DD, diZerega GS, Espinoza T, Roda N, Maldonado S, Rodgers KE. Synergistic effects of co-administration of angiotensin 1–7 and Neupogen on hematopoietic recovery in mice. Cancer Chemother Pharmacol. 2004;53:15–24. doi: 10.1007/s00280-003-0710-0. [DOI] [PubMed] [Google Scholar]
- 5.Rodgers K, Xiong S, DiZerega GS. Effect of angiotensin II and angiotensin(1–7) on hematopoietic recovery after intravenous chemotherapy. Cancer Chemother Pharmacol. 2003;51:97–106. doi: 10.1007/s00280-002-0509-4. [DOI] [PubMed] [Google Scholar]
- 6.Rodgers KE, Xiong S, diZerega GS. Accelerated recovery from irradiation injury by angiotensin peptides. Cancer Chemother Pharmacol. 2002;49:403–11. doi: 10.1007/s00280-002-0434-6. [DOI] [PubMed] [Google Scholar]
- 7.Zambidis ET, Soon Park T, Yu W, Tam A, Levine M, Yuan X, et al. Expression of angiotensin-converting enzyme (CD143) identifies and regulates primitive hemangioblasts derived from human pluripotent stem cells. Blood. 2008;112:3601–14. doi: 10.1182/blood-2008-03-144766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodgers KE, Xiong S, Steer R, diZerega GS. Effect of angiotensin II on hematopoietic progenitor cell proliferation. Stem Cells. 2000;18:287–94. doi: 10.1634/stemcells.18-4-287. [DOI] [PubMed] [Google Scholar]
- 9.Urao N, Okigaki M, Yamada H, Aadachi Y, Matsuno K, Matsui A, et al. Erythropoietin-mobilized endothelial progenitors enhance reendothelialization via Akt-endothelial nitric oxide synthase activation and prevent neointimal hyperplasia. Circ Res. 2006;98:1405–13. doi: 10.1161/01.RES.0000224117.59417.f3. [DOI] [PubMed] [Google Scholar]
- 10.Santhanam AV, d’Uscio LV, Peterson TE, Katusic ZS. Activation of endothelial nitric oxide synthase is critical for erythropoietin-induced mobilization of progenitor cells. Peptides. 2008;29:1451–5. doi: 10.1016/j.peptides.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]