Abstract
Malaria transmission occurs by intradermal deposition of Plasmodium sporozoites during the infectious bite of a female Anopheles mosquito. After formation in midgut-associated oocysts sporozoites actively enter mosquito salivary glands and subsequently invade host hepatocytes where they transform into clinically silent liver stages. To date, two sporozoite-specific transmembrane proteins have been identified that perform vital functions in natural malaria transmission. The sporozoite invasin TRAP drives sporozoite motility and target cell entry whereas the adhesin MAEBL mediates sporozoite recognition of and attachment to salivary glands. Here, we demonstrate that the sporozoite-specific transmembrane protein S6 is required for efficient malaria transmission to the vertebrate host. Targeted deletion of S6 results in severe impairment of sporozoite gliding motility and invasion of mosquito salivary glands. During sporozoite maturation S6 expression is tightly regulated by transcriptional and translational control. We propose that S6 functions together with TRAP/MIC2 family invasins to direct fast, efficient and specific cell entry and, ultimately, life cycle progression of the malaria sporozoite.
Introduction
Malaria remains the most important vector-borne infectious disease worldwide. It is caused by unicellular Plasmodium parasites that have the exceptional capacity to invade and develop within host erythrocytes. Malarial parasites are transmitted during the bloodmeal of an infected female Anopheles mosquito (Vanderberg and Frevert, 2004; Amino et al., 2006; Yamauchi et al., 2007). The contagious Plasmodium forms, sporozoites, are highly motile and actively enter the blood circulation in order to reach the liver where they undergo a dramatic transition and expansion phase. This pre-erythrocytic schizogony is clinically silent and results in the generation of thousands of pathogenic merozoites from a single sporozoite (Prudêncio et al., 2006). Plasmodium liver stage development compensates for the low numbers of transmitted sporozoites, a major bottleneck of the Plasmodium life cycle. Therefore, understanding the cellular and molecular mechanisms of sporozoite maturation, motility and invasion into host cells may assist in developing new potent intervention strategies against malaria.
Plasmodium sporozoites are formed inside oocysts, in a process termed sporogony, and share the unifying features of all apicomplexan invasive stages, i.e. they contain secretory organelles and display active locomotion (Sinden and Matuschewski, 2005). Sporozoites are covered with a dense coat made of circumsporozoite protein (CSP), the major surface coat protein (Nardin et al., 1982). Once mature, sporozoites become motile and egress from oocysts into the haemolymph (Aly and Matuschewski, 2005; Wang et al., 2005). Upon contact with their final target organ in the mosquito vector, the salivary glands, they specifically bind to and penetrate the distal portion of the lateral lobes resulting in accumulation of mature, infectious sporozoites in the salivary duct and potential transmission to the mammalian host.
The sporozoite-specific transmembrane surface protein TRAP (thrombospondin-related anonymous protein) is the founding member of a protein family that mediates cell invasion in Apicomplexan parasites (Tomley and Soldati, 2001). TRAP deficiency or mutations in key cytoplasmic and extracellular amino acid residues result in ablation of sporozoite locomotion and host cell entry (Sultan et al., 1997; Kappe et al., 1999; Wengelnik et al., 1999; Matuschewski et al., 2002a). The unifying structural features of TRAP/MIC2 family invasins are combinations of extracellular adhesive modules, i.e. the von Willebrand factor A-domain (A-domain) and the thrombospondin type I repeat (TSR), a cleavable transmembrane domain, and a cytoplasmic tail domain (CTD) that contains a penultimate tryptophan residue preceded by multiple negatively charged amino acids. According to the present model an extracellular binding event is transmitted to the cytoplasmic domain that links the transmembrane protein to the actin/myosin motor of the sporozoite (Keeley and Soldati, 2004; Schüler and Matuschewski, 2006). Up to date, TRAP remains the only known sporozoite-specific TRAP/MIC2 family protein that performs an essential role for locomotion and life cycle progression of the malaria sporozoite (Sultan et al., 1997; Matuschewski, 2006). Another sporozoite transmembrane protein, termed apical membrane antigen/erythrocyte binding-like protein (MAEBL), mediates salivary gland recognition and adhesion, but is dispensable for gliding locomotion (Kariu et al., 2002; Preiser et al., 2004; Fu et al., 2005; Sáenz et al., 2008).
In this study we characterized the in vivo function of a sporozoite-specific transmembrane protein, S6, which was initially identified in a screen for sporozoite-enriched transcripts (Kaiser et al., 2004). We show that S6 is important for efficient sporozoite locomotion and target cell entry. Apparently, Plasmodium sporozoites employ at least three stage-specific transmembrane proteins to guarantee efficient transmission to the mammalian host.
Results
S6 is specifically expressed in Plasmodium sporozoites
S6 (PF14_0404) was first discovered in a screen designed to identify Plasmodium yoelii sporozoite-specific genes that are absent in blood stages (Kaiser et al., 2004). The orthologous Plasmodium berghei S6 gene was identified in the genome database and encodes a protein of 2301 amino acid residues (Fig. 1A). The S6 protein appears to be a surface-exposed type I transmembrane protein and exhibits two main remarkable features. (i) It contains a carboxy-terminal TRAP family-like CTD, including the penultimate tryptophan and a cluster of negatively charged residues (Kaiser et al., 2004). These residues within the CTD are a hallmark of TRAP family invasins and play crucial roles during gliding locomotion (Kappe et al., 1999; Heiss et al., 2008). (ii) The large extracellular portion consists largely of low-complexity regions and lacks apparent cell adhesion modules, such as TSRs and A-domains.
Fig. 1.
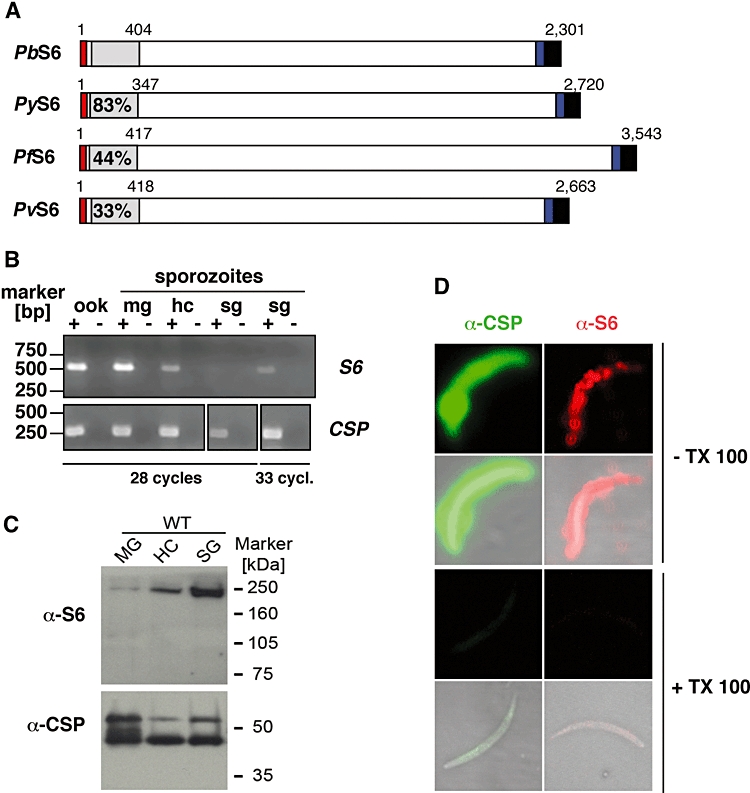
Expression of Plasmodium berghei S6 during sporozoite maturation. A. Schematic diagram of the primary structure of Plasmodium S6 proteins. The predicted signal peptide, the carboxy-terminal transmembrane span and cytoplasmic tail domain of TRAP family invasins are boxed in red, blue and black respectively. Amino acid sequence identities of the amino-terminal region (grey box) of the P. yoelii (PY04986, re-annotated), P. falciparum (PF14_0404), P. vivax (Pv118360, re-annotated) S6 orthologues are indicated as percentage of identical residues compared with the P. berghei (FJ160771) sequence. B. Downregulation of PbS6 transcripts during sporozoite maturation. Shown is a RT-PCR analysis of PbS6 mRNA in ookinetes (ook), and midgut (mg)-, haemocoel (hc)- and salivary gland (sg)-associated sporozoites. The abundant PbCSP transcript was added as a control. PbS6 and PbCSP were amplified at 28 and 24 PCR cycles respectively. Note that the S6 signal in salivary gland sporozoites can only be revealed by amplification at higher cycle number (33 cycles). C. Western blot analysis of PbS6 during sporozoite maturation. Sporozoite extracts from 100 000 wild-type oocyst, haemocoel or salivary gland sporozoites were separated on an 8% SDS-PAGE and probed with a polyclonal anti-PbS6 antiserum or the CSP monoclonal antibody as a loading control, followed by peroxidase-coupled anti-rabbit or anti-mouse antisera. D. Immunolocalization of PbS6 in sporozoites. Fixed sporozoites were incubated with a monoclonal anti-CSP antibody (green) and the polyclonal anti-PbS6 antiserum (red) and their corresponding fluorescently labelled secondary antibodies. Note that solubilization of the plasma membrane with Triton X-100 (TX 100) abolishes the strong plasma membrane labelling of both antibodies.
We first analysed S6 transcript abundance during sporozoite maturation (Fig. 1B). cDNAs generated from ookinetes, oocyst, haemocoel and salivary gland-associated sporozoites were used as templates for semi-quantitative RT-PCR. The transcript of the major sporozoite surface protein CSP served for data standardization. PbS6 expression was the highest in early mosquito stages, including ookinetes and young oocyst sporozoites. S6 was detectable in haemocoel sporozoites, but transcript levels were low in salivary gland-associated sporozoites. As observed previously (Matuschewski et al., 2002b), CSP transcription is slightly downregulated in mature salivary gland-associated sporozoites. In marked contrast, S6 can only be detected at higher cycle numbers, suggesting only residual transcript levels in mature sporozoites. In support of our findings, we independently isolated S6 as one of the most abundant transcript in a suppression subtractive hybridization screen to select for genes that are downregulated during sporozoite maturation (our unpublished data). Therefore, transcriptional control of S6 expression is markedly different from CSP and TRAP (Matuschewski et al., 2002b). Collectively, our data suggest that S6 transcription is downregulated during sporozoite maturation.
S6 expression is translationally controlled
We next wanted to examine protein expression during sporozoite formation and raised polyclonal antisera against PbS6. We first analysed protein expression during sporozoite maturation by Western blot analysis (Fig. 1C). Using the PbS6 antiserum we detected a specific signal at the expected size of ∼260 kDa. Unexpectedly, the S6 protein displays an increasing accumulation from oocyst to salivary gland sporozoites, differing substantially from the transcriptional profile, suggesting that S6 protein synthesis is delayed compared with gene transcription. This finding is reminiscent of translational repression, which has been previously observed for gametocyte-specific genes that are repressed by a DDX6 family member of DEAD-box RNA helicases, termed development of zygote inhibited (DOZI) (Mair et al., 2006).
To confirm the immunoblot analysis and detect the localization of PbS6 we next studied wild-type (WT) sporozoites by immunofluorescence microscopy. In fixed haemocoel sporozoites we detected a punctuate pattern for PbS6 that contrasted with the uniform distribution of CSP (Fig. 1D). In order to confirm the surface localization of S6 we treated sporozoites with the detergent Triton X-100 to remove the plasma membrane. Reactivity with either CSP or S6 antisera was ablated in detergent-treated sporozoites suggesting a comparable localization. Together our findings indicate that the S6 protein localizes to the sporozoite plasma membrane and its expression is under stage-specific transcriptional and translational control.
Generation of s6(−) parasites
The tight expression regulation and spatial distribution of S6 is indicative of an important cellular function that is likely restricted to sporozoites in the mosquito vector. To identify the in vivo roles of S6 in P. berghei life cycle progression we generated a Pbs6(−) parasite line by allelic exchange (Fig. 2A). Importantly, we could select for viable blood-stage parasites that contain a targeted deletion of the S6 open reading frame, in good agreement with sporozoite-specific gene expression and absence of transcripts in blood stages (Kaiser et al., 2004). The parental parasite populations were subcloned to generate clonal parasite lines, termed s6(−) (Fig. 2A).
Fig. 2.

Targeted gene disruption of P. berghei S6. A. Replacement strategy to generate s6(−) parasite lines. The wild-type (WT) S6 genomic locus was targeted with a SacII/KpnI-linearized replacement plasmid (pΔS6) containing 5′ and 3′ untranslated regions of the PbS6 open reading frame and the positive selectable marker, i.e. Toxoplasma gondii dihydrofolate reductase/thymidylate synthase (dhfr/ts). Upon a double-cross-over event, the endogenous S6 locus is replaced by the selection marker resulting in a loss-of-function parasite line, i.e. a replacement line, s6(−), in the green fluorescent PbANKA strain (Janse et al., 2006). Replacement-specific test primer combinations and corresponding fragments are indicated by arrows and grey lines respectively. B. Replacement-specific PCR analysis of the s6(−) parasite lines. The predicted gene targeting is confirmed by primer combination ‘test1’, and presence of the targeting construct by combination ‘test2’. The WT-specific test PCR confirms the absence of residual WT parasites in three representative clonal s6(−) lines. C. Depletion of S6 transcripts in s6(−) parasites. cDNAs from WT and s6(−) midgut-associated sporozoites were amplified with S6-specific primers and CSP primers as controls. D. Absence of S6 protein in s6(−) parasites. Shown is a Western blot analysis with the polyclonal anti-PbS6 antiserum and the CSP monoclonal antibody as a loading control. E. Ablation of S6 plasma membrane staining in s6(−) parasites. Fixed WT and s6(−) haemocoel sporozoites were incubated with the polyclonal anti-PbS6 antiserum (red) and their corresponding fluorescently labelled secondary antibody.
Successful gene replacement in the s6(−) clones was confirmed by integration-specific PCR (Fig. 2B) and absence of S6 transcripts by RT-PCR analysis (Fig. 2C). Western blot analysis (Fig. 2D) confirmed successful S6 depletion by the replacement strategy and the specificity of the anti-S6 antiserum. In good agreement, s6(−) haemocoel sporozoites displayed no detectable immunofluorescence staining when incubated with the S6 antiserum (Fig. 2E). In conclusion, successful generation of S6 loss-of-function parasites demonstrated that this gene is dispensable for propagation of asexual blood-stage parasites.
Impairment of mosquito salivary gland invasion in s6(−) sporozoites
We next examined the fate of the s6(−) parasite lines during Plasmodium life cycle progression. Gametocyte formation, exflagellation, transmission to mosquitoes, as well as midgut infectivity (oocyst development and morphology) of s6(−) parasites were normal when compared with WT (data not shown). In general, oocyst development is complete at day 14 after mosquito infection. At this stage, s6(−) parasites were indistinguishable from WT parasites (Fig. 3A). Quantification of midgut-associated sporozoites revealed no differences between the two parasite lines. In marked contrast, s6(−) sporozoites were severely impaired in salivary gland invasion (Fig. 3B). The quantification of isolated salivary gland sporozoites showed a dramatic decrease in s6(−) parasite numbers when compared with WT. Importantly, the observed deficiency to enter mosquito salivary glands was accompanied by substantial accumulation of viable sporozoites in the mosquito haemocoel (Fig. 3C). These findings indicate that S6 is not involved in sporozoite egress from oocysts but plays an important role prior to transmission to the mammalian host.
Fig. 3.

S6(−) sporozoites are defective in mosquito salivary gland invasion. A. WT and s6(−) parasites produce similar numbers of oocysts (left) and midgut-associated sporozoites (right). Oocysts were counted based on their GFP fluorescence and sporozoite numbers calculated per infected mosquito from at least three independent feeding experiments each. B. Deficiency in salivary gland invasion in S6 loss-of-function mutants. Shown are representative fixed salivary glands after infection (left) and quantifications of salivary gland-associated sporozoites per infected mosquito (right). Note the accumulation of haemocoel sporozoites in the s6(−) mutants, as shown by quantifications of WT and s6(−) haemocoel sporozoites.
Deficient motility in s6(−) sporozoites
The observed deficiency in salivary gland invasion resembles the phenotype of trap(−) sporozoites, which lost their ability to invade target cells and perform active gliding locomotion (Sultan et al., 1997). We therefore compared gliding motility of mutant and WT sporozoites. Because s6(−) sporozoites are severely impaired in salivary gland invasion, we tested motility of sporozoites isolated from the mosquito haemocoel by indirect immunofluorescence staining of CSP deposited into the trails of gliding sporozoites (Fig. 4A). Only rarely did we detect sporozoites with trails, which displayed a more discontinuous pattern when compared with productive motility of WT sporozoites. A quantitative analysis of gliding locomotion revealed that on average 55% of adherent WT haemocoel sporozoites showed a trail of one circle or greater (Fig. 4B). In marked contrast, only very few sporozoites (3%) displayed trail patterns that were always discontinuous.
Fig. 4.

S6(−) sporozoites are impaired in gliding locomotion. A. Representative immunofluorescence images of WT and s6(−) sporozoites, revealed with the anti-CSP antibody. WT sporozoites (left) display the typical circular continuous gliding pattern, whereas s6(−) sporozoites either are non-motile (right) or display discontinuous circular gliding (centre). B. Quantification of sporozoite adhesion and gliding trails by indirect immunofluorescence microscopy. A total of 10 000 haemocoel sporozoites of either WT or s6(−) parasites were deposited onto BSA-coated coverslips and incubated for 15 min at 37C. Trails were visualized with the anti-CSP monoclonal antibody. Shown is the mean percentage (±SD) of adherent sporozoites (shown as percentage of WT adhesion) and gliding sporozoites (trails ≥ 1 circle) from three independent experiments. C. Overlays of time-lapse micrographs of WT and s6(−) sporozoites. Shown are combined sequential images (3 s intervals) of gliding WT and s6(−) sporozoites. S6(−) sporozoites display either (i) attached waiving, (ii) incomplete circular motility or (iii) irregular movement. Note that only substrate-dependent productive movement is impaired, while attachment, bending and flexing are maintained.
The vast majority of s6(−) sporozoites were active and displayed various patterns of non-productive motility, such as attached waving, bending and flexing, and pendulum-like movements (see also Movie S1). To confirm these findings we analysed sporozoite motility by phase-contrast microscopy (Fig. 4C). This analysis confirmed that sporozoite adhesion in vitro occurs normally in the absence of S6. S6(−) haemocoel sporozoites adhere with one end and display the immature and non-productive motility patterns, but apparently lost their ability to glide over long distances (Fig. 4C). Together, our findings demonstrate a crucial role for S6 in sporozoite locomotion.
S6(−) sporozoites are impaired in transmission to the mammalian host
We finally tested whether the observed defects in gliding locomotion translate into a complete block of transmission to the mammalian host. In order to quantify the invasion capacity of s6(−) sporozoites we isolated haemocoel sporozoites by gentle perfusion of infected mosquitoes. When these sporozoites were added to subconfluent hepatoma cells and stained for mature liver stages at 48 h after sporozoite infection we consistently detected mature liver stages in s6(−)-infected hepatoma cells, albeit at greatly reduced numbers (Fig. 5A). Our quantitative analysis revealed a consistent reduction in liver stage numbers by at least an order of magnitude in vitro (Fig. 5B).
Fig. 5.

S6(−) sporozoites are defective in infectivity to the mammalian host. A. Representative immunofluorescence images of WT and s6(−) liver stages, 48 h after hepatocyte invasion. Infected hepatoma cells were fixed, permeabilized and stained with monoclonal anti-PbHSP70 antibody. B. Quantification of parasite liver stages. Haemocoel sporozoites of either WT or s6(−) parasites were added in the numbers indicated to subconfluent hepatoma cells and incubated for 48 h. Shown are total liver stages from three independent experiments (mean value ± SD). Note that WT and mutant parasites differ by at least one order of magnitude (logarithmic scale). C. Infectivity of WT and s6(−) parasites by natural malaria transmission. Highly susceptible young Sprague/Dawley rats were exposed to five infected mosquitoes. The prepatent period was determined by daily examination of Giemsa-stained blood smears (mean value ± SD). Note that a substantial portion of animals develops malaria, despite very low salivary gland infection rates.
To exclude multiple roles of S6 during hepatocyte invasion and liver stage development, we incubated haemocoel sporozoites with subconfluent hepatoma cells for 90 min and quantified intra- and extracellular sporozoites. While 18% of WT sporozoites productively invaded, only 0.5% of s6(−) sporozoites were located inside hepatoma cells (Table 1), suggesting that S6 functions exclusively during sporozoite entry and not in subsequent steps of liver stage maturation.
Table 1.
Infectivity of s6(−) haemocoel sporozoites.
| Parasite population | Invasiona | No. injected sporozoitesb | No. infected/No. injectedc | Prepatent period (days)d |
|---|---|---|---|---|
| WT | 18% | 1 000 | 10/10 | 4.7 |
| 10 000 | 8/8 | 4.6 | ||
| 100 000 | 3/3 | 3.3 | ||
| s6(−) | 0.5% | 1 000 | 1/10 | (5.0) |
| 10 000 | 8/8 | 5.1 | ||
| 100 000 | 5/5 | 3.4 |
Invasion was assessed by differential immunostaining of extra- and intracellular sporozoites.
Haemocoel sporozoites were injected intravenously at the numbers indicated.
Highly susceptible Sprague/Dawley rats were infected.
The prepatent period is the time until detection of the first blood stage by daily examination of Giemsa-stained blood smears.
When we tested natural malaria transmission through by-bite feedings with five infected s6(−) mosquitoes on naïve, young Sprague/Dawley rats, which are highly susceptible for P. berghei sporozoite infection, we detected a substantial proportion of successful, albeit delayed, blood-stage infections (Fig. 5C). This result confirms that a small proportion of s6(−) sporozoites retains the capacity to invade the salivary glands and is able to complete the entire Plasmodium life cycle. As expected, the observed defect in sporozoite locomotion and invasion to mosquito salivary glands translates into a substantial delay or absence of infection during natural transmission. To confirm that the main role of S6 is during sporozoite entry of salivary glands, we bypassed the life cycle and injected haemocoel sporozoites intravenously into susceptible animals. Similar to WT parasites, s6(−) sporozoites induced patency in a dose-dependent manner in vivo (Table 1). This finding confirms that the observed defects in sporozoite locomotion and liver stage development in vitro are compensated for in vivo. Most importantly, reduced infectivity to the mammalian host is a consequence of the reduced capacity to invade the mosquito salivary glands.
Discussion
In this study we characterized a member of the parasite motor machinery that plays an important role for sporozoite gliding motility and entry into mosquito salivary glands. Depletion of S6 by a reverse genetics approach resulted in a consistent reduction of transmission of the malaria parasite to the mammalian host. We could show that the observed decrease in infectivity is a direct consequence of a specific reduction in the loads of mature sporozoites in the salivary glands. This defect in salivary gland invasion correlates with a striking phenotype in in vitro gliding motility. Thus, the most important cellular function of S6 is in mediating sporozoite accumulation in the final target organ of the mosquito vector through its role in sporozoite gliding locomotion.
Plasmodium, like other apicomplexan parasites, forms motile extracellular stages that actively penetrate biological barriers and enter target cells. These activities are driven by the parasite's own motor machinery. Central components are transmembrane and surface proteins that link the outside world to the parasite's actin/myosin motor. The founding member of the parasite family of invasins, TRAP, is vital for sporozoite motility and cell invasion (Sultan et al., 1997) and appears to function throughout the parasite's journey from the oocysts to the mammalian liver (Sultan et al., 1997; Kappe et al., 1999; Wengelnik et al., 1999; Matuschewski et al., 2002a). The parasite apparently expresses tailor-made TRAP/MIC2 family invasins for different life cycle stages. For instance, ookinetes express the invasin circumsporozoite/TRAP-related protein (CTRP) that is essential for ookinete motility and midgut penetration (Dessens et al., 1999; Templeton et al., 2000) and can be functionally grouped into the TRAP/MIC2 family (Heiss et al., 2008). Sporozoites are arguably the most versatile extracellular parasite stages that need to breach numerous barriers along their journey (Amino et al., 2006; Baer et al., 2007; reviewed in Frevert et al., 2008). Not surprisingly, sporozoites express additional TRAP/MIC2 family proteins that together permit efficient life cycle progression. One member, TRAP-like protein (TLP), contains two extacellular adhesion domains, the TSR and the A-domain, yet plays a redundant role in all aspects of sporozoite biology (Heiss et al., 2008; Moreira et al., 2008). In marked contrast, s6(−) mutants display pronounced defects in sporozoite gliding motility and salivary gland invasion. Therefore, S6 functions appear to closely resemble those of TRAP (Sultan et al., 1997).
However, two important features differ fundamentally between the two proteins. (i) While TRAP contains classical adhesion modules, namely an A-domain and a TSR, in its extracellular portion, the extracellular region of S6 appears to be lacking any typical adhesion modules. (ii) The observed defects in the s6(−) mutants are most prominent in the mosquito phase of the Plasmodium life cycle. trap(–) mutants, in contrast, fail to infect the mammalian host in vitro and in vivo. Most strikingly, the two proteins do not have redundant but rather distinct roles. Therefore, parasite motility and host cell entry are driven by an array of extracellular proteins that each mediate individual steps from initial substrate and cell recognition to target cell penetration. S6(−) parasites largely lost their capacity to glide productively in vitro and to accumulate inside salivary glands.
Our findings show that active parasite locomotion, as displayed by haemocoel sporozoites, is necessary for salivary gland entry. In addition to the general sporozoite invasin TRAP (Sultan et al., 1997) the malaria parasite employs two stage-specific genes, MAEBL and S6, that function in salivary gland adhesion (Kariu et al., 2002) and sporozoite motility respectively. Most importantly, we identified by experimental genetics S6 as the second sporozoite-specific Plasmodium protein that has an important role in transducing the motor force from the parasite interior to the extracellular substrate.
Experimental procedures
P. berghei life cycle
Anopheles stephensi mosquitoes were raised at 28°C, 75% humidity, under a 12 h light/dark cycle, and maintained on a 10% sucrose solution during adult stages. For infections with clonal P. berghei parasites (ANKA strain, GFP-507cl; Janse et al., 2006), 4-day-old female mosquitoes were fed on anaesthetized NMRI mice, which had been infected with P. berghei WT parasites or the isogenic s6(−) parasites. Parasitaemia was determined for the presence of gametocyte-stage parasites capable of exflagellation. After infection the mosquitoes were maintained at 20°C and 80% humidity. Dissections were performed at days 10, and 14–18, to determine infectivity, and perform a detailed spatial and temporal analysis of the sporozoite populations. Oocyst, haemocoel and salivary gland-associated sporozoites were separated and analysed as described (Vanderberg, 1975).
S6 expression analysis
Detection of S6 transcripts was performed by semi-quantitative RT-PCR. A total of 5 × 105 WT sporozoites were collected from oocysts, haemocoel or salivary glands, and poly (A+) RNA was isolated using oligo dT-columns (Invitrogen). cDNA synthesis was performed after DNase I digestion (Ambion) with random decamer primers (Ambion). S6 transcript abundance was determined using primers S6RTfor (5′-GTGTTATCAACCTTCATATTATTATC-3′) and S6RTrev (5′-CTCCACTTTCGAAAAAATATACAG-3′) and CSP primers CSPfor (5′-GACGATTCTTATATCCCAAGCGC-3′) and CSPrev (5′-CCTAATGAATTGCTTACAATATTAAATATACTTG-3′) for normalization.
PbS6 gene targeting
For targeted deletion of PbS6 the gene replacement approach was followed (Thathy and Ménard, 2002). Two targeting fragments were selected from the non-coding regions of S6 and amplified with P. berghei genomic DNA as a template using the following primers: (i) S6rep1Ifor (5′-TCCCCGCGGGCACTTAATATATGCGATTATGGG-3′; SacII site is underlined) and S6repIIrev (5′-CGGGATCCTTTACTCGGTTGTCTATGAATGC-3′; BamHI site is underlined) for a 1045 bp fragment of the 5′ UTR; (ii) S6rep3for (5′-CCCCAAGCTTTATAGACATGGAACACA AAGAGGATAGC-3′; HindIII site is underlined) and S6rep4rev (5′-GGGGTACCTTCTACGAAATCATCTAGTATGCC-3′; KpnI site is underlined) for a 807 bp fragment of the 3′ UTR. Cloning of the two fragments into the P. berghei targeting vector flanking the Tgdhfr/ts-positive selection marker that provides resistance to the antifolate pyrimethamine resulted in the plasmid pMS01. The targeting vector was linearized with KpnI/SacII and parasite transfection, positive selection and parasite cloning were performed with the fluorescent P. berghei ANKA strain as described (Janse et al., 2006). Three independent s6(−) clonal parasite populations were obtained and tested for Plasmodium life cycle progression. The detailed phenotypical analysis was performed with one representative clone. Replacement-specific PCR amplifications of the s6(−) locus was performed with specific primer pairs that amplify either the WT or the mutant gene loci.
Western blotting and immunofluorescence
To generate polyclonal antisera a recombinant amino-terminally His-tagged S6 polypeptide encompassing 156 central amino acid residues, selected for favourable antigenicity, was expressed in an Escherichia coli expression vector in BL21 (DE3) cells. The purified recombinant protein was used to raise polyclonal antibodies in pre-screened rabbits housed in a SPF facility (Eurogentec, Seraing, Belgium). For Western blot analysis 105 sporozoites of the different developmental stages were homogenized in SDS sample buffer and boiled. Proteins were separated on 6% polyacrylamide gels and transferred to nitrocellulose membranes by electroblotting. Membranes were blocked, and incubated with the S6 polyclonal antisera (1:1000) or the monoclonal anti-CSP antibody (1:10 000; Potocnjak et al., 1980). Bound antibodies were detected using horseradish peroxidase-conjugated anti-rabbit and anti-mouse antibodies respectively (Sigma-Aldrich).
For sporozoite immunostaining and gliding motility assays haemocoel sporozoites of WT and s6(−) parasites were isolated by gentle perfusion in RPMI/3% bovine serum albumin (BSA) at days 15–16 post feeding. Pooled haemocoel sporozoites were transferred to BSA-precoated glass coverslips, incubated for 15 min at 37°C in a humid chamber, and fixed in 4% formaldehyde. For membrane extraction, parasites were treated with 1% Triton X-100 (Sigma) in PBS prior to fixation (Bergman et al., 2003). For permeabilization sporozoites were incubated with 0.05% Saponin (Sigma) in PBS/1% FCS. Non-permeabilized and permeabilized sporozoites were incubated with the S6 polyclonal antisera (1:100) or the monoclonal anti-CSP antibody (1:1000). Bound antibodies were detected with Alexa-Fluor 546-conjugated anti-rabbit IgG and Alexa-Fluor 488-conjugated anti-mouse IgG respectively (Molecular Probes).
Analysis of sporozoite infectivity
To determine sporozoite infectivity in vivo, s6(−) and WT haemocoel sporozoites were injected intravenously at the numbers indicated into young Sprague/Dawley rats. Patency was checked daily by Giemsa-stained blood smears for at least 14 days. The prepatent period is defined as the time until occurrence of the first blood-stage parasites.
For determination of sporozoite infectivity in vitro, haemocoel sporozoites were added to subconfluent monolayers of HuH7 cells at the numbers indicated, incubated for 90 min at 37°C and washed off. Liver stages were revealed after 48 h using primary antibodies against P. berghei HSP 70 (Tsuji et al., 1994) and Alexa-Fluor 488-conjugated anti-mouse IgG (Molecular Probes). To score sporozoite invasion a two-colour assay was performed as described previously (Rénia et al., 1988). Briefly, HuH7 cells were grown to subconfluency, incubated for 90 min with the haemocoel sporozoite suspension and washed in DMEM medium containing 10% FCS. To distinguish between extra- and intracellular parasites, extracellular parasites were labelled with the monoclonal anti-CSP antibody, followed by Alexa-Fluor 546-conjugated anti-mouse IgG (Molecular Probes). Permeabilization was performed with ice-cold methanol and re-labelling with the monoclonal anti-CSP antibody, followed by Alexa-Fluor 488-conjugated anti-mouse IgG (Molecular Probes).
Nucleotide sequence accession number
The nucleotide sequence reported in this article has been submitted to the GenBank database with the Accession No. FJ160771.
Acknowledgments
We thank Robert Ménard and Audrey Combe for continuous critical discussions and sharing unpublished data. This work was supported in part by grants from the European Commission (BioMalPar #23), the Deutsche Forschungsgemeinschaft (CRC544 #B10), the Joachim Siebeneicher Foundation and the Chica and Heinz Schaller Foundation to K.M.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Movie S1. Non-productive gliding motility of s6(−) haemocoel sporozoites on glass slides, co-incubated with 3% bovine serum albumine. Total time: 20 sec.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Aly A, Matuschewski K. A malarial cysteine protease is necessary for Plasmodium egress from oocysts. J Exp Med. 2005;202:225–230. doi: 10.1084/jem.20050545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amino R, Thiberge S, Martin B, Celli S, Shorte S, Frischknecht F, et al. Quantitative imaging of Plasmodium transmission from mosquito to mammal. Nat Med. 2006;12:220–224. doi: 10.1038/nm1350. [DOI] [PubMed] [Google Scholar]
- Baer K, Roosevelt M, Clarkson AB, van Rooijen N, Schnieder T, Frevert U. Kupffer cells are obligatory for Plasmodium yoelii sporozoite infection of the liver. Cell Microbiol. 2007;9:397–412. doi: 10.1111/j.1462-5822.2006.00798.x. [DOI] [PubMed] [Google Scholar]
- Bergman LW, Kaiser K, Fujioka H, Coppens I, Daly TM, Fox S, et al. Myosin A tail domain interacting protein (MTIP) localizes to the inner membrane complex of Plasmodium sporozoites. J Cell Sci. 2003;116:39–49. doi: 10.1242/jcs.00194. [DOI] [PubMed] [Google Scholar]
- Dessens JT, Beemtsma AL, Dimopoulos G, Wengelnik K, Crisanti A, Kafatos FC, et al. CTRP is essential for mosquito infection by malaria ookinetes. EMBO J. 1999;18:6221–6227. doi: 10.1093/emboj/18.22.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frevert U, Usynin I, Baer K, Klotz C. Plasmodium sporozoite passage across the sinusoidal cell layer. Subcell Biochem. 2008;47:182–197. doi: 10.1007/978-0-387-78267-6_15. [DOI] [PubMed] [Google Scholar]
- Fu J, Sáenz FE, Reed MB, Balu B, Singh N, Blair PL, et al. Targeted disruption of maebl in Plasmodium falciparum. Mol Biochem Parasitol. 2005;144:55–67. doi: 10.1016/j.molbiopara.2004.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss K, Nie H, Kumar S, Daly TM, Bergman LW, Matuschewski K. Functional characterization of a redundant Plasmodium TRAP family invasin, TRAP-like protein, by aldolase binding and a genetic complementation test. Eukaryot Cell. 2008;7:1062–1070. doi: 10.1128/EC.00089-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janse C, Franke-Fayard B, Mair G, Ramesar J, Thiel C, Engelmann S, et al. High efficiency transfection of Plasmodium berghei facilitates novel selection procedures. Mol Biochem Parasitol. 2006;145:60–70. doi: 10.1016/j.molbiopara.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Kaiser K, Matuschewski K, Camargo N, Ross J, Kappe SHI. Differential transcriptome profiling identifies Plasmodium genes encoding pre-erythrocytic stage-specific proteins. Mol Microbiol. 2004;51:1221–1232. doi: 10.1046/j.1365-2958.2003.03909.x. [DOI] [PubMed] [Google Scholar]
- Kappe S, Bruderer T, Gantt S, Fujioka H, Nussenzweig V, Ménard R. Conservation of a gliding motility and cell invasion machinery in Apicomplexan parasites. J Cell Biol. 1999;147:937–944. doi: 10.1083/jcb.147.5.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariu T, Yuda M, Yano K, Chinzei Y. MAEBL is essential for malaria sporozoite infection of the mosquito salivary gland. J Exp Med. 2002;195:1317–1323. doi: 10.1084/jem.20011876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley A, Soldati D. The glideosome: a molecular machinery powering motility and host-cell invasion by Apicomplexa. Trends Cell Biol. 2004;14:528–532. doi: 10.1016/j.tcb.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Mair GR, Braks JA, Garver LS, Wiegant JC, Hall N, Dirks RW, et al. Regulation of sexual development of Plasmodium by translational repression. Science. 2006;313:667–669. doi: 10.1126/science.1125129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuschewski K. Getting infectious: formation and maturation of Plasmodium sporozoites in the Anopheles vector. Cell Microbiol. 2006;8:1547–1556. doi: 10.1111/j.1462-5822.2006.00778.x. [DOI] [PubMed] [Google Scholar]
- Matuschewski K, Nunes AC, Nussenzweig V, Ménard R. Plasmodium sporozoite invasion into insect and mammalian cells is directed by the same dual binding system. EMBO J. 2002a;21:1597–1606. doi: 10.1093/emboj/21.7.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuschewski K, Ross J, Brown SM, Kaiser K, Nussenzweig V, Kappe SH. Infectivity-associated changes in the transcriptional repertoire of the malaria parasite sporozoite stage. J Biol Chem. 2002b;277:41948–41953. doi: 10.1074/jbc.M207315200. [DOI] [PubMed] [Google Scholar]
- Moreira CK, Templeton TJ, Lavazec C, Hayward RE, Hobbs CV, Kroeze H, et al. The Plasmodium TRAP/MIC2 family member, TRAP-like protein (TLP), is involved in tissue traversal by sporozoites. Cell Microbiol. 2008;10:1505–1516. doi: 10.1111/j.1462-5822.2008.01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardin EH, Nussenzweig V, Nussenzweig RS, Collins WE, Harinasuta KT, Tapchaisri P, et al. Circumsporozoite proteins of human malaria parasites Plasmodium falciparum and Plasmodium vivax. J Exp Med. 1982;156:20–30. doi: 10.1084/jem.156.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potocnjak P, Yoshida N, Nussenzweig RS, Nussenzweig V. Monovalent fragments (Fab) of monoclonal antibodies to a sporozoite surface antigen (Pb44) protect mice against malarial infection. J Exp Med. 1980;151:1504–1513. doi: 10.1084/jem.151.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiser P, Rénia L, Singh N, Balu B, Jarra W, Voza T, et al. Antibodies against MAEBL ligand domains M1 and M2 inhibit sporozoite development in vitro. Infect Immun. 2004;72:3604–3608. doi: 10.1128/IAI.72.6.3604-3608.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudêncio M, Rodriguez A, Mota MM. The silent path to thousands of merozoites: the Plasmodium liver stage. Nat Rev Microbiol. 2006;4:849–856. doi: 10.1038/nrmicro1529. [DOI] [PubMed] [Google Scholar]
- Rénia L, Mitgen F, Charoenvit Y, Ponnudurai T, Verhave JP, Collins WE, et al. Malaria sporozoite penetration. A new approach by double staining. J Immunol Methods. 1988;112:201–205. doi: 10.1016/0022-1759(88)90358-4. [DOI] [PubMed] [Google Scholar]
- Sáenz FE, Balu B, Smith J, Mendonca SR, Adams JH. The transmembrane isoform of Plasmodium falciparum MAEBL is essential for invasion of Anopheles salivary glands. PLoS ONE. 2008;3:e2287. doi: 10.1371/journal.pone.0002287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüler H, Matuschewski K. Plasmodium motility. Actin not actin' like actin. Trends Parasitol. 2006;22:146–147. doi: 10.1016/j.pt.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Sinden RE, Matuschewski K. The sporozoite. In: Sherman I, editor. Molecular Approaches to Malaria. Washington, DC: American Society for Microbiology Press; 2005. pp. 169–190. [Google Scholar]
- Sultan AA, Thathy V, Frevert U, Robson KJ, Crisanti A, Nussenzweig V, et al. TRAP is necessary for gliding motility and infectivity of Plasmodium sporozoites. Cell. 1997;90:511–522. doi: 10.1016/s0092-8674(00)80511-5. [DOI] [PubMed] [Google Scholar]
- Templeton TJ, Kaslow DC, Fidock DA. Developmental arrest of the human malaria parasite Plasmodium falciparum within the mosquito midgut via a CTRP gene disruption. Mol Microbiol. 2000;36:1–9. doi: 10.1046/j.1365-2958.2000.01821.x. [DOI] [PubMed] [Google Scholar]
- Thathy V, Ménard R. Gene targeting in Plasmodium berghei. Methods Mol Med. 2002;72:317–331. doi: 10.1385/1-59259-271-6:317. [DOI] [PubMed] [Google Scholar]
- Tomley FM, Soldati DS. Mix and match modules: structure and function of microneme proteins in apicomplexan parasites. Trends Parasitol. 2001;17:81–88. doi: 10.1016/s1471-4922(00)01761-x. [DOI] [PubMed] [Google Scholar]
- Tsuji M, Mattei D, Nussenzweig RS, Eichinger D, Zavala F. Demonstration of heat-shock protein 70 in the sporozoite stage of malaria parasites. Parasitol Res. 1994;80:16–21. doi: 10.1007/BF00932618. [DOI] [PubMed] [Google Scholar]
- Vanderberg JP. Development of infectivity by the Plasmodium berghei sporozoite. J Parasitol. 1975;61:43–50. [PubMed] [Google Scholar]
- Vanderberg JP, Frevert U. Intravital microscopy demonstrating antibody-mediated immobilisation of Plasmodium berghei sporozoites injected into skin by mosquitoes. Int J Parasitol. 2004;34:991–996. doi: 10.1016/j.ijpara.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Wang Q, Fujioka H, Nussenzweig V. Exit of Plasmodium sporozoites from oocysts is an active process that involves the circumsporozoite protein. PLoS Pathog. 2005;1:e9. doi: 10.1371/journal.ppat.0010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengelnik K, Spaccapelo R, Naitza S, Robson KJH, Janse CJ, Bistoni F, et al. The A-domain and the thrombospondin-related motif of Plasmodium falciparum TRAP are implicated in the invasion process of mosquito salivary glands. EMBO J. 1999;18:5195–5204. doi: 10.1093/emboj/18.19.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi LM, Coppi A, Snounou G, Sinnis P. Plasmodium sporozoites trickle out of the injection site. Cell Microbiol. 2007;9:1215–1222. doi: 10.1111/j.1462-5822.2006.00861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


