Abstract
The Women’s Health Initiative trials – in which more extreme adverse outcomes were observed in the medroxyprogesterone acetate (MPA) + conjugated equine estrogen (CEE) arm, as compared to the CEE only arm – suggest that the addition of MPA to estrogen treatment has undesirable consequences. An important question raised by these results is whether the adverse outcomes observed in the progestin arm can be attributed to effects that are unique to MPA or are common to all progestins. In this study we explored the potential for MPA and progesterone (P4) to differentially impact neuroendocrine function by comparing their effects on mRNA expression for the α4 subunit of GABAA receptors in the CA1 hippocampus of female rats. Prior research has shown that P4, acting through its reduced metabolite allopregnanolone (AP), can mediate α4 subunit expression, thereby altering GABAA receptor gated currents. By contrast, MPA competitively inhibits the enzymes necessary for the synthesis of AP. In this study, ovariectomized females were primed with estradiol benzoate and then treated with P4, MPA, or vehicle. Subjects were sacrificed 12 h or 24 h later and in situ hybridization was used to measure α4 mRNA in the CA1 hippocampus. At 12 h but not 24 h, α4 mRNA was reduced in the P4 group as compared to the MPA group, and as compared to the vehicle group. These results suggest that MPA, while progestational in terms of its effects in the uterus, is not a simple substitute for P4 in other systems. The relative impact of these two progestins on neuroendocrine function must be carefully explored.
Keywords: allopregnanolone, neurosteroids, GABAA receptors
1. Introduction
Medroxyprogesterone acetate (MPA) has been one of the most common progestin components of post menopausal hormone therapy in the United States [1]. As a progestin, MPA is used to protect against endometrial hyperplasia during estrogen therapy [2,3]. However, in the recently terminated Women’s Health Initiative (WHI) trials the more extreme adverse consequences of MPA + conjugated equine estrogen (CEE), as compared to CEE alone [4,5], suggest that the addition of MPA to estrogen treatment also has undesirable consequences. An important question raised by the findings of the WHI trials is whether MPA induces undesirable effects that are unique to this compound, or whether undesirable outcomes may be observed with the addition of any progestin to postmenopausal hormone therapy.
With the advent of micronized progesterone (P4) as an alternative to synthetic progestins [6], an increasing number of studies have begun to directly compare the effects of MPA and P4. Several of these studies not only suggest that poorer outcomes might be expected with MPA [but see 7,8,9], but they also have pointed to potential cellular and molecular mechanisms through which MPA may exert more extreme effects relative to P4 on breast cancer [10–12], coronary heart disease [13,14], and cognitive decline or Alzheimer’s disease [15].
Despite accumulating research comparing the effects of MPA and P4 on several measures with long-term health consequences, hormone therapy currently is prescribed most frequently for short-term control of symptoms such as hot flushes, vaginal dryness, urinary symptoms, and sleep disturbances. Nonetheless, even with short-term treatment, some research suggests that MPA may be associated with increased anxiety and irritability [16–18]. One mechanism through which MPA could have this effect is through its impact on allopregnanolone (AP), a reduced metabolite of P4 that acts as a potent positive modulator of GABAA receptors [19]. Although P4 readily is reduced to AP, MPA competitively inhibits the enzymes needed for this conversion [20–22], and in one experiment, serum and hippocampal AP levels rose after P4 treatment, but failed to show a similar increase with MPA [23]. Given that acute application of AP – either systemically [24] or directly to the CA1 hippocampus [25] – is anxiolytic, MPA may impact anxiety by reducing the availability of AP. However, fluctuations in AP following more prolonged exposure (48–72 h) also lead to changes in GABAA receptor subunit composition that have important implications for the regulation of anxiety [26–32]. Such quantitative changes in the subunit structure of GABAA receptors may serve as a good marker of potential differences between MPA and P4 in their impact on neuroendocrine function.
In this study, we tested the hypothesis that MPA and P4 may have different effects on neuroendocrine function by measuring mRNA expression for the α4 subunit of GABAA receptors in the CA1 hippocampus of female rats. Prior research has shown that changes in long-term AP exposure increase α4 subunit expression and that this change is accompanied by a rise in anxiety [29–32]. Moreover, it has been shown that α4 mRNA expression in this brain region corresponds to protein levels [31]. Therefore, determining whether MPA and P4 differentially affect α4 mRNA expression in the CA1 hippocampus is an important first step for identifying a potential mechanism through which these progestins could diverge in their impact on emotion.
2. Materials and methods
2.1. Animal care and treatment
Forty adult female Long-Evans rats weighing approximately 200–224 g were obtained from Harlan Laboratories, Indianapolis, IN. These rats were estimated to be 3 months at the start of this study, which is the age of subjects previously used by other laboratories to model the effects of hormone therapy in surgically postmenopausal animals [8,9]. Subjects were ovariectomized prior to shipment and were pair-housed on a reverse 12:12 light/dark cycle with ad libitum access to food and water. All animal care, maintenance, and treatment were conducted in accordance with the National Institutes of Health and United States Department of Agriculture guidelines, and were approved by Emory University’s Institutional Animal Care and Use Committee.
Hormone treatments began after a minimum 11-day acclimation period. Females were injected with estradiol benzoate (Product E-8518, Sigma-Aldrich, St. Louis, MO, 4 μg in 0.1 ml sesame oil, sc) followed 44 hours later by treatment with MPA (Product M-1629, Sigma-Aldrich, St. Louis, MO, 615 μg in 0.2 ml DMSO, sc), P4 (Product P-3972, Sigma-Aldrich, St. Louis, MO, 500 μg in 0.2 ml DMSO, sc), or vehicle (0.2 ml DMSO). All subjects were sacrificed by CO2 asphyxiation followed by decapitation at either 12 h (MPA: N = 7; P4: N = 7; vehicle: N = 6) or 24 h post progestin/vehicle injection (MPA: N = 7; P4: N = 6; vehicle: N = 7). The MPA and P4 doses were chosen as the molar equivalents. All brains were immediately frozen on dry ice and stored at −80°C until they were sliced into 20 μm sections on a cryostat and thaw-mounted on Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA).
2.2. In situ hybridization
A step-by-step description of the in situ hybridization procedure followed has been published previously [33]. Briefly, all slides were air-dried, fixed in 4% paraformaldehyde (4°C) for 5 min, and then rinsed in 0.1 M phosphate buffered saline (pH 7.4; 4 °C) for 2 min. Next after one rinse in 1.5% triethanolamine (TEA), slides were treated in 0.25% acetic anhydride in TEA (pH 8.0) for 10 min, and then rinsed twice with 0.03 M Na citrate in 0.3 M NaCl (2×SSC), pH 7.0. Slides were subsequently defatted by incubating them for 2 min each in 70%, 95%, and 100% ethanol, followed by 5 min in chloroform and 2 min each in 100% and 95% ethanol. At the end of this defatting sequence, the slides were air-dried and 100 μl of hybridization solution was applied to each slide, after which they were covered with parafilm and placed in a humidified incubator for 14 h at 37 °C.
The hybridization solution contained 50% formamide, 4×SSC, 1×Denhardt’s solution, 2.5 mg/ml yeast tRNA, 10% dextran sulphate (MW=50 000), 0.3 M NaCl, 10 mM Tris, 10 mM dithioxytocinhreitol (DTT), and a 45 base pair oligodeoxyribonucleoxytocinide probe for the α4 subunit (CAA GTC GCC AGG CAC AGG ACG TGC AGG AGG GCG AGG CTG ACC CCG). This probe is the reverse complement of nucleotides 133–177 of the rat α4 subunit mRNA (GenBank Accession Number L08493) [34], and was custom ordered from Invitrogen Life Technologies (Grand Island, NY). Probes were labeled at the 3′-end with 35S-dATP (Perkin Elmer, Waltham, Massachusetts, MA) using terminal deoxynucleoxytocinidyl transferase to a specific activity of 1×106/pmol, purified and mixed with the hybridization buffer at a concentration around 4 pmol/ml.
After 14 h incubation at 37 °C, the parafilm was removed. Slides were washed four times in SSC at 55 °C for 15 min each, and then once in SSC at room temperature for 60 min. Next they were dehydrated in a series of graded concentrations of ethanol. Upon completion, the rinsed and dried sections were exposed for 7 days to Kodak BioMax MR film (Eastman Kodak, Rochester, NY, USA) with 14C-labeled autoradiographic standards (Amersham Pharmacia Biotech, Arlington Heights, IL).
2.3. Image analysis
Image analysis was conducted on a PC using the AIS imaging software (Imaging Research Inc., St. Catharines, ON, Canada, version 6.0 for Windows). mRNA expression of the α4 subunit was measured bilaterally in four adjacent sections containing hippocampal CA1 neurons from Bregma −3.14 mm to −3.38 mm [35]. For each of the four sections, mRNA expression was also measured in the medial habenular nucleus, a region with low levels of α4 subunit expression [36], to control for background expression. Optical densities were converted to nCi/gm tissue equivalents using the 14C standards (Amersham Pharmacia Biotech, Arlington Heights, IL).
2.4. Data analysis
For each subject we calculated an average mRNA level for the CA1 hippocampus across the four sections in which expression was measured. Following established methods [37,38], differences in α4 subunit mRNA expression were then examined with analysis of covariance (ANCOVA) models. Progestin condition and time from treatment were entered as fixed factors in the analysis; background levels of mRNA expression from the medial habenular nucleus, again based on the average across the four sections measured for each subject, were entered as a covariate to control for confounding effects. We initially conducted a two-way ANCOVA, but because of a significant time by treatment interaction, we subsequently used two, one-way ANCOVAs to evaluate differences across progestin treatments at 12 h and 24 h. Post-hoc analyses were conducted with the Least Significant Difference test. Statistical analyses were performed using SPSS software (version 14.0 for Windows), and tests having a P ≤ 0.05 were considered significant.
Because probability estimates do not allow comparisons of the magnitude of effect between groups or studies, we calculated Cohen’s d as an indicator of effect size [39]. Cohen’s d is a measure of the magnitude of a difference between means expressed as standard deviation units and is calculated as the difference between two means divided by the average standard deviation of those means. By convention, d < 0.2 indicates a small effect, d = 0.2–0.8 indicates a moderate effect, and d > 0.8 indicates a large effect. Effect size provides an important complement to traditional probability testing because, unlike probability estimation, effect size is insensitive to sample size and offers magnitude estimates in standardized units that can be compared across groups or studies.
3. Results
The two-way ANCOVA indicated that α4 subunit mRNA expression varied significantly across treatment groups (F = 21.9, P < 0.001), with time (F = 20.9, P < 0.001), progestin condition (F = 4.24, P = 0.02), and background expression from the medial habenular nucleus (F = 13.0, P = 0.001). Because the time by treatment interaction was significant (F = 3.84, P = 0.03), we analyzed the effects of progestin treatment separately at 12 h and at 24 h.
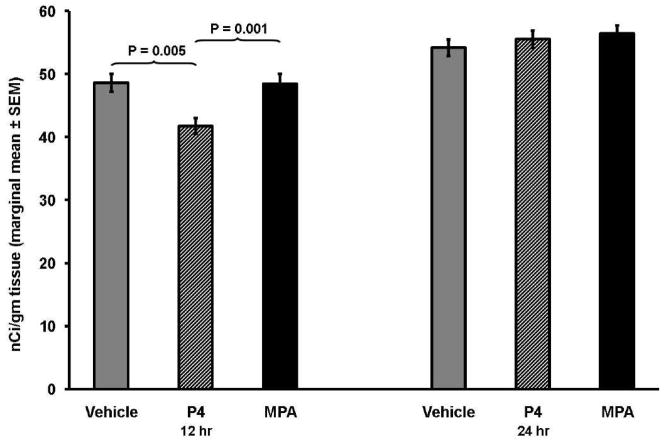
At 12 h, progestin treatment had a significant effect on α4 subunit mRNA expression (F = 9.53, P = 0.002; Fig. 1). Post-hoc analyses for this time point revealed that subjects receiving P4 had lower levels of mRNA expression in comparison to subjects receiving MPA (P = 0.001; Fig. 2) and in comparison to subject receiving vehicle (P = 0.005). Treatment with P4 had a very large effect, both in comparison to MPA (d = 2.17) or vehicle treatment (d = 2.04). By contrast, MPA treatment did not differ from vehicle treatment (P = 0.95), and there was virtually no effect difference between MPA and vehicle treatment (d = 0.04).
Fig. 1.
Levels of α4 mRNA expression in hippocampal CA1 neurons at 12 h and at 24 h among subjects treated with vehicle, P4 or MPA. Results of the ANCOVA at 12 h indicate there was a significant difference in receptor sub-unit expression across treatment conditions (P = 0.002), but there was no significant difference at 24 h (P = 0.482).
Fig. 2.
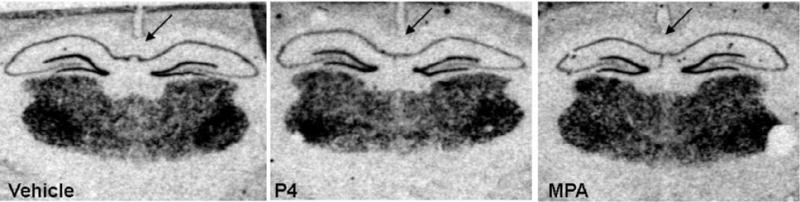
Film radiograms illustrating mRNA expression of the α4 subunit in hippocampal CA1 neurons at 12 h following treatment with vehicle, P4 or MPA. Subjects treated with P4 showed lower levels of mRNA expression in comparison to subjects treated with vehicle (P = 0.005), and especially in comparison to subjects treated with MPA (P = 0.001).
By 24h, the differential effect of P4 and MPA on α4 subunit mRNA expression had disappeared, as there was no any longer difference between progestins (F = 0.76, P = 0.48).
4. Discussion
In this study, P4 down-regulated mRNA expression for the α4 subunit of GABAA receptors in the CA1 hippocampus of female rats. MPA failed to have a similar effect. This difference was observed at the 12 h time point, but was no longer apparent at 24 h. Because changes in α4 subunit expression in the CA1 hippocampus have important implications for the regulation of anxiety [26–32], and prior research has shown that α4 mRNA expression in this brain region corresponds to protein levels [31], measurement of α4 subunit mRNA can provide a useful tool for future research evaluating how the different effects of MPA and P4 on AP metabolism may impact neuroendocrine function.
While this study suggests that MPA and P4 have different effects on α4 subunit expression, our current results apply to a single time-point. Our findings at this time are important, nonetheless, given that previous research has shown that AP modulation of α4 subunit expression is dynamic [39]. Notably, although it clearly has been demonstrated that withdrawal from long-term exposure to AP up-regulates α4 subunit expression in the CA1 hippocampus [31,32], prior research also has shown that the onset of long-term P4 exposure induces a transient rise in α4 subunit expression; this transient rise is not yet present at 24 h, but it peaks at 48–72 h, after which time α4 subunit expression returns to base-line levels [29]. Our study adds a more acute time point to this dynamic change that occurs with long-term P4 treatment and suggests that there may be an initial phase during which P4 down-regulates α4 subunit expression, which then rises over the next 60 h and eventually declines to baseline levels.
In addition to evaluating the relative effect of MPA and P4 at additional time-points, the functional implications of the difference we found need to be further explored. The α4 subunit of GABAA receptors may coexpress with either γ2 or δ subunits [40]. While the relative insensitivity of α4 containing receptors to benzodiazepines [36,41] suggests that this subunit may underlie anxiety states [39], substitution of the γ2 subunit with the δ subunit yields receptors that are highly sensitive to neurosteroids [42,43]. Moreover, changes in long-term exposure to AP have been shown to increase expression of α4βδ receptors in the CA1 hippocampus [26,27,44]. Nonetheless, while AP potentiates inward current at α4βδ receptors, it induces rapid desensitatization of outward current (inward Cl− flux) [26]. Hence, although AP may enhance inhibition in the dentate gyrus and cortex, where GABAergic current is inward [45,46] and α4βδ expression is normally high [36], it may reduce inhibition in the CA1 hippocampus, where GABAergic currents are outward [47] and α4βδ expression is normally low [36]. Thus, while acute application of AP typically is anxiolytic [25], with increases in the expression of α4βδ receptors induced through fluctuations in long-term exposure, AP may paradoxically be anxiogenic [26,30]. Hence determining whether the different effects of MPA and P4 on α4 subunit expression are accompanied by changes in the δ subunit will be important for determining the effects that might be expected with further hormonal perturbations.
In conclusion, in this study we provide evidence that MPA and P4 have different effects on mRNA expression for the α4 subunit of GABAA receptors in the CA1 hippocampus of female rats at 12 h but not 24 h post-treatment. Effects of more prolonged treatment need to be evaluated to provide a more complete time-course of progestin action on α4 subunit expression. In addition evaluating whether differences in α4 expression are accompanied by changes in δ subunit expression will be important for determining the ultimate functional consequences of these differences. Nonetheless, what is clear from this study is that MPA, while progestational in terms of its effects in the uterus, is not a simple substitute for P4 in other systems. Thus differences between these two progestins in their effect on neuroendocrine function must be carefully explored.
Acknowledgments
This research was supported by in part by the Center for Behavioral Neuroscience, an STC Program of the National Science Foundation, Agreement No IBN-9876754, and by the National Institute of Health grants RR00165, HD38917 and HD044161.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291:47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- 2.Sitruk-Ware R. Progestins in hormonal replacement therapy and prevention of endometrial disease. In: Sitruk-Ware R, Mischell DR Jr, editors. Progestins and Antiprogestins in Clincial Practice. New York: Marcel Dekker, Inc; 2000. pp. 269–77. [Google Scholar]
- 3.The Writing Group for the PEPI Trial. Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA. 1996;275:370–5. doi: 10.1001/jama.1996.03530290040035. [DOI] [PubMed] [Google Scholar]
- 4.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O’Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–12. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 5.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 6.Boothby LA, Doering PL, Kipersztok S. Bioidentical hormone therapy: a review. Menopause. 2004;11:356–67. doi: 10.1097/01.gme.0000094356.92081.ef. [DOI] [PubMed] [Google Scholar]
- 7.Koh KK, Jin DK, Yang SH, Lee SK, Hwang HY, Kang MH, Kim W, Kim DS, Choi IS, Shin EK. Vascular effects of synthetic or natural progestagen combined with conjugated equine estrogen in healthy postmenopausal women. Circulation. 2001;103:1961–6. doi: 10.1161/01.cir.103.15.1961. [DOI] [PubMed] [Google Scholar]
- 8.McNeill AM, Zhang C, Stanczyk FZ, Duckles SP, Krause DN. Estrogen increases endothelial nitric oxide synthase via estrogen receptors in rat cerebral blood vessels: effect preserved after concurrent treatment with medroxyprogesterone acetate or progesterone. Stroke. 2002;33:1685–91. doi: 10.1161/01.str.0000016325.54374.93. [DOI] [PubMed] [Google Scholar]
- 9.Sunday L, Tran MM, Krause DN, Duckles SP. Estrogen and progestagens differentially modulate vascular proinflammatory factors. Am J Physiol Endocrinol Metab. 2006;291:E261–7. doi: 10.1152/ajpendo.00550.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franke HR, Vermes I. Differential effects of progestogens on breast cancer cell lines. Maturitas. 2003;46 (Suppl 1):S55–8. doi: 10.1016/j.maturitas.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 11.Wood CE, Register TC, Lees CJ, Chen H, Kimrey S, Cline JM. Effects of estradiol with micronized progesterone or medroxyprogesterone acetate on risk markers for breast cancer in postmenopausal monkeys. Breast Cancer Res Treat. 2007;101:125–34. doi: 10.1007/s10549-006-9276-y. [DOI] [PubMed] [Google Scholar]
- 12.Seeger H, Wallwiener D, Mueck AO. The effect of progesterone and synthetic progestins on serum- and estradiol-stimulated proliferation of human breast cancer cells. Horm and Metab Res. 2003:76–80. doi: 10.1055/s-2003-39061. [DOI] [PubMed] [Google Scholar]
- 13.Simoncini T, Mannella P, Fornari L, Caruso A, Willis MY, Garibaldi S, Baldacci C, Genazzani AR. Differential signal transduction of progesterone and medroxyprogesterone acetate in human endothelial cells. Endocrinology. 2004;145:5745–56. doi: 10.1210/en.2004-0510. [DOI] [PubMed] [Google Scholar]
- 14.Otsuki M, Saito H, Xu X, Sumitani S, Kouhara H, Kishimoto T, Kasayama S. Progesterone, but not medroxyprogesterone, inhibits vascular cell adhesion molecule-1 expression in human vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2001;21:243–8. doi: 10.1161/01.atv.21.2.243. [DOI] [PubMed] [Google Scholar]
- 15.Nilsen J, Brinton RD. Divergent impact of progesterone and medroxyprogesterone acetate (Provera) on nuclear mitogen-activated protein kinase signaling. Proc Natl Acad Sci U S A. 2003;100:10506–11. doi: 10.1073/pnas.1334098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bjorn I, Bixo M, Nojd KS, Collberg P, Nyberg S, Sundstrom-Poromaa I, Backstrom T. The impact of different doses of medroxyprogesterone acetate on mood symptoms in sequential hormonal therapy. Gynecol Endocrinol. 2002;16:1–8. [PubMed] [Google Scholar]
- 17.Bjorn I, Bixo M, Nojd KS, Nyberg S, Backstrom T. Negative mood changes during hormone replacement therapy: a comparison between two progestogens. Am J Obstet Gynecol. 2000;183:1419–26. doi: 10.1067/mob.2000.107781. [DOI] [PubMed] [Google Scholar]
- 18.Sherwin BB. The impact of different doses of estrogen and progestin on mood and sexual behavior in postmenopausal women. J Clin Endocrinol Metab. 1991;72:336–43. doi: 10.1210/jcem-72-2-336. [DOI] [PubMed] [Google Scholar]
- 19.Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–7. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- 20.Jarrell J. Studies on the developmental pattern of rat ovarian 3α-hydroxysteroid dehydrogenase: inhibition of the postpubertal activity with medroxyprogesterone acetate in vivo. J Steroid Biochem. 1984;21:151–6. doi: 10.1016/0022-4731(84)90376-5. [DOI] [PubMed] [Google Scholar]
- 21.Lee TC, Miller WL, Auchus RJ. Medroxyprogesterone acetate and dexamethasone are competitive inhibitors of different human steroidogenic enzymes. J Clin Endocrinol Metab. 1999;84:2104–10. doi: 10.1210/jcem.84.6.5646. [DOI] [PubMed] [Google Scholar]
- 22.Penning TM, Sharp RB, Krieger NR. Purification and properties of 3α-hydroxysteroid dehydrogenase from rat brain cytosol. Inhibition by nonsteroidal anti-inflammatory drugs and progestins. J Biol Chem. 1985;260:15266–72. [PubMed] [Google Scholar]
- 23.Bernardi F, Pluchino N, Pieri M, Begliuomini S, Lenzi E, Puccetti S, Casarosa E, Luisi M, Genazzani AR. Progesterone and medroxyprogesterone acetate effects on central and peripheral allopregnanolone and beta-endorphin levels. Neuroendocrinology. 2006;83:348–59. doi: 10.1159/000095400. [DOI] [PubMed] [Google Scholar]
- 24.Bitran D, Shiekh M, McLeod M. Anxiolytic effect of progesterone is mediated by the neurosteroid allopregnanolone at brain GABAA receptors. J Neuroendocrinol. 1995;7:171–7. doi: 10.1111/j.1365-2826.1995.tb00744.x. [DOI] [PubMed] [Google Scholar]
- 25.Bitran D, Dugan M, Renda P, Ellis R, Foley M. Anxiolytic effects of the neuroactive steroid pregnanolone (3α-OH-5β-pregnan-20-one) after microinjection in the dorsal hippocampus and lateral septum. Brain Res. 1999;850:217–24. doi: 10.1016/s0006-8993(99)02150-2. [DOI] [PubMed] [Google Scholar]
- 26.Shen H, Gong QH, Aoki C, Yuan M, Ruderman Y, Dattilo M, Williams K, Smith SS. Reversal of neurosteroid effects at α4β2δ GABAA receptors triggers anxiety at puberty. Nat Neurosci. 2007;10:469–77. doi: 10.1038/nn1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen H, Gong QH, Yuan M, Smith SS. Short-term steroid treatment increases δ GABAA receptor subunit expression in rat CA1 hippocampus: pharmacological and behavioral effects. Neuropharmacology. 2005;49:573–86. doi: 10.1016/j.neuropharm.2005.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gulinello M, Smith SS. Anxiogenic effects of neurosteroid exposure: sex differences and altered GABAA receptor pharmacology in adult rats. J Pharmacol Exp Ther. 2003;305:541–8. doi: 10.1124/jpet.102.045120. [DOI] [PubMed] [Google Scholar]
- 29.Gulinello M, Gong QH, Li X, Smith SS. Short-term exposure to a neuroactive steroid increases α4 GABAA receptor subunit levels in association with increased anxiety in the female rat. Brain Res. 2001;910:55–66. doi: 10.1016/s0006-8993(01)02565-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith SS, Ruderman Y, Frye C, Homanics G, Yuan M. Steroid withdrawal in the mouse results in anxiogenic effects of 3α, 5β-THP: a possible model of premenstrual dysphoric disorder. Psychopharmacology. 2006;186:323–33. doi: 10.1007/s00213-005-0168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith SS, Gong QH, Hsu FC, Markowitz RS, ffrench-Mullen JM, Li X. GABAA receptor α4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998;392:926–30. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- 32.Smith SS, Gong QH, Li X, Moran MH, Bitran D, Frye CA, Hsu FC. Withdrawal from 3α-OH-5α-pregnan-20-One using a pseudopregnancy model alters the kinetics of hippocampal GABAA-gated current and increases the GABAA receptor α4 subunit in association with increased anxiety. J Neurosci. 1998;18:5275–84. doi: 10.1523/JNEUROSCI.18-14-05275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang ZX, Liu Y, Young LJ, Insel TR. Hypothalamic vasopressin gene expression increases in both males and females postpartum in a biparental rodent. J Neuroendocrinol. 2000;12:111–20. doi: 10.1046/j.1365-2826.2000.00435.x. [DOI] [PubMed] [Google Scholar]
- 34.Lauren HB, Pitkanen A, Nissinen J, Soini SL, Korpi ER, Holopainen IE. Selective changes in gamma-aminobutyric acid type A receptor subunits in the hippocampus in spontaneously seizing rats with chronic temporal lobe epilepsy. Neurosci Lett. 2003;349:58–62. doi: 10.1016/s0304-3940(03)00735-3. [DOI] [PubMed] [Google Scholar]
- 35.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. San Diego: Academic Press; 1988. [Google Scholar]
- 36.Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–62. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castensson A, Emilsson L, Preece P, Jazin EE. High-resolution quantification of specific mRNA levels in human brain autopsies and biopsies. Genome Res. 2000;10:1219–29. doi: 10.1101/gr.10.8.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lott DC, Kim SJ, Cook EH, Jr, de Wit H. Dopamine transporter gene associated with diminished subjective response to amphetamine. Neuropsychopharmacology. 2005;30:602–9. doi: 10.1038/sj.npp.1300637. [DOI] [PubMed] [Google Scholar]
- 39.Smith SS, Shen H, Gong QH, Zhou X. Neurosteroid regulation of GABAA receptors: focus on the α4 and δ subunits. Pharmacol Ther. 2007;116:58–76. doi: 10.1016/j.pharmthera.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sur C, Farrar SJ, Kerby J, Whiting PJ, Atack JR, McKernan RM. Preferential coassembly of α4 and δ subunits of the GABAA receptor in rat thalamus. Mol Pharmacol. 1999;56:110–5. doi: 10.1124/mol.56.1.110. [DOI] [PubMed] [Google Scholar]
- 41.Wafford KA, Thompson SA, Thomas D, Sikela J, Wilcox AS, Whiting PJ. Functional characterization of human GABAA receptors containing the α4 subunit. Mol Pharmacol. 1996;50:670–8. [PubMed] [Google Scholar]
- 42.Belelli D, Casula A, Ling A, Lambert JJ. The influence of subunit composition on the interaction of neurosteroids with GABAA receptors. Neuropharmacology. 2002;43:651–61. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- 43.Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABAA receptors containing the δ subunit. J Neurosci. 2002;22:1541–49. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sundstrom Poromaa I, Smith S, Gulinello M. GABA receptors, progesterone and premenstrual dysphoric disorder. Arch Womens Ment Health. 2003;6:23–41. doi: 10.1007/s00737-002-0147-1. [DOI] [PubMed] [Google Scholar]
- 45.Gulledge AT, Stuart GJ. Excitatory actions of GABA in the cortex. Neuron. 2003;37:299–309. doi: 10.1016/s0896-6273(02)01146-7. [DOI] [PubMed] [Google Scholar]
- 46.Staley KJ, Mody I. Shunting of excitatory input to dentate gyrus granule cells by a depolarizing GABAA receptor-mediated postsynaptic conductance. J Neurophysiol. 1992;68:197–212. doi: 10.1152/jn.1992.68.1.197. [DOI] [PubMed] [Google Scholar]
- 47.Staley KJ, Proctor WR. Modulation of mammalian dendritic GABAA receptor function by the kinetics of Cl− and HCO3− transport. J Physiol. 1999;519:693–712. doi: 10.1111/j.1469-7793.1999.0693n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]