Abstract
To observe the effect of the new World Health Organization (WHO) criteria on the incidence of myeloproliferative neoplasms, we performed a retrospective study of a population-based registry in the Côte d’Or area, France, from 1980 to 2007. A total of 524 myeloproliferative neoplasms were registered for the 1980–2007 period, including 135 polycythemia vera, 308 essential thrombocythemia and 81 idiopathic myelofibroses. No change in the incidence of either polycythemia vera or idiopathic myelofibrosis was observed for the 2005–2007 period, compared to 1980–2004. On the contrary, a pronounced increase in the incidence of essential thrombocythemia was noted after 2005, mainly due to the use of JAK2 mutation screening and a lower threshold of platelet count. Our study confirms the relevance of the new WHO diagnostic criteria in allowing earlier diagnosis of essential thrombocythemia.
Keywords: JAK2 mutation, myeloproliferative neoplasm, polycythemia vera, essential thrombocythemia, incidence
Introduction
The incidence of the three Philadelphia chromosome negative myeloproliferative neoplasms (MPN) has been known for a long time, with large differences in the rates according to the geographical area and/or ethnic origin:1,2 in polycythemia vera, annual incidence ranges from 0.02 to 2.8/100,000 inhabitants.3 In a previous review, Johansson described these features in relation to the wide range of annual incidence rates of polycythemia vera.4 More recently, epidemiological data from the US National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program, based on more than 18,000 MPN cases from 2001 to 2003, reported essential thrombocythemia incidence rates at lower levels5 than those generally found in other studies.4,6,7 In contrast, in previous studies, we and others noted a slight increase in the incidence of essential thrombocythemia over time, mainly related to the wider availability of platelet counts in routine examinations.4,8 In the same way, a rise in the incidence of idiopathic myelofibrosis from 1990 to 2005 has been reported recently, whereas polycythemia vera incidence remained stable during the same time.9 In 2005, the discovery of the Janus kinase 2 (JAK2) mutation (a somatic 1849G>T mutation on exon 14) called the JAK2-V617F mutation had huge consequences not only on the understanding of the physiopathology of MPN, but also on the routine practice of laboratories.10 Indeed, the JAK2-V617F mutation has been described in almost 95% of polycythemia vera, 50–70% of essential thrombocythemia and 50% of idiopathic myelofibrosis and has became a very useful diagnostic test in MPN. Moreover, more recently, other mutations (JAK2 exon 12 and MPL) have been reported in less than 5% of MPN.11,12
As a consequence of these discoveries, a complete revision of the diagnostic criteria for MPN, which now include screening for clonal markers such as the JAK2-V617F mutation, has been proposed.13 The aim of our study was to evaluate the impact of the new WHO diagnostic criteria on the incidence of MPN in a well-defined population.
Design and Methods
Population and patients
The Registry of hematologic malignancies of the Côte d’Or area, France, covers a population of 506,755 inhabitants (35% rural and 65% urban). Dijon, the main city has the only University hospital in the area, and most specialized hematologic tests, especially the JAK2-V617F mutation and progenitor growth cultures are performed in the laboratories of this hospital. All hematologic malignancies in the Côte d’Or area have been registered exhaustively since 1980 and are reviewed by a three-expert panel.
From 1980 to 2000, MPN were diagnosed according to the Polycythemia Vera Study Group criteria,14 then after 2000 according to the World Health Organization (WHO) criteria,15 including bone marrow biopsy. For the 2005–2007 period, MPN patients were classified according to the WHO 2001 classification, then according to the WHO 2008 classification,13 and the results were compared.
The JAK2-V617F mutation was screened for retrospectively on stored DNA samples from MPN patients from the year 2005, then prospectively after 2006 on samples collected at the time of diagnosis. DNA storage was performed in the biobank Centre de Ressources Biologiques Ferdinand Cabanne in Dijon, and 99 samples were tested. Purified blood granulocytes were prepared as previously described16 and genomic DNA was prepared using QIAamp DNA mini-kits (Qiagen). Levels of expression of JAK2 wild-type (WT) or mutated (V617F) were determined in duplicate using the same sensitive (0.15% JAK2-V617F) allele-specific quantitative PCRs (AS-qPCR) with the specificity based on sense forward primers.16 The JAK2 exon 12 mutation was screened for using a PCR-based sequencing approach on genomic DNA samples.
Statistical analysis
Age- and sex-specific incidence rates were calculated using population estimates from the Institut National de la Statistique et des Etudes (INSEE), the European standard population (ESP) and the World standard population (WSP). Comparison of incidence rates was based on a Poisson’s model. A mean comparison two-sided parametrical test was used to compare different groups of patients. Data were analyzed using Stata software. p<0.05 was considered statistically significant.
Results and Discussion
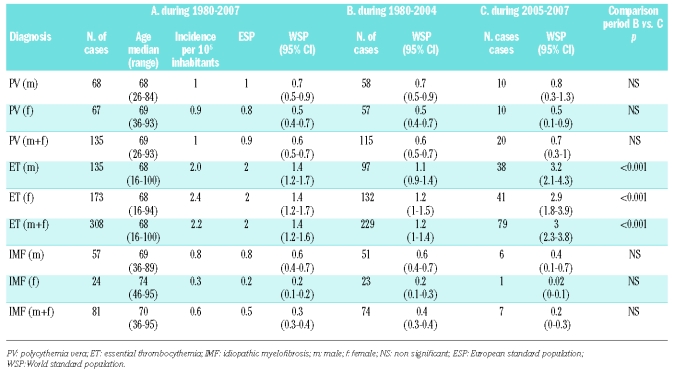
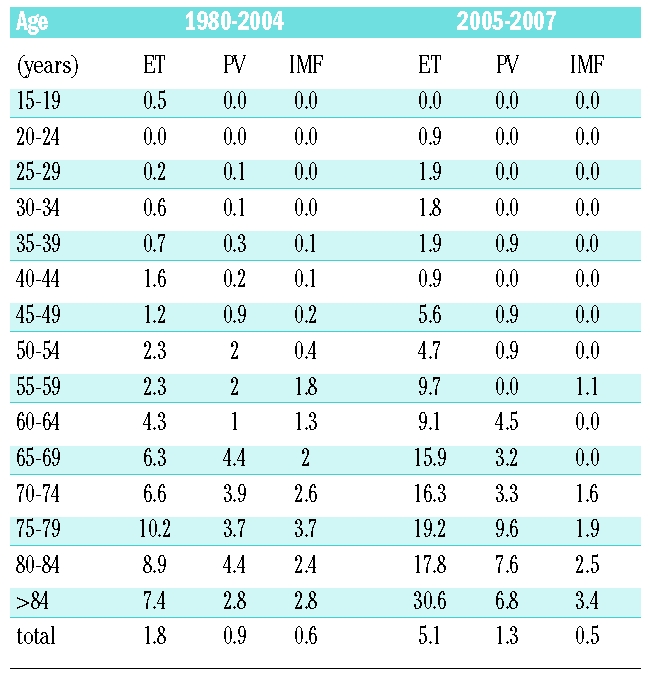
A total of 524 MPN were registered for the 1980–2007 period, including 135 polycythemia vera (68 males and 67 females), 308 essential thrombocythemia (135 males and 173 females) 81 idiopathic myelofibroses (57 males and 24 females) (Table 1). Depending on the ESP or on the WSP, the 1980–2007 incidence rates per 100,000 inhabitants per year were respectively 0.9 and 0.6 for polycythemia vera, 2 and 1.4 for essential thrombocythemia, and 0.5 and 0.3 for idiopathic myelofibrosis, (Table 1). In essential thrombocythemia, females were predominant (56%) whereas in idiopathic myelofibrosis, males were predominant (70%). In polycythemia vera, the sex ratio was 1 (Table 1). The incidence rates increased with age for all MPN (Table 2). This contrasts with a previous report which observed a peak in the incidence of essential thrombocythemia in young age;17 however, a recent large-scale epidemiological study did not confirm this finding.7
Table 1.
Incidence rates in the Côte d’Or area, 1980–2007 for myeloproliferative neoplasms.
Table 2.
Annual age-standardized incidence rates (per 100,000) for essential thrombocythemia (ET), polycythemia vera (PV) and idiopathic myelofibrosis (IMF) in the Côte d’Or area.
When analyzed according to the WHO 2001 classification, the incidence of essential thrombocythemia before 2005 was not statistically different from that after 2005 (WSP 1.2 versus 1.52 respectively, p>0.05). However, as previously suspected,7 based on the recent WHO 2008 diagnostic criteria, taking into account both clonal markers, mainly the JAK2-V617F mutation, and a lower threshold of platelet values (450×109/L), the incidence of essential thrombocythemia in the 2005–2007 period was more than double that in 1980–2004, in both males and females: it increased from 1.2 to 3 (Figure 1).
Figure 1.
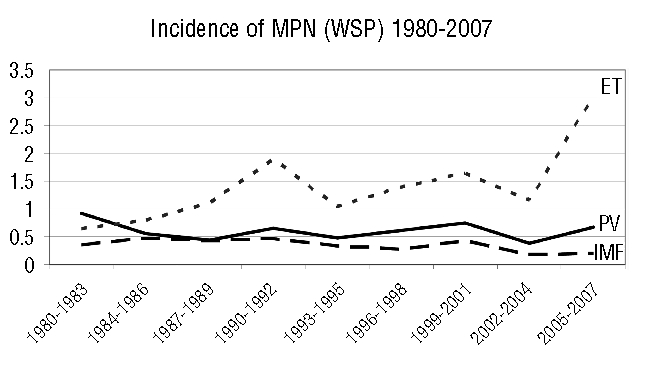
Evolution of incidence of myeloproliferative neoplasms (MPN), incidence rates from 1980 to 2007 in the Côte d’Or area registry. There was a significant increase in the incidence of essential thrombocytemia (p<0.001) in 2005–2007 compared to 1980–2004 when diagnosed according to the WHO 2008 criteria. Polycythemia vera (PV), essential thrombocythemia (ET) and idiopathic myelofibrosis (IMF).
One explanation of the increase in the number of essential thrombocythemia cases from 2005 is a change in the diagnostic strategy: firstly, according to the new WHO criteria, the threshold platelet count at which essential thrombocythemia would be suspected decreased from 600×109/L to 450×109/L. Secondly, the JAK2 test has been widely used as a screening test in first intention in case of thrombocytosis. In contrast, until 2005, diagnosis of essential thrombocythemia was mainly based on the exclusion of all other causes of chronic thrombocytosis, and apart from bone marrow histology features, no positive criteria were available.15 Moreover, it must be pointed out that the results of bone marrow biopsy were dependent on the experience of the hemopathologist, and sometimes in difficult cases, no clear diagnosis was made.
As a consequence, the mean number of essential thrombocythemia registered every year in 1980–2004 was 9, whereas it rose to 27 in 2005, 26 in 2006 and 26 in 2007. Of the 79 cases of newly diagnosed essential thrombocythemia from 2005 to 2007, 13 patients had previously been followed for thrombocytosis without certain diagnosis. As soon as the JAK2 test was available, a lot of unexplained thrombocytoses were tested, leading to new further exams and confirming the MPN in some cases. In these cases, the presence of the JAK2-V617F mutation led to the retrospective diagnosis of essential thrombocythemia, but later than the beginning of the increase in the platelet count. This caused, at least in part, an artificial increase (estimated at +0.4) in the incidence of essential thrombocythemia during the 2005–2007 period.
Essential thrombocythemia patients diagnosed after 2005 also had a lower platelet count than did those diagnosed before 2005 (835,203 versus 945,716×109/L, p<0.05). This point is related to the high sensitivity of the JAK2 test, even at the beginning of the disease, and also supports the fact that in the future, essential thrombocythemia patients will probably be diagnosed at an earlier phase than previously, at least for most of them. However, with regard to the median age at the time of diagnosis, no difference between the two periods was noticed. Previously, in order to explore thrombocytosis, the threshold sustained platelet count was 600×109/L. In confirmation of our observation, the new 2008 WHO criteria lowered the platelet threshold from 600×109/L to 450×109/L. The argument was that the previous platelet threshold compromised the detection of early-phase essential thrombocythemia.13 In fact, this change seems to be a good decision, because in our series, 24% of recently essential thrombocythemia diagnosed after 2004 had less than 600×109/L platelets. In contrast with essential thrombocythemia, no significant modification in the incidence of polycythemia vera or idiopathic myelofibrosis was noted over the same period (Table 1). In fact, unlike essential thrombocythemia, the diagnosis of polycythemia vera and idiopathic myelofibrosis have been based on positive criteria for a long time, and screening for the JAK2 mutation did not considerably change the incidence of these diseases. In our study, before 2005, the diagnosis of polycythemia vera was based on the absence of cause of secondary erythrocytosis, and the presence of splenomegaly, endogenous erythroid colony formation, thrombocytosis, a low erythropoietin (EPO) level or bone marrow biopsy.15 However, screening for the JAK2 mutation completely transformed the diagnostic approach for this disease: current guidelines in suspected polycythemia vera recommend screening for this mutation in peripheral blood together with concomitant determination of EPO.6,13 For idiopathic myelofibrosis, the incidence was close to that in other reports, with no significant change over time.3 This is in contrast with Hemminki et al. who found an increase in the incidence of idiopathic myelofibrosis, related to probable indolent forms of idiopathic myelofibrosis reported.9 In the 20 polycythemia vera diagnosed after 2004, 17 were positive for the JAK2-V617F mutation, with a mean JAK2-V617F allele burden of 38.6%. There were 3 JAK2 exon 12 positive polycythemia vera patients that is a higher proportion (15%) of polycythemia vera patients compared to the low percentage of such a mutation usually observed in large series.18,19 This discrepancy may be due to our relatively small number of polycythemia vera (n=20) registered since 2005. Among the 79 essential thrombocythemia diagnosed after 2004, 76% were JAK2-V617F positive, with a mean allele burden of 19%. Seven idiopathic myelofibroses were diagnosed until 2005, and the JAK2-V617F mutation was positive in 5 of them, with a mean level of JAK2 of 43.9%. The percentages of JAK2-V617F positive patients with essential thrombocythemia, polycythemia vera and idiopathic myelofibrosis were close to those we described previously,16 and have been classically observed.20–22 In the same way, as generally noted, the median JAK2-V617F allele burden was higher both in polycythemia vera and idiopathic myelofibrosis than in essential thrombocythemia.16 Of note, the high proportion of JAK2-V617F positive essential thrombocythemia patients (76%), related to both a relatively small number of essential thrombocythemia (n=79) and performing the JAK2 mutation with a high sensitivity test on purified granulocytes, at the time of diagnosis, prior to any cytoreductive therapy: recent studies have shown the ability of hydroxyurea or interferon to reduce and even to make the mutated clone undetectable.23–25 These hypotheses could explain, at least in part, the high proportion of JAK2-V617F positive essential thrombocythemia patients. Despite weaknesses in our study, mainly due to the relatively small size of the studied population (500,000 inhabitants in the Côte d’Or area), our work presents some strong and reliable methodological features: (i) the Côte d’Or area has a stable population with a low rate of people moving away from the region; (ii) most specialized hematologic tests are performed in the hematology laboratory of the only University hospital in the area, and very few tests for hematologic malignancies are performed outside this center; (iii) all hematologic malignancies in the Côte d’Or area have been exhaustively registered since 1980 and have been reviewed not only by epidemiologists but also by the same three-expert panel of hematologists. In conclusion, routine use of the new WHO diagnostic criteria allows accurate and earlier diagnosis of MPN, especially essential thrombocythemia, and is associated with a significant increase in the incidence of essential thrombocythemia. In terms of public health, our study is a particularly clear example of the usefulness of molecular epidemiology in order to produce more accurate incidence rates and to evaluate the consequences at the population level. Prospective studies are needed to evaluate whether earlier diagnosis and treatment of essential thrombocythemia will be associated with fewer side effects, particularly thrombotic events.
Acknowledgments
we wish to thank Mrs Dominique Bouchot, Martine Courtois, Isabelle Helot, Aurélie Herry for excellent technical help, and Dr Sylvie Hermouet for relevant discussion.
Footnotes
Authorship and Disclosures
FG and CS designed the study; FG analyzed the data and wrote the paper; MM performed statistical analyses; GB, IJ, PMC and CS collected the data; SC performed the JAK2 exon 12 tests; IL and EF enrolled patients; MM enrolled patients and revised the manuscript.
All the individuals listed as co-authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship as detailed in the ICMJE website: http://www.icmje.org/#author.
The authors reported no potential conflicts of interest.
Funding: this work was supported by a grant from the Ligue contre le Cancer, Comité de Côte d’Or.
References
- 1.Chaiter Y, Brenner B, Aghai E, Tatarsky I. High incidence of myeloproliferative disorders in Ashkenazi Jews in northern Israel. Leuk Lymphoma. 1992;7:251–5. doi: 10.3109/10428199209053630. [DOI] [PubMed] [Google Scholar]
- 2.Ania BJ, Suman VJ, Sobell JL, Codd MB, Silverstein MN, Melton LJ., 3rd Trends in the incidence of polycythemia vera among Olmsted County, Minnesota residents, 1935–1989. Am J Hematol. 1994;47:89–93. doi: 10.1002/ajh.2830470205. [DOI] [PubMed] [Google Scholar]
- 3.Johansson P, Kutti J, Andreasson B, Safai-Kutti S, Vilen L, Wedel H, et al. Trends in the incidence of chronic Philadelphia chromosome negative (Ph-) myeloproliferative disorders in the city of Goteborg, Sweden, during 1983–99. J Intern Med. 2004;256:161–5. doi: 10.1111/j.1365-2796.2004.01357.x. [DOI] [PubMed] [Google Scholar]
- 4.Johansson P. Epidemiology of the myeloproliferative disorders polycythemia vera and essential thrombocythemia. Semin Thromb Hemost. 2006;32:171–3. doi: 10.1055/s-2006-939430. [DOI] [PubMed] [Google Scholar]
- 5.Rollison DE, Howlader N, Smith MT, Strom SS, Merritt WD, Ries LA, et al. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001–2004, using data from the NAACCR and SEER programs. Blood. 2008;112:45–52. doi: 10.1182/blood-2008-01-134858. [DOI] [PubMed] [Google Scholar]
- 6.Girodon F, Lippert E, Mossuz P, Dobo I, Boiret-Dupre N, Lesesve JF, et al. JAK2V617F detection and dosage of serum erythropoietin: first steps of the diagnostic work-up for patients consulting for elevated hematocrit. Haematologica. 2007;92:431–2. doi: 10.3324/haematol.10660. [DOI] [PubMed] [Google Scholar]
- 7.Phekoo KJ, Richards MA, Moller H, Schey SA. The incidence and outcome of myeloid malignancies in 2,112 adult patients in southeast England. Haematologica. 2006;91:1400–4. [PubMed] [Google Scholar]
- 8.Girodon F, Jooste V, Maynadie M, Favre B, Schaeffer C, Carli P. Incidence of chronic Philadelphia chromosome negative (Ph-) myeloproliferative disorders in the Cote d’Or area, France, during 1980–99. J Intern Med. 2005;258:90–1. doi: 10.1111/j.1365-2796.2005.01505.x. [DOI] [PubMed] [Google Scholar]
- 9.Hemminki K, Zhang H, Sundquist J, Lorenzo Bermejo J. Myeloproliferative disorders in Sweden: Incidence trends and multiple tumors. Leuk Res. 2009;33:e14–6. doi: 10.1016/j.leukres.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 10.James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–8. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 11.Pikman Y, Lee BH, Mercher T, McDowell E, Ebert BL, Gozo M, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006;3:e270. doi: 10.1371/journal.pmed.0030270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott LM, Tong W, Levine RL, Scott MA, Beer PA, Stratton MR, et al. AK2J exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med. 2007;356:459–68. doi: 10.1056/NEJMoa065202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tefferi A, Vardiman JW. Classification and diagnosis of myeloproliferative neoplasms: the 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia. 2008;22:14–22. doi: 10.1038/sj.leu.2404955. [DOI] [PubMed] [Google Scholar]
- 14.Berlin NI. Diagnosis and classification of the polycythemias. Semin Hematol. 1975;12:339–51. [PubMed] [Google Scholar]
- 15.Jaffe ESHN, Stein H, Vardiman JW. World Health Organization Classification of Tumours of Hematopoietic and Lymphoid Tissues. IARC press ed. Lyon, France: World Health Organisation; 2001. [Google Scholar]
- 16.Lippert E, Boissinot M, Kralovics R, Girodon F, Dobo I, Praloran V, et al. The JAK2- V617F mutation is frequently present at diagnosis in patients with essential thrombocythemia and polycythemia vera. Blood. 2006;108:1865–7. doi: 10.1182/blood-2006-01-013540. [DOI] [PubMed] [Google Scholar]
- 17.Jensen MK, de Nully Brown P, Nielsen OJ, Hasselbalch HC. Incidence, clinical features and outcome of essential thrombocythaemia in a well defined geographical area. Eur J Haematol. 2000;65:132–9. doi: 10.1034/j.1600-0609.2000.90236.x. [DOI] [PubMed] [Google Scholar]
- 18.Cazzola M. Somatic mutations of AK2 exon 12 as a molecular basis of J erythrocytosis. Haematologica. 2007;92:1585–9. doi: 10.3324/haematol.11506. [DOI] [PubMed] [Google Scholar]
- 19.Scott LM, Beer PA, Bench AJ, Erber WN, Green AR. Prevalance of JAK2 V617F and exon 12 mutations in polycythaemia vera. Br J Haematol. 2007;139:511–2. doi: 10.1111/j.1365-2141.2007.06806.x. [DOI] [PubMed] [Google Scholar]
- 20.Campbell PJ, Scott LM, Buck G, Wheatley K, East CL, Marsden JT, et al. Definition of subtypes of essential thrombocythaemia and relation to polycythaemia vera based on JAK2 V617F mutation status: a prospective study. Lancet. 2005;366:1945–53. doi: 10.1016/S0140-6736(05)67785-9. [DOI] [PubMed] [Google Scholar]
- 21.Vannucchi AM, Antonioli E, Guglielmelli P, Longo G, Pancrazzi A, Ponziani V, et al. Prospective identification of high-risk polycythemia vera patients based on JAK2(V617F) allele burden. Leukemia. 2007;21:1952–9. doi: 10.1038/sj.leu.2404854. [DOI] [PubMed] [Google Scholar]
- 22.Barosi G, Bergamaschi G, Marchetti M, Vannucchi AM, Guglielmelli P, Antonioli E, et al. JAK2 V617F mutational status predicts progression to large splenomegaly and leukemic transformation in primary myelofibrosis. Blood. 2007;110:4030–6. doi: 10.1182/blood-2007-07-099184. [DOI] [PubMed] [Google Scholar]
- 23.Kiladjian JJ, Cassinat B, Turlure P, Cambier N, Roussel M, Bellucci S, et al. High molecular response rate of polycythemia vera patients treated with pegylated interferon α-2a. Blood. 2006;108:2037–40. doi: 10.1182/blood-2006-03-009860. [DOI] [PubMed] [Google Scholar]
- 24.Ricksten A, Palmqvist L, Johansson P, Andreasson B. Rapid decline of JAK2V617F levels during hydroxyurea treatment in patients with polycythemia vera and essential thrombocythemia. Haematologica. 2008;93:1260–1. doi: 10.3324/haematol.12801. [DOI] [PubMed] [Google Scholar]
- 25.Girodon F, Schaeffer C, Cleyrat C, Mounier M, Lafont I, Santos FD, et al. Frequent reduction or absence of detection of the JAK2-mutated clone in JAK2V617F-positive patients within the first years of hydroxyurea therapy. Haematologica. 2008;93:1723–7. doi: 10.3324/haematol.13081. [DOI] [PubMed] [Google Scholar]