Abstract
Annually, premature birth is a major public health problem accounting for over 13,000 deaths and 30,000 surviving infants with life-long morbidity. Preterm premature rupture of the membranes is the initiating event leading to preterm birth of 40% of these premature infants. Fetal membrane (FM) rupture is a catastrophic tissue failure, a unique event in normal physiology; other tissue failures (bone breaks, aneurism ruptures) are pathological processes. The mechanisms which cause FM failure and thereby rupture are not understood. A full understanding of FM failure process requires a complete characterization of structural and biomechanical behavior at near/full term under sub-failure (forces well below that which induce rupture) and failure conditions as well as elucidating the biological factors which modulate its failure. The relatively, highly loaded stated of the FM in vivo may also facilitate its susceptibility to enzymatic degradation, which was shown to be augmented with increased load in collagenous tissues. Indeed, this last observation may help to provide the link between biomechanical degradation and premature mechanical failure in the FM. This integrated approach will further the understanding of this unique physiological event and thereby provide insight into how to anticipate and when appropriate, intervene to prevent preterm FM rupture.
Keywords: intact fetal membrane, planar biaxial mechanical testing, sub-failure mechanical and structural properties
1. Introduction
Premature birth is a major public problem accounting for over 13,000 deaths per year and 30,000 surviving infants with life-long morbidity yearly [1–4]. Premature failure of the fetal membrane (FM), prior to full gestation, accounts for one third of all premature human births and affects 3% of all pregnancies [4]. Normal pregnancy requires that the physical integrity of the FM be maintained until term delivery. The FM is the membrane surrounding the fetus during gestation and is a structurally soft tissue critical for maintaining a successful pregnancy and delivery [5]. The membrane is subjected to applied stresses during pregnancy and must support the bulk loads of the fetus and amniotic fluid as well as tolerate local deformation associated with fetal movement [5].
The intact FM is a bilayer structure composed of both the amnion layer and the choriodecidua layer. The choriodecidua layer is thicker than the amnion layer and is cellular [5]. The amnion layer is stiff and strong and only accounts for approximately 20% of the FM thickness [5]. However, the amnion layer dominates the mechanical response of the intact FM [5]. The amnion layer is avascular and is composed of various sublayers. The sublayer nearest the fetus is composed of epithelial cells which secrete collagen, Types III and IV, and glycoproteins forming the subsequent basement membrane [6]. Below the basement membrane is the compact sublayer, which forms the fibrous framework of the amnion and is thought to contribute to the structural integrity of FM [6]. The compact sublayer is formed by the mesenchymal cells in the subsequent fibroblast layer, and contains both Type I and III collagens [6]. The spongy sublayer lies below the fibroblast sublayer and is composed of proteoglycans and glycoproteins along with a meshwork of mostly Type III collagen [6]. The spongy sublayer separates the amnion and the chorion layers and allows the amnion to slide on the underlying chorion [6]. The chorion layer contains a connective tissue sublayer and cytotrophoblasts and is firmly adhered to the maternal decidua. When the FM separates from the uterus at delivery, some adherent uterine tissue, part of the decidua, remains attached to the chorion [7]. The resulting layer is the “choriodecidua” layer.
2. Physiological failure of the FM
Tissue failure, as occurs when the FM ruptures, is a unique event in normal human physiology, whereas other tissue failures (e.g. tendon/ligament failures, bone fracture, skin tears, vascular aneurysms) are all pathological processes. FM rupture is likely a direct consequence of a programmed, biochemically mediated weakening process [6, 8] that may work in coordination with physical forces. However, in order to understand FM failure, the relationship between physical rupture and programmed, biochemically mediated FM weakening must be established. These two mechanisms are discussed in detail in the following.
2.1 Biochemically mediated processes which lead to programmed weakening of the FM
Traditionally, membrane rupture was thought to be a consequence of physical stress alone, particularly during labor. However, in 10% of term and 40% of preterm deliveries, membrane rupture precedes contractions [6]. It has thus become clear that rupture of the FM, term or preterm, is not solely the result of the stretch and shear forces of uterine contractions, but in significant part the consequence of a programmed, biochemically mediated, weakening process [6, 8]. Animal data clearly documented gestational changes in the FM consistent with weakening [6, 9–11]. A homogeneous remodeling process over the entire FM with degradation of collagen Type I as a major component was seen in the rat amnion at end gestation [12]. Attempts to document similar gestational changes in human FM were initially unsuccessful, however [13]. Using a meticulous approach, the Bell group identified a region of high morphological change at the rupture site in term vaginal deliveries [14]. The same group later identified a similar region overlaying the cervix in patients that delivered by cesarean section without labor [15]. This area of altered morphology was characterized by increased matrix metalloproteinase (MMP) activity [16]. Apoptosis, particularly in the chorion trophoblast layer, was also reported in this para-cervical region of human FM by them and thereafter by several other groups [15–19].
Three groups have now confirmed that a zone of altered morphology exists in the region overlying the cervix [14, 17, 19]. El Khwad et. al, however, was the first to demonstrate that these biochemical and histological changes are associated with physical weakening of the FM [17]. It was demonstrated that a discrete zone of weakness (“weak zone”) is present in term, prelabor FM in the region overlying the cervix. In addition to decreased rupture strength (1/3 of that in other areas of the FM), the “weak zone” showed deceased work to rupture, stiffness, and ductility (displacement at the time of rupture). Concomitant with these differences in biophysical properties, it was demonstrated that the “weak zone” FM exhibited biochemical characteristics consistent with tissue remodeling and apoptosis. MMP 9 was increased in the “weak zone” relative to the remaining FM, and tissue inhibitor of metalloproteinase (TIMP) 3 was decreased in the “weak zone” compared with the rest of the FM. Poly ADP-Ribose Polymerase (PARP) cleavage, a marker of apoptosis was also increased in the “weak zone”. Finally, it was demonstrated that the “weak zone” contained the same histological changes noted in areas of high morphological change by Malak et. al [14, 17]. In another report, El Khwad et. al demonstrated that FMs delivered vaginally also had a “weak zone” with identical biochemical markers as the Cesarean FM [18]. Furthermore, in FMs with spontaneous rupture, the “weak zone” was bisected by the rupture tear line [18].
The above data is consistent with a programmed biochemical process characterized by extracellular matrix (ECM) remodeling and apoptosis acting focally upon the FM region overlying the cervix to create the “weak zone”. Further, the “weak zone” contains the rupture initiation site. In addition, Kumar et. al also demonstrated that in vitro incubation of FM with either TNFα or IL-1β can transform “non-weak” FM into weak membranes with the same biochemical markers of ECM remodeling and apoptosis seen in the naturally occurring “weak zone” [20].
2.2 Biomechanical studies of the FM failure behavior
Many investigators performed in vitro biomechanical studies in an effort to understand FM failure [5, 8, 17, 21–31]. Three types of testing techniques were utilized (Table 1):
Table 1.
Summary of mechanical tests performed on the intact FM. This table provides the values at which the membrane ruptures along with relevant details about the testing techniques. Note that the fixture diameter is the diameter of the circular opening in the center specimen holder in both the burst and puncture devices. This opening in the specimen holder allows the air/water of the burst device and the probe of the puncture device to impinge on the secured FM specimen. The probe diameter is the diameter of the metal probe/plunger in the puncture tests.
| Study | Failure Value | Details |
|---|---|---|
| Uniaxial Test | ||
| 1 [23, 33] | 0.95 N (237.7 N/m) | Specimen Dimensions: 4mm×15mm |
| Burst (Inflation) Test | ||
| 2 [33, 53] | 393 mmHg (261.98 N/m) | FD: 20 mm |
| 3 [34] | 15 N(152.06 N/m) | FD: 15.7 mm |
| 4 [25] | 0.205kg/cm (201.105 N/m) | FD: 54 mm |
| 5 [28] | 60 mmHg (152.39 N/m) | FD: 76.2 mm |
| 6 [29] | 40 mmHg (101.59 N/m) | FD: 76.2 mm |
| 7 [30] | 100 mmHg(253.98 N/m) | FD: 76.2 mm |
| Puncture Test | ||
| 10 [35] | 11.34 N (150.40 N/m) | FD: 15.88 mm; PD: 12 mm |
| 11 [17] | 9.07±2.61N (144.35 N/m) | FD: 25 mm; PD: 10 mm |
| 12 [5] | 4.15±1.27N (206.40 N/m) | FD: 20 mm; PD: 3.2 mm |
| 13 [36] | 9.71±2.42N (154.54 N/m) | FD:25 mm; PD: 10 mm |
FD = Fixture Diameter
PD = Probe Diameter
Tensile testing, in which the membrane is placed between two grips and is gradually pulled apart while the resulting forces and strains are monitored [21–24, 31].
Burst testing, in which a section of membrane is clamped in a circular ring and increasing pressure is applied, either via air or fluid, to membrane until failure occurs while the height of the membrane is monitored [25–30].
Puncture testing, in which a spherically tipped metal probe displaces the central portion of a membrane, which is clamped in a ring, perpendicular to the plane of its surface [8], while the resultant force is measured [5, 8, 17].
Note that in order to efficiently compare values from each mode of testing, each failure value was converted to a membrane tension (Table 1). For tensile testing, the reported force was converted to membrane tension by dividing the force by the width of the specimen (T=Force/width). For burst testing, the reported pressures were converted to membrane tension using the Law of LaPlace. Specifically, the Law of LaPlace states the relationship between pressure, surface tension, and curvature of the surface of the FM and can be expressed as:
| (1) |
where T is the mean membrane tension, P is internal pressure, and a is the estimated radius of the FM [32]. The radius, a, was estimated from the radius of the fixture diameter. For puncture testing and for burst testing where only force was provided, the reported force was first converted to pressure by dividing the force by the surface area of a hemisphere (P = Force/Surface Area). After conversion to pressure, equation 1, above, was used to determine the membrane tension. It is important to note that these methods were only used to provide an estimate of the membrane tensions.
In each mode of testing, various mechanical parameters (i.e. stiffness and rupture strength of the membranes) were determined [8, 23, 33]. Of these methodologies, tensile testing does not provide a physiological testing state as the stresses applied are uniaxially, whereas the FM is physiologically loaded under a complex biaxial state. Tensile testing is used because standard stress-strain calculations are simple to apply. Burst testing better mimics the physiologic loading state. However, it is logistically difficult and requires relatively large pieces of membrane. In puncture tests, the plunger impinges on the FM resulting in an approximately planar state of tension applied to the FM and can be carried out quickly on relatively with small pieces of membrane. The resultant force measurement is dependent on the size of the metal probe and tissue holder. However, Schober et al. demonstrated that data obtained by puncture testing can be directly related to that which would be obtained using the more physiological burst testing methodology [8, 9, 34, 35]. As a result, several recent biomechanical investigations of the FM were conducted using this method [5, 8, 17, 18].
There are major differences in conclusions by different investigators due to various reasons. First, different investigators were lead to different conclusions because of the use of different testing methodologies (Table 1). Also, the heterogeneity of the tissue biomechanical properties of the FM surface resulted in different conclusions. Investigators were not aware of the existence of a “weak zone” prior to reports by El Khwad et. al [17]. In addition, there was confusion as to which layer of the FM was stronger (amnion or choriodecidua) and which component ruptures first. Recent studies by Arikat et. al included video-recording of the rupture process and demonstrated that the choriodecidua component of the intact FM did rupture first [36].
3. Changes in collagen architecture under mechanical loading
Before proceeding, we present a short description of the key biomechanical terms.
Force: the physical force exerted from an external source, such as a plunger, to the FM.
Stress (P): Similar to a pressure, is the force per original cross sectional area.
Membrane tension (T): An alternative stress measure expressed as force/unit length (N/m), often used when thickness measurements are difficult and/or of low accuracy.
Stretch (λ): A measure of deformation defined as λ=L/Lo, where L is the current length of the fiber and Lo is the original length.
Strain (ε): Another common measure of deformation defined as ε=λ−1 and usually expressed in percent.
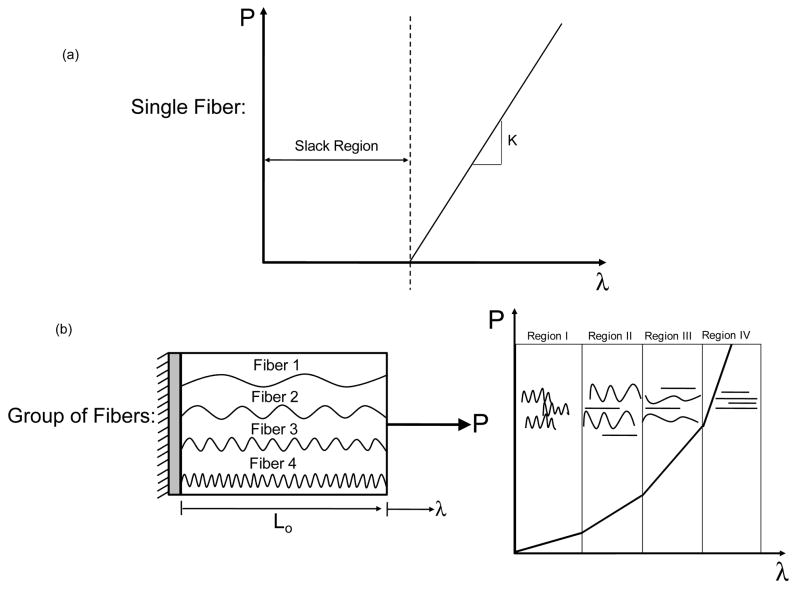
The FM is a dense collagenous membrane, whose mechanical behavior resembles that of other soft tissues rich in Type I collagen. Collagen Type I is known to provide structural integrity to collagenous tissues and is inherently crimped [37]. It is found in the compact layer of the amnion and is initially undulated in the stress-free state. To gain insight into the tissue failure process, consider a single undulated collagen fiber that is initially crimped and cannot bear load (Fig. 1a). As increasing λ is applied, the fiber begins to straighten and bear load (Fig. 1a). At this point, the fiber is considered to be linearly elastic (Fig. 1a) with slope K, also known as the elastic constant.
Figure 1.
(a) Single collagen fiber. Traditional recruitment modeling assumes that a collagen fiber does not bear load until it is fully straightened. (b) Group of collagen fibers. Gradual recruitment of collagen fibers results in a non-linear stress strain relationship (Regions I, II, III). Once all collagen fibers become straighten the stress-stretch curve transitions into a linear region (Region IV).
We next consider a group of collagen fibers, with each fiber having a separate degree of undulation and are thus recruited at different levels of λ (Fig 1b). For example, Fiber 1 has the least amount of collagen crimp, and thus it straightens first. With increasing λ, Fiber 2 becomes straightened, followed by Fibers 3 and then 4 (Fiber 1b). At the bulk tissue level, this phenomenon translates to a non-linear P-λ response (Fig 1b). In Region I of the P-λ response, most of the collagen fibers are crimped. However, with increasing λ, more and more fibers straighten and begin to support load (Regions II and III, Fig. 1b). Once all collagen fibers are straightened, the P-λ curve transitions into a linear region (Region IV, Fig. 1b).
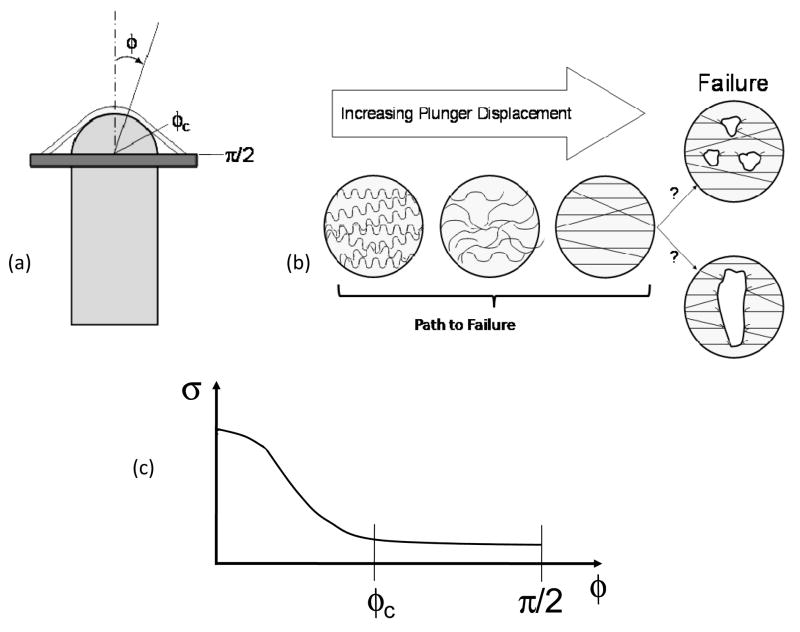
We can next see how this tissue level behavior is reflected in the puncture tests (Fig. 2). Initially, in the unloaded state, the collagen fibers of the FM are crimped (Fig. 2b). With increasing stretch induced by the plunger displacement, the amnion and the choriodecidua distend, and the crimped collagen fibers begin to straighten (Fig. 2a, b). As the plunger continues to stretch the tissue, the collagen fibers continue to straighten and eventually become taught (Fig. 2b). Arikat et al. demonstrated that the choriodecidua always ruptured before the amnion layer [36]. It was reported that after significant distension a linear fracture appears in the choriodecidua, while the amnion layer remained intact [36].
Figure 2.
(a) Schematic of puncture device and the FM being deformed. φ is the angle between the tip of the plunger and an arbitrary point along the membrane and is defined from 0 to π/2. At the tip of the plunger, φ is 0. φc is the angle in which the FM loses contact with the plunger. (b) Idealization of collagen behavior during puncture studies. Initially, the collagen fibers are crimped. With increasing plunger displacement, the collagen fibers begin to bear load and becomes straightened. However, it is unclear whether failure initiates as small defects, which leads to complete failure, or failure is catastrophic. (c) Due to the geometry of the plunger, the stress field over the surface of the FM during puncture studies is heterogeneous, resulting in a complex data analysis. At 0, which is the tip of the plunger, the stress is the greatest. With increasing φ, the stress decreases over the surface of the FM and eventually becomes homogenous.
However, it is unclear how the amnion, the layer attributed to the mechanical strength of the FM [5, 31], actually ruptures. There are two possible scenarios describing the amnion tissue failure (Fig. 2b). In scenario 1, the collagen fibers are recruited more gradually. Then, with increased stretched induced by the plunger displacement, the collagen fibers begin to fail at different stretches, resulting in a gradual failure of the collagen fibers. This would result in small point defects which would ultimately lead to total failure. In scenario 2, all collagen fibers are recruited and straightened rapidly with increased plunger displacement, and all collagen fibers will then fail soon after so that failure is catastrophic.
The importance of biomechanics in FM failure is also reflected in the relation between biomechanical and biochemical processes. It has been demonstrated that proteolytic enzymes play a role in membrane rupture [8] and perhaps work synergistically with mechanical forces to induce tissue failure. This speculation is supported by the known interplay between mechanical deformation and collagen degradation in soft collagenous tissues [38–40]. For example, Lee et. al demonstrated that tensile loading accelerates the proteolysis of bovine pericardium subjected to collagenase [38]. Also, another study demonstrated that the degradation rate of collagen increased with stretch [40].
These observationscould be caused by two possible mechanisms [40]. Collagen fibers are composed of three polypeptide chains (tropocollagens) intertwined to form a triple helix or collagen molecule. The stretching of the collagen molecule may result in the opening of new sites making it more susceptible to enzymatic degradation [40]. If only certain sites were exposed during stretch, then only these collagen fibers would be more susceptible to failure and thus, not all the collagen fibers will fail at once. Scenario 1 may be explained by this phenomenon (Fig 2b). Another possible mechanics for the increased degradation rate could be that the enzymatic breakdown of a given collagen molecule caused the remaining stress in the tissue to be transferred to a neighboring molecule, which may then rupture. If this is a widespread event, rapid break down of the collagen fibers could ultimately result in a catastrophic failure of the tissue [40]. Scenario 2 may be explained by this mechanism (Fig. 2b). While the exact mechanism is unknown, it may be that mechanical stress may facilitate weakening of the collagen fibers by attacking the molecules that organize collagen Type 1, such as decorin, biglycan, the fibulin family. Irrespective of the exact mechanisms, we speculate that enzymatic degradation of the FM collagen may be accentuated by mechanical stress.
4. Understanding sub-failure mechanical behavior is a necessary prerequisite for understanding failure in the FM
Most in vitro mechanical studies have focused on the failure behavior of the FM, whereas minimal data exists for the sub-failure mechanical properties. In order to fully understand FM failure, it is first necessary to characterize the sub-failure response of the FM. By taking this approach, the entire stress-strain response of the FM from the stress-free state (i.e. unloaded) up to failure occurs is known. With this information, one can develop a sub-failure structural model of the FM response, which then can be extrapolated to rupture conditions. One approach to modeling tissue mechanics are structural constitutive (i.e. stress-strain) models that can help elucidate the correlation between the structural components and the resulting mechanical behavior of tissues. In addition to the need for modeling, it is also imperative to gain more insight into the effects of stretch on the FM failure, which can only be investigated under sub-failure conditions.
Our understanding of FM failure biomechanics would be greatly enhanced by utilizing physiologic biaxial testing approaches to characterize the sub-failure state and delineate the effects of ECM degradation due to a programmed, biochemically mediated, weakening process. In contrast, simple interpretations of puncture-mode failure tests have been implemented to investigate FM failure, only providing a limited understanding of the FM ECM failure process. For example, in a puncture test, a stress “amplification” is induced at the tip of the plunger [41]. Specifically, the stress in the FM is greatest at the tip of the plunger (Fig. 2c), and with increasing values of φ, which is the angle between the tip of the plunger and an arbitrary point along the membrane, the stress is reduced, until the stress field eventually becomes homogenous. The angle in which the FM loses contact with the plunger is designated by φc. Calculations using an average stress would cause an underestimation of the failure stresses.
Thus, current failure testing techniques provide limited information on FM tissue mechanics. To better understand the physiologic behavior of the FM, mechanical testing techniques need to be more amenable to the determination of FM sub-failure structural mechanical properties. In particular, the central target region of the test specimen should contain uniform stress and strain fields permitting data analysis to be simply performed [42]. Also, the target region should be small and located away from the outer edges of the test specimen to avoid the effects of specimen grips or tethers [42].
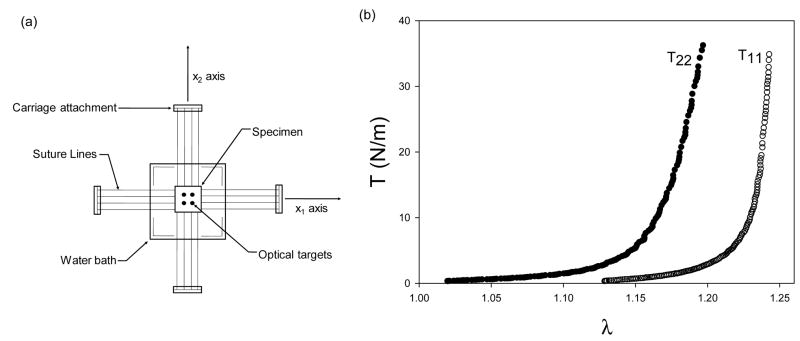
Planar biaxial mechanical testing can provide these necessary boundary conditions. Planar biaxial mechanical testing is one approach for studying sub-failure mechanical properties. In a planar biaxial mechanical test, membrane tension (T) is applied along each axis (the x1 and x2 device axes). Physiological membrane tension for the intact FM can be estimated using a simple Law of LaPlace expressed by equation 1, above. P was assumed to be 10 mmHg and was based on volume of the FM at term [43, 44]. This results in an estimated mean in vivo FM stress of 35 N/m.
Using this approximation, we conducted mechanical studies of the FM sub-failure properties under planar biaxial tension using well established approaches [42]. Our current results demonstrated that the FM exhibited modest mechanical anisotropy, meaning that the FM’s response to stretch was different along the x1 and x2 axes. Moreover, the resulting T-λ curves displayed a “toe” region followed by a transition into a highly linear, stiff region (Fig. 3). The intact FM is constantly loaded in vivo, and thus, the physiologic operating range is in the stiff linear region and not in the “toe” region.
Figure 3.
(a) Biaxial device. (b) Equi-biaxial tension responses of the intact FM.
These initial results suggest that the FM collagen fibers become fully loaded and are straightened well below physiological loading levels. This indicates that the FM collagen fibers have little or no structural reserve. In contrast, physiological operating ranges of other soft tissues (e.g. pericardium, heart valves, ligaments) are either in the early linear region or in the “toe” region of the stress-strain curves [45–50]. The FM appears to be loaded proportionally much closer to its failure stress. However, other soft tissues have a large structural reserve, indicating that their physiological loading ranges are well below their failure strengths [48–50]. The structural reserves in these tissues prevent them from being deformed past their failure strength. If failure does occur in these tissues, it is usually due to fatigue or a pathological condition. In contrast, the FM is intrinsically designed to fail at a prescribed time, and since the FM has minimal structural reserve in comparison to other soft tissue, failure is probably easily facilitated during labor.
5. Structural properties of the FM
Soft connective tissues, such as the FM, can efficiently bear tensile forces due to their collagen fibers, and the tissue can endure the greatest tensile stresses in the direction in which the collagen fibers are oriented. Thus, understanding the gross collagen fiber architecture can contribute to a better understanding and predictability of the mechanical properties of soft tissues. To obtain insight into the structure of the FM, we applied small angle light scattering (SALS) to quantify the FM fiber structure [51, 52]. A detailed description of the SALS technique has been previously presented [51, 52]. Briefley, a 4 mW HeNe continuous unpolarized laser (λ = 632.8 nm) is passed through the tissue specimen. The laser is then scattered according to the collagen fiber structure. An orientation index (OI) can be obtained from the scatter pattern and is an indicator of the degree of fiber alignment. A normalized orientation index (NOI) was calculated using:
| (2) |
where NOI ranges from 0% for a complete random network to 100% for a perfectly aligned network [51].
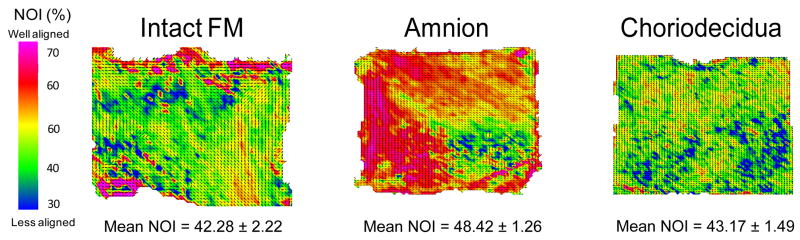
In the stress free state, it was observed that the gross collagen fiber architecture of the FM and the separated layers were not homogenously aligned but did exhibit small regions of fiber alignment (Fig. 4). The intact FM and the choriodecidua exhibited low fiber alignment (low NOI value) and were not statistically different, suggesting that both the intact FM and the choriodecidua are effectively isotropic (i.e. contain no preferred collagen direction) (Fig. 4). These findings are reasonable because the intact FM should be able to accommodate all fetal movements during gestation, and when the FM is deformed by fetal movement, the collagen fibers can freely rotate to support the loading. If the FM could only withstand stress in one direction, the FM could rupture easily if stress was applied by the fetus in any other direction other than the preferred direction. However, the amnion layer did exhibit regions of high fiber alignment (higher NOI value) and was significantly more aligned than the intact (p<0.001) and the choriodecidua (p=0.002) (Fig. 4). The modest alignment of the amnion layer probably contributed to the modest anisotropic mechanical behavior of the intact FM.
Figure 4.
Gross fiber architecture of the intact and separated tissues in a stress free state. The gross collagen fiber architecture of the intact FM and the separated layers exhibited small regions of alignment, but the overall fiber architecture was not homogenously aligned. The amnion layer contains the greatest degree of collagen fiber alignment.
6. Summary and Conclusions
Membrane rupture appears not to be entirely a result of physical forces alone, since in 10% of term labor and 40% of premature labor membrane rupture occurs before contractions begin. It has been established that FMs have a “weak zone” characterized by collagen remodeling and apoptosis in the region overlying the cervix which is present prior to labor [17, 18]. Furthermore, it has been shown that non weak membranes can be transformed into weak membranes by in vitro incubation with cytokines. These in vitro weakened FMs have the identical biomarkers of remodeling and apoptosis seen in the naturally occurring “weak zone” [20]. Although the biochemical processes affecting the FM have been somewhat clarified, the concomitant effects of mechanical processes that lead to tissue failure are less clear. The manner in which physical stretch forces act upon the biochemically weakened membranes which overlay the cervix is not known, but rupture initiation is postulated to occur in this region [18]. The relatively high loaded state of the FM in vivo may also facilitate its susceptibility to enzymatic degradation, which has been shown to increase with increased stretch [40]. Indeed, this last observation may help to provide the link between biomechanical degradation and premature mechanical failure in the FM.
Thus, in order to develop a rational basis for treatment and prevention of premature FM failure, we need first to understand FM structural and biomechanical behavior. This includes it constituent layers at near/full term under normal physiological loading states. In addition, understanding the gross collagen fiber architecture (i.e. collagen fiber alignment) can contribute to a better understanding and predictability of the biomechanical properties of the FM. Once these properties are established, we can then better formulate how the tissue transitions to the ability to fail at full term.
Acknowledgments
This work is supported by a grant from the Nation Institute of Health Beginning Grant-in-Aid (HD48467) for JJM and the University of Pittsburgh Provost’s Development Fund Pre-doctoral Fellowship to EMJ. The authors would like to thank Robert Moore, Jill Novak, Deepak Kumar and the Obstetrical Service at MetroHealth Medical Center, Cleveland, OH for assistance procuring fetal membranes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.(CDC), C.f.D.C.a.P. Infant Mortality-United States, 1992. Morbidity and Moratality Weekly Report (MMWR) 1994;43(49):905–9. [PubMed] [Google Scholar]
- 2.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Munson ML. Births: final data for 2003. Natl Vital Stat Rep. 2005;54(2):1–116. [PubMed] [Google Scholar]
- 3.Mathews TJ, MF M. National vital statistics reports. National Center for Health Statistics; Hyattsville, MD: 2006. Infant mortality statistics from the 2003 period linked birth/infant death data set. [PubMed] [Google Scholar]
- 4.Mercer BM. Preterm Premature Rupture of the Membranes. Obstetrics and Gynecology. 2003;101(1):178–93. doi: 10.1016/s0029-7844(02)02366-9. [DOI] [PubMed] [Google Scholar]
- 5.Oyen ML, Cook RF, Calvin SE. Mechanical failure of human fetal membrane tissues. Journal of Materials Science: Materials in Medicine. 2004;15:651–658. doi: 10.1023/b:jmsm.0000030205.62668.90. [DOI] [PubMed] [Google Scholar]
- 6.Parry S, Strauss JF. Premature Rupture of The Fetal Membranes. New England Journal of Medicine. 1998;338(10):663–670. doi: 10.1056/NEJM199803053381006. [DOI] [PubMed] [Google Scholar]
- 7.Kellicot WE. Outlines of Chordate Development. New Your: Henry Holt and Company; 1913. p. 471. [Google Scholar]
- 8.Moore RM, Mansour JM, Redline RW, Mercer BM, Moore JJ. The Physiology of Fetal Membrane Rupture: Insight Gained from the Determination of Physical Properties. Placenta. 2006;27:1037–1051. doi: 10.1016/j.placenta.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Lei HV-OF, Paavola LG, Strauss JF. 92-kDa gelatinase (matrix metalloproteinase-9) is induced in rat amnion immediately prior to parturition. Biology of Reproduction. 1995;53:339–44. doi: 10.1095/biolreprod53.2.339. [DOI] [PubMed] [Google Scholar]
- 10.Lei M, Ghezzo H, Chen MF, Eidelman DH. Airway smooth muscle orientation in intraparenchymal airways. J Appl Physiol. 1997;82(1):70–7. doi: 10.1152/jappl.1997.82.1.70. [DOI] [PubMed] [Google Scholar]
- 11.Paavola L, Furth EE, Delgado V, Boyd CO, Jacobs CC, Lei H, Strauss JF. Striking changes in the structure and organization of rat fetal membranes precede parturition. Biology of Reproduction. 1995;53:321–338. doi: 10.1095/biolreprod53.2.321. [DOI] [PubMed] [Google Scholar]
- 12.Lei HFFE, Kalluri R, Chiou T, Tilly K, Tilly JL, Elkon KB, Jeffrey JJ, Strauss JF. A program of cell death and extracellular matrix degradation is activated in the amnion before the onset of labor. J Clin Invest. 1997;98:1971–8. doi: 10.1172/JCI119001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manabe YHN, Fukumoto M. Tensile strength and collagen content of amniotic membrane do not change after the second trimester or during delivery. Obstet Gynecol. 1991;78(1):24–7. [PubMed] [Google Scholar]
- 14.Malak TMBS. Structural characteristics of term human fetal membranes: a novel zone of extreme morhological alteration within the rupture site. Br J Obstet Gynaecol. 1994;101:375–86. doi: 10.1111/j.1471-0528.1994.tb11908.x. [DOI] [PubMed] [Google Scholar]
- 15.McLaren J, Taylor DJ, Bell SC. Increased incidence of apoptosis in non-labor-affected cytotrophoblast cells in term fetal membranses the cervix. Human Reproduction. 1999;14:2895–2900. doi: 10.1093/humrep/14.11.2895. [DOI] [PubMed] [Google Scholar]
- 16.McLaren J, Taylor DJ, Bell SC. Increased Concentration of pro-matrix metalloproteinase 9 in term fetal membranes overylying the cervix befor labor: implications for membrane remodeling and rupture. American Journal of Obstetrics and Gynecology. 2000;182:409–416. doi: 10.1016/s0002-9378(00)70232-8. [DOI] [PubMed] [Google Scholar]
- 17.El Khwad M, Stetzer B, Moore RM, Kumar D, Mercer B, Arikat S, Redline RW, Mansour JM, Moore JJ. Term Human Fetal Membranes Have a Weak Zone Overlying the Lower Uterine Pole and Cervix Before Onset of Labor. Biology of Reproduction. 2005;72:720–726. doi: 10.1095/biolreprod.104.033647. [DOI] [PubMed] [Google Scholar]
- 18.El Khwad M, Pandey V, Stetzer B, Mercer B, Kumar D, Moore RM, Fox J, Redline RW, Mansour JM, Moore JJ. Fetal Membranes From Term Vaginal Deliveries Have a Zone of Weakness Exhibiting Characteristics of Apoptosis and Remodeling. Journal for the Society of Gynecologic Investigation. 2006;13(3):191–5. doi: 10.1016/j.jsgi.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 19.Reti NG, Lappas M, Riley C, Wlodek M, Permezel M, Walker S, Rice GE. Why do membranes rupture at term? Evidence of increased cellular apoptosis in the supracervical fetal membranes. American Journal of Obstetrics and Gynecology. 2007;196:484.e1–484.e10. doi: 10.1016/j.ajog.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 20.Kumar D, Fung W, Moore RM, Pandey V, Fox J, Stetzer B, Mansour JM, Mercer BM, Redline RW, Moore JJ. Proinflammartory Cytokines Found in Amniotic Fluid Induce Collagen Remodeling, Apoptosis, and Biophysical Weakening of Cultured Human Fetal Membranes. Biology of Reproduction. 2006;74:29–34. doi: 10.1095/biolreprod.105.045328. [DOI] [PubMed] [Google Scholar]
- 21.Artal RSR, Neuman M, Burstein AH, Stojkov J. The mechanical properties of prematurely and non--prematurely ruptured membranes. Am J Obstet Gynecol. 1976;125(5):655–9. doi: 10.1016/0002-9378(76)90788-2. [DOI] [PubMed] [Google Scholar]
- 22.Artal RBR, Hobel CJ, Hollister D. An in vitro model for the study of enzymatically mediated biomechanical changes in the chorioamniotic membranes. Am J Obstet Gynecol. 1979;133:656–9. doi: 10.1016/0002-9378(79)90014-0. [DOI] [PubMed] [Google Scholar]
- 23.Oxlund H, Helmig R, Halaburt JT, Uldbjerg N. Biomechanical analysis of human chorioamniotic membranes. Eur J Obstet Gynecol Reprod Biol. 1990;34(3):247–55. doi: 10.1016/0028-2243(90)90078-f. [DOI] [PubMed] [Google Scholar]
- 24.Helmig ROH, Petersen LK, Uldbjerg N. Different biomechanical properties of human fetal membranes obtained before and after delivery. Eur J Obstet Gynecol Reprod Biol. 1993;48(3):183–9. doi: 10.1016/0028-2243(93)90086-r. [DOI] [PubMed] [Google Scholar]
- 25.Polishuk WZKS, Peranio A. The physical properties of fetal membranes. Obstet Gynecol. 1962;20:2004–10. [PubMed] [Google Scholar]
- 26.Parry-Jones EPS. A study of the elasticity and tension of fetal membranes and of the relation of the area of the gestational sac to the area of the uterine cavity. Br J Obstet Gynaecol. 1976;83(3):205–12. doi: 10.1111/j.1471-0528.1976.tb00810.x. [DOI] [PubMed] [Google Scholar]
- 27.Al-Zaid NSB-RM, Goldspink G. Bursting pressure and collagen content of fetal membranes and their relation to premature rupture of the membranes. Br J Obstet Gynaecol. 1980;87(3):227–9. doi: 10.1111/j.1471-0528.1980.tb04524.x. [DOI] [PubMed] [Google Scholar]
- 28.Lavery JPMC. The viscoelastic nature of chorioamniotic membranes. Obstet Gynecol. 1977;50(4):467–72. [PubMed] [Google Scholar]
- 29.Lavery JPMC. Deformation and creep in the human chorioamniotic sac. Am J Obstet Gynecol. 1979;134(4):366–75. doi: 10.1016/s0002-9378(16)33077-0. [DOI] [PubMed] [Google Scholar]
- 30.Lavery JPMC, Knight RD. The effect of labor on the rheologic response of chorioamniotic membranes. Obstet Gynecol. 1982;60(1):87–92. [PubMed] [Google Scholar]
- 31.Oyen ML, Cook RF, Stylianopoulos T, Barocas VH, Calvin SE, Landers DV. Uniaxial and biaxial mechanical behaviour of human amnion. Journal of Materials Research. 2005;20(11):2902–2909. [Google Scholar]
- 32.Fung YC. Biomechanics: Mechanical Properties of Living Tissues. 2. New York: Springer Verlag; 1993. p. 568. [Google Scholar]
- 33.Calvin SE, Oyen ML. Microstructure and Mechanics of the Chorioamnion Membrane with an Emphasis on Fractrue Properties. Ann NY Acad Sci. 2007;1101:166–185. doi: 10.1196/annals.1389.009. [DOI] [PubMed] [Google Scholar]
- 34.Schober EA, Kusy RP, Whitley JQ, Savitz DA. Effect of thickness on the fracture characteristics of fetal membranes. Journal of Materials Science: Materials in Medicine. 1994;5:130–137. [Google Scholar]
- 35.Schober EA, Kusy RP, Savitz DA. Resistance of fetal membranes to concentrated force applications and reconciliation of puncture and burst testing. Ann Biomed Eng. 1994;22(5):540–8. doi: 10.1007/BF02367090. [DOI] [PubMed] [Google Scholar]
- 36.Arikat S, Novince RW, Mercer BM, Kumar D, Fox JM, Mansour JM, Moore JM. Separation of amnion from choriodecidua is an integral event to the rupture of normal term fetal membranes and constitutes a significant component of the work required. American Journal of Obstetrics and Gynecology. 2006;194(1):211–7. doi: 10.1016/j.ajog.2005.06.083. [DOI] [PubMed] [Google Scholar]
- 37.Nimni ME. Biochemistry and Biomechanics. In: Nimni ME, editor. Collagen Volume II Biochemistry and Biomechanics. CRC Press; Boca Raton, FL: 1988. [Google Scholar]
- 38.Ellsmere JC, Khanna RA, Lee JM. Mechanical loading of bovine pericardium accelerates enzymatic degradation. Biomaterials. 1999;20:1143–1150. doi: 10.1016/s0142-9612(99)00013-7. [DOI] [PubMed] [Google Scholar]
- 39.Coulson WF. THE EFFECT OF PROTEOLYTIC ENZYMES ON THE TENSILE STRENGTH OF WHOLE AORTA AND ISOLATED AORTIC ELASTIN. BIOCHIMICA ET BIOPHYSICA ACTA. 1971;237:378–386. doi: 10.1016/0304-4165(71)90334-5. [DOI] [PubMed] [Google Scholar]
- 40.Huang C, I, Yannas V. Mechanochemical Studies of Enzymatic Degradation of Insoluble Collagen Fibers. Journal of Biomedical Material Research Symposium. 1977;8:137–154. doi: 10.1002/jbm.820110113. [DOI] [PubMed] [Google Scholar]
- 41.Freytes DO, Rundell AE, Vande Geest J, Vorp DA, Webster TJ, Badylak SF. Analytically derived material properties of multilaminated extracellualr matrix devices using the ball-burst test. Biomaterial. 2005;26:5518–5531. doi: 10.1016/j.biomaterials.2005.01.070. [DOI] [PubMed] [Google Scholar]
- 42.Sacks MS. Biaxial mechanical evaluation of planar biological materials. Journal of Elasticity. 2000;61:199–246. [Google Scholar]
- 43.Cunningahm FG, McDonald PC, Leveno KS, Gant NF, Gilstrap J. In: William’s Obstetrics. Lang Aa., editor. Norwalk: Conneticut; 1993. [Google Scholar]
- 44.Kubik-Huch RA, Wildermuth S, Gettuzzi L, Rake A, Siefert B, Chaoui R, Marincek B. Fetus and Uteroplacental Unit: Fast MR Imaging with Three-dimensional Reconstructuion and Volumetry - Feasibility Study. Radiology. 2001;219(2):567–573. doi: 10.1148/radiology.219.2.r01ma24567. [DOI] [PubMed] [Google Scholar]
- 45.Reece IJ, van Noort R, Martin TR, Black MM. The physical properties of bovine pericardium: a study of the effects of stretching during chemical treatment in glutaraldehyde. Ann Thorac Surg. 1982;33(5):480–5. doi: 10.1016/s0003-4975(10)60789-8. [DOI] [PubMed] [Google Scholar]
- 46.Crofts CE, Trowbridge EA. The tensile strength of natural and chemically modified bovine pericardium. Journal of Biomedical Materials Research. 1988;22:89–98. doi: 10.1002/jbm.820220202. [DOI] [PubMed] [Google Scholar]
- 47.Zioupos P, Barbenel JC, Fisher J. Mechanical and Optical Anisotropy of Bovine Pericardium. Medical and Biological Engineering and Computing. 1992;30:76–82. doi: 10.1007/BF02446197. [DOI] [PubMed] [Google Scholar]
- 48.Sacks MS, Mirnajafi A, Sun W, Schmidt P. Bioprosthetic heart valve heterograft biomaterials: structure, mechanical behavior and computational simulation. Expert Rev Med Devices. 2006;3(6):817–34. doi: 10.1586/17434440.3.6.817. [DOI] [PubMed] [Google Scholar]
- 49.Thorton GM, Schwab TD, Oxlan TR. Cyclic loading causes faster rupture and strain rate than static loading in medial collateral ligament at high stress. Clinical Biomechanics. 2007;22:932–940. doi: 10.1016/j.clinbiomech.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 50.Bartel DL, Davy DT, Keaveny TM. Orthopaedic Biomechanics Mechanics and Design in Musculoskeletal Systems. Upper Saddle River: Pearson Education, Inc; 2006. p. 371. [Google Scholar]
- 51.Sacks MS, Smith DB, Hiester ED. A small angle light scattering device for planar connective tissue microstructural analysis. Ann Biomed Eng. 1997;25(4):678–89. doi: 10.1007/BF02684845. [DOI] [PubMed] [Google Scholar]
- 52.Sacks MS. Small-angle light scattering methods for soft connective tissue structural analysis. Encyclopedia of Biomaterials and Biomedical Engineering. 2004 [Google Scholar]
- 53.MacLachlan TB. A method for the investigation of the strength of hte fetal membranes. American Journal of Obstetrics and Gynecology. 1965;91:309–313. doi: 10.1016/0002-9378(65)90243-7. [DOI] [PubMed] [Google Scholar]