Abstract
Breast cancer is the most common noncutaneous malignancy among every major ethnic group of women in the United States. Anthracyclines and taxanes are the most active and widely used chemotherapeutic agents for breast cancer, but the increased use of these agents at an early stage of disease often renders tumors resistant to these drugs by the time the disease recurs, thereby reducing the number of treatment options for metastatic disease. Moreover, even when these agents can be used in the metastatic setting, treatment failure occurs in most cases, and as a result the 5-year survival rates of patients with metastatic breast cancer are low. This outcome underscores the need for new, effective treatments of metastatic breast cancer and has led to investigation of novel ways to overcome the problem of drug resistance. This article reviews the current treatment options for breast cancer resistant to anthracycline and taxane and provides recommendations for disease management. Published sources for this review were found by searching PubMed (https://www.ncbi.nlm.nih.gov/pubmed) and congress Web sites.
ABC = ATP-binding cassette; BCRP = breast cancer resistance protein; CI = confidence interval; EGFR = epidermal growth factor receptor; FDA = Food and Drug Administration; HER2 = human epidermal growth factor receptor 2; IRR = independent radiology review; MBC = metastatic breast cancer; MDR = multidrug resistance protein; NCCN = National Comprehensive Cancer Network; ORR = overall response rate; OS = overall survival; PARP-1 = poly-ADP-ribose polymerase 1; PFS = progression-free survival; TN = triple negative; TTP = time to progression; VEGF = vascular endothelial growth factor
Breast cancer is the most common noncutaneous malignancy among every major ethnic group of women in the United States, annually causing nearly 40,000 deaths in the United States and more than 400,000 deaths worldwide.1,2 Anthracyclines and taxanes are the most active and widely used chemotherapeutic agents for treating breast cancer in hormone receptor-negative patients and those whose disease progresses while they are taking hormone therapy.3 These agents are commonly used in the adjuvant setting, either in combination or sequentially.4 A meta-analysis of 13 clinical trials involving nearly 23,000 women with high-risk, early-stage breast cancer showed that incorporating taxanes into anthracycline-based regimens significantly improves disease-free survival and overall survival (OS) rates.5,6 This benefit is evident regardless of hormone receptor status, degree of nodal involvement, age, menopausal status, and type of taxane or administration schedule. Anthracyclines and taxanes, either alone or in combination, are also the preferred option for hormone receptor-negative patients with metastatic breast cancer (MBC).7 Response rates of 25% to 69% have been reported when taxanes (paclitaxel or docetaxel) are used as first-line treatment of MBC.8-10 In several phase 2 studies, Perez et al11-13 demonstrated the activity and beneficial therapeutic ratio of weekly paclitaxel as a single agent or in combination with chemotherapy and biological therapy for MBC.
Because of the increase in use of anthracyclines and taxanes as therapy for early-stage breast cancer, many patients' tumors are resistant to these agents by the time of disease recurrence, thereby reducing the number of treatment options for MBC. Moreover, even when these agents can be used to treat MBC, treatment failure occurs in most cases; as a result, the 5-year survival rate of patients with MBC is only 27%.1 These data underscore the need for new, effective treatments of MBC and have led to the investigation of novel methods for overcoming the problem of drug resistance. Resistance to anthracycline and taxane may be defined clinically as disease recurrence within 6 months of completion of adjuvant or neoadjuvant treatment with these agents or tumor progression that occurs during treatment or within 3 months of the last dose of treatment.14 This article reviews the main treatment options for anthracycline- and taxane-resistant breast cancer and provides recommendations for disease management.
METHODS
Findings of published abstracts, primary manuscripts, secondary papers, and reviews are summarized in this review. These sources were found by searching PubMed (https://www.ncbi.nlm.nih.gov/pubmed) and congress Web sites (American Society of Clinical Oncology, San Antonio Breast Cancer Symposium). Search terms included “Breast Neoplasms” [MeSH] OR “breast neoplasm*” OR “breast cancer” OR “breast tumor*” OR “breast tumour*” OR “cancer of the breast” plus “Neoplasm Recurrence, Local” [MeSH] OR “Recurrence” [MeSH] OR “recurrent” OR “recurrence” OR “refractory” OR “Drug Resistance” [MeSH] OR “Drug Resistance, Neoplasm” [MeSH] OR “resistant” OR “resistance” OR “pre-treated” OR “pre treated” OR “pretreated” OR “metastatic.” Search terms used to find reports about triple-negative breast cancer were “triple negative” OR “triple-negative” OR “basal-like” OR “HER-2 negative” OR “HER2-negative” AND “estrogen-receptor negative” OR “ER-negative” AND “progesterone-receptor negative” OR “PR-negative.” No date limitations were imposed on the searches. The studies selected were phase 2 or phase 3 trials, particularly those supporting drug approvals in the MBC setting (where applicable).
MECHANISMS OF ANTHRACYCLINE AND TAXANE RESISTANCE
Anthracyclines form complexes with DNA and topoisomerase II to impair DNA replication and repair, thereby promoting apoptosis via p53 DNA damage sensors and caspase mechanisms. They also act via oxidant mechanisms that lead to cellular apoptosis.15 Taxanes bind reversibly to β-tubulin to stabilize microtubule complexes and promote microtubule polymerization, causing cell-cycle arrest and apoptosis.16
Preclinical evidence suggests that resistance to anthracyclines may develop via multiple cellular mechanisms.17 Overexpression of P-glycoprotein and other adenosine triphosphate (ATP)-binding cassette (ABC) transport proteins confers multidrug resistance; these pumps promote drug efflux and thereby reduce drug concentrations within tumor cells.18 P-glycoprotein is the most important member of the ABC family because it confers the strongest resistance to the widest variety of anticancer drugs, including both anthracyclines and taxanes (Table).18,19 The breast cancer resistance protein (BCRP) is another member of the ABC family that is involved in drug resistance in the absence of overexpression of known multidrug resistance transporters, such as P-glycoprotein or the multidrug resistance protein (MDR). The BCRP was first identified in the breast cancer cell line MCF7/AdrVp,20 and its overexpression confers resistance to anthracyclines but not to taxanes. Anthracycline resistance may also be mediated by antioxidant defense mechanisms, topoisomerase II mutations, overexpression of transcription-linked DNA repair mechanisms, and alterations in apoptotic signaling.15 Besides P-glycoprotein overexpression, taxane resistance may be caused by β-tubulin gene mutations, overexpression of class III β-tubulin or microtubule-associated proteins, or alterations in mitotic checkpoint signaling proteins.16 Class III β-tubulin overexpression has been associated with lower response rates and shorter survival times in response to taxane therapy.21
TABLE.
Resistance Proposed to be Conferred by Overexpression of P-glycoprotein and Other ABC Transportersa
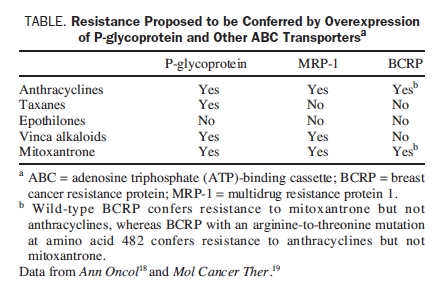
Although preclinical evidence demonstrating anthracycline and taxane resistance is substantial and has increased our understanding of the underlying mechanisms responsible for this resistance, relatively few data are available to show how resistance actually develops in the clinical situation, within individuals or groups of patients with breast cancer. Further research in this area will be of great interest.
TREATMENT OPTIONS AFTER EXPOSURE TO ANTHRACYCLINE AND TAXANE
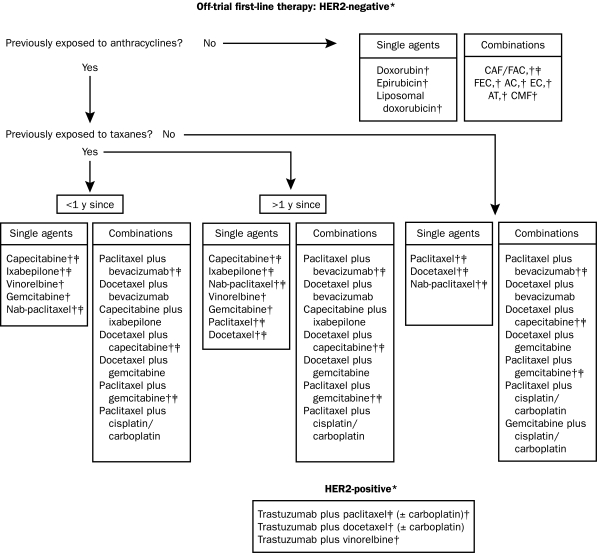
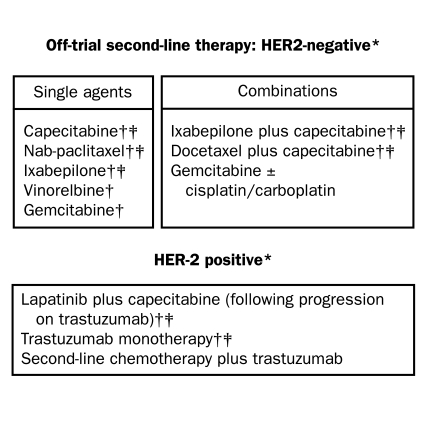
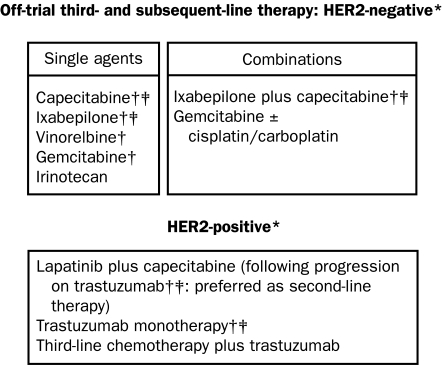
Treatment options for recurrent breast cancer and MBC are shown in Figures 1, 2 and 3.3,22 The choice of therapy for each patient depends on several interlinking factors, including tumor type, disease stage, functional status, and treatment history, particularly any previous adjuvant therapy for early breast cancer. Anthracycline or taxane regimens are still relevant first-line therapies for recurrent breast cancer or MBC if the tumor has progressed after previous (eg, adjuvant) exposure to one but not both types of drug, leaving the remaining class of therapy open to the clinician and patient.23 Additionally, it may still be possible to derive subsequent benefit from administration of a second taxane if tumor progression has occurred during treatment with the first taxane (eg, retreatment with paclitaxel if previous failure of first-line paclitaxel therapy occurred >1 year earlier; or second-line treatment with docetaxel after previous treatment with paclitaxel; or second-line treatment with albumin-bound paclitaxel after previous docetaxel therapy).23-25
FIGURE 1.
First-line options for recurrent or metastatic breast cancer resistant to anthracyclines and/or taxanes. AC = doxorubicin/cyclophosphamide; AT = doxorubicin plus docetaxel or paclitaxel; CAF/FAC = cyclophosphamide, doxorubicin plus fluorouracil; CMF = cyclophosphamide, methotrexate, fluorouracil; EC = epirubicin plus cyclophosphamide; FEC = fluorouracil, epirubicin plus cyclophosphamide; HER2 = human epidermal growth factor receptor 2.
*Regimens are supported by clinical trial data and/or under clinical investigation (source: www.clinicaltrials.gov).
†These regimens are also recommended by the National Comprehensive Cancer Network for recurrent or metastatic breast cancer (line of therapy unspecified).
‡These regimens are Food and Drug Administration approved as discussed in the text.
FIGURE 2.
Second-line options for recurrent or metastatic breast cancer resistant to anthracyclines and/or taxanes.
*Regimens are supported by clinical trial data and/or under clinical investigation (source: www.clinicaltrials.gov).
†These regimens are also recommended by the National Comprehensive Cancer Network (line of therapy unspecified).
‡These regimens are Food and Drug Administration approved as discussed in the text.
FIGURE 3.
Third- and subsequent-line options for recurrent or metastatic breast cancer resistant to anthracyclines and/or taxanes.
*Regimens are supported by clinical trial data and/or under clinical investigation (source: www.clinicaltrials.gov).
†These regimens are also recommended by the National Comprehensive Cancer Network (line of therapy unspecified).
‡These regimens are Food and Drug Administration approved as discussed in the text.
TREATMENT OPTIONS FOR RESISTANT BREAST CANCER
Chemotherapies
For patients with breast tumors that do not express human epidermal growth factor receptor 2 (HER2), the preferred chemotherapy options (although not all have been approved by regulatory agencies such as the Food and Drug Administration [FDA]) for patients whose tumors have progressed during therapy with anthracycline and/or taxane are capecitabine (Xeloda), gemcitabine (Gemzar), vinorelbine (Navelbine), liposomal doxorubicin (pegylated [Doxil] or nonpegylated [Caelyx]), albumin-bound paclitaxel (Abraxane), and the recently approved epothilone B analog ixabepilone (Ixempra), both as single agents and in combination regimens3,7,22,24 (Figures 1, 2 and 3).
Capecitabine. Capecitabine is an oral prodrug that is converted by thymidine phosphorylase—an enzyme found at higher levels in tumor cells than in normal tissues—into the antimetabolite 5-fluorouracil.26,27 In phase 2 studies involving patients with MBC who had been previously treated with anthracycline and taxane, single-agent capecitabine produced overall response rates (ORRs) of 15% to 29%, median times to progression (TTP) of 2.8 to 6.2 months, and median OSs of 8.1 to 15.2 months.27,28 Dose-limiting adverse effects associated with capecitabine included hand-foot syndrome (grade 3 in 5%-22% of patients) and diarrhea (grade 3/4 in 5%-19% of patients).28 In a phase 3 study of patients with advanced breast cancer who had previously been treated with anthracycline, capecitabine plus docetaxel achieved significantly better ORRs (42% vs 30%; P=.006), longer median TTPs (6.1 vs 4.2 months; P<.001), and longer median OSs (14.5 vs 11.5 months; P<.001) than docetaxel alone.29 Capecitabine is currently approved by the US FDA as monotherapy for patients with MBC whose tumors are resistant to paclitaxel and an anthracycline or resistant to paclitaxel in which an anthracycline-based regimen is contraindicated. Capecitabine in combination with docetaxel is also indicated for MBC after failure of previous anthracycline therapy.30
Gemcitabine. Gemcitabine is an antimetabolite that is incorporated into DNA. Phase 2 studies of single-agent gemcitabine as treatment of MBC have demonstrated response rates of 14% to 37% for chemotherapy-naïve patients and 12% to 30% for patients previously treated with anthracycline or taxane.31 In a global phase 3 study involving 529 patients with advanced breast cancer who had previously been treated with anthracycline, paclitaxel was administered as first-line therapy every 3 weeks either alone or in combination with gemcitabine. The combination produced higher ORRs (41.4% vs 26.2%; P=.0002), longer median TTPs (6.1 vs 4.0 months; P=.0002), and longer OSs (18.6 vs 15.8 months; P=.049) than paclitaxel alone.32 However, only 15% of patients in the paclitaxel-only arm crossed over to gemcitabine when their disease progressed,33 and the reported median OS for both treatment arms was lower than currently expected for patients with newly diagnosed MBC (18-36 months).34-37 Nevertheless, on the basis of the results of this phase 3 trial, gemcitabine combined with paclitaxel was approved in the United States as first-line treatment of patients with MBC after failure of previous adjuvant chemotherapy containing anthracycline.38
Gemcitabine has also shown efficacy in treating patients with MBC when given in combination with docetaxel. A European randomized phase 3 study involving 295 patients who had been previously treated with anthracycline demonstrated that gemcitabine plus docetaxel was as effective (ORR, 27%; 95% confidence interval [CI], 18.4%-34.7%) as capecitabine plus docetaxel (ORR, 31%; 95% CI, 22.6%-39.5%) but with significantly less nonhematological toxicity.39 A recent systematic meta-analysis of 83 clinical trials concluded that, although available findings do not support the use of gemcitabine as a standard option beyond the second-line treatment setting, this agent is effective when administered with a taxane as first-line or second-line therapy for MBC.40
Vinorelbine. Vinorelbine is a semisynthetic vinca alkaloid that is active as a single agent and in combination with other chemotherapy or biologic agents in the treatment of MBC.41,42 A study involving 40 patients with MBC demonstrated that weekly treatment with vinorelbine, after failure of anthracycline and taxane regimens, achieved an ORR of 25%, with a median TTP of 6 months (range, 4-12 months), and a median OS of 6 months (range, 2 to ≥18 months).43 In a phase 3 study involving patients with MBC who had been previously treated with anthracycline and taxane, the combination of vinorelbine plus gemcitabine achieved longer median progression-free survival (PFS) times than did vinorelbine alone (6.0 vs 4.0 months; P=.0028), with a trend toward increased ORR (36% vs 26%; P=.09), but it did not prolong OS (15.9 vs 16.4 months; P=.80). The incidence of hematologic toxicity was also higher with the combination regimen.44 Most recently, an open-label, prospective phase 2 study assessed the efficacy of vinorelbine in combination with capecitabine for 31 patients with MBC who had undergone previous treatment with anthracyclines and taxanes. The combination therapy achieved an ORR of 49% (95% CI, 30%-67%), a median TTP of 7.6 months (95% CI, 5.7-9.8 months), and a median OS of 27.2 months.45 Of note, although vinorelbine may be used fairly commonly, it has not been approved by US regulatory agencies for treatment of patients with breast cancer.
Liposomal Doxorubicin. Liposomal doxorubicin is a formulation of anthracycline doxorubicin in which the parent drug is enclosed (encapsulated) in a fatty coating known as a liposome. This formulation was developed so that the tissue distribution and pharmacokinetics of the parent drug could be altered, thereby improving the therapeutic index (ie, better separation between efficacy and toxicity).46,47 Both pegylated and nonpegylated liposomal doxorubicin formulations have been developed; they differ in lipid composition, size, and loading method. Clinical trials show that liposomal doxorubicin is as effective as conventional doxorubicin but is associated with significantly less cardiotoxicity in the first-line treatment of MBC.48,49 However, the pegylated liposomal doxorubicin formulation is associated with higher incidences of hand-foot syndrome and stomatitis or mucositis.50 In a phase 3 trial involving patients with taxane-resistant tumors, pegylated liposomal doxorubicin was shown to be as effective as a comparator regimen (either vinorelbine or the combination of mitomycin C plus vinblastine). In a subset of anthracycline-naïve patients, liposomal doxorubicin achieved longer PFS times than the comparator (5.8 vs 2.1 months; P=.01).51 Liposomal doxorubicin has not yet received FDA approval for MBC, although several trials of this agent as single therapy or in combination with other chemotherapy regimens are under way (www.clinicaltrials.gov).
Albumin-Bound Paclitaxel. Albumin-bound paclitaxel is a solvent-free formulation of paclitaxel consisting of albumin-based nanoparticles (mean size, 120-130 nm). This drug eliminates the need for premedications and lessens the risk of hypersensitivity. It was developed to improve the solubility and pharmacokinetic profile of paclitaxel, and it demonstrated increased tumor distribution in preclinical models.52 Albumin-bound paclitaxel is approved for the treatment of breast cancer after failure of anthracycline-based combination chemotherapy for MBC or after relapse within 6 months of adjuvant chemotherapy.53 Its approval was based on the results of a phase 3 study (N=454) that compared albumin-bound paclitaxel (260 mg/m2 every 3 weeks) with a standard dose of castor oil-based paclitaxel (175 mg/m2 every 3 weeks).54 Albumin-bound paclitaxel achieved significantly higher response rates than standard paclitaxel (33% vs 19%, respectively; P=.001) and a significantly longer time to tumor progression (23.0 vs 16.9 weeks; hazard ratio, 0.75; P=.006). The use of albumin-bound paclitaxel was also associated with a lower incidence of grade 4 neutropenia (9% vs 22%; P<.001), even though the paclitaxel dose was 49% higher. Febrile neutropenia was uncommon (<2%), and its incidence did not differ between the 2 study arms. Grade 3 sensory neuropathy was more common in the albumin-bound paclitaxel arm than in the standard paclitaxel arm (10% vs 2%; P<.001) but was easily managed and improved rapidly (median, 22 days). Weekly schedules of albumin-bound paclitaxel given on days 1, 8, and 15 of a 28-day cycle were tested in two phase 2 trials including patients with taxane-refractory MBC. The results showed an ORR of 14% at a dose of 100 mg/m2 (clinical benefit rate [ORR plus stable disease ≥16 weeks], 26%) and an ORR of 16% at a dose of 125 mg/m2 (clinical benefit rate, 37%).55 The results of a subsequent randomized phase 2 study suggested that PFS times achieved with albumin-bound paclitaxel at a dose of 150 mg/m2 given weekly or 300 mg/m2 given every 3 weeks were superior to those achieved with docetaxel56 as first-line therapy for MBC, but these findings have not yet been published in a peer-reviewed article.
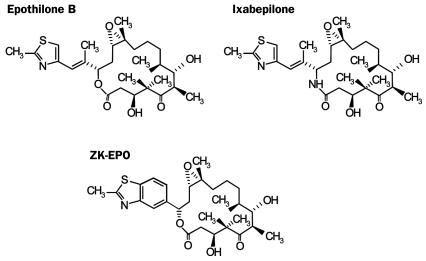
Ixabepilone. Ixabepilone (BMS-247550), a semisynthetic analog of epothilone B, is the first member of the epothilone class to be approved for treating breast cancer. Other epothilones are currently in clinical development, including epothilone B (also known as patupilone or EPO906) and the epothilone B derivative sagopilone (ZKEPO).57 Chemical structures of epothilones are shown in Figure 4.57
FIGURE 4.
Chemical structures of ixabepilone and other epothilones in clinical development. From the Oncologist,57 with permission from the AlphaMed, Company, Inc.
The epothilones are a new class of microtubule-stabilizing drugs that were initially identified as cytotoxic metabolites produced by the myxobacterium Sorangium cellulosum.58 Epothilones bind to β-tubulin to stabilize microtubule polymers, thereby inducing cell-cycle arrest and triggering tumor cell apoptosis.57,59 However, the epothilones bind to a different site on β-tubulin than do the taxanes,60 and they induce apoptosis by distinct epothilone-selective mechanisms.61 These factors together may explain why these agents remain effective against tumors refractory or resistant to taxane.59,62,63 Moreover, epothilones are generally not influenced by resistance mechanisms that reduce the activity of taxanes, anthracyclines, and other drugs, including P-glycoprotein overexpression59,63 (Table).18,19
Ixabepilone demonstrated antitumor activity against both paclitaxel-sensitive and paclitaxel-refractory xenograft models.63,64 Notably, ixabepilone can bind to a wider variety of β-tubulin isoforms than can the taxanes; therefore, ixabepilone retains activity in cells for which the predominant β-tubulin isotype is class III, such as the paclitaxel-resistant cell line Pat-21 breast.63,64 Single-agent ixabepilone has been evaluated in a series of phase 2 studies across the spectrum of advanced breast cancer, from first-line treatment of taxane-naïve patients to treatment of patients with tumors resistant to anthracycline and taxane.65-69 Administering ixabepilone monotherapy (40 mg/m2) as a 3-hour intravenous infusion every 3 weeks to 65 patients with MBC previously treated with an adjuvant anthracycline only achieved an ORR of 41.5%, a median duration of response of 8.2 months, and a median survival of 22.0 months.68 Phase 2 trials of ixabepilone (40-50 mg/m2 given by infusion over 1-3 hours every 3 weeks) achieved ORRs of 57% in patients with taxane-naïve disease and 12% in patients with taxane-refractory MBC.65,69 The largest phase 2 study of single-agent ixabepilone involved 126 patients whose tumors were resistant to an anthracycline, a taxane, and capecitabine; 88% of the study cohort had previously received at least 2 lines of chemotherapy for metastatic disease.67 Single-agent ixabepilone achieved ORRs in 12% of patients and stable disease in an additional 50%; the median duration of response was 5.7 months, and the median PFS time was 3.1 months. Treatment was generally well tolerated; the most common grade 3 or 4 events were peripheral sensory neuropathy (14%), fatigue (13%), and myalgia (8%).
The activity of single-agent ixabepilone, combined with preclinical evidence showing synergy between ixabepilone and capecitabine in tumor xenografts,70 led to a study that compared ixabepilone plus capecitabine14 with capecitabine alone for patients with anthracycline- and taxane-resistant MBC (defined as disease recurrence within 6 months after completion of adjuvant or neoadjuvant treatment or tumor progression during treatment or within 3 months of the last dose). In this pivotal phase 3 trial, 742 patients previously treated with as many as 3 chemotherapy regimens were randomly assigned to treatment with the combination of ixabepilone plus capecitabine or with capecitabine alone.14 The combination achieved a significantly higher PFS than capecitabine alone (independent radiology review [IRR], 5.8 vs 4.2 months, P=.0003; investigator assessment, 5.3 vs 3.8 months, P=.0011). Combination therapy was also associated with a significantly higher ORR (IRR, 35% vs 14%; P<.0001; investigator assessment, 42% vs 23%). A subset analysis showed that combination therapy extended PFS for patients in all age groups and regardless of estrogen receptor or HER2 status, performance status, or number of disease sites (0-2 vs ≥3). The results of this phase 3 trial have been supported by a confirmatory phase 3 study involving 1221 patients with MBC (approximately 50% of whom had tumors resistant to anthracyclines and taxanes).71
The pivotal phase 3 trial14 showed that the toxicity profiles of ixabepilone and capecitabine do not overlap; adding ixabepilone to the regimen did not increase capecitabine-related toxic effects, such as hand-foot syndrome. However, treatment with ixabepilone combined with capecitabine was associated with a 23% rate of grade 3 and 4 peripheral neuropathy, whereas capecitabine alone was not associated with this adverse effect. However, the duration of symptoms was short; the median time to resolution to grade 1 or baseline peripheral neuropathy was 6 weeks. The incidence of grade 3 or 4 neutropenia was significantly higher in the combination arm (68%) than in the single-therapy arm (11%; P =.0001). Although the incidence of febrile neutropenia was only slightly higher in the combination arm (4% vs 1%; P=.001), the risk of toxicity and neutropenia-related death was higher when the ixabepilone and capecitabine combination was used to treat patients with hepatic dysfunction. Therefore, the combination of ixabepilone and capecitabine must not be given to patients with aspartate aminotransferase or alanine aminotransferase activity higher than 2.5 times the upper limit of normal or a bilirubin concentration higher than 1 times the upper limit of normal,72 a caveat fairly similar to the guidelines associated with the use of docetaxel.
On the basis of clinical data, the FDA approved ixabepilone in combination with capecitabine for the treatment of locally advanced breast cancer or MBC after failure of an anthracycline and a taxane or as single-agent therapy after failure of an anthracycline, a taxane, and capecitabine.72 Ixabepilone is currently undergoing phase 2 evaluation in combination with a variety of agents, including cetuximab, trastuzumab, carboplatin, bevacizumab, liposomal doxorubicin, and epirubicin.
Targeted Agents
Bevacizumab. Bevacizumab, an antiangiogenic vascular endothelial growth factor inhibitor and recombinant monoclonal antibody, is approved in combination with paclitaxel as first-line therapy for HER2-negative MBC.73 This approval was based on results of the Intergroup E2100 clinical trial,37 which enrolled 772 patients with MBC, 269 (39.9%) of whom had previously received anthracycline and 108 (16.0%) of whom had previously received taxanes in the adjuvant setting. Patients were randomly assigned to receive weekly paclitaxel (on days 1, 8, and 15) with or without bevacizumab (on days 1 and 15 of a 28-day cycle) as first-line therapy. This combination regimen achieved significantly better results (median PFS, 11.8 months; hazard ratio, 0.60; ORR, 36.9%) than paclitaxel alone (median PFS, 5.9 months; ORR, 21.2%; P<.001). The OS rate was similar in the 2 groups (combination regimen, 26.7 months; paclitaxel alone, 25.2 months; hazard ratio, 0.88; P=.16). The PFS was also better for the bevacizumab-containing regimen than for the paclitaxel regimen for those patients who had previously received anthracyclines (5.6 months vs 10.7 months) or taxanes (3.0 months vs 12.0 months). Results fairly consistent with those of E2100 were found by the European AVADO trial,74 although the absolute improvements were much smaller that those achieved in E2100. The AVADO trial was a randomized, double-blind, placebo-controlled phase 3 study involving 705 patients with newly diagnosed MBC. Patients were randomly assigned to receive docetaxel plus placebo or 1 of 2 doses of bevacizumab (7.5 or 15 mg/kg) every 21 days; 54.3% of patients had previously been treated in the adjuvant setting with anthracycline, and 15.6%, with taxanes. Preliminary results showed that the PFS for women taking either dose of bevacizumab plus docetaxel was significantly higher than that for women taking docetaxel alone (stratified hazard ratio, 0.69, P=.0035 for the low dose; stratified hazard ratio, 0.61, P=.0001 for the higher dose). The median PFS was 8.0 months with docetaxel alone, 8.7 months with docetaxel plus low-dose bevacizumab, and 8.8 months with docetaxel plus higher dose bevacizumab. As in the E2100 trial, the PFS was also higher (although modestly so) for patients taking the bevacizumab-containing regimen than for those who had previously received chemotherapy (hazard ratio for docetaxel plus low-dose bevacizumab, 0.67; hazard ratio for docetaxel plus higher-dose bevacizumab, 0.65) and for those who were previously treated with taxanes (hazard ratio 0.59 and 0.42, for low dose and higher dose, respectively).
The improvement in PFS for patients treated with bevacizumab and those treated with paclitaxel or docetaxel alone in the E2100 study (5.9 months) and the AVADO study (0.7 or 0.8 months) is intriguing, as is the fact that there appears to be no statistically significant difference in PFS between the group treated with a low dose of bevacizumab and the group treated with a standard dose. A report of the findings from other studies (such as the RIBBON-1 trial) in the first-line setting is expected in June 2009. Results of other first-line studies of bevacizumab combined with chemotherapy are particularly interesting, including the 34% ORR achieved by bevacizumab plus capecitabine in the XCALIBr trial75 and the 53% ORR achieved by addition of trastuzumab to docetaxel plus bevacizumab in the N0432 study.76 The only randomized study of second-line therapies reported to date showed no improvement in PFS when bevacizumab was added to capecitabine.77 The combination of bevacizumab plus chemotherapy (taxane, capecitabine, vinorelbine, or gemcitabine) is currently being evaluated as second-line therapy for MBC in the RIBBON-2 trial, and the results are expected sometime in 2009.
Trastuzumab and Lapatinib. Trastuzumab and lapatinib provide additional treatment options for advanced or metastatic HER2-positive breast tumors, which affect an estimated 20% to 25% of patients. These patients are a clinically important subgroup because HER2-positive tumors tend to exhibit a more aggressive disease course with a poorer prognosis than HER2-negative tumors.78-80
Trastuzumab is a recombinant monoclonal antibody that attaches to the extracellular domain of HER2 to block HER2-mediated signaling.81 It has been approved as first-line treatment in combination with paclitaxel for HER2-positive MBC; approval was based on the results of a phase 3 trial, in which the median TTP was 6.9 months for patients receiving the combination therapy and 3.0 months for patients receiving paclitaxel alone (P<.001).82 Trastuzumab is also indicated as a single agent for patients who have received 1 or more chemotherapy regimens for MBC.83 This indication was based on the results of a phase 2 open-label study involving 222 patients, most of whom had previously experienced disease progression while receiving therapy with anthracycline (97%) or taxane therapy (67%).84 The median TTP was 3.1 months, and the ORR was 15% (evaluated by an independent committee).
Some evidence suggests that trastuzumab may retain its activity across subsequent lines of therapy. Nonrandomized retrospective studies demonstrated that treatment with 2 or more trastuzumab-based regimens was advantageous for OS or TTP.85-87 Response to trastuzumab beyond progression was supported by an extension study that was part of a phase 3 trial of therapy for MBC.88 Furthermore, a prospective randomized study involving 112 patients with MBC showed that trastuzumab plus capecitabine after initial progression during trastuzumab-based therapy achieved better results (PFS, 8.5 months; OS, 20.3 months) than with capecitabine alone (PFS, 5.6 months; OS, 19.9 months).89 However, further controlled trials are needed to confirm the efficacy of repeated trastuzumab treatment after tumor relapse.
Lapatinib is a small-molecule inhibitor that binds intracellularly to HER2 and the epidermal growth factor receptor (EGFR; HER1), thereby blocking phosphorylation, activation, and downstream signaling.90 Lapatinib is approved in combination with capecitabine for MBC that has progressed after previous treatment with a taxane, an anthracycline, and trastuzumab, and it is considered the preferred targeted therapy for HER2-positive patients whose disease progresses during treatment with trastuzumab.
Approval of lapatinib was based on the results of a phase 3 trial that demonstrated that the median TTP is 23.9 weeks with lapatinib and capecitabine and 18.3 weeks with capecitabine alone (P<.001).91 Additionally, new findings have shown that a benefit is achieved when lapatinib is added to paclitaxel as first-line treatment of HER2-positive MBC.92 Furthermore, for postmenopausal women with estrogen receptor-positive and HER2-positive MBC, the addition of lapatinib to the aromatase inhibitor letrozole increased the PFS time from 3.0 months (letrozole alone) to 8.2 months (lapatinib plus letrozole; hazard ratio, 0.71; 95% CI, 0.53-0.96).93
Research studies are increasing our understanding of the mechanisms underlying resistance to trastuzumab and of the HER2 pathway and its interactions with other signal transduction pathways94,95; however, a comprehensive discussion of trastuzumab-refractory HER2-positive MBC is beyond the scope of this article. This increased understanding of trastuzumab resistance has led to the development of novel agents that hold promise for the treatment of patients with HER2-positive MBC who have been treated with trastuzumab and lapatinib.
Trastuzumab-DM1 is a novel antibody-drug conjugate in which the trastuzumab antibody is covalently linked to the microtubule inhibitor DM1 (derived of maytansine-1) so that trastuzumab-associated anti-HER2 activity can be coupled with targeted DM1 internalization. A recent phase 1 dose-finding study involving patients with HER2-positive MBC found that trastuzumab-DM1 produced objective tumor responses at doses less than or equal to the maximum tolerated dose.96
Pertuzumab, like trastuzumab, is an anti-HER2 antibody; however, pertuzumab binds to a distinct epitope of HER2 and acts to block its interaction with other HER family members. In a phase 2 study involving patients with trastuzumab-resistant HER2-positive MBC, the combination of pertuzumab and trastuzumab produced a tangible clinical benefit (complete or partial response or stable disease) for half of the patients (33/66) without significant negative effects on cardiac function.97
Like lapatinib, neratinib is a dual HER2 and EGFR inhibitor, but unlike lapatinib it binds irreversibly to these receptors. Neratinib has proved effective for treating patients with HER2-positive MBC, particularly those who have not previously undergone treatment with trastuzumab.98 A phase 3 trial comparing neratinib with the combination of lapatinib plus capecitabine for patients with HER2-positive MBC began in November 2008 (NCT00777101; www.clinicaltrials.gov). In addition, Hsp90 inhibitors such as tanespimycin, mammalian target of rapamycin inhibitors such as temsirolimus, insulin growth factor receptor 1 inhibitors, and newer antiangiogenesis agents such as pazopanib, sorafenib, and sunitinib are currently under investigation.99,100
TRIPLE-NEGATIVE MBC
Breast cancer that is estrogen receptor-negative, progesterone receptor-negative, and HER2-negative (called triple-negative [TN]) deserves particular attention because these tumors are typically aggressive and are associated with a poorer prognosis than other breast cancer tumors. The overall survival rate of patients with early TN breast cancer has been estimated at 67%, whereas that of patients with other types of breast cancer is 75%; in addition, TN disease is associated with a higher rate of visceral and soft-tissue metastasis.101,102 Therapies for patients with TN disease are based on standard chemotherapy regimens because hormonal and HER2-targeted therapies are unsuitable; in the recurrent or MBC setting, treatment choices may be further constrained by the tumor's resistance to anthracyclines and taxanes. Improved treatment options are therefore needed.
Neoadjuvant studies with regimens containing anthracycline and taxanes have demonstrated that, although TN tumors respond better to these agents than do lesions belonging to the so-called luminal subtype of breast cancer, TN disease is associated with poorer disease-free survival and OS.103,104 Preclinical studies have found several molecular and pathologic features classically associated with this phenotype that may be targetable. These features include overexpression or activation (or both) of EGFR/HER1, vascular endothelial growth factor (VEGF), p63 (p53-related transcription factor), caveolin-1, the Src kinase pathway, the mitogen-activated protein kinase pathway, the Akt pathway, the phosphoinositol-3-kinase pathway, and the poly-ADP (adenosine diphosphate)-ribose polymerase 1 (PARP-1) pathway.105-108
Two phase 2 studies investigated the activity of cetuximab, a monoclonal anti-EGFR antibody, in patients with TN disease. The first phase 2 study, which administered a combination of cetuximab plus carboplatin to 102 patients with previously treated TN MBC, achieved an ORR of 18% and an overall clinical benefit rate of 27%. Unfortunately, TTP was short (2 months), and OS was only 12 months.109 The second study, a randomized phase 2 trial investigating the combination of irinotecan and carboplatin with or without cetuximab in 103 patients with MBC (72 of whom had previous treatment for TN MBC), demonstrated that the response rate was higher for the patients with TN disease who received the EGFR inhibitor (49% vs 30%). These findings suggest that cetuximab may improve the antitumor activity of the chemotherapy, but this agent was also associated with a much greater incidence of grade 3 and 4 toxic effects.110
Several studies are currently investigating the role of VEGF inhibitors such as bevacizumab in the treatment of TN breast cancer. A subset analysis of the pivotal E2100 clinical trial (paclitaxel with or without bevacizumab for patients with MBC; >90% of patients had HER2-negative disease) demonstrated that the benefit seen in the combination arm was maintained in patients with estrogen-receptor/progesterone-receptor-negative disease (hazard ratio, 0.53; 95% CI, 0.40-0.70).37 Ongoing trials are addressing the role of bevacizumab for TN breast cancer in the neoadjuvant and adjuvant settings; these include the CALGB 40603 clinical trial (NCT00861705) and the BEATRICE clinical trial (NCT00528567; www.clinicaltrials.org). A recent phase 2 trial of oral dasatinib (70 mg/d) administered to 43 evaluable patients with advanced TN MBC (66% had been previously treated in the metastatic setting) demonstrated that this Src kinase inhibitor as a single agent exerts modest activity (clinical benefit, 9.3%).111 Another phase 2 trial is evaluating the efficacy of a dose of 100 mg/d in this setting (NCT00817531; www.clinicaltrials.gov), and additional trials of oral dasatinib in combination with chemotherapy are being planned. Similar studies of the oral multi-kinase inhibitor sunitinib are currently ongoing. The results of phase 2 trials have indicated that gemcitabine combinations may be effective for and well tolerated by patients with TN MBC, including those previously treated with an anthracycline or a taxane. In one study (N0234; N=20), combination therapy with gemcitabine plus erlotinib was associated with an ORR of 25%112; in a second phase 2 trial, the combination of gemcitabine plus carboplatin and BSI-201, a PARP-1 inhibitor, was found to have no substantial safety concerns, and the clinical benefit rate of this combination is being evaluated.113
The suggestion that TN tumors may be more sensitive than other tumors to platinum agents provided the rationale for multiple phase 2 studies in the metastatic setting, as well as for neoadjuvant trials in early-stage breast cancer. We expect that ongoing phase 3 trials specifically designed to test therapies for TN disease will be able to determine the true role of platinum compounds in relationship to that of other chemotherapy agents in treating patients with TN tumors. Data from phase 3 studies evaluating therapies for TN MBC remain scarce, but a pooled analysis of data from 399 such patients from 2 phase 3 trials of ixabepilone plus capecitabine (most of whom had previously been treated with anthracyclines and taxanes) demonstrated that this regimen achieved an ORR of 31% and a median PFS time of 4.2 months, whereas capecitabine monotherapy achieved an ORR of 15% and a median PFS time of 1.7 months.114 These results are similar to those obtained from the overall trial populations (non-TN MBC).14,71 Finally, the acknowledgment that the biology of TN MBC differs from that of HER-2 positive or luminal MBC and the understanding of the aberrant pathways of these tumors should lead to the evaluation of newer drugs, including the DNA-damaging chemotherapy brostallicin and novel targeted agents such as PARP-1 inhibitors ABT-888 and AZD2281, angiogenesis inhibitors such as VEGF-Trap (AVE0005), multi-kinase inhibitors such as sunitinib, mammalian target of rapamycin inhibitors such as RAD-001, hypomethylating agents, and histone acetylating inhibitors such as suberoylanilide hydroxamic acid (Vorinostat), checkpoint kinase 1 inhibitors such as UCN-01, transforming growth factor-beta inhibitors, androgen receptor blockers, and tumor necrosis factor-related apoptosis-inducing ligand receptor agonists.
CONCLUSION
Because anthracycline and taxanes are now being used more commonly in the adjuvant setting, the resistance of breast cancer to these agents has become an increasingly important issue. The optimal choice of therapy for resistant breast cancer is inextricably influenced by each patient's clinical and treatment history, in both the adjuvant and the metastatic settings.115 Prior experience complicates and restricts the available management options. No single regimen has been found optimal for patients with resistant or refractory HER2-negative breast cancer, and few biological markers exist that can determine which patients will benefit from particular treatments (no such markers exist for chemotherapy). The management of resistant breast cancer is complicated by the absence of clear evidence-based guidelines for clinicians, because National Comprehensive Cancer Network recommendations are targeted toward the broader population of patients with recurrent or metastatic disease.
It remains fairly clear that some patients may be treated with combination therapy and others with sequential single-agent chemotherapy regimens; the choice of therapy will be based on the patient's condition, pace of tumor growth, and extent of disease.23 Several chemotherapy agents—capecitabine, gemcitabine, vinorelbine, liposomal doxorubicin, albumin-bound paclitaxel, and ixabepilone—offer a limited duration of therapeutic activity to patients whose tumors have progressed to become resistant to anthracyclines, taxanes, or both. On the basis of the findings of the E2100 and AVADO trials, it is reasonable to consider treating patients with newly diagnosed MBC with a regimen consisting of a taxane plus bevacizumab, even if they have already undergone adjuvant therapy with a regimen containing anthracycline, taxane, or both. Those findings also indicate that continued research is important for optimizing treatment options. Moreover, it seems that the current standard of care for patients with MBC who require second-line or third-line therapy is capecitabine alone or perhaps preferably in combination with ixabepilone. Ixabepilone is the only FDA-approved epothilone, and single-agent ixabepilone therapy is appropriate for patients considered eligible for first-line or second-line treatment who have previously undergone therapy with anthracyclines, taxanes, and capecitabine.
Targeted biologic agents are a welcome additional option for patients with resistant breast cancer. The management of HER2-postive MBC is challenging because the disease is associated with a particularly poor prognosis, but trastuzumab and lapatinib have helped overcome the problem of chemotherapeutic resistance. A chemotherapy regimen containing trastuzumab is the preferred first-line therapy for HER2-positive MBC, but promising results have also been achieved with lapatinib in combination with agents such as capecitabine, paclitaxel, or letrozole. However, further research is needed to determine ideal dosing schedules, optimal duration of therapy, implications of trastuzumab resistance, and effective treatment combinations. Lapatinib, in combination with capecitabine, is currently the preferred second or third choice for treating HER2-positive tumors that have progressed during treatment with trastuzumab. Emerging findings suggest that reasonable approaches (not yet approved by regulatory agencies) for subsequent lines of therapy are trastuzumab plus a chemotherapeutic agent or another biologic agent. The approved option for these patients is a combination of capecitabine and lapatinib. Numerous trials are under way to evaluate newer biologic agents in combination with chemotherapy, hormone treatment, trastuzumab, or lapatinib for advanced or metastatic HER2-positive breast cancer. Analysis of available tumor tissue is imperative for further elucidating the mechanisms of tumor sensitivity and resistance to treatment and for optimizing patient care.
Acknowledgments
We acknowledge StemScientific, funded by Bristol-Myers Squibb, United States, as well as Melisa Walker (Mayo Clinic's site in Jacksonville, FL) for providing writing and editing support. Neither Bristol-Myers Squibb nor StemScientific influenced the content of the manuscript, nor did the authors receive financial compensation for the manuscript.
REFERENCES
- 1.American Cancer Society Web site http://www.cancer.org/downloads/STT/2008CAFFfinalsecured.pdf. Cancer Facts & Figures 2008. Accessed April 14, 2009.
- 2.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24(14):2137-2150 [DOI] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network Web site http://www.nccn.org/professionals/physician_gls/PDF/breast.pdf. NCCN Practice Guidelines in Oncology—v.1.2009: Breast Cancer. doi: 10.6004/jnccn.2009.0070. Accessed May 1, 2009. [DOI] [PubMed]
- 4.Dean-Colomb W, Esteva FJ. Emerging agents in the treatment of anthracycline- and taxane-refractory metastatic breast cancer. Semin Oncol. 2008;35(2)(suppl 2):S31-S38 [DOI] [PubMed] [Google Scholar]
- 5.De Laurentiis M, Cancello G, D'Agostino D, et al. Taxane-based combinations as adjuvant chemotherapy of early breast cancer: a meta-analysis of randomized trials. J Clin Oncol. 2008;26(1):44-53 [DOI] [PubMed] [Google Scholar]
- 6.Peto R, Early Breast Cancer Trialists' Collaborative Group The worldwide overview: new results for systemic adjuvant therapies Presented at the 30th Annual San Antonia Breast Cancer Symposium; San Antonio, TX; December13-16, 2007 [Google Scholar]
- 7.Kataja V, Castiglione M, ESMO Guidelines Working Group Locally recurrent or metastatic breast cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2008;19(2 suppl):ii11-ii13 [DOI] [PubMed] [Google Scholar]
- 8.Conlin AK, Seidman AD. Taxanes in breast cancer: an update. Curr Oncol Rep. 2007;9(1):22-30 [DOI] [PubMed] [Google Scholar]
- 9.Eniu A, Palmieri FM, Perez EA. Weekly administration of docetaxel and paclitaxel in metastatic or advanced breast cancer. Oncologist 2005;10(9):665-685 [DOI] [PubMed] [Google Scholar]
- 10.Perez EA. Paclitaxel in breast cancer. Oncologist 1998;3(6):373-389 [PubMed] [Google Scholar]
- 11.Perez EA, Hillman DW, Stella PJ, et al. A phase II study of paclitaxel plus carboplatin as first-line chemotherapy for women with metastatic breast carcinoma. Cancer 2000;88(1):124-131 [DOI] [PubMed] [Google Scholar]
- 12.Perez EA, Vogel CL, Irwin DH, Kirshner JJ, Patel R. Multicenter phase II trial of weekly paclitaxel in women with metastatic breast cancer. J Clin Oncol. 2001;19(22):4216-4223 [DOI] [PubMed] [Google Scholar]
- 13.Perez EA, Vogel CL, Irwin DH, Kirshner JJ, Patel R. Weekly paclitaxel in women age 65 and above with metastatic breast cancer. Breast Cancer Res Treat 2002;73:85-88 [DOI] [PubMed] [Google Scholar]
- 14.Thomas ES, Gomez HL, Li RK, et al. Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol. 2007November20;25(33):5210-5217 Epub 2007 Oct 29 [DOI] [PubMed] [Google Scholar]
- 15.Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56(2):185-229 [DOI] [PubMed] [Google Scholar]
- 16.McGrogan BT, Gilmartin B, Carney DN, McCann A. Taxanes, microtubules and chemoresistant breast cancer. Biochim Biophys Acta. 2008April;1785(2):96-132 Epub 2007 Nov 12 [DOI] [PubMed] [Google Scholar]
- 17.Chien AJ, Moasser MM. Cellular mechanisms of resistance to anthracyclines and taxanes in cancer: intrinsic and acquired. Semin Oncol. 2008;35(2, suppl 2):S1-S14 [DOI] [PubMed] [Google Scholar]
- 18.Fojo T, Menefee M. Mechanisms of multidrug resistance: the potential role of microtubule-stabilizing agents. Ann Oncol. 2007;18(suppl 5):v3-v8 [DOI] [PubMed] [Google Scholar]
- 19.Brooks TA, Minderman H, O'Loughlin KL, et al. Taxane-based reversal agents modulate drug resistance mediated by P-glycoprotein, multidrug resistance protein, and breast cancer resistance protein. Mol Cancer Ther. 2003;2(11):1195-1205 [PubMed] [Google Scholar]
- 20.Faneyte IF, Kristel PM, Maliepaard M, et al. Expression of the breast cancer resistance protein in breast cancer. Clin Cancer Res. 2002;8(4):1068-1074 [PubMed] [Google Scholar]
- 21.Sève P, Dumontet C. Is class III beta-tubulin a predictive factor in patients receiving tubulin-binding agents? Lancet Oncol. 2008;9(2):168-175 [DOI] [PubMed] [Google Scholar]
- 22.Moreno A. Management of metastatic breast cancer: chemotherapy, anti-angiogenesis, other targets Paper presented at: 18th Annual Hematology/Oncology Reviews; Amelia Island, FL; August4-8, 2008 [Google Scholar]
- 23.Jones SE. Metastatic breast cancer: the treatment challenge. Clin Breast Cancer 2008;8(3):224-233 [DOI] [PubMed] [Google Scholar]
- 24.Gralow J, Rugo H, Gradishar W, et al. Novel taxane formulations in the treatment of breast cancer: a thought leader discussion and consensus roundtable. Clin Breast Cancer 2008;8(1):33-37 [DOI] [PubMed] [Google Scholar]
- 25.Moreno-Aspitia A, Perez EA. Nanoparticle albumin-bound paclitaxel (ABI-007): a newer taxane alternative in breast cancer. Future Oncol. 2005;1(6):755-762 [DOI] [PubMed] [Google Scholar]
- 26.Wagstaff AJ, Ibbotson T, Goa KL. Capecitabine: a review of its pharmacology and therapeutic efficacy in the management of advanced breast cancer. Drugs 2003;63(2):217-236 [DOI] [PubMed] [Google Scholar]
- 27.Walko CM, Lindley C. Capecitabine: a review. Clin Ther. 2005;27(1):23-44 [DOI] [PubMed] [Google Scholar]
- 28.Jones L, Hawkins N, Westwood M, Wright K, Richardson G, Riemsma R. Systematic review of the clinical effectiveness and cost-effectiveness of capecitabine (Xeloda) for locally advanced and/or metastatic breast cancer. Health Technol Assess 2004;8(5):iii, xiii-xvi,1-143 [DOI] [PubMed] [Google Scholar]
- 29.O'Shaughnessy J, Miles D, Vukelja S, et al. Superior survival with capecitabine plus docetaxel combination therapy in anthracycline-pretreated patients with advanced breast cancer: phase III trial results. J Clin Oncol. 2002;20(12):2812-2823 [DOI] [PubMed] [Google Scholar]
- 30.Xeloda (capecitabine) [package insert] Nutley, NJ: Roche Pharmaceuticals; 2006. http://www.rocheexchange.com/oncology/productinformation/xeloda?gclid=CP3XivCsmJcCFRHxDAodTFfxXQ#Product_Information Accessed April 14, 2009 [Google Scholar]
- 31.Silvestris N, Cinieri S, La Torre I, et al. Role of gemcitabine in metastatic breast cancer patients: a short review. Breast 2008June;17(3):220-226 Epub 2007 Nov 26 [DOI] [PubMed] [Google Scholar]
- 32.Albain KS, Nag SM, Calderillo-Ruiz G, et al. Gemcitabine plus paclitaxel versus paclitaxel monotherapy in patients with metastatic breast cancer and prior anthracycline treatment. J Clin Oncol. 2008;26(24):3950-3957 [DOI] [PubMed] [Google Scholar]
- 33.Albain KS, Nag S, Calderillo-Ruiz G, et al. Global phase III study of gemcitabine plus paclitaxel (GT) vs. paclitaxel (T) as frontline therapy for metastatic breast cancer (MBC): first report of overall survival [abstract 510]. J Clin Oncol. 2004;22(14S)(July15suppl):510 [Google Scholar]
- 34.Ellis MJ, Hayes DF, Lippman ME. Treatment of metastatic breast cancer. In: Harris JR, Lippman ME, Morrow M, et al., eds. Diseases of the Breast 2nd ed.Philadelphia, PA: Lippincott Williams & Wilkins; 2000:749-797 [Google Scholar]
- 35.Fountzilas G, Dafni U, Dimopoulos MA, et al. A randomized phase III study comparing three anthracycline-free taxane-based regimens, as first line chemotherapy, in metastatic breast cancer: a Hellenic Cooperative Oncology Group study. Breast Cancer Res Treat. doi: 10.1007/s10549-008-0047-9. [published online ahead of print May 16, 2008] doi 10.1007/s10549-008-0047-9. [DOI] [PubMed] [Google Scholar]
- 36.Harris JR, Morrow M, Norton L. Malignant tumors of the breast. In: De Vita VT, Jr, Hellman S, Rosenberg SA, eds. Cancer: Principles and Practice of Oncology 5th ed.Philadelphia, PA: Lippincott; 1997:1557-1616 [Google Scholar]
- 37.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357(26):2666-2676 [DOI] [PubMed] [Google Scholar]
- 38.Gemzar (gemcitabine HCI for injection) [package insert] Indianapolis, IN: Eli Lilly & Company; 2007. http://www.fda.gov/cder/foi/label/2006/020509s039lbl.pdf Accessed April 14, 2009 [Google Scholar]
- 39.Chan S, Romieu G, Huober J, et al. Gemcitabine plus docetaxel (GD) versus capecitabine plus docetaxel (CD) for anthracycline-pretreated metastatic breast cancer (MBC) patients (pts): results of a European Phase III study [abstract 581]. J Clin Oncol. 2005;23(suppl 16, pt 1):24s [Google Scholar]
- 40.Dent S, Messersmith H, Trudeau M. Gemcitabine in the management of metastatic breast cancer: a systematic review. Breast Cancer Res Treat 2008April;108(3):319-331 Epub 2007 May 26 [DOI] [PubMed] [Google Scholar]
- 41.Domenech GH, Vogel CL. A review of vinorelbine in the treatment of breast cancer. Clin Breast Cancer 2001;2(2):113-128 [DOI] [PubMed] [Google Scholar]
- 42.Mano M. Vinorelbine in the management of breast cancer: new perspectives, revived role in the era of targeted therapy. Cancer Treat Rev. 2006April;32(2):106-118 Epub 2006 Feb 13 [DOI] [PubMed] [Google Scholar]
- 43.Zelek L, Barthier S, Riofrio M, et al. Weekly vinorelbine is an effective palliative regimen after failure with anthracyclines and taxanes in metastatic breast carcinoma. Cancer 2001;92(9):2267-2272 [DOI] [PubMed] [Google Scholar]
- 44.Martín M, Ruiz A, Muñoz M, et al. Gemcitabine plus vinorelbine versus vinorelbine monotherapy in patients with metastatic breast cancer previously treated with anthracyclines and taxanes: final results of the phase III Spanish Breast Cancer Research Group (GEICAM) trial. Lancet Oncol. 2007;8(3):219-225 [DOI] [PubMed] [Google Scholar]
- 45.Estévez LG, Batista N, Sánchez-Rovira P, et al. A Phase II study of capecitabine and vinorelbine in patients with metastatic breast cancer pretreated with anthracyclines and taxanes. Clin Breast Cancer 2008;8(2):149-154 [DOI] [PubMed] [Google Scholar]
- 46.Lorusso V, Manzione L, Silvestris N. Role of liposomal anthracyclines in breast cancer. Ann Oncol. 2007;18(suppl 6):vi70-vi73 [DOI] [PubMed] [Google Scholar]
- 47.Rivera E. Liposomal anthracyclines in metastatic breast cancer: clinical update. Oncologist 2003;8(suppl 2):3-9 [DOI] [PubMed] [Google Scholar]
- 48.Batist G, Ramakrishnan G, Rao CS, et al. Reduced cardiotoxicity and preserved antitumor efficacy of liposome-encapsulated doxorubicin and cyclophosphamide compared with conventional doxorubicin and cyclophosphamide in a randomized, multicenter trial of metastatic breast cancer. J Clin Oncol. 2001;19(5):1444-1454 [DOI] [PubMed] [Google Scholar]
- 49.Harris L, Batist G, Belt R, et al. TLC D-99 Study Group Liposome-encapsulated doxorubicin compared with conventional doxorubicin in a randomized multicenter trial as first-line therapy of metastatic breast carcinoma. Cancer 2002;94(1):25-36 [DOI] [PubMed] [Google Scholar]
- 50.O'Brien ME, Wigler N, Inbar M, et al. CAELYX Breast Cancer Study Group Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol. 2004;15(3):440-449 [DOI] [PubMed] [Google Scholar]
- 51.Keller AM, Mennel RG, Georgoulias VA, et al. Randomized phase III trial of pegylated liposomal doxorubicin versus vinorelbine or mitomycin C plus vinblastine in women with taxane-refractory advanced breast cancer. J Clin Oncol. 2004;22(19):3893-3901 [DOI] [PubMed] [Google Scholar]
- 52.Desai N, Trieu V, Yao Z, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel [published correction appears in Clin Cancer Res. 2006;12(12): 3869] Clin Cancer Res. 2006;12(4):1317-1324 [DOI] [PubMed] [Google Scholar]
- 53.Abraxane (albumin-bound paclitaxel) [package insert] Los Angeles, CA: Abraxis Bioscience, Inc.May2007. http://www.fda.gov/cder/foi/label/2008/021660s013lbl.pdf Accessed November, 2008 [Google Scholar]
- 54.Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005November;23(31):7794-7803 Epub 2005 Sep 19 [DOI] [PubMed] [Google Scholar]
- 55.Blum JL, Savin MA, Edelman G, et al. Phase II study of weekly albumin-bound paclitaxel for patients with metastatic breast cancer heavily pretreated with taxanes. Clin Breast Cancer 2007;7(11):850-856 [DOI] [PubMed] [Google Scholar]
- 56.Gradishar W, Krasnojon D, Cheporov S, Makhson A, Manikhas G, Hawkins G. A randomized phase 2 trial of qw or q3w ABI-007 (ABX) vs. q3W solvent-based docetaxel (TXT) as first-line therapy in metastatic breast cancer (MBC) [abstract 46]. Breast Cancer Res Treat 2006;100(suppl 1):S21 [Google Scholar]
- 57.Cortes J, Baselga J. Targeting the microtubules in breast cancer beyond taxanes: the epothilones. Oncologist 2007;12(3):271-280 [DOI] [PubMed] [Google Scholar]
- 58.Gerth K, Bedorf N, Höfle G, Irschik H, Reichenbach H. Epothilons A and B: antifungal and cytotoxic compounds from Sorangium cellulosum (Myxobacteria): production, physico-chemical and biological properties. J Antibiot (Tokyo) 1996;49(6):560-563 [DOI] [PubMed] [Google Scholar]
- 59.Goodin S, Kane MP, Rubin EH. Epothilones: mechanism of action and biologic activity. J Clin Oncol. 2004;22(10):2015-2025 [DOI] [PubMed] [Google Scholar]
- 60.Bode CJ, Gupta ML, Jr, Reiff EA, Suprenant KA, Georg GI, Himes RH. Epothilone and paclitaxel: unexpected differences in promoting the assembly and stabilization of yeast microtubules [published correction appears in Biochemistry. 2002;41(24);7858] Biochemistry 2002;41(12):3870-3874 [DOI] [PubMed] [Google Scholar]
- 61.Vahdat L. Ixabepilone: a novel antineoplastic agent with low susceptibility to multiple tumor resistance mechanisms. Oncologist 2008;13(3):214-221 [DOI] [PubMed] [Google Scholar]
- 62.Lee FY, Borzilleri R, Fairchild CR, et al. BMS-247550: a novel epothilone analog with a mode of action similar to paclitaxel but possessing superior antitumor efficacy. Clin Cancer Res. 2001;7(5):1429-1437 [PubMed] [Google Scholar]
- 63.Lee FY, Smykla R, Johnston K, et al. Preclinical efficacy spectrum and pharmacokinetics of ixabepilone. Cancer Chemother Pharmacol. 2009January;63(2):201-212 Epub 2008 Mar 19 [DOI] [PubMed] [Google Scholar]
- 64.Jordon M, Miller H, Ni L, et al. The Pat-21 breast cancer model derived from a patient with primary Taxol resistance recapitulates the phenotype of its origin, has altered beta-tubulin expression and is sensitive to ixabepilone [abstract LB-280]. Proc Am Assoc Cancer Res. 2006;47:73 [Google Scholar]
- 65.Denduluri N, Low JA, Lee JJ, et al. Phase II trial of ixabepilone, an epothilone B analog, in patients with metastatic breast cancer previously untreated with taxanes. J Clin Oncol. 2007August10;25(23):3421-3427 Epub 2007 Jul 2 [DOI] [PubMed] [Google Scholar]
- 66.Low JA, Wedam SB, Lee JJ, et al. Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, in metastatic and locally advanced breast cancer. J Clin Oncol. 2005;23(12):2726-2734 [DOI] [PubMed] [Google Scholar]
- 67.Perez EA, Lerzo G, Pivot X, et al. Efficacy and safety of ixabepilone (BMS-247550) in a phase II study of patients with advanced breast cancer resistant to an anthracycline, a taxane, and capecitabine. J Clin Oncol. 2007August10;25(23):3407-3414 Epub 2007 Jul 2 [DOI] [PubMed] [Google Scholar]
- 68.Roché H, Yelle L, Cognetti F, et al. Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, as first-line therapy in patients with metastatic breast cancer previously treated with anthracycline chemotherapy. J Clin Oncol. 2007August10;25(23):3415-3420 Epub 2007 Jul 2 [DOI] [PubMed] [Google Scholar]
- 69.Thomas E, Tabernero J, Fornier M, et al. Phase II clinical trial of ixabepilone (BMS-247550), an epothilone B analog, in patients with taxane-resistant metastatic breast cancer. J Clin Oncol. 2007August10;25(23):3399-3406 Epub 2007 Jul 2 [DOI] [PubMed] [Google Scholar]
- 70.Lee FY, Camuso A, Castenada S, et al. Preclinical efficacy evaluation of ixabepilone (BMS-247550) in combination with cetuximab or capecitabine in human colon and lung carcinoma xenografts. [abstract 12017]. J Clin Oncol. 2006;24(suppl 18)(pt 1):597s [Google Scholar]
- 71.Hortobagyi GN, Perez E, Vrdoljak E, et al. Analysis of overall survival (OS) among patients (pts) with metastatic breast cancer (MBC) receiving either ixabepilone (I) plus capecitabine (C) or c alone: results from two randomized phase III trials [abstract 186]. In: Proceedings from ASCO Breast Cancer Symposium 2008 Washington, DC: September5-7, 2008. http://www.asco.org/ASCO/Abstracts+%26+Virtual+Meeting/Abstracts?&vmview=abst_detail_view&confID=58&abstractID=40389 Accessed April 14, 2009 [Google Scholar]
- 72.Ixempra kit (ixabepilone) for injection [package insert] Princeton, NJ: Bristol-Myers Squibb Company; 2007. http://packageinserts.bms.com/pi/pi_ixempra.pdf Accessed April 14, 2009 [Google Scholar]
- 73.Avastin (bevacizumab) [package insert] San Francisco, CA: Genentech, Inc; 2008. http://www.gene.com/gene/products/information/pdf/avastin-prescribing.pdf Accessed April 14, 2009 [Google Scholar]
- 74.Miles D, Chan A, Romieu G, et al. Randomized, double-blind, placebo-controlled, phase III study of bevacizumab with docetaxel or docetaxel with placebo as first-line therapy for patients with locally recurrent or metastatic breast cancer (mBC): AVADO [abstract LBA1011]. J Clin Oncol. 2008;26(suppl 15):43s [Google Scholar]
- 75.Sledge G, Miller K, Moise C, Gradishar W. Safety and efficacy of capecitabline (C) plus bevacizumab (B) as first-line in metastatic breast cancer [abstract 1013]. J Clin Oncol. 2007;25(suppl 18):35s [Google Scholar]
- 76.Perez EA, Hillman DW, Kugler JW, Steen PD, Fitch TR, Rowland KM. North Central Cancer Treatment Group (NCCTG) N0432: phase II trial of docetaxel with capecitabine and bevacizumab as first line chemotherapy for patients with metastatic breast cancer [abstract 2069]. Breast Cancer Res Treat 2006;100(suppl 1):S104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miller KD, Chap LI, Holmes FA, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23(4):792-799 [DOI] [PubMed] [Google Scholar]
- 78.Owens MA, Horten BC, Da Silva MM. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin Breast Cancer 2004;5(1):63-69 [DOI] [PubMed] [Google Scholar]
- 79.Sjögren S, Inganäs M, Lindgren A, Holmberg L, Bergh J. Prognostic and predictive value of c-erbB-2 overexpression in primary breast cancer, alone and in combination with other prognostic markers. J Clin Oncol. 1998;16(2):462-469 [DOI] [PubMed] [Google Scholar]
- 80.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235(4785):177-182 [DOI] [PubMed] [Google Scholar]
- 81.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127-137 [DOI] [PubMed] [Google Scholar]
- 82.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783-792 [DOI] [PubMed] [Google Scholar]
- 83.Herceptin (trastuzumab) [package insert] San Francisco, CA: Genentech Inc; 2009. http://www.gene.com/gene/products/information/pdf/herceptin-prescribing.pdf Accessed April 15, 2009 [Google Scholar]
- 84.Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17(9):2639-2648 [DOI] [PubMed] [Google Scholar]
- 85.Fountzilas G, Razis E, Tsavdaridis D, et al. Continuation of trastuzumab beyond disease progression is feasible and safe in patients with metastatic breast cancer: a retrospective analysis of 80 cases by the hellenic cooperative oncology group. Clin Breast Cancer 2003;4(2):120-125 [DOI] [PubMed] [Google Scholar]
- 86.Gelmon KA, Mackey J, Verma S, et al. Use of trastuzumab beyond disease progression: observations from a retrospective review of case histories. Clin Breast Cancer 2004;5(1):52-58 [DOI] [PubMed] [Google Scholar]
- 87.Stemmler HJ, Kahlert S, Siekiera W, Untch M, Heinrich B, Heinemann V. Prolonged survival of patients receiving trastuzumab beyond disease progression for HER2 overexpressing metastatic breast cancer (MBC). Onkologie 2005;28(11):582-586 [DOI] [PubMed] [Google Scholar]
- 88.Tripathy D, Slamon DJ, Cobleigh M, et al. Safety of treatment of metastatic breast cancer with trastuzumab beyond disease progression. J Clin Oncol. 2004;22(6):1063-1070 [DOI] [PubMed] [Google Scholar]
- 89.Von Mickwitz G, Zielinski C, Maarteense E, et al. Capecitabine vs. capecitabine + trastuzumab in patients with HER2-positive metastatic breast cancer progressing during trastuzumab treatment: the TBP phase III study (GBG 26/BIG 3-05) [abstract 1025]. J Clin Oncol. 2008;26(suppl 15):47s [Google Scholar]
- 90.Moy B, Goss PE. Lapatinib: current status and future directions in breast cancer. Oncologist 2006;11(10):1047-1057 [DOI] [PubMed] [Google Scholar]
- 91.Tykerb (lapatinib) tablets [package insert] Research Triangle Park, NC: GlaxoSmithKline; 2008. http://us.gsk.com/products/assets/us_tykerb.pdf Accessed April 15, 2009 [Google Scholar]
- 92.Di Leo A, Gomez HL, Aziz Z, et al. Phase III, double-blind, randomized study comparing lapatinib plus paclitaxel with placebo plus paclitaxel as first-line treatment for metastatic breast cancer [published correction appears in J Clin Oncol. 2009;27(11):1923] J Clin Oncol. 2008December1;26(34):5544-5552 Epub 2008 Oct 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Johnston S, Pegram M, Press M, et al. Lapatinib combined with letrozole vs. letrozole alone for front line postmenopausal hormone receptor positive (HR+) metastatic breast cancer (MBC): final results from the EGF30008 trial [abstract 46]. Cancer Res. 2009;69(suppl 2). [Google Scholar]
- 94.Bender LM, Nahta R. Her2 cross talk and therapeutic resistance in breast cancer. Front Biosci. 2008;13:3906-3912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nahta R, Yu D, Hung MC, Hortobagyi GN, Esteva FJ. Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol. 2006;3(5):269-280 [DOI] [PubMed] [Google Scholar]
- 96.Beeram M, Burris HA, Modi S, et al. A phase I study of trastuzumab-DM1 (T-DM1), a first-in-class HER2 antibody-drug conjugate (ADC), in patients (pts) with advanced HER2+ breast cancer (BC) [abstract 1028]. J Clin Oncol. 2008;26(suppl 15):48s [Google Scholar]
- 97.Baselga J, Imadalou K, Paton V, Gray D, Swain S. Efficacy, safety and tolerability of dual monoclonal antibody therapy with pertuzumab + trastuzumab in HER2+ metastatic breast cancer patients previously treated with trastuzumab [abstract 3138]. Cancer Res. 2009;69(suppl 2):249s [Google Scholar]
- 98.Burstein HJ, Sun Y, Tan AR, et al. Neratinib (HKI-272), an irreversible pan erbB receptor tyrosine kinase inhibitor: phase 2 results in patients with advanced HER2+ breast cancer [abstract 37]. Cancer Res. 2009;69(suppl) 2:73s 19117979 [Google Scholar]
- 99.Whenham N, D'Hondt V, Piccart MJ. HER2-positive breast cancer: from trastuzumab to innovatory anti-HER2 strategies. Clin Breast Cancer 2008;8(1):38-49 [DOI] [PubMed] [Google Scholar]
- 100.Widakowich C, Dinh P, de Azambuja E, Awada A, Piccart-Gebhart M. HER-2 postitive breast cancer: what else beyond trastuzumab-based therapy? Anti-Cancer Agents Med Chem 2008;8(5):488-496 http://www.benthamdirect.org/pages/content.php?ACAMC/2008/00000008/00000005/0005W.SGM Accessed April 15, 2009 [DOI] [PubMed] [Google Scholar]
- 101.Haffty BG, Yang Q, Reiss M, et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early stage breast cancer. J Clin Oncol. 2006December20;24(36):5652-5657 Epub 2006 Nov 20 [DOI] [PubMed] [Google Scholar]
- 102.Smid M, Wang Y, Zhang Y, et al. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008;68(9):3108-3114 [DOI] [PubMed] [Google Scholar]
- 103.Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13(8):2329-2334 [DOI] [PubMed] [Google Scholar]
- 104.Rouzier R, Perou CM, Symmans WF, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005;11(16):5678-5685 [DOI] [PubMed] [Google Scholar]
- 105.Cleator S, Heller W, Coombes RC. Triple-negative breast cancer: therapeutic options. Lancet Oncol. 2007;8(3):235-244 [DOI] [PubMed] [Google Scholar]
- 106.Finn RS, Dering J, Ginther C, et al. Dasatinib, an orally active small molecule inhibitor of both the src and abl kinases, selectively inhibits growth of basal-type/“triple-negative” breast cancer cell lines growing in vitro. Breast Cancer Res Treat 2007November;105(3):319-326 Epub 2007 Feb 1 [DOI] [PubMed] [Google Scholar]
- 107.Kreike B, van Kouwenhove M, Horlings H, et al. Gene expression profiling and histopathological characterization of triple-negative/basal-like breast carcinomas. Breast Cancer Res. 2007;9(5):R65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rakha EA, Reis-Filho JS, Ellis IO. Basal-like breast cancer: a critical review. J Clin Oncol. 2008;26(15):2568-2581 [DOI] [PubMed] [Google Scholar]
- 109.Carey LA, Rugo HS, Marcom PK, et al. TBCRC 001: EGFR inhibition with cetuximab added to carboplatin in metastatic triple-negative (basal-like) breast cancer [abstract 1009]. J Clin Oncol. 2008;26(suppl 15):43s [Google Scholar]
- 110.O'Shaughnessy J, Weckstein DJ, Vukelja SJ. Preliminary results of a randomized phase II study of weekly irinotecan/carboplatin with or without cetuximab in patients with metastatic breast cancer [abstract 308]. Breast Cancer Res Treat 2007;106(suppl 1):S32 [Google Scholar]
- 111.Finn RS, Bengala C, Ibrahim N, et al. Phase II trial of dasatinib in triple-negative breast cancer: results of study CA180059 [abstract 3118] Poster presented at: 31st San Antonio Breast Cancer Symposium San Antonio, TX: December11, 2008 http://www.posters2view.com/sabcs08/viewp.php?nu=3118 Accessed April 15, 2009 [Google Scholar]
- 112.Thome S, Hobday T, Hillman D, et al. Translational correlates, including outcome for patients with ER-/PR-/HER2-(triple negative [TNeg]) disease from N0234, a phase II trial of gemcitabine and erlotinib for pts with previously treated metastatic breast cancer (MBC) [abstract 1071]. J Clin Oncol. 2007;25(suppl 18):49s [Google Scholar]
- 113.O'Shaughnessy J, Osborne C, Blum J, et al. Triple negative breast cancer: a phase 2, multi-center, open-label, randomized trial of gemcitabine/carboplatin (G/C), with or without BSI-201, a PARP inhibitor [abstract 2120]. Cancer Res. 2009;69(suppl 2):194s [Google Scholar]
- 114.Rugo HS, Roché H, Thomas E, et al. Ixabepilone plus capecitabine vs capecitabine in patients with triple negative tumors: a pooled analysis of patients from two large phase III clinical studies [abstract 3057]. Cancer Res. 2009;69(suppl 18):225s [Google Scholar]
- 115.Michaud LB. Treatment-experienced breast cancer. Am J Health Syst Pharm. 2008;65(10)(suppl 3):S4-S9 [DOI] [PubMed] [Google Scholar]