Abstract
OBJECTIVE: To determine the rate of medical complications from long-term video-electroencephalographic (EEG) monitoring for epilepsy.
PATIENTS AND METHODS: We reviewed the medical records of 428 consecutive adult patients with epilepsy who were admitted for diagnostic scalp video-EEG monitoring at Mayo Clinic's site in Arizona from January 1, 2005, to December 31, 2006; 149 met inclusion criteria for the study. Seizure number and type as well as timing and presence of seizure-related adverse outcomes were noted.
RESULTS: Of the 149 adult patients included in the study, seizure clusters occurred in 35 (23%); 752 seizures were recorded. The mean time to first seizure was 2 days, with a mean length of stay of 5 days. Among these patients, there was 1 episode of status epilepticus, 3 potentially serious electrocardiographic abnormalities, 2 cases of postictal psychosis, and 4 vertebral compression fractures during a generalized convulsion, representing 11% of patients with a recorded generalized tonic-clonic seizure. No deaths, transfers to the intensive care unit, falls, dental injuries, or pulmonary complications were recorded. An adverse event requiring intervention or interfering with normal activity occurred in 21% of these patients. Length of stay was not affected by occurrence of adverse events.
CONCLUSION: Prolonged video-EEG monitoring is an acceptably safe procedure. Adverse events occur but need not result in substantial morbidity or increase length of hospitalization. Appropriate precautions must be in place to prevent falls and promptly detect and treat seizure clusters, status epilepticus, serious electrocardiographic abnormalities, psychosis, and fractures.
Prolonged video-electroencephalographic monitoring is an acceptably safe procedure; adverse events occur but need not result in substantial morbidity or increase length of hospitalizations. Appropriate precautions must be in place to prevent falls and promptly detect and treat seizure clusters, status epilepticus, serious electrocardiographic abnormalities, psychosis, and fractures.
CPS = complex partial seizure; ECG = electrocardiogram; EEG = electroencephalographic; EMU = epilepsy monitoring unit; GTC = generalized tonic-clonic; SUDEP = sudden unexplained death in epilepsy
Inpatient video-electroencephalographic (EEG) monitoring is a valuable tool for diagnosis of recurrent spells, epilepsy classification, and evaluation for epilepsy surgery in patients with intractable partial epilepsy. The yield of video-EEG monitoring is highly dependent on the recording of seizures. To increase the likelihood of capturing events in a timely fashion, it is standard practice to use activating procedures such as reduction of antiepileptic drugs, sleep deprivation, hyperventilation, and photic stimulation. With these provocative maneuvers, patients may experience seizures of greater frequency and/or severity than their norm, potentially increasing risk of seizure-related injury. A recent widely publicized death during video-EEG monitoring has raised awareness among neurologists and the general public of the risks inherent in video-EEG monitoring.1,2
Persons with epilepsy are at greater risk of accidental injury and death than the general population.3-5 Although this greater risk in part reflects underlying or comorbid medical conditions, between one-fourth and one-third of reported injuries are the direct result of seizures or seizure-related falls. In population-based studies, commonly described seizure-related traumas include head injuries, soft tissue injuries, dental injuries, fractures, burns, drowning, and motor vehicle crashes.5 However, surprisingly little is known about the frequency of these complications during video-EEG monitoring. The risk of harm from seizures in the inpatient setting could be lower than in the community because safety precautions can be in place to address increased seizure activity and to avoid falls. Furthermore, many potentially dangerous situations faced in daily life, such as driving, cooking, bathing, and swimming, are of course absent in the hospital setting.
For editorial comment, see page 493
The increased rates of morbidity and mortality in persons with epilepsy argue for the use of diagnostic video-EEG monitoring to potentially improve diagnosis and treatment and thereby minimize future injury. However, the lack of information on risks inherent in video-EEG monitoring may make it more difficult to counsel patients considering admission for epilepsy monitoring and to obtain their informed consent. Furthermore, the lack of benchmark information hinders the assessment of the adequacy and efficacy of safety measures used in epilepsy monitoring units (EMUs), which are not currently standardized.6 Our study sought to determine the frequency of prolonged or severe seizures and seizure-related injuries in patients undergoing diagnostic video-EEG monitoring at a tertiary epilepsy referral center.
PATIENTS AND METHODS
After approval by the Mayo Clinic Institutional Review Board, the records of all consecutive adult patients admitted for video-EEG monitoring at Mayo Clinic's site in Arizona from January 1, 2005, to December 31, 2006, were reviewed. Only patients admitted with a diagnosis of recurrent spells of indeterminate etiology or for a presurgical evaluation and with a final discharge diagnosis of partial or generalized epilepsy were included. Pediatric patients were excluded. Patients with both epilepsy and psychogenic nonepileptic seizures were included, but only their recorded epileptic seizures were used in the analysis. Patients with exclusively psychogenic nonepileptic events or who had undergone intracranial EEG studies were excluded. Simple partial seizures, complex partial seizures (CPSs), and primary and secondary generalized tonic-clonic (GTC) seizures were analyzed. Absence (petit mal), myoclonic, and psychogenic nonepileptic seizures were not included in the analysis. The number, type, and timing of recorded seizures were determined from the hospital record, as was the presence of seizure-related adverse outcomes, including development of seizure cluster or status epilepticus, postictal psychosis, falls, orthopedic injury, aspiration pneumonia, pulmonary edema, cardiac arrest, or any complication requiring transfer to an intensive care unit. Seizures were defined by the presence of both clinical symptoms and electrographic activity. Seizure clusters were defined as 3 or more CPSs or GTC seizures in a 4-hour or 24-hour period. Simple partial seizures were not included in the cluster analysis. Prolonged seizures were defined as those lasting between 5 and 30 minutes. Status epilepticus was defined as seizure activity lasting more than 30 minutes. Statistical comparisons were performed using the Fisher exact test for categorical variables and the 2-tailed t test for continuous variables. Statistical significance was set at P<.05.
The EMU at Mayo Clinic's site in Arizona is a 6-bed unit integrated into the general neurology floor. As such, an unused bed designated for the EMU can be made available to other patients. The unit is staffed by nurses with specialized training who have passed EMU-specific competencies. Three full-time board-certified staff epileptologists rotate through the EMU with 1 full-time fellow. Four board-certified EEG technologists are responsible for the technical aspects of the EMU in addition to other hospital duties and are on call 24 hours a day. At Mayo Clinic's site in Arizona, standard safety precautions in 2005-2006 included 24-hour continuous observation of both live video and EEG monitoring by a trained technologist for all patients admitted to the EMU. Live visual EEG review by the technologist is supplemented by computerized real-time seizure and spike detection software. When events are noted, the technologist triggers an audible and visible alarm to activate nursing response. Registered nurses with specific EMU competencies provide 24-hour coverage with a maximum patient-to-nurse ratio of 4:1. Nursing staff provide bedside care and test neurologic function during the ictal and postictal period per standardized protocols. All video-EEG recordings include a single-channel electrocardiogram (ECG) monitor. Independently monitored formal cardiac telemetry and monitoring of pulse oximetry are available at physician discretion. Further safety measures include specialized padded bed railings, a voluntary safety belt while in bed, and nurse-supervised limited patient mobility while out of bed. Antiepileptic medications are tapered or discontinued on a case-by-case basis according to physician judgment, not per protocol. Usual practice is tapering of medication during the course of several days. Standard admission orders include a rescue oral or intravenous benzodiazepine for a prolonged (>10 minutes) GTC seizure and for clusters of 2 GTC seizures or 4 CPSs in 24 hours, unless a specific contraindication is identified. Intravenous access is secured at the time of admission. Standard orders also include notifying physicians of these events and of falls. A staff epileptologist, who is present during standard business hours and is on call 24 hours a day, reviews video-EEG recordings daily.
RESULTS
In 2005 and 2006, 428 patients were admitted for video-EEG monitoring. The indication for admission was recurrent spells of indeterminate etiology in 24 patients (56%) and presurgical evaluation of medically intractable epilepsy in 105 (25%). The discharge diagnosis was partial epilepsy in 188 patients (44%), generalized epilepsy in 33 (8%), pure psychogenic nonepileptic seizure in 103 (24%), and mixed epileptic and psychogenic seizure in 13 (3%). The 13 patients (3%) admitted for treatment of preexisting partial status epilepticus were excluded from further analysis. Eighteen patients (4%) had physiologic events unrelated to epilepsy (eg, symptomatic hypoglycemia, rapid eye movement sleep disorder, basilar migraine). Sixty admissions (14%) were nondiagnostic because of failure to record a typical event.
A total of 149 admitted patients met the criteria for inclusion in this study. Age ranged from 18 to 88 years, with a median of 44 years. Men (74 [49.7%]) and women (75 [50.3%]) were equally represented. Of the 149 study patients, 59 (40%) were referred for recurrent spells of indeterminate etiology and 90 (60%) for presurgical evaluation. On the basis of the discharge diagnosis after video-EEG monitoring, 124 patients (83%) had partial epilepsy, 12 (8%) had generalized epilepsy, and 13 (9%) had mixed epileptic and psychogenic seizures. Length of stay ranged from 2.0 to 18.0 days, with a mean of 5.7 days.
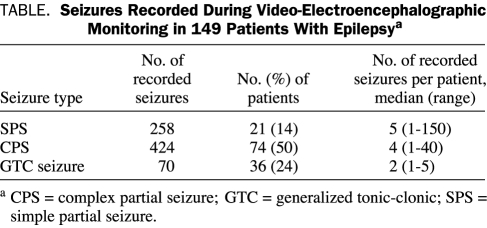
Seizures were recorded in 109 (73%) of the study patients. For these 109 patients, mean time to first seizure was 2.09 days (95% confidence interval, 1.70-2.48 days; range, 1-14 days). In total, 752 seizures were recorded. The mean ± SE number of seizures per person was 6.89±1.49 in those with recorded events and 5.04±1.11 in all 149 study patients. Details on seizure type are summarized in the Table. Of the 36 patients with a recorded GTC seizure, 35 (97%) reported it as a habitual seizure type. The remaining patient had a history of a single GTC seizure that was the presenting seizure, with subsequent continued partial seizures refractory to medication.
TABLE.
Seizures Recorded During Video-Electroencephalographic Monitoring in 149 Patients With Epilepsya
Twenty-four hour seizure clusters occurred in 35 (23%) of the included admissions and in 35 (32%) of the 109 patients in whom seizures were recorded, whereas 4-hour seizure clusters occurred in only 14 admissions (9%), representing 13% (14/109) of those with recorded seizures. Clusters were not predicted by the presence of a GTC seizure (P=.65 for 24-hour clusters; P=.19 for 4-hour clusters; Fisher exact test). Of the 52 clusters, 13 (25%) were treated with a rescue medication, 10 (19%) with intravenous benzodiazepine, and 3 (6%) with an intravenous antiepileptic medication. The remaining patients required no intervention.
Unusually prolonged seizures were very rare, occurring in only 2 of the admitted patients. Status epilepticus (partial complex) occurred in 1 patient and responded to treatment with an intravenous benzodiazepine. The second prolonged seizure was a GTC seizure lasting between 5 and 30 minutes that terminated with administration of an intravenous antiepileptic drug.
Seizure-related injuries were also rare. No patient required intubation, cardiopulmonary resuscitation, or transfer to the intensive care unit. No deaths occurred. There were no falls, dental injuries, or lacerations (other than occasional minor tongue lacerations sustained during convulsive seizure activity). Three (2.75%) of the 109 patients with recorded seizures were found to have potentially serious cardiac abnormalities during the peri-ictal period; all had inpatient cardiology consultation. One patient had ictal asystole that was subsequently treated with an implanted cardiac pacemaker. The second had a brief ventricular tachycardia that resolved spontaneously and required no intervention. The third had acute ST-segment depression that rapidly resolved and required no intervention. No complications of aspiration pneumonia or neurogenic pulmonary edema were noted. Psychotic symptoms were exhibited by 2 patients after seizure activity: one after a single GTC seizure in a patient with a history of postictal psychosis and the second after a seizure cluster with 3 CPSs and 1 GTC seizure. In the latter case, it was unclear whether the psychosis was a reaction to seizure or to administration of intravenous levetiracetam for treatment of the cluster. In both cases, the psychosis resolved without intervention and did not delay hospital discharge. Orthopedic injuries occurred in 4 (3.7%) of the patients with recorded seizures. All were vertebral compression fractures presenting with acute thoracic back pain that developed after a GTC seizure. Of the 70 recorded GTC seizures in this series, 4 (5.7%) led to a recognized vertebral fracture. The patients with vertebral compression fractures all had intractable partial epilepsy treated with long-term anticonvulsant medication (11-45 years). Two had been previously evaluated for antiepileptic drug-induced bone disease and were known to have osteopenia as determined by dual-energy x-ray absorptiometry for which they were receiving prophylactic calcium and vitamin D supplementation. The other 2 were found to have osteopenia by dual-energy x-ray absorptiometry after discharge. One of these was receiving prophylactic calcium and vitamin D supplementation before hospital admission. Two of the four were found to have underlying chronic compression fractures as well. All 4 required narcotic pain medication beyond hospital discharge. No other fracture types and no shoulder or hip dislocations were reported.
Overall, 21 (14%) of the 149 admitted patients whose records were reviewed and 21 (19%) of the 109 patients with a recorded seizure had an adverse event of at least moderate severity, defined as requiring medical treatment or interfering with normal activity. No difference was noted in length of stay between those with and without a complication (P=.22; t test).
DISCUSSION
Successful diagnostic video-EEG monitoring requires a balance between seizure-associated risk and the need to gain diagnostic information in a timely fashion. Our findings of a mean time from admission to seizure activity of 2 days and a mean total length of stay of 5 to 6 days are in line with those of earlier reports on outcome of video-EEG monitoring.7-9 Procedures to increase the likelihood of recording seizures, such as sleep deprivation and medication withdrawal, are known to increase the risk of seizure clusters, prolonged seizures, and status epilepticus. Seizure clusters, defined as 3 or more CPSs or GTC seizures within 24 hours, have been reported to occur in 48% to 61% of patients admitted for video-EEG monitoring.7,8,10 In the current study, seizure clusters were present in one-fourth of patients. In the EMU of Mayo Clinic's site in Arizona, patients are under continuous 24-hour supervision and have standard orders in place from admission for administration of rescue medications if clusters or prolonged seizure activity develops. Only 13 (25%) of the 52 clusters noted in this study required intravenous benzodiazepine or anticonvulsant medication therapy. Furthermore, the presence of a cluster did not predict a longer hospital stay. Our results show clusters to be frequent but generally mild. However, close monitoring is justified because clusters are difficult to predict. Clusters were not predicted by the presence of generalized seizure activity in this study. Earlier evaluations of clusters showed no difference in the frequency of seizure clusters in patients undergoing rapid vs a more gradual withdrawal of antiepileptic drugs.10
Generalized tonic-clonic seizures have been reported to occur for the first time or after a prolonged remission during video-EEG monitoring, presumably as a result of medication withdrawal.8,11 In our series, this was observed in only 1 of the 36 patients in whom convulsive seizures were recorded. The development of de novo generalization is notable because GTC seizures in particular have been correlated with higher injury rates.12 Prolonged CPSs or secondary generalized seizures have been previously noted in less then 3% of patients undergoing video-EEG monitoring.7,13 Short of death, status epilepticus is the most serious potential complication of video-EEG monitoring. Reported rates of partial complex or generalized status epilepticus, which range from 0% to 3% of admissions for video-EEG monitoring,7,8 are much higher than would be expected on the basis of an estimated incidence in the general population of 8 to 41 cases per 100,000 person-years.14-16 Prolonged seizure activity and status epilepticus were observed in less than 1% of cases in this series. Because increased seizure activity is not uncommon and occurs unpredictably, seizure activity should be closely monitored at all times and treatment should be readily available. With appropriate monitoring and care, increased seizure activity in the EMU need not result in substantial morbidity or increased length of hospitalization.
Even brief seizures may result in injury or even death as a result of trauma and falls. Orthopedic injury is a concern for patients with epilepsy, in both the community and the hospital setting. The relative risk of fracture for this group is twice that of the general population.17,18 Persons with epilepsy may be at increased risk of fracture because of seizure-related trauma and falls, antiepileptic drug-related gait instability, and antiepileptic drug-related bone disease. Both generalized and partial seizures can result in falls. The EMU of Johns Hopkins Hospital reported a rate of 8 falls per 1000 patient-days, 60% of which resulted in abrasions or contusions.19 Serious orthopedic injuries can result from falls or as a result of intense muscle contraction during seizures. The only previous study to examine orthopedic injuries during video-EEG monitoring focused on shoulder dislocations, which were observed in 5 of 806 patients.20 All 5 occurred during a GTC seizure with the patient supervised in bed. In the current case series, the only orthopedic injury of note was vertebral compression fracture, which occurred in 4 patients (11%) with a recorded GTC seizure. Of all noted complications, only vertebral fractures were considered severe; all 4 required additional evaluation and treatment for chronic pain months after discharge from the EMU. Although vertebral fracture is a recognized complication of seizure, little is known about the rate of occurrence during video-EEG monitoring or in the general epilepsy population.21 Of note, despite a high index of suspicion in the time frame beyond that included in this study, we have observed only 1 vertebral compression fracture acquired during video-EEG monitoring (K.H.N. and J.F.D., unpublished data, 2009). The low rate of orthopedic injury and absence of any dental injuries in this series may in part reflect the absence of falls. As noted by Sanders et al,19 falls are an important potential source of injury during video-EEG monitoring. Falls may occur as a result of seizure or sedation and ataxia resulting from administered medications. Precautions to limit or prohibit independent ambulation, supervision in the bathroom, and use of padding in the bed or floor may all be helpful in minimizing orthopedic trauma in the EMU. Clinicians should also be particularly alert to the potential for fracture in patients experiencing generalized convulsions in bed during video-EEG monitoring.
Death during video-EEG monitoring has occasionally been reported anecdotally; however, no data on the true frequency of this rare, troubling outcome are available. Sudden unexplained death in epilepsy (SUDEP) is a potential cause of death in the EMU. SUDEP is estimated to account for between 2% and 17% of epilepsy-related deaths, with an incidence of 0.35 per 1000 person-years in the epilepsy population.22,23 Only 4 cases of SUDEP or near-SUDEP have been reported during video-EEG monitoring, suggesting that this feared complication is rare in the monitored setting.24-27 Although the mechanism of SUDEP has not been established, cardiac arrhythmia, pulmonary edema, and central apnea secondary to a convulsive seizure are among the proposed etiologies. The most commonly noted ictal ECG change is benign sinus tachycardia, but arrhythmias as well as conduction and repolarization abnormalities have also been observed.28-31 Potentially serious peri-ictal ECG changes, such as asystole, atrial fibrillation, supraventricular or ventricular tachycardia, and ST-segment changes, have been observed in 5% to 14% of seizures recorded during video-EEG monitoring.28,30-32 Serious ECG abnormalities are more likely with prolonged or generalized seizures.30-32 In a seizure-by-seizure analysis, ECG changes were observed in less than 1% of recorded CPSs or GTC seizures. The unexpectedly low rate of ECG changes in our series (<1% on a seizure-by-seizure analysis) may reflect an underascertainment of true ECG changes because this assessment was based on information in the video-EEG report rather than on a retrospective evaluation of the original ECG tracings. Although ECG changes would likely be seen with habitual seizures, the fact that seizures in the EMU may be longer and more likely to generalize may increase risk of serious abnormalities. At a minimum, patients undergoing video-EEG monitoring should likely have a single-channel ECG monitor with resources available to address any life-threatening abnormalities detected. At our institution, we are increasingly moving to formal cardiac telemetry monitoring for all patients in the EMU.
Postictal psychosis has been described in 6% of patients undergoing video-EEG monitoring, often in patients with no history of psychosis.33,34 Potential risk factors unique to the EMU setting may include increased seizure frequency and clustering, increased seizure generalization, and the psychiatric adverse effects of antiepileptic drug withdrawal.35 Postictal psychosis may increase the risk of developing chronic psychosis and is associated with increased mortality.33,36 Furthermore, preparations should be made to manage developing psychosis, agitation, and combativeness during video-EEG monitoring to prevent injury to the patient, family, and staff. Necessary interventions may include simple reassurance, reduction of environmental stimuli, use of restraints, and administration of sedative or antipsychotic medications.
Overall, our study found that moderate to severe adverse events requiring intervention occurred in 14% of all admissions and in 19% of those in whom seizures were recorded. Although seizure clusters were common, most were not clinically important and required no intervention. No deaths and no transfers to a higher level of care occurred. Only vertebral fractures resulted in clear adverse sequelae persisting beyond hospital discharge.
Our study is limited by its retrospective design, which may have led to underascertainment of complication rates. Because of the sample size, rarely occurring events, such as SUDEP, were not adequately measured. Although good outcomes were observed in this series, the frequency of noted adverse events underscores the importance of appropriate close monitoring for seizures and potential injury. Outcomes may be influenced by safety and monitoring procedures, which are not standardized even among large epilepsy referral centers. In an international survey of 42 EMUs, only half had 24-hour dedicated nursing staff coverage, less than half had patients under continuous surveillance by staff, and only two-thirds had continuous ECG monitoring.37
CONCLUSION
Although the risks of video-EEG monitoring are relatively low, the procedure is not risk free. Video-EEG monitoring is increasingly available outside of traditional tertiary epilepsy centers. Smaller community-based hospitals and physician offices providing video-EEG monitoring may be unable to offer the level of care from experienced nursing and EEG technologists available at a larger referral center that is prepared for complex cases and higher-risk invasive EEG studies. Further study is needed to facilitate development of standardized guidelines for monitoring and safety strategies to minimize potential morbidity and mortality across centers offering video-EEG monitoring services.
REFERENCES
- 1.Phillips LA. Death in epilepsy monitoring unit raises questions about safety policies and practice standards. Neurol Today 2008;8(16):1,15 [Google Scholar]
- 2.Maass B. Monitoring mistake leads to hospital death. Channel CBS 4 Web site. Denver, Colorado www.cbs4denver.com/investigates/Charles.Gray.death.2.768551.html Published July 10, 2008. Accessed February 25, 2009
- 3.Cockerell OC, Johnson AL, Sander JW, Hart YM, Goodridge DM, Shorvon SD. Mortality from epilepsy: results from a prospective population-based study. Lancet 1994;344(8927):918-921 [DOI] [PubMed] [Google Scholar]
- 4.Nilsson L, Tomson T, Farahmand BY, Diwan V, Persson PG. Cause-specific mortality in epilepsy: a cohort study of more than 9000 patients once hospitalized for epilepsy. Epilepsia 1997;38(10):1062-1068 [DOI] [PubMed] [Google Scholar]
- 5.Wirrell EC. Epilepsy-related injuries. Epilepsia 2006;47(suppl 1):79-86 [DOI] [PubMed] [Google Scholar]
- 6.Velis D, Plouin P, Gotman J, da Silva FL, ILAE DMC Subcommittee on Neurophysiology Recommendations regarding the requirements and applications for long-term recordings in epilepsy. Epilepsia 2007;48(2):379-384 [DOI] [PubMed] [Google Scholar]
- 7.Rose AB, McCabe PH, Gilliam FG, et al. Consortium for Research in Epilepsy (CoRE) Occurrence of seizure clusters and status epilepticus during inpatient video-EEG monitoring. Neurology 2003;60(6):975-978 [DOI] [PubMed] [Google Scholar]
- 8.Yen DJ, Chen C, Shih YH, et al. Antiepileptic drug withdrawal in patients with temporal lobe epilepsy undergoing presurgical video-EEG monitoring. Epilepsia 2001;42(2):251-255 [DOI] [PubMed] [Google Scholar]
- 9.Eisenman LN, Attarian H, Fessler AJ, Vahle VJ, Gilliam F. Self-reported seizure frequency and time to first event in the seizure monitoring unit. Epilepsia 2005;46(5):664-668 [DOI] [PubMed] [Google Scholar]
- 10.Haut SR, Swick C, Freeman K, Spencer S. Seizure clustering during epilepsy monitoring. Epilepsia 2002;43(7):711-715 [DOI] [PubMed] [Google Scholar]
- 11.Azar NJ, Wright AT, Wang L, Song Y, Abou-Khalil BW. Generalized tonic-clonic seizures after acute oxcarbazepine withdrawal. Neurology 2008;70(22):2187-2188 [DOI] [PubMed] [Google Scholar]
- 12.Lawn ND, Bamlet WR, Rakhakrishnan K, O'Brien PC, So EL. Injuries due to seizures in persons with epilepsy. Neurology 2004;63(9):1565-1570 [DOI] [PubMed] [Google Scholar]
- 13.Jennsen S, Gracely EJ, Sperling MR. How long do most seizures last? A systematic comparison of seizures recorded in the epilepsy monitoring unit. Epilepsia 2006;47(9):1499-1503 [DOI] [PubMed] [Google Scholar]
- 14.DeLorenzo RJ, Hauser WA, Towne AR, et al. A prospective, population-based epidemiological study of status epilepticus in Richmond, Virginia. Neurology 1996;46(4):1029-1035 [DOI] [PubMed] [Google Scholar]
- 15.Hesdorffer DC, Logroscino G, Cascino G, Annegers JF, Hauser WA. Incidence of status epilepticus in Rochester, Minnesota, 1965-1984. Neurology 1998;50(3):735-741 [DOI] [PubMed] [Google Scholar]
- 16.Coeytaux A, Jallon P, Galobrades B, Morabia A. Incidence of status epilepticus in French speaking Switzerland (EPISTAR). Neurology 2000;55(5):693-697 [DOI] [PubMed] [Google Scholar]
- 17.Vestergaard P. Epilepsy, osteoporosis and fracture risk—a meta-analysis. Acta Neurol Scand 2005;112(5):277-286 [DOI] [PubMed] [Google Scholar]
- 18.Souverein PC, Webb DJ, Petri H, Weil J, Van Staa TP, Egberts T. Incidence of fractures among epilepsy patients: a population-based retrospective cohort study in the General Practice Research Database. Epilepsia 2005;46(2):304-310 [DOI] [PubMed] [Google Scholar]
- 19.Sanders PT, Cysyk BJ, Bare MA. Safety in long-term EEG/video monitoring. J Neurosci Nurs. 1996;28(5):305-313 [DOI] [PubMed] [Google Scholar]
- 20.DeToledo JC, Lowe MR. Seizures, lateral decubitus, aspiration, and shoulder dislocation: time to change the guidelines? Neurology 2001;56(3):290-291 [DOI] [PubMed] [Google Scholar]
- 21.Schrader S, Noe K, Drazkowski J, Sirven J. Unexpected vertebral compression fractures in young men with epilepsy. Neurology 2007;68(suppl 1):A149 [Google Scholar]
- 22.Ficker DM, So EL, Shen WK, et al. Population based study of the incidence of sudden unexplained death in epilepsy. Neurology 1998;51(5):1270-1274 [DOI] [PubMed] [Google Scholar]
- 23.Ficker DM. Sudden unexplained death and injury in epilepsy. Epilepsia 2000;41(suppl 2):S7-S12 [DOI] [PubMed] [Google Scholar]
- 24.Bird JM, Dembny KAT, Sandeman D, Butler S. Sudden unexplained death in epilepsy: an intracranially monitored case. Epilepsia 1997;38(suppl 11):S52-S56 9092961 [Google Scholar]
- 25.Lee M. Video-EEG recording of a sudden unexpected death in epilepsy (SUDEP) [abstract]. Epilepsia 1998;39(suppl 6):S123-S124 [Google Scholar]
- 26.So EL, Sam MC, Lagerlund TL. Postictal central apnea as a cause of SUDEP: evidence from a near-SUDEP incident. Epilepsia 2000;41(11):1494-1497 [DOI] [PubMed] [Google Scholar]
- 27.Tavee J, Morris H., III Severe postictal laryngospasm as a potential mechanism for sudden unexpected death in epilepsy: a near miss in an EMU. Epilepsia 2008December;49(12):2113-2117 Epub 2008 Sep 17 [DOI] [PubMed] [Google Scholar]
- 28.Keilson MJ, Hauser WA, Magrill JP, Goldman M. ECG abnormalities in patients with epilepsy. Neurology 1987;37(10):1624-1626 [DOI] [PubMed] [Google Scholar]
- 29.Keilson MJ, Hauser WA, Magrill JP. Electrocardiographic changes during electrographic seizures. Arch Neurol. 1989;46(11):1169-1170 [DOI] [PubMed] [Google Scholar]
- 30.Nei M, Ho RT, Sperling MR. EKG abnormalities during partial seizures in refractory epilepsy. Epilepsia 2000;41(5):542-548 [DOI] [PubMed] [Google Scholar]
- 31.Zijlmans M, Flanagan D, Gotman J. Heart rate changes and ECG abnormalities during epileptic seizures: prevalence and definition of an objective clinical sign. Epilepsia 2002;43(8):847-854 [DOI] [PubMed] [Google Scholar]
- 32.Opherk C, Coromilas J, Hirsch LJ. Heart rate and EKG changes in 102 seizures: analysis of influencing factors. Epilepsy Res. 2002;52(2):117-127 [DOI] [PubMed] [Google Scholar]
- 33.Kanner AM, Stagno S, Kotagal P, Morris HH. Postictal psychiatric events during prolonged video-electroencephalographic monitoring studies. Arch Neurol. 1996;53(3):258-263 [DOI] [PubMed] [Google Scholar]
- 34.Alper K, Devinsky O, Westbrook L, et al. Premorbid psychiatric risk factors for postictal psychosis. J Neuropsychiatry Clin Neurosci. 2001;13(4):492-499 [DOI] [PubMed] [Google Scholar]
- 35.Devinsky O, Abramson H, Alper K, et al. Postictal psychosis: a case control series of 20 patients and 150 controls. Epilepsy Res. 1995;20(3):247-253 [DOI] [PubMed] [Google Scholar]
- 36.Logsdail SJ, Toone BK. Post-ictal psychosis: a clinical and phenomenological description. Br J Psychiatry 1988;152:246-252 [DOI] [PubMed] [Google Scholar]
- 37.Fitzsimons M, Browne G, Kirker J, Staunton H. An international survey of long-term video/EEG services. J Clin Neurophysiol. 2000;17(1):59-67 [DOI] [PubMed] [Google Scholar]