Recent advances in optical imaging with single molecules beyond the diffraction limit (e.g., PALM, FPALM, STORM)1-3 have introduced a new requirement for fluorescent labels: fluorophores must be actively controlled (usually via photoswitching or photoactivation) to ensure that only a single emitter is switched on at a time in a diffraction-limited region. The location of each of these sparse molecules is precisely determined, and a super-resolution image is obtained from the summation of many successive rounds of photoactivation. The ultimate spatial resolution is determined by a number of factors, most importantly the total number of photons detected from each individual molecule.4 Super-resolution imaging by these methods, and cell imaging in general, often use photoswitchable fluorescent proteins, which have the advantage of being genetically targeted;5,6 however, they provide 10-fold fewer photons before photobleaching than good small-molecule emitters.7 Bright organic fluorophores that can be turned on and/or off are therefore attractive; also smaller labels might be less perturbative than fluorescent proteins, their chemistries and photophysics can be more readily tailored, and they can be targeted using schemes actively under development.8-12
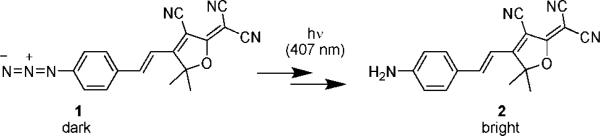
Here, we report a new, bright photoactivatable organic fluorophore that can be imaged at the single-molecule level in living cells. We use the DCDHF class of single-molecule cellular labels,13-15 in which an amine donor is connected to a dicyanomethylenedihydrofuran acceptor via a conjugated π-bonded network. We have reengineered a red-emitting DCDHF to produce the fluorogen 1, a molecule that is dark until photoactivated with a short burst of low-intensity violet light. Photoactivation leads to conversion of the azide moiety to an amine, which shifts the absorption to long wavelengths and creates a bright, red emitter that is photostable enough to be imaged on the single-molecule level in living cells. Our proof-of-principle demonstration shows that photoactivation of an azide-based DCDHF fluorogen provides a new class of labels that would be useful for super-resolution imaging schemes that require active control of single molecules.
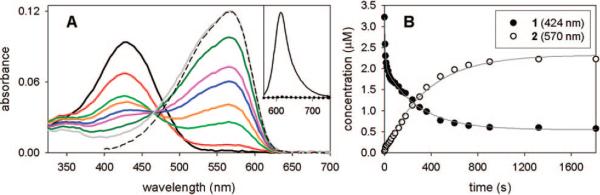
A fluorescent label useful for live-cell imaging must be pumped at wavelengths >500 nm to avoid cellular autofluorescence. Figure 1A shows that activating the fluorogen 1 at 407 nm leads to loss of the azido-DCDHF absorption at 424 nm and the formation of long-wavelength absorption at 570 nm from 2. The fluorogen does not absorb at 594 nm, but this wavelength does strongly pump the emissive photoproduct 2. Thus, imaging with 594 nm produces no emission until 407 nm is used to turn-on of fluorescence.
Figure 1.
(A) Absorption curves in ethanol (bubbled with N2) showing photoactivation of 1 (λabs = 424 nm) over time to fluorescent product 2 (λabs = 570 nm). Different colored curves represent 0, 10, 90, 150, 240, 300, 480, and 1320 s of illumination by 3.1 mW/cm2 of diffuse 407-nm light. The sliding isosbestic point may indicate a build-up of reaction intermediates.18 Dashed line is the absorbance of pure, synthesized 2. (Inset) Dotted line is weak pre-activation fluorescence of 1 excited at 594 nm; solid line is strong post-activation fluorescence resulting from exciting 2 at 594 nm, showing >100-fold turn-on ratio. (B) Photoactivation kinetics from data in A. The total yield of the reaction ([2]f/[1]i) is 69%. Photoconversion data for 1 were fit using two exponentials (τ = 7.4 and 291 s); data for 2 were fit using one exponential (τ = 353 s).
Drawing from the extensive work on the photochemistry of aryl azides,16 we expected that photoelimination of N2 would produce a highly reactive nitrene, which could subsequently react either by inserting into bonds of surrounding molecules (2 and Supporting Information (SI)) or intramolecularly via a ring-expansion to an azepine. Product 2 is an amine, as is required to produce a fluorescent red-shifted DCDHF; minor nonfluorescent products are discussed in SI; no azepine products were found. Azide-based fluorogens have been reported previously, but they require short wavelengths and are not photostable enough to be applied to single-molecule imaging.17
Figure 1 and Scheme 1 may be rationalized as follows. First, in 1, replacing the usual amine donor group in DCDHF fluorophores with the mildly electron-withdrawing azide (Hammett constants, Table S1) disrupts the donor–π–acceptor push–pull character. This explains the large (∼150-nm) blue-shift of the absorption wavelength relative to the amine donor DCDHF. Second, electron-withdrawing substituents (i.e., the DCDHF acceptor) on an aryl azide stabilize the nitrene intermediate in an alcohol solvent, and yield aniline products rather than azepines.19 In fact, HPLC–MS and NMR analysis of the photoproducts of 1 confirm structure 2 as the major product in ethanol (see SI).
Scheme 1.
Photochemical Activation of the Azido-DCDHF Fluorogen
The efficiency of azide photolysis is measured by the quantum yield of photoconversion (ΦP), which is calculated from the rate at which 1 disappears (Figure 1B and SI); photoconversion of 1 is thousands of times more likely than bleaching pathways of the photoproduct fluorophore 2 (ΦP >> ΦB, Table 1). Photoconversion of 1 requires only very mild illumination by violet light (407 nm), which is several orders of magnitude lower irradiance than required for activating PA-GFP or EYFP, and only slightly higher than required for Dronpa or the Cy3/Cy5 photoswitch (Table S2).5,6,12,20 This is important, because high doses of short-wavelength light can kill cells, alter morphology, or create unwanted phenotypes.
Table 1.
Photophysical Properties of 1 and 2 in Ethanol (unless Otherwise Stated)a
| λabs (nm), ε (M−1cm−1) | λfl (nm) | ΦF | ΦPb | ΦBc | SM Ntot,ed | |
|---|---|---|---|---|---|---|
| 1 | 424, 29100 | 552 | n/a | 0.0059 | n/a | n/a |
| 2 | 570, 54100 | 613 | 0.025, 0.39e | n/a | 4.1 × 10−6 | 2.3 × 106 |
See SI for details on measurements and calculations.
Quantum yield of photoconversion from azide with 407-nm illumination (see SI).
Bulk quantum yield of permanent photobleaching, measured in aqueous gelatin.
Average number of photons emitted per molecule in gelatin.
Fluorescence quantum yield in ethanol and PMMA, respectively; rigidification increases the brightness.13
Imaging single molecules in living cells has stringent prerequisites, and foremost among them is photostability. An emitter that delays permanent photobleaching will be bright longer and thus be easier to image and precisely locate.4 As metrics of photostability, we report both the probability of photobleaching per photon absorbed (ΦB) and the total number of photons emitted per molecule (Ntot,e), which is an average from hundreds of individual copies of 2 (see SI). Most DCDHFs are photostable,13-15 and the photoproduct 2 is no exception: each molecule emits millions of photons on average before bleaching.
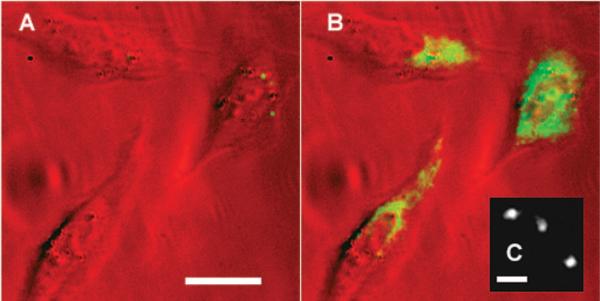
For quantitative analysis, single molecules were easily activated and imaged in polymer films and aqueous gelatin. But a crucial test is whether this fluorogen can also be photoactivated in living cells. Figure 2 shows three Chinese hamster ovary (CHO) cells growing on a glass slide and incubated with 1, which easily inserts into and penetrates the plasma membrane; fluorescence in the cytosol turns on only after a short flash of diffuse violet light. A fraction of the fluorophores remained stationary at the activation site (movie S1), presumably bioconjugated to relatively static biomolecules (via nitrene insertion into C–C bonds). The remaining untethered fraction was free to move throughout the cell: single molecules were visible diffusing in the cell. (Figure S1 shows the two-dimensional tracking of a single molecule.)
Figure 2.
(A) Three CHO cells incubated with fluorogen 1 are dark before activation. (B) The fluorophore 2 lights up in the cells after activation with a 10-s flash of diffuse, low-irradiance (0.4 W/cm2) 407-nm light. (False color: red is the white-light transmission image and green shows the fluorescence images, excited at 594 nm.) Scalebar: 20 μm. (C) Single molecules of activated 2 in a cell under higher magnification. Background was subtracted and the image was smoothed with a 1-pixel Gaussian. Scalebar: 800 nm.
The photoactivatable DCDHF single-molecule fluorogen presented here is but one example of a larger class based on replacing a donor group in a push–pull chromophore with a photoactivatable azide group. Unlike the Cy3/Cy5 photoswitching system, photoactivating the azido-DCDHF does not require other additives (i.e., oxygen-scavengers and exogenous thiol)12,21,22 and thus may find greater ease of use in living systems. The next step we are pursuing with these photoactivatable DCDHFs is to apply specific targeting schemes to direct the label to desired locations. These molecules may also be used for fluorogenic photoaffinity labeling;23 assuming a binding pocket is engineered for the fluorogen, a flash of blue light can simultaneously turn on fluorescence and form a covalent bond between the DCDHF and the biomolecule. The azido-DCDHF fluorogen described here is an example of a rich new class of photoactivatable molecules, which should be a powerful tool for single-molecule studies in the chemically and optically complex medium of the cell.
Supplementary Material
Acknowledgment
This work was supported in part by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant No. P20-HG003638-02.
Footnotes
Supporting Information Available: Experimental procedures, chemical analysis, additional figures and movies, plus comparisons to other photoswitchable fluorophores. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Betzig E, Patterson GH, Sougrat R, Lindwasser OW, Olenych S, Bonifacino JS, Davidson MW, Lippincott-Schwartz J, Hess HF. Science. 2006;313:1642–645. doi: 10.1126/science.1127344. [DOI] [PubMed] [Google Scholar]
- 2.Hess ST, Girirajan TPK, Mason MD. Biophys. J. 2006:194–4272. doi: 10.1529/biophysj.106.091116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rust MJ, Bates M, Zhuang X. Nat. Methods. 2006;3:793–795. doi: 10.1038/nmeth929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson RE, Larson DR, Webb WW. Biophys. J. 2002;82:2775–2783. doi: 10.1016/S0006-3495(02)75618-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ando R, Mizuno H, Miyawaki A. Science. 2004;306:1370–1373. doi: 10.1126/science.1102506. [DOI] [PubMed] [Google Scholar]
- 6.Patterson GH, Lippincott-Schwartz J. Science. 2002;297:1873–1877. doi: 10.1126/science.1074952. [DOI] [PubMed] [Google Scholar]
- 7.Harms GS, Cognet L, Lommerse PHM, Blab GA, Schmidt T. Biophys. J. 2001;80:2396–2408. doi: 10.1016/S0006-3495(01)76209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen I, Ting A. Curr. Opin. Biotechnol. 2005;16:35–40. doi: 10.1016/j.copbio.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Prescher JA, Bertozzi CR. Nat. Chem. Biol. 2005;1:13–21. doi: 10.1038/nchembio0605-13. [DOI] [PubMed] [Google Scholar]
- 10.Adams SR, Campbell RE, Gross LA, Martin BR, Walkup GK, Yao Y, Llopis J, Tsien RY. J. Am. Chem. Soc. 2002;124:6063–6076. doi: 10.1021/ja017687n. [DOI] [PubMed] [Google Scholar]
- 11.Fölling J, Belov V, Kunetsky R, Medda R, Schönle A, Egner A, Eggeling C, Bossi M, Hell SW. Angew. Chem., Int. Ed. 2007;46:6266–6270. doi: 10.1002/anie.200702167. [DOI] [PubMed] [Google Scholar]
- 12.Bates M, Blosser TR, Zhuang X. Phys. Rev. Lett. 2005;94:108101-1–108101-4. doi: 10.1103/PhysRevLett.94.108101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willets KA, Nishimura SY, Schuck PJ, Twieg RJ, Moerner WE. Acc. Chem. Res. 2005;38:549–556. doi: 10.1021/ar0401294. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura SY, Lord SJ, Klein LO, Willets KA, He M, Lu ZK, Twieg RJ, Moerner WE. J. Phys. Chem. B. 2006;110:8151–8157. doi: 10.1021/jp0574145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lord SJ, Lu Z, Wang H, Willets KA, Schuck PJ, Lee H-LD, Nishimura SY, Twieg RJ, Moerner WE. J. Phys. Chem. A. 2007;111:8934–8941. doi: 10.1021/jp0712598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schriven EFV, editor. Azides and Nitrenes: ReactiVity and Utility. Academic Press; Orlando, FL: 1984. [Google Scholar]
- 17.Dockter ME. J. Biol. Chem. 1979;254:2161–2164. [PubMed] [Google Scholar]
- 18.Muller P. Pure Appl. Chem. 1994;66:1077–1184. [Google Scholar]
- 19.Soundararajan N, Platz MS. J. Org. Chem. 1990;55:2034–2044. [Google Scholar]
- 20.Dickson RM, Cubitt AB, Tsien RY, Moerner WE. Nature. 1997;388:355–358. doi: 10.1038/41048. [DOI] [PubMed] [Google Scholar]
- 21.Heilemann M, Margeat E, Kasper R, Sauer M, Tinnefeld P. J. Am. Chem. Soc. 2005;127:3801–3806. doi: 10.1021/ja044686x. [DOI] [PubMed] [Google Scholar]
- 22.Rasnik I, McKinney SA, Ha T. Nat. Methods. 2006;3:891–893. doi: 10.1038/nmeth934. [DOI] [PubMed] [Google Scholar]
- 23.Kotzyba-Hibert F, Kapfer I, Goeldner M. Angew. Chem., Int. Ed. Engl. 1995;34:1296–1312. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.