Abstract
Rationale
There has been controversy over the abuse potential of methylphenidate (MPH) in the context of treatment for attention deficit hyperactivity disorder (ADHD).
Objective
The objective of this study was to compare the reinforcing and subjective effects of oral MPH in adults with and without ADHD.
Materials and methods
Following screening, 33 adults (n= 16 with ADHD; n=17 free from psychiatric diagnoses) completed four pairs of experimental sessions, each of which included a sampling session and a self-administration session. During sampling sessions, subjects received in randomized order 0 (placebo), 20, 40, and 60 mg MPH. During self-administration sessions, subjects completed a progressive ratio (PR) task to earn portions of the dose received on the corresponding sampling session. Subjective effects were recorded throughout all sessions. The main outcome measure for the study was the number of ratios completed on the PR task. Secondary measures included peak subjective effects and area-under-the-curve values for subjective effects.
Results
Compared to the control group, the ADHD group completed more ratios on the PR task. Both groups showed robust effects of methylphenidate on subjective endpoints. Main effects of group were noted on subjective effects involving concentration and arousal.
Conclusions
Compared to placebo, MPH produced reinforcing effects only for the ADHD group and not for the control group. Increases in stimulant-related subjective effects in non-ADHD subjects were not associated with drug reinforcement.
Keywords: Methylphenidate, Abuse potential, Drug reinforcement, ADHD, Subjective effects
Introduction
Methylphenidate (MPH) is commonly prescribed for the treatment of attention deficit hyperactivity disorder (ADHD), with hundreds of trials demonstrating efficacy in managing the disorder in children and adults (Elia et al. 1999; Goldman et al. 1998). Consequent increases in use of MPH in both the USA and other countries have led to significant controversy regarding the abuse, misuse, and diversion of MPH (Berbatis et al. 2002; Fogelman and Kahan 2001; Ivis and Adlaf 1999; Lin et al. 2005; Robison et al. 1999; Romano et al. 2002; Safer et al. 1996; Zito et al. 2000). MPH has high potential for abuse and is considered a schedule II drug by the Drug Enforcement Administration. A number of single-case studies describe inappropriate intranasal or intravenous use of orally prescribed MPH (Garland 1998; Levine et al. 1986; Massello and Carpenter 1999). In addition, the majority of laboratory-based studies with both nonhumans and humans suggest that MPH exhibits a behavioral profile similar to other drugs of abuse, such as amphetamine and cocaine (Kollins 2003; Kollins et al. 2001). Other studies have shown that a substantial minority of young people report lifetime and past year use of prescription stimulants and that diversion is a common problem (Johnston et al. 2006; Kroutil et al. 2006).
In spite of the controversy surrounding the abuse and diversion of MPH, relatively few studies have systematically assessed MPH abuse potential in adults with and without ADHD under controlled laboratory conditions. Such laboratory studies may be useful to help identify mechanisms underlying the reported misuse and diversion of MPH and other stimulants by determining the extent to which individuals with or without ADHD will self-administer MPH and how the subjective effects of the drug influence this behavior. Several studies have assessed the reinforcing effects of MPH—considered to be an important determinant of abuse potential (Brady 1988)—in non-ADHD subjects. Two studies used a choice procedure and reported that presumably non-ADHD adults did not choose to take MPH (10–40 mg) over placebo or the option to take no capsules under normal conditions (Chait 1994; Roehrs et al. 1999). However, when participants were limited to 4 h of sleep, 10 mg/kg MPH was reliably selected (88%) over placebo (Roehrs et al. 1999). Another study with healthy adults used a progressive ratio procedure and demonstrated that 40 mg oral MPH significantly increased break points compared to placebo and was comparable to 10 and 20 mg d-amphetamine (Rush et al. 2001). A series of more recent studies reported that MPH functioned as a reinforcer when administered intranasally (10–30 mg) or when administered prior to a high-demand task (Stoops et al. 2003, 2005). However, these studies also showed that when administered prior to a relaxation condition or when administered in moderately high doses (48 mg) to stimulant abusers, MPH did not function as a reinforcer (Stoops et al. 2004, 2005).
To date, only two studies have evaluated the reinforcing effects of MPH in individuals diagnosed with ADHD. These studies showed that both children and young adults with ADHD reliably chose MPH over placebo capsules but that these effects were not associated with typical stimulant and/or euphorigenic subject-rated effects (Fredericks and Kollins 2004; MacDonald Fredericks and Kollins 2005). These studies were comparatively small, did not control for previous MPH or other stimulant exposure, did not use a non-ADHD comparison group, and did not evaluate a range of doses of MPH. The purpose of the present study, therefore, was to compare the reinforcing and subjective effects of orally administered MPH in adults with and without ADHD under controlled laboratory conditions.
Materials and methods
Subjects
Thirty-three subjects participated in the study after providing informed consent approved by the local Institutional Review Board. Sixteen were diagnosed with ADHD, and 17 were free from all psychiatric disorders. There were no significant differences between the groups with respect to demographic variables (Table 1). Inclusion criteria for both groups included age between 18 and 45 years and body mass index between 18 and 30. For the ADHD group, subjects had to meet Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for ADHD, any subtype, and have elevated scores on relevant subscales of both self report and observer versions of the Conners’ Adult ADHD Rating Scales (CAARS; Conners et al. 1998). Exclusion criteria for both groups included: (a) a history of adverse reactions to stimulant medication; (b) resting heart rate or systolic blood pressure>100 bpm or 160 mm/Hg, respectively; (c) history of seizures, heart palpitations, or other significant medical conditions; (d) estimated IQ of less than 70; (e) current DSM-IV axis I or II disorder (except for nicotine dependence); (f) current psychoactive drug use; and (g) history of clinical or recreational use of stimulant drugs, including MPH, amphetamine, cocaine, and 3,4 methylenedioxymethamphetamine. For the Control group, subjects were excluded from participation if they scored highly (T score≥60) on any of the relevant subscales on self-report or observer versions of the CAARS. All of these inclusion/exclusion criteria have been used in previous studies in our laboratory and in other studies. For the ADHD group, the recruitment of individuals with no history of stimulant drug treatment could represent a challenge with respect to generalizability of findings. However, since only approximately 10% of adults meeting criteria for ADHD are currently treated (Kessler et al. 2006), this concern was mitigated.
Table 1.
Demographic and screening information
| ADHD | Control | p value | |
|---|---|---|---|
| Total N | 16 | 17 | |
| Male | 8 | 9 | |
| Female | 8 | 8 | |
| Ethnicity (%Caucasian) | 81.3 | 76.5 | NS |
| Age (years) | 28.6 | 24.9 | NS |
| Vital signs | |||
| Height (in.) | 68.0 | 67.9 | NS |
| Weight (lbs) | 164.1 | 162.2 | NS |
| Body mass index | 24.7 | 24.4 | NS |
| Heart rate (bpm) | 75.9 | 68.6 | NS |
| Systolic blood pressure (mmHg) | 122.6 | 127.0 | NS |
| Diastolic blood pressure (mmHg) | 74.1 | 73.3 | NS |
| CAARS self-report | |||
| Inattentive (T score) | 78.9 | 44.8 | <0.001 |
| Hyperactive impulsive (T score) | 66.6 | 41.1 | <0.001 |
| ADHD index (T score) | 65.5 | 41.4 | <0.001 |
| CAARS observer report | |||
| Inattentive (T score) | 69.7 | 47.2 | <0.001 |
| Hyperactive impulsive (T score) | 66.3 | 46.1 | <0.001 |
| ADHD index (T score) | 66.2 | 46.4 | <0.001 |
| Cognitive functioning | |||
| KBIT composite | 111.7 | 111.8 | NS |
| KBIT vocabulary | 108.6 | 109.8 | NS |
| KBIT matrices | 112.4 | 111.5 | NS |
CAARS Conners’ Adult ADHD Rating Scale; KBIT Kaufman Brief Intelligence Test
Psychiatric functioning was assessed using the Conners’ Adult ADHD Interview for DSM-IV (CAADID; Epstein et al. 2000), a screening version of the Structured Clinical Interview for DSM-IV (SCID; First et al. 1996), and a semi-structured clinical interview with a trained clinician. Subjects underwent a physical examination and medical and developmental histories were obtained. Current and previous drug use, including alcohol and cigarettes, was also measured. There were no differences between the groups with respect to frequency or history of alcohol, cigarette, marijuana, or other drug use.
General procedures
Following screening, subjects completed four pairs of experimental sessions, for a total of eight sessions. Subjects abstained from food and beverage 4 h prior to scheduled sessions and abstained from alcohol 12 h prior to scheduled sessions. Subjects were told that the purpose of the study was to measure the effects on mood and behavior of various drugs used to treat ADHD (MPH, amphetamine, atomoxetine), and that they could receive these drugs or placebo. Other than receiving this general information, participants were unaware of the type of drug administered.
Each pair of experimental sessions consisted of a sampling session and a self-administration session. On each of the sampling sessions, subjects were exposed to one of four fixed doses of MPH (0, 20, 40, and 60 mg). These doses were administered in eight identical capsules each sampling session, such that subjects received eight capsules containing 0, 2.5, 5 mg, or 7.5 mg each for the 0, 20, 40, and 60 mg conditions, respectively. All eight capsules were ingested at the same time (see Drug Dose and Administration below) On subsequent self-administration sessions, subjects had the opportunity to work for the same divided doses of drug they received on corresponding sampling sessions. For example, for the 20-mg condition, subjects received eight capsules of 2.5 mg each on the sampling session. During the subsequent self-administration session, subjects could earn zero to eight capsules of 2.5 mg MPH, depending on their performance on the progressive ratio (PR) task (see below). Subjects were instructed that each capsule earned during the self-administration session corresponded to the same dose as each capsule taken during the preceding sampling session.
Across all sessions, subjects were provided a standardized meal at the beginning of the session. Subjects provided urine samples each day that were screened for excluded drugs using InstaCup Drug Screens (Columbia Laboratory Supplies). Breath alcohol levels (BAL) were assessed each morning with a handheld breathalyzer (ALERT model; Columbia Laboratory Supplies), and participants were required to record a BAL of 0.0. No subjects were excluded for noncompliance with drug and alcohol requirements. Throughout all sessions when behavioral testing was not being conducted, subjects were allowed to participate in a variety of sedentary activities, including watching television/movies, playing video games, reading, or completing puzzles.
Behavioral testing was conducted in a separate sound-attenuated room located adjacent to the general laboratory area. The testing area consisted of a desk and chair, a microcomputer, computer monitor, keyboard, computer mouse, and physiological monitoring equipment. Responses to all subject-rated measures and the Progressive Ratio Task were collected via computer. Sampling sessions lasted approximately 4.5 h, and Self-Administration sessions lasted approximately 6 h. Subjects were able to earn a total of $1,030 for participation in this experiment.
Sampling session procedures
On each sampling session, subjects first completed all pre-drug subject-rated questionnaires, and vital signs were assessed. MPH or Placebo capsules (n=8 capsules each day) were then simultaneously administered orally, and subjects were informed that in subsequent sessions they would have the opportunity to work on a computer task to earn the drug dose they received during the sampling session. They were explicitly informed that they would be able to earn zero to eight capsules in the corresponding self-administration session. Subject-rated drug effects were evaluated 15 min prior to capsule administration and every 30 (Adjective Rating Scale [ARS], Drug Effect Questionnaire [DEQ], Side Effects) or 60 min (Addiction Research Center Inventory [ARCI]) thereafter. Heart rate and blood pressure were assessed using a Vital Check 4200 digital monitor (Ivac Corp., San Diego, CA, USA) 15 min prior to capsule ingestion and every 30 min thereafter.
Self-administration session procedures
Self-administration sessions took place 1–2 days after the corresponding sampling sessions. They were similar, except that after arrival at the laboratory and completion of the subjective effects questionnaires, subjects completed the progressive ratio task (see below) in which they had the chance to work to receive up to the total dose of MPH administered during the corresponding sampling session. The PR task lasted 60 min, after which whatever portion of the MPH dose earned was administered. Subjects then remained in the laboratory for 4 h and behavioral testing took place as described in the sampling sessions. Subjects were informed that even if they chose not to complete any ratios they would still remain in the laboratory for the full duration of the session.
Dependent measures—drug reinforcement
Progressive ratio task
During this task, subjects were instructed that they would have the opportunity to work for doses of the medications received during the previous sampling session. A total of eight divided doses MPH (or placebo) were available to subjects during each self-administration session, the total of which was equal to the dose administered during the sampling session that immediately preceded it.
The PR session lasted 60 min and was administered via computer (Rush et al. 2001; Stoops et al. 2003, 2004). The computer screen first displayed the question: “Do you want to work to earn one of the capsules from the previous sampling session?” If the participant selected “No,” they were required to remain in the testing room for the duration of the task, but no capsules were subsequently administered. If a subject responded “Yes”, a response box appeared in the center of the computer screen and they were instructed to click the mouse within the box in order to earn access to the drug. Subjects were not instructed as to how many times the button had to be pressed. The PR task was programmed such that the first ratio required 75 clicks, and each subsequent ratio required a number of clicks equal to 1.75 times the previous ratio (131, 230, 402, 703, 1,231, 2,154, and 3,770 responses, for the remaining seven ratios). As soon as a ratio was completed, the screen went blank, and a message appeared indicating that they had earned a capsule. Subjects were then given the option to continue working for additional capsules. They also had the option to choose not to respond for additional capsules. At the end of the 60 min, subjects were given the number of capsules (zero to eight) corresponding to the number of ratios they completed. The primary outcome measure was the number of ratios completed.
Dependent measures—subject-rated effects
The Adjective Rating Scale (Oliveto et al. 1992), the Addiction Research Center Inventory (Martin et al. 1971), the Drug-Effect Questionnaire (Rush et al. 2001; Stoops et al. 2004), and a Side Effects Questionnaire (SEQ; Rapoport et al. 1980) were used to assess subjective effects. The ARS consists of 32 items and contains two 16-item subscales: Sedative and Stimulant. The Stimulant scale from the ARS has been shown to be sensitive to the effects of MPH in the dose range used in the present study (Rush et al. 2001). The ARCI is commonly used to assess abuse liability of a variety of drug classes and contains five major subscales: Morphine-benzedrine group (MBG; a measure of euphoria); pentobarbital, chlorpromazine, alcohol group (PCAG; a measure of sedation); lysergic acid diethylamide (LSD; a measure of dysphoria); and benzedrine group and amphetamine scales (BG, A; empirically derived stimulant sensitive scales). The DEQ consists of eleven 100 mm visual analog scales presented on the computer screen one at a time. Subjects are instructed to rate each item on the basis of how they feel at the present time. Each visual analog scale was anchored with the descriptors “not at all,” “some,” and “an awful lot.” The DEQ items were: “I feel the medicine’s effect,” “I feel good effects of the medicine,” I feel bad effects from the medicine,” “I like the medicine,” “I feel friendly,” “I feel confused,” “I can concentrate right now,” “I feel excited,” “I feel alert,” “I feel relaxed,” and “I would like to take this medicine again.” Subjects used the mouse to position a cross-line on the analog scale to indicate their response. The SEQ is an eight-item scale that has been used to assess common side effects of stimulant drugs (e.g., Rapoport et al. 1980). The items are as follows: Worried/Anxious, Tired, Headache, Stomachache, Irritable, Sad, Loss of Appetite, and Uninterested in being around others. Participants rated each of the items on a four-point scale (1=None; 2=Mild; 3=Moderate; 4=Severe).
Drug dose and administration
All drugs were administered under double-blind conditions. Sequences of dose conditions were randomized and letter codes used to identify the conditions were counterbalanced across participants. All drugs were prepared by a research pharmacy, which also prepared and dispensed capsules. Medical oversight for the protocol was the responsibility of the study physician (AKC). During each session, participants orally ingested eight capsules (sampling sessions) or zero to eight capsules (self-administration sessions) with 150 ml water. During both sampling and self-administraton sessions, all capsules for each given day were administered at the same time. During the sampling sessions, the eight capsules were administered at the beginning of the session, and during the self-administration sessions, all earned capsules were administered at the end of the PR task. Doses were prepared by encapsulating commercially available immediate-release MPH hydrochloride and lactose filler. Placebo capsules contained only lactose. The total dose administered for each of the four sampling sessions was 0 (placebo), 20, 40, or 60 mg MPH. The amount of drug in each of the eight capsules was 0, 2.5, 5, and 7.5 mg, respectively, for these sessions. During self-administration sessions, dosing varied depending on subject performance during the PR task. Drug administration procedures were designed to ensure that participants swallow the capsules and did not open them in their mouths and taste the contents (Abreu and Griffiths 1996).
Data analysis
Analyses were conducted using Stata SE/9.0 for Macintosh (Stata Corp., College Station, TX, USA). The primary outcome measure for the study was the mean number of ratios completed on the PR task. We initially evaluated group differences on this measure using a three-way mixed model analysis of variance (ANOVA) with Group (ADHD versus Control) and Sex (Male versus Female) as the between-subjects factors and MPH Dose (placebo, 20 mg, 40 mg, 60 mg) as the within-subjects factor. Since there were no main effects or interactions with the Sex factor, men and women were combined for subsequent analyses. When main effects of interactions were observed, planned post hoc comparisons were conducted using Tukey’s honestly significant difference method to further explore main effects and interactions. We did not apply any statistical corrections to control for familywise error rate but instead used the conventional alpha level of 0.05 to determine statistical significance.
Data from sampling sessions only were used for analysis of subjective effects, since these sessions ensured standardized dosing across all participants. Peak effects for each day were calculated as the highest value post drug administration. Area under the curve (AUC) was calculated for each measure using the trapezoid method1, which integrates the time course of subjective effects across each session. Both peak effects and AUC data were subsequently analyzed in the same manner as described above for the PR task using mixed model ANOVA. Pearson correlations were also calculated between peak subjective effects from the DEQ during sampling sessions and PR performance for the corresponding self-administration session to determine whether there were specific subjective effects that were associated with MPH reinforcement. These correlations were calculated separately for ADHD and Control groups to determine whether the pattern of associations differed between groups.
Results
Progressive ratio task
For the mean number of PR ratios completed, there was a significant main effect for dose (F=3.67, p=0.02) and a significant dose × group interaction (F=3.82, p=0.01; see Fig. 1). Planned comparisons revealed that the ADHD group completed significantly more ratios for the 20-mg dose compared to the Control group (p<0.01), and a trend was noted (p<0.10) for the ADHD group to complete more ratios for the 40- and 60-mg doses. A trend was also noted for the Control subjects to complete more ratios for the placebo dose. The difference in the number of ratios completed under placebo versus active drug conditions across groups likely accounted for the significant interaction effect.
Fig. 1.

Progressive ratio task performance as a function of MPH dose for Control and ADHD subjects
Subject-rated effects
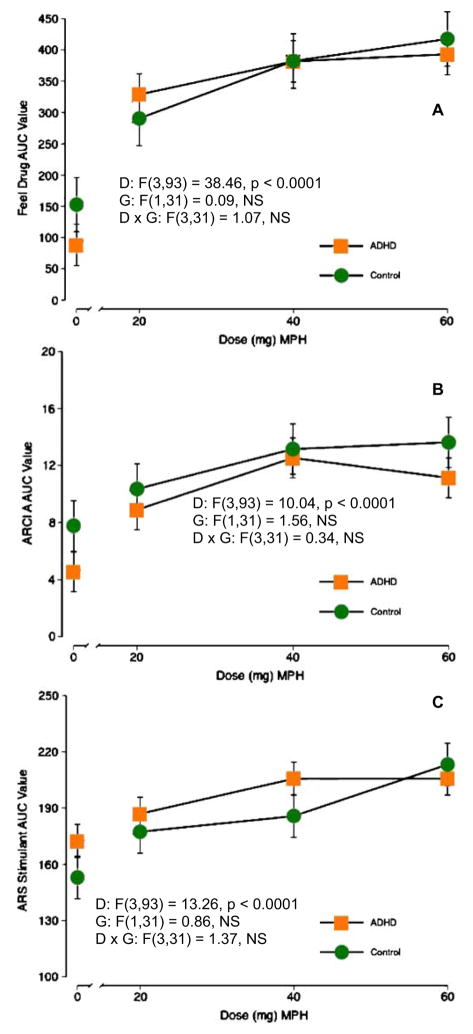
Figures 2 and 3 display representative items for subject-rated items. AUC analyses are shown since they represent the full time course of drug effects throughout the day. Peak effects and AUC results were largely similar (see below). Figure 2 shows results from prototypical stimulant drug effects (Feel Drug, ARCI A scale, ARS stimulant scale). Figure 3 shows items for which significant main effects for Group were observed. Specific details of each scale are described below.
Fig. 2.
Area-under-the-curve figures for selected subject-rated effects as a function of MPH dose for Control and ADHD subjects. Items were selected on the basis of being prototypical items for which stimulants produce robust effects and for showing significant dose effects, but no group or group × dose interaction effects. D dose, G group. a DEQ feel drug, b ARCI A scale, c ARS Stimulant scale
Fig. 3.
AUC figures for selected subject-rated items as a function of MPH dose for Control and ADHD subjects. Items were selected on the basis of showing significant effects of Group. D dose, G group. a DEQ Concentrate Item, b ARS Sedative scale
ARS
There was a main effect of dose on the AUC analysis of the Stimulant subscale (F=13.26, p<0.01), with all active MPH doses higher than placebo and the 60 mg dose higher than 20 mg. The AUC analyses also revealed significant main effects of dose (F=2.76, p<0.05) and group (F=4.63, p<0.05) for the Sedative subscale of the ARS. Post-hoc comparisons showed that the 40-mg dose was significantly lower than placebo and that the ADHD group scored significantly higher than the Control group. Only a significant main effect of dose for peak effects on the Stimulant subscale of the ARS (F=14.67, p<0.001) was observed (20, 40, 60 mg>placebo; 60>20 mg).
ARCI
There were significant main effects of dose for all five subscales in the AUC analysis (F values=4.74–10.36, p’s< 0.01). The 40- and 60-mg doses were significantly different from placebo for all subscales, and the 20-mg dose was significantly different from placebo for the BG and PCAG subscales. There was also a significant main effect of group for the BG scale (F=4.9, p<0.05) with the Control group reporting higher ratings than the ADHD group. For peak effects analyses, significant main effects of MPH dose were identified for four of the five ARCI subscales (A, BG, MBG, LSD; F values=7.69–18.12, p’s<0.001). For these scales, there were generally dose-dependent increases in scores, and all active doses of drug were statistically different from placebo.
DEQ
AUC analyses of DEQ items resulted in significant main effects for eight of 11 subscales (all items except Bad Effects, Confused, Relaxed; F values=3.99–38.46, p’s< 0.01), with active doses of MPH generally higher than placebo. There were also significant main effects of group for the Confused, Concentrate, and Alert items (F values= 4.40–11.85, p’s<0.05) with the Control group scoring higher than the ADHD group for the Concentrate and Alert items and the opposite (ADHD > Control) for the Confused item. Finally, there was a significant dose × group interaction for the Concentrate item, and post hoc comparisons revealed that the Control group scored higher under the placebo, 20, and 60 mg conditions. The pattern of findings for peak effects analyses were largely the same as for the AUC analyses of DEQ items.
SEQ
The AUC analyses of SEQ items revealed main effects of dose for the Worried–Anxious, Tired, and Loss of Appetite items (F values=4.30–14.32, p’s<0.01). Pairwise comparisons revealed that the 20, 40, and 60 mg doses were all higher than placebo for the Loss of Appetite item, and the 60-mg dose was higher than placebo for the Worried–Anxious item. The 40 mg MPH dose was lower than placebo for the Tired item. There were also main effects of group for the Irritable and Tired items with the ADHD group scoring higher (F values=4.12–4.59, p’s<0.05). In addition, a main effect of group was observed for the Sad item with ADHD individuals reporting higher scores compared to the Control group (F=7.23, p<0.05). Peak effects analyses of the SEQ items resulted in a similar pattern of findings.
Relationships between PR task and subjective effects
Table 2 shows the DEQ items by group and dose condition that were significantly correlated with PR ratios completed. As can be seen, the pattern of associations differed between the two groups—there were significant correlations between the Alert and Concentrate subjective effects items and PR ratios completed for the ADHD group only under the 20 and 40 mg dose conditions. The only DEQ items that were correlated with PR performance in the Control condition were Like Drug and Take Again for the placebo and 20-mg conditions.
Table 2.
DEQ items significantly correlated (p<0.05) with progressive ratio task performance by group and dose
| Group/dose condition | Placebo | 20 mg MPH | 40 mg MPH | 60 mg MPH |
|---|---|---|---|---|
| ADHD | – | Friendly (r=0.60); alert (r=0.54) | Good effects (r=0.54); concentrate (r=0.49); take again (r=0.52) | – |
| Control | Like drug (r=0.55); feel drug (r=0.63) | Like drug (r=0.49); feel drug (r=0.57) | – | – |
Discussion
The present study is the first to directly compare the reinforcing and subjective effects of MPH in individuals with and without ADHD. Several noteworthy findings were observed. First, the reinforcing effects of MPH as measured by the PR task were significantly higher in adults diagnosed with ADHD compared to a group of nondiagnosed adults. Second, the only group differences that were observed for the subject rated effects measures were on items generally assessing cognitive status (Concentration and Confused items from the DEQ) or affect/arousal (Sedative subscale of the ARS; Irritable, Sad, Tired from the SEQ; Alert from the DEQ). Since other measures of subject-rated effects did not differ between groups, it is possible that the comparative reinforcing effects of MPH in ADHD versus non-ADHD adults may be more related to the effects of the drug on these endpoints rather than the euphoria-producing effects that are commonly associated with drug reinforcement. Finally, among healthy, non-ADHD adults in the present study, MPH doses as high as 60 mg failed to increase PR break points above placebo levels. This was observed in spite of the fact that robust main effects of MPH were found for typical stimulant subjective effects (e.g., Like Drug, Good Effects, Excited, Would Like to Take Again, ARCI A, and ARCI BG.). This suggests that the reinforcing effects of MPH are not isomorphic with the subjective effects of the drug.
These findings are consistent with previous work that showed children and young adults with ADHD reliably chose MPH over placebo, with a lack of significant effects for subjective effects (Fredericks and Kollins 2004; MacDonald Fredericks and Kollins 2005). In addition, other studies have reported that MPH failed to produce reinforcing effects in non-ADHD adults when administered under routine laboratory conditions, although when environmental conditions are manipulated (i.e., sleep deprivation, high demand tasks), MPH exhibits more robust reinforcing effects (Chait 1994; Roehrs et al. 1999; Stoops et al. 2005).
ADHD is hypothesized to be the result of disrupted dopaminergic transmission in corticostriatal circuits, which in turn gives rise to the characteristic deficits in executive functioning observed in ADHD patients (Grace 2001; Solanto 2002). This hypothesis is supported by studies showing differences in dopamine transporter (DAT) density in relevant striatal areas in ADHD patients compared to controls (Cheon et al. 2003; Dougherty et al. 1999; Dresel et al. 2000; Krause et al. 2000, 2002, 2003; Larisch et al. 2006; Spencer et al. 2005; Volkow et al. 2007). Although these studies have reported discrepant findings with respect to the direction of DAT density change (i.e., some report higher levels, some report lower levels in ADHD), collectively, they suggest associations of DAT density and its consequent effects on DA neurotransmission with the clinical condition of ADHD. Since MPH increases DA neurotransmission through blockade of the DAT, at the clinically relevant oral doses used in the present study, the drug may reinforce behavior more strongly in patients with ADHD not because it produces euphorigenic effects but rather because it restores DA activity to comparable rates as non-ADHD individuals. These effects are noticeable by observed changes in self-reported increases in concentration, alertness, and decreases in confusion. As such, the reinforcing effects of the drug in individuals with ADHD may be mediated most saliently by improvements in cognition and executive functioning. Preliminary support for this hypothesis comes from the differential pattern of correlations observed between items from the DEQ and PR task performance (Table 2).
There was discordance in the present study between the reinforcing and subjective effects of MPH among the non-ADHD subjects. None of the active doses of MPH occasioned PR responding above placebo levels for the Control group. By contrast, even when considered independently of the ADHD group, a number of prototypical stimulant drug subjective effects showed main effects of MPH dose for the Control group (e.g., Good Effects, Like Drug, Take Again, ARCI A; data not shown). These findings highlight the fact that multiple behavioral indices of abuse liability are required to characterize the potential for a drug to be self-administered. A more fine-grained analysis of these data may be required to better understand the findings. For example, previous studies of drug reinforcement have conducted “responder analyses” by examining subjects who worked for drug administration versus those who did not to help identify those individual difference and drug factors that are most strongly associated with reinforcement (Perkins et al. 1997). For example, several studies have shown that the personality trait of sensation seeking is positively associated with the reinforcing effects of amphetamine (Kelly et al. 2006; Stoops et al. 2007). Although not measured in the present study, similar personality traits may help to explain our lack of reinforcing effects in the control group and the observed between group differences
The lack of reinforcing effects of MPH in the non-ADHD individuals in the present study deserves comment given the well-established abuse potential of MPH (Kollins et al. 2001). This observation is not unprecedented in the literature. Several previous studies have failed to show that MPH functions as a reinforcer under routine laboratory conditions (Chait 1994; Roehrs et al. 1999; Stoops et al. 2005). In studies that have shown MPH to function as a reinforcer, it often occurs under specific environmental conditions, such as sleep deprivation or prior to a high demand task (Roehrs et al. 1999; Stoops et al. 2005). The methods for this study did not require any specific demands following the PR task, other than remaining in the laboratory for several hours and participating in sedentary activities. As such, the present findings are comparable to those studies that failed to report reinforcing effects of MPH in presumably non-ADHD adults. Participants in the present study were also stimulant naïve and were therefore receiving MPH for the first time in the laboratory. The reinforcing effects of MPH may have been different in the non-ADHD group if individuals who had recreational experience with stimulant drugs were included.
The present study had several limitations. First, we did not include objective measures of attention, concentration, or other executive functions, and thus, the interpretation that MPH reinforcement was mediated in individuals with ADHD by improvements in concentration and alertness is based solely on self-report. Moreover, the observed mediation is only a correlation and cannot be interpreted as causal. It will be important for future work to evaluate the association between improvements in cognitive functioning and drug reinforcement in individuals with ADHD. The dose range of MPH evaluated in the present study (20–60 mg) was also somewhat narrow and may explain the lack of reinforcing effects in the non-ADHD participants and, in general, the lack of dose-dependent effects across endpoints. Also, there were no explicit task manipulations following MPH administration, and this may have also led to lower reinforcing effects among non-ADHD subjects. Given that group differences in drug reinforcement were still observed, however, the dose range and post-administration tasks might best be viewed as independent variables to be manipulated in subsequent studies, rather than outright limitations of this study. Our sample ascertainment strategy may limit generalizability of the findings. We excluded those participants in both groups who exhibited any DSM-IV Axis I or II psychopathology, or who had any history of recreational or clinical stimulant use. Comorbidity in adult ADHD is common and among non-ADHD individuals, those with no psychiatric history may be least likely to misuse MPH (Kessler et al. 2006). Epidemiological surveys suggest that illicit use of prescription stimulants like MPH is more likely to occur in individuals with more extensive drug use history (McCabe et al. 2005). It would be interesting in future studies to include a more diverse sample of both ADHD and non-ADHD individuals to determine whether psychiatric comorbidity or stimulant use history moderate the reinforcing effects of the drug. We also did not apply any corrections to our statistical tests, and given the number of comparisons that were conducted, it is possible that some of the observed effects occurred by chance. However, since the majority of significant effects converged in a similar direction, this concern is mitigated to some degree. Finally, our use of immediate-release MPH precludes conclusions about the reinforcing or subjective effects of longer-acting formulations of MPH, which are commonly used. At least two studies have shown that longer-acting formulations produce lower magnitude subjective effects in non-ADHD individuals, and future work should clarify whether similar formulation differences are observed for reinforcing measures (Kollins et al. 1998; Spencer et al. 2006).
In spite of these limitations, the present study adds to the literature on abuse liability of MPH, especially in the context of clinical use of the drug for the management of ADHD in adults. Individuals with ADHD responded more for MPH capsules than their nondiagnosed peers, and these reinforcing effects were not related to euphorogenic subjective effects. MPH and other stimulants continue to be a mainstay of effective management of ADHD across the lifespan. Still, better understanding of the risk profile of the drug with respect to abuse liability will improve the care we are able to provide to our patients.
Acknowledgments
This work was funded by grant R01DA017196 from the National Institute on Drug Abuse (SHK).
Footnotes
The trapezoid method sums post drug administration values according to the following formula: [1/2×(time 1 value)+sum (time 2 value+time 3 value+…time n−1 value)+1/2×(time n value)]. This method has been used previously to describe time course effects of drugs in laboratory studies (Kollins and Rush 1999).
References
- Abreu ME, Griffiths RR. Drug tasting may confound human drug discrimination studies. Psychopharmacology (Berl) 1996;125:255–257. doi: 10.1007/BF02247336. [DOI] [PubMed] [Google Scholar]
- Berbatis CG, Sunderland VB, Bulsara M. Licit psychostimulant consumption in Australia, 1984–2000: international and jurisdictional comparison. Med J Aust. 2002;177:539–543. doi: 10.5694/j.1326-5377.2002.tb04948.x. [DOI] [PubMed] [Google Scholar]
- Brady J. The reinforcing functions of drugs and assessment of abuse liability. NIDA Res Monogr. 1988;81:440–456. [PubMed] [Google Scholar]
- Chait LD. Reinforcing and subjective effects of methylphenidate in humans. Behav Pharmacol. 1994;5:281–288. doi: 10.1097/00008877-199406000-00005. [DOI] [PubMed] [Google Scholar]
- Cheon KA, Ryu YH, Kim YK, Namkoong K, Kim CH, Lee JD. Dopamine transporter density in the basal ganglia assessed with [123I]IPT SPET in children with attention deficit hyperactivity disorder. Eur J Nucl Med Mol Imaging. 2003;30:306–311. doi: 10.1007/s00259-002-1047-3. [DOI] [PubMed] [Google Scholar]
- Conners CK, Erhardt D, Sparrow EP. Conners Adult ADHD Rating Scale. Multi-Health Systems, Inc; 1998. [Google Scholar]
- Dougherty DD, Bonab AA, Spencer TJ, Rauch SL, Madras BK, Fischman AJ. Dopamine transporter density in patients with attention deficit hyperactivity disorder. Lancet. 1999;354:2132–2133. doi: 10.1016/S0140-6736(99)04030-1. [DOI] [PubMed] [Google Scholar]
- Dresel S, Krause J, Krause KH, LaFougere C, Brinkbaumer K, Kung HF, Hahn K, Tatsch K. Attention deficit hyperactivity disorder: binding of [99mTc]TRODAT-1 to the dopamine transporter before and after methylphenidate treatment. Eur J Nucl Med. 2000;27:1518–1524. doi: 10.1007/s002590000330. [DOI] [PubMed] [Google Scholar]
- Elia J, Ambrosini PJ, Rapoport JL. Treatment of attention-deficit-hyperactivity disorder. N Engl J Med. 1999;340:780–788. doi: 10.1056/NEJM199903113401007. [DOI] [PubMed] [Google Scholar]
- Epstein JN, Johnston DE, Conners CK. Conners Adult ADHD Interview for DSM-IV. Multi-Health Systems; 2000. [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW. User’s guide for the Structured Clinical Interview for DSM-IV Axis I Disorders—Research Version. Biometrics Research Department; 1996. [Google Scholar]
- Fogelman Y, Kahan E. Methylphenidate use for attention deficit hyperactivity disorder in northern Israel–a controversial issue. Isr Med Assoc J. 2001;3:925–927. [PubMed] [Google Scholar]
- Fredericks EM, Kollins SH. Assessing methylphenidate preference in ADHD patients using a choice procedure. Psychopharmacology (Berl) 2004;175:391–398. doi: 10.1007/s00213-004-1838-2. [DOI] [PubMed] [Google Scholar]
- Garland EJ. Intranasal abuse of prescribed methylphenidate [letter] J Am Acad Child Adolesc Psych. 1998;37:1242–1243. doi: 10.1097/00004583-199812000-00003. [DOI] [PubMed] [Google Scholar]
- Goldman LS, Genel M, Bezman RJ, Slanetz PJ. Diagnosis and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Council on Scientific Affairs, American Medical Association. JAMA. 1998;279:1100–1107. doi: 10.1001/jama.279.14.1100. [DOI] [PubMed] [Google Scholar]
- Grace AA. Psychostimulant actions on dopamine and limbic system function: Relevance to the pathophysiology and treatment of ADHD. In: Solanto MV, Arnsten AFT, Castellanos FX, editors. Stimulant Drugs & ADHD: Basic and Clinical Neuroscience. Oxford University Press; Oxford: 2001. pp. 134–157. [Google Scholar]
- Ivis FJ, Adlaf EM. Prevalence of methylphenidate use among adolescents in Ontario. Can J Public Health. 1999;90:309–312. doi: 10.1007/BF03404516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Secondary school students. I. National Institute on Drug Abuse; Bethesda, MD: 2006. Monitoring the Future national survey results on drug use, 1975–2005. (NIH Publication No. 06-5883) [Google Scholar]
- Kelly TH, Robbins G, Martin CA, Fillmore MT, Lane SD, Harrington NG, Rush CR. Individual differences in drug abuse vulnerability: d-amphetamine and sensation-seeking status. Psychopharmacology (Berl) 2006;189:17–25. doi: 10.1007/s00213-006-0487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, Faraone SV, Greenhill LL, Howes MJ, Secnik K, Spencer T, Ustun TB, Walters EE, Zaslavsky AM. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163:716–723. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollins SH. Comparing the abuse potential of methylphenidate versus other stimulants: a review of available evidence and relevance to the ADHD patient. J Clin Psychiatry. 2003;64(Suppl 11):14–18. [PubMed] [Google Scholar]
- Kollins SH, Rush CR. Effects of training dose on the relationship between discriminative-stimulus and self-reported drug effects of d-amphetamine in humans. Pharmacol Biochem Behav. 1999;64:319–329. doi: 10.1016/s0091-3057(99)00084-2. [DOI] [PubMed] [Google Scholar]
- Kollins SH, Rush CR, Pazzaglia PJ, Ali JA. Comparison of acute behavioral effects of sustained-release and immediate-release methylphenidate. Exp Clin Psychopharmacol. 1998;6:367–374. doi: 10.1037//1064-1297.6.4.367. [DOI] [PubMed] [Google Scholar]
- Kollins SH, MacDonald EK, Rush CR. Assessing the abuse potential of methylphenidate in nonhuman and human subjects: a review. Pharmacol Biochem Behav. 2001;68:611–627. doi: 10.1016/s0091-3057(01)00464-6. [DOI] [PubMed] [Google Scholar]
- Krause KH, Dresel SH, Krause J, Kung HF, Tatsch K. Increased striatal dopamine transporter in adult patients with attention deficit hyperactivity disorder: effects of methylphenidate as measured by single photon emission computed tomography. Neurosci Lett. 2000;285:107–110. doi: 10.1016/s0304-3940(00)01040-5. [DOI] [PubMed] [Google Scholar]
- Krause KH, Dresel SH, Krause J, Kung HF, Tatsch K, Ackenheil M. Stimulant-like action of nicotine on striatal dopamine transporter in the brain of adults with attention deficit hyperactivity disorder. Int J Neuropsychopharmacol. 2002;5:111–113. doi: 10.1017/S1461145702002821. [DOI] [PubMed] [Google Scholar]
- Krause KH, Dresel SH, Krause J, la Fougere C, Ackenheil M. The dopamine transporter and neuroimaging in attention deficit hyperactivity disorder. Neurosci Biobehav Rev. 2003;27:605–613. doi: 10.1016/j.neubiorev.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Kroutil LA, Van Brunt DL, Herman-Stahl MA, Heller DC, Bray RM, Penne MA. Nonmedical use of prescription stimulants in the United States. Drug Alcohol Depend. 2006;84:135–143. doi: 10.1016/j.drugalcdep.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Larisch R, Sitte W, Antke C, Nikolaus S, Franz M, Tress W, Muller HW. Striatal dopamine transporter density in drug naive patients with attention-deficit/hyperactivity disorder. Nucl Med Commun. 2006;27:267–270. doi: 10.1097/00006231-200603000-00010. [DOI] [PubMed] [Google Scholar]
- Levine B, Caplan YH, Kauffman G. Fatality resulting from methylphenidate overdose. J Anal Toxicol. 1986;10:209–210. doi: 10.1093/jat/10.5.209. [DOI] [PubMed] [Google Scholar]
- Lin SJ, Crawford SY, Lurvey PL. Trend and area variation in amphetamine prescription usage among children and adolescents in Michigan. Soc Sci Med. 2005;60:617–626. doi: 10.1016/j.socscimed.2004.06.002. [DOI] [PubMed] [Google Scholar]
- MacDonald Fredericks E, Kollins SH. A pilot study of methylphenidate preference assessment in children diagnosed with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2005;15:729–741. doi: 10.1089/cap.2005.15.729. [DOI] [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, Jasinski DR. Physiologic, subjective and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12(2):245–258. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- Massello W, 3rd, Carpenter DA. A fatality due to the intranasal abuse of methylphenidate (Ritalin) J Forensic Sci. 1999;44:220–221. [PubMed] [Google Scholar]
- McCabe SE, Knight JR, Teter CJ, Wechsler H. Non-medical use of prescription stimulants among US college students: prevalence and correlates from a national survey. Addiction. 2005;100:96–106. doi: 10.1111/j.1360-0443.2005.00944.x. [DOI] [PubMed] [Google Scholar]
- Oliveto AH, Bickel WK, Hughes JR, Shea PJ, Higgins ST, Fenwick JW. Caffeine drug discrimination in humans: acquisition, specificity and correlation with self-reports. J Pharmacol Exp Ther. 1992;261:885–894. [PubMed] [Google Scholar]
- Perkins KA, Grobe JE, Caggiula A, Wilson AS, Stiller RL. Acute reinforcing effects of low-dose nicotine nasal spray in humans. Pharmacol Biochem Behav. 1997;56:235–241. doi: 10.1016/s0091-3057(96)00216-x. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Buchsbaum MS, Weingartner H, Zahn TP, Ludlow C, Mikkelsen EJ. Dextroamphetamine. Its cognitive and behavioral effects in normal and hyperactive boys and normal men. Arch Gen Psychiatry. 1980;37:933–943. doi: 10.1001/archpsyc.1980.01780210091010. [DOI] [PubMed] [Google Scholar]
- Robison LM, Sclar DA, Skaer TL, Galin RS. National trends in the prevalence of attention-deficit/hyperactivity disorder and the prescribing of methylphenidate among school-age children: 1990–1995. Clin Pediatr (Phila) 1999;38:209–217. doi: 10.1177/000992289903800402. [DOI] [PubMed] [Google Scholar]
- Roehrs T, Papineau K, Rosenthal L, Roth T. Sleepiness and the reinforcing and subjective effects of methylphenidate. Exp Clin Psychopharmacol. 1999;7:145–150. doi: 10.1037//1064-1297.7.2.145. [DOI] [PubMed] [Google Scholar]
- Romano E, Baillargeon RH, Wu HX, Robaey P, Tremblay RE. Prevalence of methylphenidate use and change over a two-year period: a nationwide study of 2- to 11-year-old Canadian children. J Pediatr. 2002;141:71–75. doi: 10.1067/mpd.2002.125399. [DOI] [PubMed] [Google Scholar]
- Rush CR, Essman WD, Simpson CA, Baker RW. Reinforcing and subject-rated effects of methylphenidate and d-amphetamine in non-drug-abusing humans. J Clin Psychopharmacol. 2001;21:273–286. doi: 10.1097/00004714-200106000-00005. [DOI] [PubMed] [Google Scholar]
- Safer DJ, Zito JM, Fine EM. Increased methylphenidate usage for attention deficit disorder in the 1990s. Pediatrics. 1996;98:1084–1088. [PubMed] [Google Scholar]
- Solanto MV. Dopamine dysfunction in AD/HD: integrating clinical and basic neuroscience research. Behav Brain Res. 2002;130:65–71. doi: 10.1016/s0166-4328(01)00431-4. [DOI] [PubMed] [Google Scholar]
- Spencer TJ, Biederman J, Madras BK, Faraone SV, Dougherty DD, Bonab AA, Fischman AJ. In vivo neuroreceptor imaging in attention-deficit/hyperactivity disorder: a focus on the dopamine transporter. Biol Psychiatry. 2005;57:1293–1300. doi: 10.1016/j.biopsych.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Spencer TJ, Biederman J, Ciccone PE, Madras BK, Dougherty DD, Bonab AA, Livni E, Parasrampuria DA, Fischman AJ. PET study examining pharmacokinetics, detection and likeability, and dopamine transporter receptor occupancy of short- and long-acting oral methylphenidate. Am J Psychiatry. 2006;163:387–395. doi: 10.1176/appi.ajp.163.3.387. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Glaser PE, Rush CR. Reinforcing, subject-rated, and physiological effects of intranasal methylphenidate in humans: a dose–response analysis. Drug Alcohol Depend. 2003;71:179–186. doi: 10.1016/s0376-8716(03)00131-5. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Glaser PE, Fillmore MT, Rush CR. Reinforcing, subject-rated, performance and physiological effects of methylphenidate and d-amphetamine in stimulant abusing humans. J Psychopharmacol. 2004;18:534–543. doi: 10.1177/0269881104047281. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Fillmore MT, Glaser PE, Rush CR. Reinforcing effects of methylphenidate: influence of dose and behavioral demands following drug administration. Psychopharmacology (Berl) 2005;177:349–355. doi: 10.1007/s00213-004-1946-z. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Robbins CG, Martin CA, Rush CR, Kelly TH. The reinforcing, subject-rated, performance, and cardiovascular effects of d-amphetamine: influence of sensation-seeking status. Addict Behav. 2007;32:1177–1188. doi: 10.1016/j.addbeh.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Newcorn J, Fowler JS, Telang F, Solanto MV, Logan J, Wong C, Ma Y, Swanson JM, Schulz K, Pradhan K. Brain dopamine transporter levels in treatment and drug naive adults with ADHD. Neuroimage. 2007;34:1182–1190. doi: 10.1016/j.neuroimage.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Zito JM, Safer DJ, dosReis S, Gardner JF, Boles M, Lynch F. Trends in the prescribing of psychotropic medications to preschoolers. JAMA. 2000;283:1025–1030. doi: 10.1001/jama.283.8.1025. [DOI] [PubMed] [Google Scholar]