Abstract
Despite the premature and somewhat infamous rise and fall of psychosurgery in the mid-20th century, the current era of functional neuromodulation proffers immense opportunity for surgical intervention in treatment-resistant psychiatric disorders. On the basis of recent successes with novel, focused, less invasive, and reversible treatment strategies for movement disorders, several therapeutic trials have been conducted to investigate the effectiveness of deep brain stimulation (DBS) in treatment-resistant depression, obsessive-compulsive disorder (OCD), and Tourette syndrome. The many anatomic targets for these psychiatric disorders are indicative of both the system-wide effects of DBS and the network-level dysfunction mediating the emotional and cognitive disturbances. To gain insight into the application of neuromodulation therapies and their further advancement, we must elucidate neuroanatomic networks involved in refractory psychiatric illness, the neurophysiological anomalies that contribute to disordered information processing therein, and the local and system-wide modulatory effects of DBS. This review discusses the history of psychosurgical procedures, recent DBS clinical data, current anatomic models of psychopathology, and possible therapeutic mechanisms of action of DBS neuromodulation. Our search criteria for PubMed included combinations of the following terms: neuromodulation, DBS, depression, OCD, Tourette syndrome, mechanism of action, and history. Dates were not restricted. As clinical and basic scientific investigations probe the neuromodulatory effects of DBS in the treatment of refractory neuropsychiatric illness, our knowledge of these disorders and our potential to treat them are rapidly expanding. Indeed, this modern era of neuromodulation may provide the key that unlocks many of the mysteries pertaining to the biological basis of disordered emotional neurocircuitry.
CBF = cerebral blood flow; Cg25 = Brodmann Area 25 in the subgenual cingulate cortex; CM/Pf = centre médian/parafascicular; DBS = deep brain stimulation; MRI = magnetic resonance imaging; OCD = obsessive-compulsive disorder; PANAS = Positive and Negative Affect Scales; TS = Tourette syndrome; VC = ventral anterior internal capsule; VS = ventral striatum
The term psychosurgery is rich in emotional valence. Many negative connotations are conjured up by descriptions of historical experimental neurosurgical procedures for derangements in behavior and thinking. However, with the success of novel, relatively noninvasive, more focused, and reversible treatment strategies for movement disorders, such as deep brain stimulation (DBS), the current era of functional neurosurgery proffers immense opportunity for surgical treatment of refractory psychiatric illness. Given its turbulent past, care must be taken in incorporating psychiatric neuromodulatory intervention into mainstream medicine; profound medical, ethical, and spiritual issues should be carefully negotiated by the neurosurgeon, psychiatrist, and neurologist, in conjunction with the patient and patient's family. With strict ethical guidelines, meticulous patient-screening programs, and selective, image-guided anatomic targeting based on proven neuropathophysiology, we cautiously stand on the verge of a modern era of neuropsychiatric neuromodulation. Yet it is only with the highest standards in clinical and scientific endeavor that we can maximize the potential of neuromodulatory surgery to offer substantial relief from serious treatment-resistant psychiatric conditions and avoid revisiting the devastating mistakes of the past.
For editorial comment, see page 493
THE EARLY DAYS OF PSYCHIATRIC NEUROSURGERY
The mid-20th century witnessed the premature rise and fall of psychosurgery within mainstream medicine. Today, a new era of neurosurgical intervention for psychiatric illness is emerging within the context of modern neuromodulation technologies that are much more focused and much less invasive and destructive. The disease burden of treatment-resistant mental illness for patients, their family, and society and the potential to relieve this burden through neuromodulatory technologies demand that we carefully and methodically explore these therapeutic options with the highest degree of scientific rigor. Developing an appreciation of the somewhat tainted history of psychiatric neurosurgery will help to ensure that we avoid repeating past errors and safeguard future patients and families. In doing so, we must remain mindful of the important differences between the medicine of today and that of the early days of psychosurgery. Psychiatric neurosurgery was introduced in an era void of psychoactive medications, one in which the only treatment option available was institutionalization. The desperate need for alternatives to incarceration and physical restraint during these times can, in part, explain the hasty enthusiasm with which psychosurgical interventions were embraced.1
Although psychiatric neurosurgery may have been conducted as early as 5100 BC,2 Gottlieb Burckhardt's 1891 attempt to placate 6 severely agitated psychiatric patients by surgically extracting sections of their frontal lobes represents the first psychosurgery trial of modern medicine. Although he considered these surgeries relatively successful, further attempts were abandoned under pressure from colleagues.1,3 Some 44 years later, John Farquhar Fulton and Carlyle Jacobsen's research investigating how specific portions of the cerebral cortex modulate behavioral and physiological function ignited interest in the potential of neurosurgery for the treatment of psychiatric conditions.4,5 This research, which showed that bilateral removal of the frontal lobes profoundly reduced the expression of anxiety and “frustrational behavior” in chimpanzees, is thought to have inspired Egas Moniz and Walter Freeman to surgically treat anxiety states in human patients.1
After attending the 1935 International Neurological Congress in London, where this primate neurophysiology work was presented, Moniz enlisted the expertise of Portuguese neurosurgeon Almeida Lima to perform the first frontal leucotomy on a human patient.1 During this surgical procedure, the fiber tracts from the frontal lobes were destroyed with an injection of alcohol.6 Shortly thereafter, in September 1936, Freeman and the neurosurgeon James Watts started their prefrontal lobotomy program.1 They used radiographic guidance and skeletal landmarks to locate the white matter tracts of interest. However, borrowing from a technique reported in 1937 by Italian psychiatrist Amarro Fiamberti, Freeman soon streamlined this procedure, introducing the transorbital leucotomy in 1946. This now infamous technique involved inserting an ice pick underneath the eyelid through the roof of the orbit and maneuvering it to sever the white matter fiber tracts; the key “advantage” touted for this procedure was that it could be done almost anywhere.1 The period between 1945 and 1955 saw intense media support for the procedure and for the work of such figures as Moniz and Freeman, with approximately 50,000 lobotomies performed in the United States at this time.6,7 Indeed, the height of social acceptance came in 1949, when Egas Moniz shared the Nobel Prize for medicine for his discovery of the therapeutic value of prefrontal leucotomies.1 Although this technique fell out of favor in the late 1950s, Freeman remained a strong proponent of the frontal lobotomy, despite the advent of effective pharmacotherapies, until his death in 1972.6
Published in the late 1940s, the first long-term follow-up studies of patients who had undergone lobotomy indicated that, despite improvements in agitation and disruptive behavior, “successfully treated” patients also typically experienced serious cognitive, affective, and psychomotor deficits.8,9 More selective targeting procedures were soon devised to minimize such negative outcomes. William Beecher Scoville provided an important first step toward developing minimally invasive strategies for psychiatric neurosurgery with his introduction of selective cortical undercutting procedures.10 This concept was advanced by the introduction to neurosurgery of the stereotactic frame, which enables the precise localization of anatomic targets in the brain.1,5 More localized lesioning procedures, including the anterior capsulotomy and stereotactic subcaudate tractotomy, were in turn made possible.1
The US Congress considered a ban on psychosurgery as part of the National Research Act of 1974. Although the driving force behind this legislation was the institution of a ban on psychosurgery, the investigation by Congress provided evidence of the efficacy of modern localized lesioning procedures, such as the cingulotomy and anterior capsulotomy, and led to the call for further research into similar treatments.1 Within the current framework of neuromodulation technologies such as DBS, we are beginning to see a renaissance of neurosurgical intervention for severe psychiatric disorders.6,11 Although the effects of high-frequency DBS are functionally equivalent to the creation of a lesion, DBS has the advantage of being reversible as well as adjustable.12 Other emerging technologies, including transcranial magnetic stimulation, gene therapy, stem cell transplant, and vagal nerve stimulation, may also potentially play an important role in the nondestructive modification of neural pathways.1,7,13,14 This review, however, focuses on the rapidly evolving use of DBS neurosurgical interventions to treat obsessive-compulsive disorder (OCD), treatment-resistant depression, and Tourette syndrome (TS). Our search criteria for PubMed included combinations of the following terms: neuromodulation, DBS, depression, OCD, TS, mechanism of action, and history. Dates were not restricted.
DEEP BRAIN STIMULATION
The DBS surgical targets for the treatment of psychiatric disorders include placement of an electrode in the white matter adjacent to Brodmann Area 25 in the subgenual cingulate cortex (Cg25) for depression,15 in the anterior internal capsule for OCD,16 and in the thalamic centre médian/parafascicular (CM/Pf) nucleus for TS.17 Although the actual surgical procedure may vary somewhat from institution to institution, all combine stereotactic techniques with detailed image guidance. Commonly, a stereotactic head frame is placed on the patient while he or she is receiving local anesthesia, and magnetic resonance imaging (MRI) is obtained to identify the anterior commissure, posterior commissure, and the midcommissural point. Well-established x, y, and z target coordinates, relative to the midcommissural point, are used for planning electrode placement. Commercially available planning software may be used to determine the target coordinates and entry point for a safe electrode trajectory that avoids blood vessels and ventricles.
Once imaging has been completed and a safe electrode trajectory established, the patient is returned to the operating room, where, under sterile conditions and while receiving local anesthesia, surgery commences (Figure 1). Burr holes are placed in the skull at the predetermined entry point. Although microelectrode recording is commonly performed during DBS surgery for movement disorders, the relative usefulness of obtaining extracellular unit activity for psychiatric indications remains to be determined. After placement of electrodes, test stimulation is conducted using a temporary external stimulator. The patient remains awake so that verbal feedback can be obtained to ensure that unwanted adverse effects do not occur. For example, thalamic stimulation may produce paresthesias, indicating current spread into the somatosensory thalamus. If such unwanted adverse effects are noted, the electrode may be moved to another location. Confirmation of accurate electrode placement is usually performed first with intraoperative fluoroscopy and then postoperative MRI or computed tomography (Figure 2). Once the electrode placement is confirmed and trial stimulation is deemed successful, a pulse generator is placed in the chest area under the clavicle. Common stimulation parameters used for neuromodulation of psychiatric illness include frequencies of 65 to 185 Hz, amplitudes of 1 to 8 V, and pulse widths of 60 to 450 μs.18
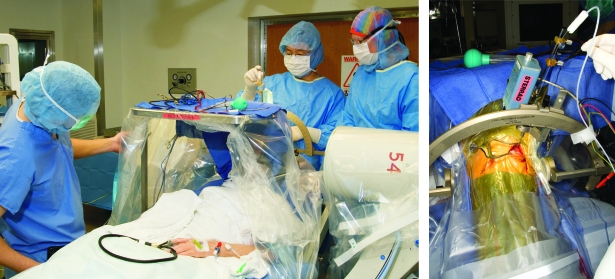
FIGURE 1.
Left, Intraoperative photograph during deep brain stimulation (DBS) surgery. The patient is awake with stereotactic head frame in place, and the DBS electrode lead has been placed by the neurosurgeon. Fluoroscopy machine is lateral to the patient's head and helps to confirm that the leads are at target. The neurophysiologist (front) is giving test stimulation with an external hand-held stimulator to make sure there are no adverse effects. The patient does not feel pain as the brain is without pain receptors. Right, Close-up photograph of the stereotactic arc, burr hole, and DBS lead in place connected to the external pulse generator via connector wire.
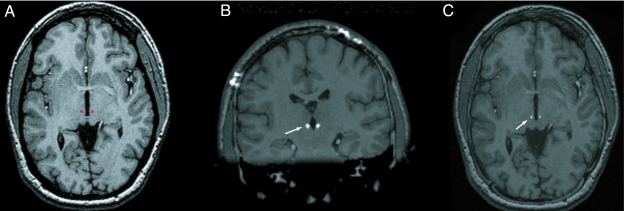
FIGURE 2.
A, Preoperative magnetic resonance imaging with a surgical plan of a patient with Tourette syndrome, in which the centre médian/parafascicular thalamic nucleus was targeted (red dots). Coronal (B) and axial (C) postoperative computed tomogram fused with preoperative magnetic resonance imaging showing Medtronic 3387 lead in the centre médian/parafascicular nucleus of thalamus (white arrows).
CLINICAL INDICATIONS
Depression
Major depressive disorder is a medical illness with substantial personal morbidity and mortality. In the developed world, major depression is second only to cardiovascular disease in premature mortality and time lived with disability; in persons aged 15 to 44 years, depression is the most disabling medical illness.19 In the United States, the prevalence of major depression, known to be a chronic remitting and relapsing illness, is approximately 17%, affecting almost 1 in 5 persons.20 Although pharmacotherapy and evidence-based psychotherapy can effectively treat many patients with major depression, up to 20% of patients fail to respond to these first-line therapeutic interventions.21 Currently, no standardized, clinically applicable definition of treatment-resistant depression exists; however, it is generally agreed that the concept emphasizes adequate dosing and duration of different antidepressant treatments or augmentation interventions without substantial or sustained benefit.
Depression is unlikely to be a disease of a specific brain region or to result from a single neurotransmitter deficit. Instead, as reviewed extensively elsewhere,22-24 the underlying neurobiology of major depression is thought to be a “systems-level” or circuitry disorder affecting cortical, subcortical, and limbic brain regions. Genetic vulnerability, developmental insult or aberrancy, environmental stressors, and interactions of the 3 can negatively affect this cortical-subcortical-limbic network. Early hypothesis-driven research by Mayberg et a115 focused on the Cg25 area. The subgenual cingulate cortex has critical projections to and from the VS, nucleus accumbens, and limbic cortical loop.25 Early studies implicated Cg25 involvement in sadness and antidepressant effects.26,27 In this regard, a decrease in Cg25 activity has been associated with immediate clinical response to a number of antidepressant treatments, including serotonin reuptake inhibitor therapy,28 electroconvulsive therapy,29 transcranial magnetic stimulation,30 and ablative surgery.31 Whether longer-term sustained response (ie, prevention of relapse or recurrence) correlates with Cg25 activity remains to be determined. Against this scientific and clinical background, early work in the field focused on long-term DBS of Cg25 to modulate associated gray matter and downstream targets in treatment-resistant depression.
A 6-month proof-of-concept study was published both as a preliminary report (6 patients)15 and a final report (20 patients).32 With MRI guidance, DBS electrodes were implanted bilaterally in Cg25 white matter. Blind sequential stimulation of each electrode was conducted to assess for spontaneous report or behavioral effect. As opposed to an immediate sense of happiness or euphoria, the consistent observation with all 6 patients in the preliminary report was a “disappearance of the void,” “sudden calmness or lightness,” “connectedness,” and a greater sensory awareness. These observations were noted for specific DBS electrode contact points, were reproducible, and were not observed in sham or subthreshold stimulation. Abrupt behavioral changes were captured using the Positive and Negative Affect Scales (PANAS). All patients endorsed dose-dependent adverse effects of psychomotor slowing and light-headedness at high settings (>7.0 V), most commonly in the superior (Nos. 3 and 7) electrodes.
During the 5-day postoperative period, before placement of the pulse generator, variable DBS parameters were tested daily. Reproducible, specific, abrupt behavioral changes were again observed along with a reduction in the negative subcomponents and an increase in the positive subcomponents of PANAS; these changes were not observed during sham stimulation (0 V or subthreshold) or off periods. Longer stimulation periods were associated with longer carry-over of beneficial behavioral effects. A long-term stimulation phase, involving a 4-week period of parameter optimization and a 6-month follow-up phase, was thus trialed.15 At study end point, response was achieved in 4 (67%) of the 6 patients, with 3 patients achieving remission or near-remission status. The response (60%) and remission (35%) rates were similar in the final study.32 After 6 months, blind discontinuation of DBS resulted in the return of depressive symptoms and blind reinstitution of DBS in the normalization of these symptoms. One interim post hoc analysis of 15 patients suggested a higher response rate for patients with treatment-resistant depression (8 of 12 patients) vs atypical depression (0 of 3 patients).33 Otherwise, no demographic, clinical, or image-based predictors of response were identified. Preliminary analyses have suggested no difference in electrode location within Cg25 in patients who respond vs those who do not.32
Positron emission tomographic cerebral blood flow (CBF) studies have shown that, before DBS, 5 depressed patients had elevated blood flow to Cg25 and decreased blood flow to the prefrontal cortex (BA9/46), premotor (BA6), dorsal anterior cingulate (BA24), and anterior insular regions compared with age-matched healthy volunteers. High-frequency DBS of Cg25 induced CBF decreases in Cg25 blood flow and increases in blood flow to the prefrontal cortex, which correlated with substantial improvement in depression in patients with previous treatment resistance. The lack of response with sham or subthreshold stimulation, reversal and re-response of effect with off-on-off-on design, sustained 6-month improvement, and change in CBF are compelling, converging scientific observations supporting the effectiveness of Cg25 DBS for treatment of depression. A randomized placebo-controlled trial is currently under way to attempt replication of this proof-of-concept study (M.A.F., oral communication, St. Jude Medical, 2008).
A second brain region of potential interest for DBS and depression is the ventral anterior internal capsule/ventral striatum (VC/VS). This target was chosen on the basis of previous clinical research reporting a reduction of obsessive-compulsive and depressive symptoms in treatment-resistant OCD.34 Recently, Malone et al35 reported a multisite trial of DBS of the VC/VS in 15 patients with treatment-resistant depression. Mean follow-up was just under 2 years. Similar response rates were observed with the Montgomery-Åsberg or Hamilton Rating Scales for Depression: approximately 50% at 3 months, 45% at 6 months, and 53% at last follow-up. Remission rates were 27%, 24%, and 37%, respectively. No neuropsychological deficits were associated with DBS. Of note, 1 patient with treatment-resistant bipolar I depression experienced hypomania on 2 occasions that resolved after modification of stimulation parameters.
As reviewed by Schlaepfer et al,36 the VS (in particular the accumbens) plays a key role in processing information related to normal reward or pleasure, pathologic reward (ie, addiction craving), and anhedonia (pathologic lack of pleasurable reward). In this third target proof-of-concept study, 3 patients with treatment-resistant depression underwent bilateral implantation of the nucleus accumbens (1 electrode each in the core and shell regions). Clinical ratings of depression improved in all 3 patients when the stimulator was on and worsened when the stimulator was turned off. Significant inverse correlation was found between increased stimulation and decreased depression ratings. A case of bilateral DBS of the accumbens for severe anxiety and secondary depression has been reported.37 Although mood and anxiety did not improve, the patient's alcohol dependence was reduced remarkably. Preclinical animal studies are now investigating the potential role of accumbens DBS for use in the treatment of addiction, and preliminary results are promising.38,39
Obsessive-Compulsive Disorder
Obsessive-compulsive disorder, which is characterized by recurring, anxiety-provoking thoughts (obsessions) and repetitive behaviors (compulsions), affects approximately 2% to 3% of the general population.40 Severe cases of OCD that are refractory to medical therapy can be extremely disabling. On the basis of the successful history of lesioning procedures for OCD, early DBS targets have focused on the ventral aspect of the anterior limb of the VC/VS.16,41-48 The nucleus accumbens is the primary component of the VS and lies at the ventral end of the internal capsule. The positive findings of several clinical trials assessing the efficacy of DBS for patients with OCD led the US Food and Drug Administration to grant DBS a humanitarian device exemption for OCD in 2009.
In the earliest study, bilateral DBS of the ventral aspects of the anterior limbs of the VC/VS was trialed in 4 patients with severe OCD.16 Of the 4 patients, 3 were reported to have received some benefit from stimulation, including a sudden change in anxiety for 1 patient. In a subsequent trial, DBS of the anterior limb resulted in substantial clinical improvement, including the return to normal activities, for 2 of 3 patients with OCD 15 to 33 months after surgery.49 A blinded on-off study paradigm has also been used to assess the effects of bilateral DBS of the ventral aspects of the anterior limbs of the VC/VS. In one such study, 2 of 4 patients were reported to have received meaningful relief from OCD symptoms. The suicide of 1 patient was also reported.50 In each of these studies, stimulation of the VC/VS required the use of high-amplitude voltages (5 to 10.5 V) for therapeutic benefit,51 suggesting that the actual therapeutic target could be slightly removed from the electrode site.52
Deep brain stimulation of the right nucleus accumbens (shell region) has also been trialed for refractory OCD and anxiety.48 In this instance, effective stimulation amplitudes were lower (2.0 to 6.5 V) than those in the already discussed VC/VS trials and resulted in near complete recovery, without any reported adverse effects, for 3 of the 4 patients in this study. The follow-up period was 24 to 30 months, with clinical improvements noted as early as a few days to several weeks after DBS initiation. However, this improvement was qualitatively observed rather than objectively quantified. Another potential therapeutic target of interest, particularly for individuals with comorbid movement disorders, is the subthalamic nucleus. Deep brain stimulation of the subthalamic nucleus is well known to be effective for treatment of Parkinson disease; however, psychiatric adverse effects have been noted, particularly when electrodes are positioned in the anterior region.53-60 When subthalamic nucleus DBS has been used for patients with comorbid Parkinson disease and OCD, unintentional psychiatric benefits have been observed,61,62 including substantial improvement of OCD symptoms. The efficacy of this target for OCD is supported by a recent animal study in which high-frequency stimulation of the anterior subthalamic nucleus significantly reduced repetitive behaviors in a primate animal model of OCD and TS.63 In another case report,64 ventral caudate DBS was used for a patient with comorbid OCD and major depression. In this instance, remission of depressive symptoms was observed at 6 months and remission of OCD symptoms at 12 to 15 months after surgery.
Functional imaging data strongly implicate hyperactivity of the VS, medial thalamus, and orbitofrontal cortex in OCD pathology. This has been observed during both the neutral and provoked state and after successful conventional treatments. The neutral or resting condition is the state during which patients with OCD are symptomatic and experience intrusive obsessive thoughts and compulsive urges. The provoked state occurs when the patient with OCD is presented with a stimulus known to exacerbate his or her OCD profile. The critical role of the VS, medial thalamus, and orbitofrontal cortex in mediating the pathology of this disease is highlighted by their increased activity when symptoms are exacerbated65-67 and their reduced activity after successful pharmacological,68 cognitive-behavioral,69 or neurosurgical70 therapeutic intervention. Imaging techniques have shown that successful DBS treatment of patients with OCD correlates with lower activity in frontal cortical areas.71,72 Animal studies have also investigated the neurophysiological effects of DBS of the accumbens in rats. Thirty minutes of high-frequency stimulation of this OCD target effectively inhibited the activity of nearly all orbitofrontal neurons via mechanisms mediated by γ-aminobutyric acid (GABA).73 Although further research must be conducted to better elucidate the system-wide effects of DBS in OCD, the preliminary work of McCracken and Grace,73 together with clinical imaging data, indicate that DBS-mediated modulation of neuronal network activity, including upstream neurotransmission, is an important therapeutic mechanism of action.
Tourette Syndrome
Tourette syndrome is characterized by repetitive, stereotyped, involuntary movements and vocalizations called tics. Simple motor tics include eye blinking, facial grimacing, shoulder shrugging, repetitive throat-clearing, sniffing, and grunting. These are typically sudden, brief, repetitive movements incorporating a limited number of muscle groups. Patients may also have complex tics that might include facial grimacing combined with a head twist and a shoulder shrug, sniffing, or touching of objects. Vocal tics, including coprolalia (uttering swear words) or echolalia (repeating the words or phrases of others), are also relatively common in TS. The early symptoms of TS are almost always noticed first in childhood, with an average age of onset of 7 to 10 years.74 The estimated worldwide prevalence of TS is 4 to 5 in 10,000.74 Although in most cases the disorder is self-limited or can be treated by medication or behavioral therapy, its symptoms can be intractable to any conservative treatment in a small percentage of patients.75 Thus, since the 1950s, various attempts have been made to treat such patients through neurosurgical procedures, including ablative surgeries and, more recently, DBS.
The ablative neurosurgical target sites are diverse and include the frontal lobe (prefrontal lobotomy and bimedial frontal leucotomy), the limbic system (limbic leucotomy and anterior cingulotomy), the thalamus, and the cerebellum.75-77 Combined ablative approaches have also been tried, such as anterior cingulotomies plus infrathalamic lesions. The results have often been unsatisfactory, or major adverse effects such as hemiplegia or dystonia have occurred. Thus, in 1999, DBS was trialed as a new approach for intractable TS.78
Maciunas et al17 have conducted the only prospective double-blind crossover trial of DBS; in their study of 5 adults with TS, bilateral thalamic electrodes were implanted into the CM/Pf nucleus. In the randomized phase of the trial, a statistically significant reduction in the modified Rush Video-Based Tic Rating Scale score was identified with bilateral stimulation. Improvement was noted in motor and sonic tic counts on the Yale Global Tic Severity Scale, TS Symptom List scores, and quality of life indices. Further, the benefit was persistent after 3 months of open stimulator programming. From these results, the authors concluded that bilateral thalamic DBS reduces tic frequency and severity in some patients with TS who have exhausted other available means of treatment.17
In another study, 18 patients with TS underwent DBS placed bilaterally in the CM/Pf nucleus and ventralis oralis complex of the thalamus.77 Patients were evaluated postsurgery, with formal assessments at least every 3 months thereafter, including “on-off” and “sham-off” in the first 9 patients. All patients responded well to DBS, although to differing degrees; the duration of follow-up assessments ranged from 3 to 18 months. Comorbid symptoms of OCD, self-injurious behaviors, anxiety, and premonitory sensations decreased after treatment with DBS. These authors concluded that the CM/Pf nucleus and ventralis oralis complex of the thalamus may be a good DBS target for TS.77
The globus pallidus may be another effective DBS target for TS. Diederich et al79 reported on a 14-month follow-up study of a patient with intractable TS who underwent bilateral DBS of the internal globus pallidus. They found that tic frequency decreased by 73% in the postoperative phase and that vocal tics became less intense.79 In support of this, Ackermans et al76 have described the effects of bilateral pallidal stimulation in a patient with intractable TS. They implanted DBS electrodes in the posteroventral part of the globus pallidus internus and found that DBS resulted in substantial reduction of tics and compulsions. Furthermore, DBS of the nucleus accumbens and VC/VS has also been shown to improve TS.80 Taken together, these data suggest that there may be multiple DBS targets for patients with TS and that the pathophysiology of TS involves distributed neural networks that may be modified by DBS.
NEURONAL NETWORKS
The many anatomic targets for treatment-resistant psychiatric disorders currently being trialed with DBS are indicative of both the system-wide effects of DBS and the network-level dysfunction mediating the emotional and cognitive disturbances.15,52,81-84 Elucidating the neuroanatomic networks involved in treatment-resistant psychiatric illness, the neurophysiological anomalies that contribute to disordered information processing therein, and the local and system-wide modulatory effects of DBS is critical for gaining insight into how to apply and further develop neuromodulation therapies. Current anatomic models of psychopathology focus on the neural networks of the basal ganglia, thalamus, frontal cortex, and subcortical structures.
Thalamocortical loops are considered a key anatomic substrate for the expression of any behavior, and thus dysfunction of information processing therein is likely to underlie, at least in part, the pathophysiology of depression, OCD, and TS.81 Thalamocortical loops convey information from specific regions of the cerebral cortex to localized targets within the thalamus and on to the basal ganglia, including the striatum and globus pallidum. The basal ganglia, in turn, is critically implicated in filtering information flow from cortical and limbic regions to modulate the expression of attention, motivated behaviors, and motor function.85-87 Dysfunction of information flow through the limbic components of the cortico-striatal-pallido-thalamocortical loops can result in disordered behavioral and emotional processing. This system is thus rich in anatomic targets for the surgical modulation of severe neuropsychiatric conditions. In fact, DBS targets for treatment of movement disorders, including Parkinson disease, tremor, and dystonia, also lie within this circuitry, and neuropsychiatric adverse effects have been noted when stimulation encroaches on limbic processes.53-55 The most pertinent aspects of the cortico-striatal-pallido-thalamocortical loops for psychiatric illness include the prefrontal and orbitofrontal cortices and the mesolimbic system (Figure 3).51,88,89
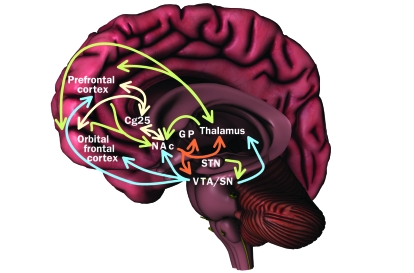
FIGURE 3.
Neuronal networks implicated in psychiatric illness. The prefrontal cortex, orbital frontal cortex, nucleus accumbens (NAc), and thalamus receive dopaminergic inputs from the ventral tegmental area (VTA) and substantia nigra (SN) regions of the midbrain. Reciprocal excitatory glutamatergic pathways connect the thalamus to the prefrontal and orbital frontal cortices. The prefrontal cortex, orbital frontal cortex, and thalamus project glutamatergic inputs to the NAc, which in turn activates inhibitory output neurons for γ-aminobutyric acid (GABA) that project to components of the basal ganglia, including the globus pallidum (GP) and VTA/SN, as well as to the thalamus. The subthalamic nucleus (STN) provides excitatory glutamatergic inputs to the VTA and SN. Connections also exist between Brodmann Area 25 in the subgenual cingulate cortex (Cg25), the NAc, and prefrontal and orbital frontal cortices; however, the neurotransmitter(s) involved remain to be confirmed. Blue = dopamine; green = glutamate; orange = GABA; yellow = unknown.
Dysregulation of information flow within and across these networks appears to contribute to psychopathology. The ability of DBS of the nucleus accumbens to mediate each of the 3 aforementioned psychiatric disorders may relate to its role as an interface for limbic and cortical processing within the cortico-striatal-pallido-thalamocortical loop.86 Indeed, the accumbens can be viewed as a “motivation gateway,” linking emotional limbic and cortical information systems with subsequent motor control systems. The mechanisms by which DBS at least partly reverses this dysfunction remain to be determined; however, several lines of evidence indicate that high-frequency stimulation can affect multiple brain regions to mediate information transfer throughout this interconnected network.
MECHANISM OF ACTION OF DBS
The extracellular stimulation paradigm of the high-frequency stimulation used in DBS consists of short pulses (60-450 μs) regularly applied at a frequency of 65 to 185 Hz.18,90 Although the clinical efficacy of DBS has been established, the biological mechanisms of action remain to be elucidated. Three primary explanations have been proposed for the biological mechanisms of DBS: (1) it silences stimulated neurons, (2) it modulates neuronal network activity and neurotransmission, and (3) it induces long-term synaptic changes (plasticity).52,90 The first explanation is based on the observation that, functionally, DBS induces a similar therapeutic effect to that of a lesion of the stimulated area. Evidence for the second hypothesis has been provided by animal and imaging studies showing that DBS-evoked activity propagates throughout the associated neuronal network to modulate neuronal activity and neurotransmitter efflux in distal nuclei. The third has been inferred from the delayed time course of therapeutic effects of stimulation. Clarification of these potential biological mechanisms of action is imperative to help maximize the therapeutic efficacy of DBS and minimize unwanted adverse effects.
The 3-dimensional electrical field generated by DBS is a complex phenomenon.91 Neurons surrounding the electrode are subject to both depolarizing (activating) and hyperpolarizing (inhibiting) effects, depending on their position relative to the electrode and the specific stimulation parameters applied.92,93 The local effects of stimulation on neuronal activity, in turn, affect the flow of information throughout the network.94-97 As illustrated in Figure 4, the types of neurons affected by DBS include local cells (cells with cell bodies located close to the electrode), afferent inputs (neurons that project axon terminals to the site of stimulation and synapse with local cells), and fibers of passage (cells that project axons through, or nearby, the site of stimulation).52 Local glial cells can also be affected by DBS, although the physiological effect of electrical stimulation on these cell types is only beginning to be considered. How DBS affects each of these cell types to ultimately contribute to the overall therapeutic benefits experienced by the patient still remains to be determined, and much basic and clinical research is now being directed toward this end.
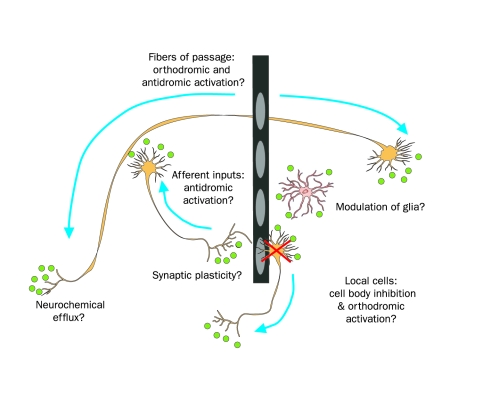
FIGURE 4.
Possible therapeutic mechanisms of action of deep brain stimulation. High-frequency stimulation is thought to inhibit local cell body activity and possibly stimulate orthodromic action potentials, antidromically activate afferent neuronal inputs, and stimulate neurons passing near the electrode. Local glial cell activity may also be modulated. Modulation of neurochemical efflux, including neurotransmitters, neuropeptides, and retrograde messengers, may occur both locally and distally. In addition to these short-term changes, long-term adaptations likely occur, including the formation of new synapses and/or regulation of receptor expression.
Our current understanding of the network-wide actions of DBS in neuropsychiatric conditions is particularly limited. The delayed time course of therapeutic effects of stimulation indicates that sudden disruption of pathological network activity may provide only a minor component of the therapeutic mechanism of action.52 Although some therapeutic benefit is observed shortly after treatment, a substantial proportion of symptom improvement occurs only after months of long-term stimulation.15,34,98 The cumulative nature of this process suggests that therapeutic benefits may result from underlying long-term changes (plasticity) occurring within the neuronal network.52 This is supported by observations that metabolic changes observed with DBS therapy linger after long-term stimulation ceases, with a concordant progressive worsening of symptoms occurring after DBS.15,72 It has thus been hypothesized that DBS for neuropsychiatric disease has both short- and long-term components resulting from complementary but distinct mechanisms of action. That is, sudden symptom reprieve results from the immediate disruption of pathologic activity in cortico-striatal-pallido-thalamocortical circuitry, whereas enduring improvements occur only after long-term changes in synaptic effectiveness and/or connectivity have had time to take effect.52 Thus, the normalization of abnormal (hyper/hypo) metabolic changes may result from activity-dependent mechanisms that work together to mediate neuronal plasticity (including formation of new synapses, growth of new connections, and up- or down-regulation of information flow at the synapse). Over time, this may ultimately result in global changes in neuronal network processes that mediate the enduring therapeutic effects of DBS.52
CONCLUSION
The effective use of high-frequency DBS for treatment of various neurologic diseases is now well established, and its therapeutic scope is extending into the realm of neuropsychiatric conditions. Indeed, the disease burden of refractory mental illness for patients, their families, and society and the potential for neuromodulation technologies to relieve this burden require that we evaluate these therapeutic options with the highest degree of scientific rigor. Coupled with ongoing advances in DBS technologies is a budding field of scientific endeavor that continues to advance our understanding of psychiatric neurobiology. As we stand at the threshold of this evolving field, it is imperative that those who provide patient care stay at the very forefront of clinical and scientific progress in psychiatric neuromodulation therapies, with a clear appreciation of the strengths and limitations inherent in this technology.
Acknowledgments
We thank Stephan Goerss, BS, and Deborah Gorman, BSN, for their assistance with figure preparation.
Footnotes
This work was supported by a National Institutes of Health K08 NS 52232 award and a Mayo Foundation 2008-2010 Research Early Career Development Award for Clinician Scientists (K.H.L.) and an American Australian Association Sir Keith Murdoch Fellowship (S.J.T.).
REFERENCES
- 1.Heller AC, Amar AP, Liu CY, Apuzzo ML. Surgery of the mind and mood: a mosaic of issues in time and evolution. Neurosurgery 2006;59(4):720-733 [DOI] [PubMed] [Google Scholar]
- 2.Alt KW, Jeunesse C, Buitrago-Téllez CH, Wächter R, Boës E, Pichler SL. Evidence for stone age cranial surgery [published correction appears in Nature. 1997;387(6635)768] [letter] Nature 1997;387(6631):360 [DOI] [PubMed] [Google Scholar]
- 3.Kopell BH, Greenberg BD. Anatomy and physiology of the basal ganglia: implications for DBS in psychiatry. Neurosci Biobehav Rev. 2008;32(3):408-422 Epub 2007 Aug 7 [DOI] [PubMed] [Google Scholar]
- 4.Horwitz NH, John F. Fulton (1899-1960). Neurosurgery 1998;43(1):178-184 [DOI] [PubMed] [Google Scholar]
- 5.Kopell BH, Rezai AR. Psychiatric neurosurgery: a historical perspective. Neurosurg Clin N Am. 2003;14(2):181-197, vii [DOI] [PubMed] [Google Scholar]
- 6.Feldman RP, Goodrich JT. Psychosurgery: a historical overview. Neurosurgery 2001;48(3):647-659 [DOI] [PubMed] [Google Scholar]
- 7.Mashour GH, Walker EE, Martuza RL. Psychosurgery: Past, present, and future. Brain Res Rev. 2005;48(3):409-419 [DOI] [PubMed] [Google Scholar]
- 8.Moore BE, Friedman S, Simon B, Farmer J, Connecticut Lobotomy Committee A cooperative clinical study of lobotomy. Res Publ Assoc Nerv Ment Dis. 1948;27(1):769-794 [PubMed] [Google Scholar]
- 9.Wilson I, Warland EH. Pre-frontal leucotomy in a thousand cases London, Great Britain: Board of Control, His Majesty's Stationery Office; 1947. http://www.mdx.ac.uk/WWW/STUDY/7.htm#1957 Accessed March 23, 2009 [Google Scholar]
- 10.Scoville WB. Selective cortical undercutting as a means of modifying and studying frontal lobe function in man: preliminary report of forty-three operative cases. J NeuroSurg. 1949;6(1):65-73 [DOI] [PubMed] [Google Scholar]
- 11.Fins JJ. From psychosurgery to neuromodulation and palliation: history's lessons for the ethical conduct and regulation of neuropsychiatric research. Neurosurg Clin N Am. 2003;14(2):303-319, ix-x [DOI] [PubMed] [Google Scholar]
- 12.Lee KH, Blaha CD, Bledsoe JM. Mechanisms of action of deep brain stimulation: a review. In: Krames E, Peckham PH, Rezai A, eds. Neuromodulation Burlington, MA: Elsevier; In press [Google Scholar]
- 13.George MS, Rush AJ, Sackeim HA, Marangell HB. Vagus nerve stimulation (VNS): utility in neuropsychiatric disorders. Int J Neuropsychopharmacol. 2003;6(1):73-83 [DOI] [PubMed] [Google Scholar]
- 14.Pascual-Leone A, Catalá MD, Pascual-Leone Pascual A. Lateralized effect of rapid-rate transcranial magnetic stimulation of the prefrontal cortex on mood. Neurology 1996;46(2):499-502 [DOI] [PubMed] [Google Scholar]
- 15.Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron 2005;45(5):651-660 [DOI] [PubMed] [Google Scholar]
- 16.Nuttin B, Cosyns P, Demeulemeester H, Gybels J, Meyerson B. Electrical stimulation in anterior limbs of internal capsules in patients with obsessive-compulsive disorder [letter]. Lancet 1999;354(9189):1526 [DOI] [PubMed] [Google Scholar]
- 17.Maciunas RJ, Maddux BN, Riley DE, et al. Prospective randomized double-blind trial of bilateral thalamic deep brain stimulation in adults with Tourette syndrome. J NeuroSurg. 2007;107(5):1004-1014 [DOI] [PubMed] [Google Scholar]
- 18.Larson PS. Deep brain stimulation for psychiatric disorders. Neurotherapeutics 2008;5(1):50-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: global burden of disease study. Lancet 1997;349(9064):1498-1504 [DOI] [PubMed] [Google Scholar]
- 20.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication [published correction appears in Arch Gen Psychiatry. 2005;62(7):709. Merikangas KR added] Arch Gen Psychiatry 2005;62(6):617-627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Psychiatric Association Practice guideline for the treatment of patients with major depressive disorder (revision). Am J Psychiatry 2000;157(4)(suppl):1-45 [PubMed] [Google Scholar]
- 22.Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9(3):471-481 [DOI] [PubMed] [Google Scholar]
- 23.Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7(5):541-547 [DOI] [PubMed] [Google Scholar]
- 24.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron 2002;34(1):13-25 [DOI] [PubMed] [Google Scholar]
- 25.Hauptman JS, DeSalles AA, Espinoza R, Sedrak M, Ishida W. Potential surgical targets for deep brain stimulation in treatment-resistant depression. Neurosurg Focus 2008;25(1):E3 [DOI] [PubMed] [Google Scholar]
- 26.Mayberg HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry 1999;156(5):675-682 [DOI] [PubMed] [Google Scholar]
- 27.Seminowicz DA, Mayberg HS, McIntosh AR, et al. Limbic-frontal circuitry in major depression: a path modeling metanalysis. Neuroimage 2004;22(1):409-418 [DOI] [PubMed] [Google Scholar]
- 28.Mayberg HS, Brannan SK, Tekell JL, et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry 2000;48(8):830-843 [DOI] [PubMed] [Google Scholar]
- 29.Nobler MS, Oquendo MA, Kegeles LS, et al. Decreased regional brain metabolism after ECT. Am J Psychiatry 2001;158(2):305-308 [DOI] [PubMed] [Google Scholar]
- 30.Mottaghy FM, Keller CE, Gangitano M, et al. Correlation of cerebral blood flow and treatment effects of repetitive transcranial magnetic stimulation in depressed patients. Psychiatry Res. 2002;115(1-2):1-14 [DOI] [PubMed] [Google Scholar]
- 31.Dougherty DD, Weiss AP, Cosgrove GR, et al. Cerebral metabolic correlates as potential predictors of response to anterior cingulotomy for treatment of major depression. J NeuroSurg. 2003;99(6):1010-1017 [DOI] [PubMed] [Google Scholar]
- 32.Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry 2008;64(6):461-467 Epub 2008 Jul 18 [DOI] [PubMed] [Google Scholar]
- 33.Giacobbe P, Kennedy SH, Fulton K, Lozano AM, Mayberg HS. Predictors of response to deep brain stimulation for treatment resistant depression Poster presented at: 45th Annual Meeting of the American College of Neuropsychopharmacology; December3-7, 2006; Hollywood, FL [Google Scholar]
- 34.Greenberg BD, Malone DA, Friehs GM, et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder [published correction appears in Neuropsychopharmacology. 2006;319110: 2394] Neuropsychopharmacology 2006;31(11):2384-2393 Epub 2006 Jul 19 [DOI] [PubMed] [Google Scholar]
- 35.Malone DA, Jr, Dougherty DD, Rezai AR, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry 2009;65(4):267-275 Epub 2008 Oct 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schlaepfer TE, Cohen MX, Frick C, et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology 2008;33(2):368-377 Epub 2007 Apr 11 [DOI] [PubMed] [Google Scholar]
- 37.Kuhn J, Lenartz D, Huff W, et al. Remission of alcohol dependency following deep brain stimulation of the nucleus accumbens: valuable therapeutic implications? J Neurol Neurosurg Psychiatry 2007;78(10):1152-1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu HY, Jin J, Tang JS, et al. Chronic deep brain stimulation in the rat nucleus accumbens and its effect on morphine reinforcement. Addict Biol. 2008;13(1):40-46 [DOI] [PubMed] [Google Scholar]
- 39.Vassoler FM, Schmidt HD, Gerard ME, et al. Deep brain stimulation of the nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug seeking in rats. J Neurosci. 2008;28(35):8735-8739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stein DJ. Obsessive-compulsive disorder. Lancet 2002;360(9330):397-405 [DOI] [PubMed] [Google Scholar]
- 41.Hodgkiss AD, Malizia AL, Bartlett JR, Bridges PK. Outcome after the psychosurgical operation of stereotactic subcaudate tractotomy, 1979-1991. J Neuropsychiatry Clin Neurosci. 1995;7(2):230-234 [DOI] [PubMed] [Google Scholar]
- 42.Jenike MA. Neurosurgical treatment of obsessive-compulsive disorder. Br J Psychiatry Suppl. 1998;173(35):79-90 [PubMed] [Google Scholar]
- 43.Lippitz BE, Mindus P, Meyerson BA, Kihlström L, Lindquist C. Lesion topography and outcome after thermocapsulotomy or gamma knife capsulotomy for obsessive-compulsive disorder: relevance of the right hemisphere. Neurosurgery 1999;44(3):452-458 [DOI] [PubMed] [Google Scholar]
- 44.Cosgrove GR. Surgery for psychiatric disorders. CNS Spectr. 2000;5(10):43-52 [DOI] [PubMed] [Google Scholar]
- 45.Dougherty DD, Baer L, Cosgrove GR, et al. Prospective long-term follow-up of 44 patients who received cingulotomy for treatment-refractory obsessive-compulsive disorder. Am J Psychiatry 2002;159(2):269-275 [DOI] [PubMed] [Google Scholar]
- 46.Montoya A, Weiss AP, Price BH, et al. Magnetic resonance imaging-guided stereotactic limbic leukotomy for treatment of intractable psychiatric disease. Neurosurgery 2002;50(5):1043-1049 [DOI] [PubMed] [Google Scholar]
- 47.Greenberg BD, Price LH, Rauch SL, et al. Neurosurgery for intractable obsessive-compulsive disorder and depression: critical issues. Neurosurg Clin N Am. 2003;14(2):199-212 [DOI] [PubMed] [Google Scholar]
- 48.Sturm V, Lenartz D, Koulousakis A, et al. The nucleus accumbens: a target for deep brain stimulation in obsessive-compulsive- and anxiety-disorders. J Chem Neuroanat. 2003;26(4):293-299 [DOI] [PubMed] [Google Scholar]
- 49.Gabriëls L, Cosyns P, Nuttin B, Demeulemeester H, Gybels J. Deep brain stimulation for treatment-refractory obsessive-compulsive disorder: psychopathological and neuropsychological outcome in three cases. Acta Psychiatr Scand 2003;107(4):275-282 [PubMed] [Google Scholar]
- 50.Abelson JL, Curtis GC, Sagher O, et al. Deep brain stimulation for refractory obsessive-compulsive disorder. Biol Psychiatry 2005;57(5):510-516 [DOI] [PubMed] [Google Scholar]
- 51.Lipsman N, Neimat JS, Lozano AM. Deep brain stimulation for treatment-refractory obsessive-compulsive disorder: the search for a valid target. Neurosurgery 2007;61(1):1-11 [DOI] [PubMed] [Google Scholar]
- 52.Lujan JL, Chaturvedi A, McIntyre CC. Tracking the mechanisms of deep brain stimulation for neuropsychiatric disorders. Front Biosci. 2008;13:5892-5904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krack P, Batir A, Van Blercom N, et al. Five-year follow-up of bilateral subthalamic stimulation in advanced Parkinson's disease. N Engl J Med. 2003;349(20):1925-1934 [DOI] [PubMed] [Google Scholar]
- 54.Doshi PK, Chhaya N, Bhatt MH. Depression leading to attempted suicide following bilateral subthalamic nucleus stimulation for Parkinson's disease. Mov Disord 2002;17(5):1084-1085 [DOI] [PubMed] [Google Scholar]
- 55.Bejjani BP, Damier P, Arnulf I, et al. Transient acute depression induced by high-frequency deep brain stimulation. N Engl J Med. 1999;340(19):1476-1480 [DOI] [PubMed] [Google Scholar]
- 56.Temel Y, Kessels A, Tan S, Topdag A, Boon P, Visser-Vandewalle V. Behavioural changes after bilateral subthalamic stimulation in advanced Parkinson disease: a systematic review. Parkinsonism Relat Disord 2006;12(5):265-272 Epub 2006 Apr 18 [DOI] [PubMed] [Google Scholar]
- 57.Houeto JL, Mesnage V, Mallet L, et al. Behavioural disorders, Parkinson's disease and subthalamic stimulation. J Neurol Neurosurg Psychiatry 2002;72(6):701-707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Romito LM, Raja M, Daniele A, et al. Transient mania with hypersexuality after surgery for high frequency stimulation of the subthalamic nucleus in Parkinson's disease. Mov Disord 2002;17(6):1371-1374 [DOI] [PubMed] [Google Scholar]
- 59.Castelli L, Perozzo P, Genesia ML, et al. Sexual well being in Parkinsonian patients after deep brain stimulation of the subthalamic nucleus. J Neurol Neurosurg Psychiatry 2004;75(9):1260-1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Doshi P, Bhargava P. Hypersexuality following subthalamic nucleus stimulation for Parkinson's disease. Neurol India 2008;56(4):474-476 [DOI] [PubMed] [Google Scholar]
- 61.Mallet L, Mesnage V, Houeto JL, et al. Compulsions, Parkinson's disease, and stimulation. Lancet 2002;360(9342):1302-1304 [DOI] [PubMed] [Google Scholar]
- 62.Fontaine D, Mattei V, Borg M, et al. Effect of subthalamic nucleus stimulation on obsessive-compulsive disorder in a patient with Parkinson disease: case report. J NeuroSurg. 2004;100(6):1084-1086 [DOI] [PubMed] [Google Scholar]
- 63.Baup N, Grabli D, Karachi C, et al. High-frequency stimulation of the anterior subthalamic nucleus reduces stereotyped behaviors in primates. J Neurosci. 2008;28(35):8785-8788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aouizerate B, Cuny E, Martin-Guehl C, et al. Deep brain stimulation of the ventral caudate nucleus in the treatment of obsessive-compulsive disorder and major depression: case report. J NeuroSurg. 2004;101(4):682-686 [DOI] [PubMed] [Google Scholar]
- 65.McGuire PK, Bench CJ, Frith CD, Marks IM, Frackowiak RS, Dolan RJ. Functional anatomy of obsessive-compulsive phenomena. Br J Psychiatry 1994;164(4):459-468 [DOI] [PubMed] [Google Scholar]
- 66.Breiter HC, Rauch SL. Functional MRI and the study of OCD: from symptom provocation to cognitive-behavioral probes of cortico-striatal systems and the amygdala. Neuroimage 1996;4(3, pt 3):S127-S138 [DOI] [PubMed] [Google Scholar]
- 67.Breiter HC, Rauch SL, Kwong KK, et al. Functional magnetic resonance imaging of symptom provocation in obsessive-compulsive disorder. Arch Gen Psychiatry 1996;53(7):595-606 [DOI] [PubMed] [Google Scholar]
- 68.Swedo SE, Pietrini P, Leonard HL, et al. Cerebral glucose metabolism in childhood-onset obsessive-compulsive disorder: revisualization during pharmacotherapy. Arch Gen Psychiatry 1992;49(9):690-694 [DOI] [PubMed] [Google Scholar]
- 69.Schwartz JM, Stoessel PW, Baxter LR, Jr, Martin KM, Phelps ME. Systematic changes in cerebral glucose metabolic rate after successful behavior modification treatment of obsessive-compulsive disorder. Arch Gen Psychiatry 1996;53(2):109-113 [DOI] [PubMed] [Google Scholar]
- 70.Mindus P, Ericson K, Greitz T, Meyerson BA, Nyman H, Sjögren I. Regional cerebral glucose metabolism in anxiety disorders studied with positron emission tomography before and after psychosurgical intervention: a preliminary report. Acta Radiol Suppl. 1986;369:444-448 [PubMed] [Google Scholar]
- 71.Nuttin BJ, Gabriëls LA, Cosyns PR, et al. Long-term electrical capsular stimulation in patients with obsessive-compulsive disorder. Neurosurgery 2003;52(6):1263-1272 [DOI] [PubMed] [Google Scholar]
- 72.Van Laere K, Nuttin B, Gabriels L, et al. Metabolic imaging of anterior capsular stimulation in refractory obsessive-compulsive disorder: a key role for the subgenual anterior cingulate and ventral striatum. J Nucl Med. 2006;47(5):740-747 [PubMed] [Google Scholar]
- 73.McCracken CB, Grace AA. High-frequency deep brain stimulation of the nucleus accumbens region suppresses neuronal activity and selectively modulates afferent drive in rat orbitofrontal cortex in vivo. J Neurosci. 2007;27(46):12601-12610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ackermans L, Temel Y, Visser-Vandewalle V. Deep brain stimulation in Tourette's syndrome. Neurotherapeutics 2008;5(2):339-344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Temel Y, Visser-Vandewalle V. Surgery in Tourette syndrome. Mov Disord 2004;19(1):3-14 [DOI] [PubMed] [Google Scholar]
- 76.Ackermans L, Temel Y, Cath D, et al. Dutch Flemish Tourette Surgery Study Group Deep brain stimulation in Tourette's syndrome: two targets? Mov Disord 2006;21(5):709-713 [DOI] [PubMed] [Google Scholar]
- 77.Servello D, Porta M, Sassi M, Brambilla A, Robertson MM. Deep brain stimulation in 18 patients with severe Gilles de la Tourette syndrome refractory to treatment: the surgery and stimulation. J Neurol Neurosurg Psychiatry 2008;79(2):136-142 Epub 2007 Sep 10 [DOI] [PubMed] [Google Scholar]
- 78.Vandewalle V, van der Linden C, Groenewegen HJ, Caemaert J. Stereotactic treatment of Gilles de la Tourette syndrome by high frequency stimulation of thalamus [letter]. Lancet 1999;353(9154):724 [DOI] [PubMed] [Google Scholar]
- 79.Diederich NJ, Kalteis K, Stamenkovic M, Pieri V, Alesch F. Efficient internal pallidal stimulation in Gilles de la Tourette syndrome: a case report. Mov Disord 2005;20(11):1496-1499 [DOI] [PubMed] [Google Scholar]
- 80.Kuhn J, Lenartz D, Mai JK, et al. Deep brain stimulation of the nucleus accumbens and the internal capsule in therapeutically refractory Tourette-syndrome [letter]. J Neurol. 2007;254(7):963-965 Epub 2007 Apr 6 [DOI] [PubMed] [Google Scholar]
- 81.Kopell BH, Greenberg B, Rezai AR. Deep brain stimulation for psychiatric disorders. J Clin Neurophysiol. 2004;21(1):51-67 [DOI] [PubMed] [Google Scholar]
- 82.Greenberg BD, Rezai AR. Mechanisms and the current state of deep brain stimulation in neuropsychiatry. CNS Spectr. 2003;8(7):522-526 [DOI] [PubMed] [Google Scholar]
- 83.Modell JG, Mountz JM, Curtis GC, Greden JF. Neurophysiologic dysfunction in basal ganglia/limbic striatal and thalamocortical circuits as a pathogenetic mechanism of obsessive-compulsive disorder. J Neuropsychiatry Clin Neurosci. 1989;1(1):27-36 [DOI] [PubMed] [Google Scholar]
- 84.Lerner A, Bagic A, Boudreau EA, et al. Neuroimaging of neuronal circuits involved in tic generation in patients with Tourette syndrome. Neurology 2007;68(23):1979-1987 [DOI] [PubMed] [Google Scholar]
- 85.Llinas RR, Leznik E, Urbano FJ. Temporal binding via cortical coincidence detection of specific and nonspecific thalamocortical inputs: a voltage-dependent dye-imaging study in mouse brain slices. Proc Natl Acad Sci U S A 2002;99(1):449-454 Epub 2002 Jan 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Goto Y, Grace AA. Limbic and cortical information processing in the nucleus accumbens. Trends Neurosci. 2008;31(11):552-558 Epub 2008 Sep 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Redgrave P, Gurney K, Reynolds J. What is reinforced by phasic dopamine signals? Brain Res Brain Res Rev. 2008;58(2):322-339 Epub 2007 Oct 26 [DOI] [PubMed] [Google Scholar]
- 88.Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119-146 [PubMed] [Google Scholar]
- 89.Rauch SL. Neuroimaging and neurocircuitry models pertaining to the neurosurgical treatment of psychiatric disorders. Neurosurg Clin N Am. 2003;14(2):213-223, vii-viii [DOI] [PubMed] [Google Scholar]
- 90.Hammond C, Ammari R, Bioulac B, Garcia L. Latest view on the mechanism of action of deep brain stimulation. Mov Disord 2008;23(15):2111-2121 [DOI] [PubMed] [Google Scholar]
- 91.McIntyre CC, Mori S, Sherman DL, Thakor NV, Vitek JL. Electric field and stimulating influence generated by deep brain stimulation of the subthalamic nucleus. Clin Neurophysiol. 2004;115(3):589-595 [DOI] [PubMed] [Google Scholar]
- 92.McIntyre CC, Grill WM. Excitation of central nervous system neurons by nonuniform electric fields. Biophys J 1999;76(2):878-888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rattay F. The basic mechanism for the electrical stimulation of the nervous system. Neuroscience 1999;89(2):335-346 [DOI] [PubMed] [Google Scholar]
- 94.Lee KH, Blaha CD, Harris BT, et al. Dopamine efflux in the rat striatum evoked by electrical stimulation of the subthalamic nucleus: potential mechanism of action in Parkinson's disease. Eur J Neurosci. 2006;23(4):1005-1014 [DOI] [PubMed] [Google Scholar]
- 95.Grill WM, Cantrell MB, Robertson MS. Antidromic propagation of action potentials in branched axons: implications for the mechanisms of action of deep brain stimulation. J Comput Neurosci. 2008;24(1):81-93 Epub 2007 Jun 12 [DOI] [PubMed] [Google Scholar]
- 96.Windels F, Carcenac C, Poupard A, Savasta M. Pallidal origin of GABA release within the substantia nigra pars reticulata during high-frequency stimulation of the subthalamic nucleus. J Neurosci. 2005;25(20):5079-5086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Galati S, Mazzone P, Fedele E, et al. Biochemical and electrophysiological changes of substantia nigra pars reticulata driven by subthalamic stimulation in patients with Parkinson's disease. Eur J Neurosci. 2006;23(11):2923-2928 [DOI] [PubMed] [Google Scholar]
- 98.Cosyns P, Gabriels L, Nuttin B. Deep brain stimulation in treatment refractory obsessive compulsive disorder. Verh K Acad Geneeskd Belg. 2003;65(6):385-399 [PubMed] [Google Scholar]