Table 1.
Names, structures, and photophysical parameters of various DCDHFs and standard fluorophores. The structures are generalized: the methyl groups on the amine and dihydofuran ring may vary in length and composition; the data presented is representative of chromophores with varying R1–R4 groups, because varying the length of the alkyl chains has little effect on the photophysics. See text for details on photophysical parameters. (Data from refs. [19,20], and [24].)
| Compound | Structure | εmax [m−1 cm−1][a] | λabs [nm][a] | λem [nm][a] | ΦF in toluene {PMMA} | ΦB in gelatin {PMMA} (× 10−6) | Ntot,e in PMMA (× 106) |
|---|---|---|---|---|---|---|---|
| DCDHF-P | 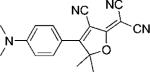 |
71 000 | 486 | 505 | 0.044 {0.92} | 6.6 | 2.4 |
| DCDHF-P with 1 constraining ring | 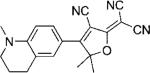 |
95 900[b] | 495 | 515 | 0.10 | ||
| DCDHF-P with 2 constraining rings | 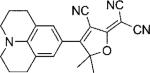 |
90 400[b] | 503 | 527 | 0.21 | 1.1 | |
| DCDHF-V-P | 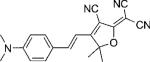 |
45 500 | 562 | 603 | 0.02 {0.39} | 2.8 | ∼1.9 |
| DCDHF-T | 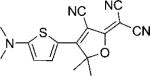 |
100 000 | 514 | 528 | 0.11 | ∼0.91 | |
| DCDHF-V-T | 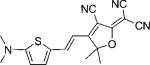 |
114 000 | 614 | 646 | 0.02 | ∼0.23 | |
| DCDHF-N | 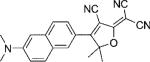 |
42 000 | 526 | 579 | 0.85 {0.98} | 3.4 | 1.1 |
| DCDHF-V-N | 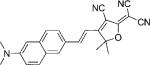 |
58 100[b] | 574 | 671 | 0.01 | ||
| DCDHF-V-(1,4)N | 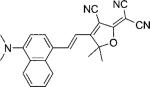 |
22 200[b] | 538 | 661 | 0.01 | ||
| DCDHF-A | 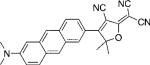 |
35 000 | 585 | 689 | 0.54 | 1.7 | 2.2 |
| DCDHF-P-P | 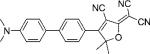 |
31 000 | 506 | 623 | 0.82 | 4.5 | |
| DCDHF-T-T | 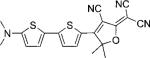 |
71 800 | 634 | 679 | 0.50 | {2.1} | |
| DCDHF-V-T-T | 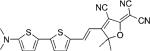 |
49 800 | 708 | 779 | 0.13 | {3.4} | |
| DCDHF-P-T | 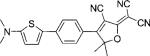 |
44 000 | 591 | 663 | 0.21 | 6.4 | |
| DCDHF-T-P | 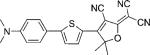 |
22 000 | 575 | 631 | 0.74 | 2.9 | |
| DCDHF-V-T-P | 47 300 | 611 | 723 | 0.07 | {0.13} | ||
| DCDHF-P-T-P | 28 000 | 541 | 709 | 0.34 | 0.91 | ||
| Rhodamine 6G | 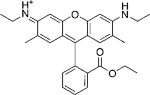 |
105 000[c] | 530[c] | 556[c] | 0.95[c] | 3.5 | 1.4 |
| Fluorescein | 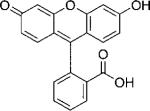 |
92 300[c] | 483[c] | 515[c] | 0.79[c] | 64 |
In toluene.
In dichloromethane.
In ethanol.
