Abstract
Aims
Patients with paroxysmal atrial fibrillation (AF) often present with typical angina pectoris and mildly elevated levels of cardiac troponin (non ST-segment elevation myocardial infarction) during an arrhythmic event. However, in a large proportion of these patients, significant coronary artery disease is excluded by coronary angiography. Here we explored the potential underlying mechanism of these events.
Methods and results
A total of 14 pigs were studied using a closed chest, rapid atrial pacing (RAP) model. In five pigs RAP was performed for 7 h (600 b.p.m.; n = 5), in five animals RAP was performed in the presence of angiotensin-II type-1-receptor (AT1-receptor) inhibitor irbesartan (RAP+Irb), and four pigs were instrumented without intervention (Sham). One-factor analysis of variance was performed to assess differences between and within the three groups. Simultaneous measurements of fractional flow reserve (FFR) and coronary flow reserve (CFR) before, during, and after RAP demonstrated unchanged FFR (P = 0.327), but decreased CFR during RAP (RAP: 67.7 ± 7.2%, sham: 97.2 ± 2.8%, RAP+Irb: 93.2 ± 3.3; P = 0.0013) indicating abnormal left ventricular (LV) microcirculation. Alterations in microcirculatory blood flow were accompanied by elevated ventricular expression of NADPH oxidase subunit Nox2 (P = 0.039), lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1, P = 0.004), and F2-isoprostane levels (P = 0.008) suggesting RAP-related oxidative stress. Plasma concentrations of cardiac troponin-I (cTn-I) increased in RAP (RAP: 613.3 ± 125.8 pmol/L vs. sham: 82.5 ± 12.5 pmol/L; P = 0.013), whereas protein levels of eNOS and LV function remained unchanged. RAP+Irb prevented the increase of Nox2, LOX-1, and F2-isoprostanes, and abolished the impairment of microvascular blood flow.
Conclusion
Rapid atrial pacing induces AT1-receptor-mediated oxidative stress in LV myocardium that is accompanied by impaired microvascular blood flow and cTn-I release. These findings provide a plausible mechanism for the frequently observed cTn-I elevation accompanied with typical angina pectoris symptoms in patients with paroxysmal AF and normal (non-stenotic) coronary arteries.
Keywords: Angina pectoris, Angiotensin, Atrial fibrillation, Microvascular flow, Oxidative stress
Introduction
Angina pectoris is a typical symptom in patients with paroxysmal atrial fibrillation (AF). In most of these patients, angina pectoris is associated with mildly elevated cardiac troponin (cTn) levels suggesting a non-ST-segment elevation myocardial infarction. However, in a large proportion of these patients, significant coronary artery disease can be excluded by coronary angiography despite clinical symptoms.1,2
Although the elevated ventricular rate during AF may contribute to the symptoms of angina pectoris,1 angina pectoris develops also in patients with slow ventricular rate and most patients tolerate fast ventricular rates in sinus rhythm without any clinical symptoms.3,4
Chronic atrial tachycardia impairs ventricular function by causing myocardial ischaemia with abnormalities in Ca2+ handling and contractility. It further leads to alterations in extracellular matrix and tissue structure and tachycardia-induced cardiomyopathy.5 Recent reports suggest that myocardial blood flow is reduced, whereas coronary vascular resistance is elevated in patients with AF.6,7 One potential link between AF, abnormal ventricular perfusion, and cardiomyocyte dysfunction is the renin–angiotensin system. Atrial tachycardia or AF increases systemic and cardiac tissue angiotensin-II (ANG-II) levels.8,9 ANG-II activates nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase)10–12 and induces oxidative stress10 that may impair ventricular microvascular blood flow causing myocardial ischaemia, release of cTn, and ventricular dysfunction. Moreover, repetitive episodes of AF-induced ventricular ischaemia may contribute to the development of a pathologic vicious cycle combining AF and left ventricular (LV) dysfunction.
The present study addressed the molecular basis of the discrete LV alterations induced by short-term rapid atrial pacing (RAP). Specifically, we studied whether RAP increases oxidative stress in the LV and impairs coronary and microvascular blood flow through ANG-II-dependent mechanisms.
Methods
Rapid atrial pacing model
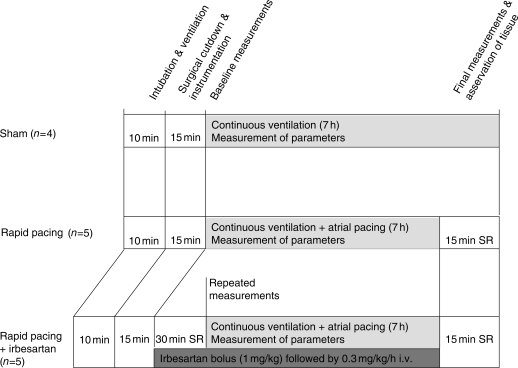
A total of 14 pigs (23 ± 3 kg) were studied (Figure 1). Pigs were pre-medicated, intubated, and instrumented as previously described.8 Rapid atrial pacing was performed in five animals (Pacing Group; RAP) at a rate of 600 b.p.m. (twice diastolic threshold, 2 ms pulse duration) for 7 h. In five additional animals, RAP was performed in the presence of irbesartan infusion (1 mg/kg bolus followed by 0.3 mg/kg/h i.v.; Irbesartan Group; RAP+Irb), and four pigs were instrumented without further interventions (sham; Figure 1). After 7 h of RAP, the chest and the pericardial sac were opened and the heart exposed. Parts of the anterior wall of the LV were cross-clamped, excised, and immediately frozen in liquid nitrogen.
Figure 1.
Study conduct of the in vivo experiments. Sham-operated animals served as control; SR, sinus rhythm.
Pigs were randomly assigned to the study groups. No animal was excluded from the study. The experiments were done in a repetitive order with each sham followed by one RAP+Irb and one RAP. Molecular analyses with regard to protein expression and determination of plasma levels of cardiac markers were done by investigators blinded to the in vivo results. The animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Magdeburg.
Measurement of coronary flow reserve
The coronary flow reserve (CFR) measurements were performed using a pressure temperature sensor-tipped guidewire (Radi Medical System), which also allows the simultaneous determination of the fractional flow reserve (FFR). The CFR measurements are influenced by flow abnormalities in the epicardial arteries and the microcirculation. In contrast, reduced FFR is specific to epicardial lesions. Thus, a normal FFR (non-obstructed epicardial vessels) in the presence of reduced CFR is indicative of microvascular pathology.11,12 Angiography of the left coronary artery was performed using a 6F guiding catheter. Thereafter, a coronary pressure wire (Radi Medical System) was advanced into the distal left anterior descending (LAD) artery. Coronary flow reserve was calculated using the Radi Medical System software package. In brief, the software allows the pressure sensor, located 3 cm from the tip of the pressure wire, to act also as a distal temperature sensor, whereas the shaft of the wire acts as a proximal temperature sensor. Thus, the transit time of an injectant can be calculated. Three millilitre of saline (room temperature) was injected by hand into the LAD three times. The mean transit time was recorded after each injection and then averaged. Thereafter, hyperaemia was induced by systemic application of adenosine at a dose of 140 µg/kg/min and the measurements of the transit time were repeated as described above. The CFR was calculated by the ratio between the baseline and hyperaemic values. Simultaneously to the CFR, the FFR was calculated by the software. Measurements of FFR and CFR were performed during normal sinus rhythm before and 15 min after 7 h pacing (Figure 1). In addition, parameters were also assessed during pacing immediately before its termination. Baseline FFR value was 0.98 ± 0.08 and CFR baseline value was 1.6 ± 0.09. Baseline values were used for normalization and set to 100%. Baseline values were comparable to previous reports obtained in pigs (FFR, 0.97 ± 0.03), in which it was shown that CFR values (mean 2.0 ± 0.9; range 0.7–4.5) are often lower than 2 and show a larger variability compared with humans.12,13
Western blotting
Extracted LV proteins (30 µg per lane) were separated by 10% PAGE, followed by transfer to nitrocellulose membranes (Schleicher and Schuell, Dassel, Germany). Membranes were blocked with Roti-Block (Roth, Karlsruhe, Germany). The primary antibodies against human oxidized low-density lipoprotein receptor-1 (LOX-1)/SR-E1 (polyclonal goat IgG, dilution in TBS 1:1000, R&D Systems, Wiesbaden, Germany), endothelial nitric oxide synthase (eNOS; sc-654 polyclonal rabbit IgG, dilution in TBS 1:1000, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, MAB374 mouse monoclonal IgG, 1:1000, Chemicon Europe, Hamshire, UK) together with the secondary anti-goat (1:10000, Dianova, Hamburg, Germany), anti-mouse, or anti-rabbit (1:2000, Cell Signaling Technology, Inc., Danvers, MA, USA) horseradish-peroxidase-conjugated antibodies, and the SuperSignal West Dura Extended Duration substrate (Pierce, Rockford, IL, USA) were used for detection.
For detection of the NADPH oxidase subunits Nox1, Nox2, and p67phox, proteins were isolated as previously described.14,15 Membranes were incubated with primary antibodies against Nox1 (Mox1) (1:1000, Santa Cruz Biotechnology, Inc.), Nox2 (p91phox) (1:1000, BD Transduction Laboratories, Franklin Lakes, NJ, USA) or p67phox (1:500, BD Transduction Laboratories) and then with secondary horseradish peroxidase conjugated anti-rabbit IgG or anti-mouse IgG (1:2000 or 1:5000; GE Healthcare, Freiburg, Germany). Subsequently, the same membranes were normalized to GAPDH (1:5000, Abcam, Cambridge, UK). The protein expression was detected with Western Lightning Chemiluminescence Reagent Plus (PerkinElmer Life Sciences, Boston, MA, USA) and quantified using AIDA Image Analyzer software (Raytest, Berlin, Germany).
RNA isolation, reverse transcription, and quantitative PCR
Total RNA was prepared from ventricular tissue by applying the method of Chomczynski and Sacchi as recently described.16 One microgram of total RNA was transcribed into cDNA using AMV reverse transcriptase (Promega, Mannheim, Germany) as described previously.17 The iCycler (BioRad, Munich, Germany) was used for quantitative PCR. All samples were analysed in triplicate.
Determination of tissue concentrations of F2-isoprostanes
Frozen LV samples were sliced by scissors and subsequently homogenized by ultraturrax and Dounce homogenizer to yield 10% homogenates in 180 mM KCL, 10 mM EDTA, 0.1 mM BHT, pH 7.4. To hydrolyse esterified F2-isoprostanes, the heart homogenates (100 µL) were treated with 4 M KOH at 40°C for 30 min and neutralized by addition of 4 M HCl (pH adjusted to 2.0 with 0.1 M HCl). One nanogram of 9α,11α-PGF2α-d4 (Cayman Chem. Co., Ann Arbor, MI, USA) was used as internal standard. The samples were centrifuged at 5000 g for 15 min and the supernatant was applied onto a C18 cartridge. Solid phase extraction and derivatization steps were performed as previously described.18 F2-isoprostanes were separated and measured by gas chromatography negative-ion chemical ionization mass spectrometry assay (DSQ/Trace GC Ultra, Thermo Fischer Scientific, Dreieich, Germany) with ammonia as reagent gas using selected ion monitoring of the carboxylate anion [M-181] at m/z 569 and m/z 573 for F2-isoprostanes and the deuterated internal standard. The F2-isoprostane peak co-eluted with authentic 8-iso-PGF2α, but it is known that other F2-isoprostane isomers occur at the same retention time.19 All analyses were performed in triplicate for each tissue sample. The protein content was determined using the method of Bradford.20
Determination of plasma concentrations of NT-pro-BNP and cardiac Tn-I
N-terminal pro-B-type natriuretic peptide (NT-pro-BNP) was measured on the E-module of the Cobas 6000 analyzer (ROCHE Diagnostics GmbH, Mannheim, Germany) using the sandwich ECLIA immunoassay (electrochemiluminescence) for the in vitro quantitative determination in human serum and plasma. Measuring range varied between 0.6 pmol/L (lower detection limit) and 4130 pmol/L (maximum of the master curve). In vitro tests with 51 commonly used pharmaceuticals showed no interference. Daily quality control was performed using Elecsys PreciControl Cardiac 1 and 2 (ROCHE Diagnostics GmbH).
Cardiac Tn-I (cTn-I) was measured on the Axsym analyzer (Abbott Laboratories, Abbott Park, IL, USA) using the three-step MEIA (Microparticle enzyme immunoassay) for the in vitro quantitative determination in serum and plasma. Measuring range varied between 0.02 and 22 ng/mL (linearity). Cross reactivity with muscle Tn-I was ≤0.1%, with troponin-T and troponin-C <1%. Daily quality control was performed using AxSym Troponin I ADV controls (Abbott Laboratories Ltd., Cootehill, Ireland).
Statistical analysis
The parameters analysed are summarized as mean + SEM in Table 1. Haemodynamic parameters are presented as mean values averaged from repetitive measurements within each animal throughout the study period. After 7 h pacing, the three groups (sham; RAP; RAP+Irb) were compared with one-factor analysis of variance (ANOVA, Table 1). In case of a significant global test, the Tukey test was used for pairwise group comparison (Table 2).
Table 1.
Group comparisons (ANOVA)
| Parameter | Sham | RAP | RAP+Irb | P-value of ANOVA |
|---|---|---|---|---|
| Haemodynamic parameters | x̄±sError | x̄±sError | x̄±sError | |
| Right atrial pressure (mmHg) | 5.3 ± 0.9 | 6.2 ± 0.7 | 5.8 ± 0.9 | 0.816 |
| Systolic right ventricular pressure (mmHg) | 23.7 ± 2.3 | 26.6 ± 1.4 | 24.2 ± 2.4 | 0.576 |
| Pulmonary capillary wedge pressure (mmHg) | 6.3 ± 0.3 | 7.0 ± 0.9 | 5.8 ± 0.7 | 0.535 |
| Systolic left ventricular pressure (mmHg) | 80 ± 4 | 74 ± 4 | 79 ± 2 | 0.375 |
| LV end-diastolic pressure (mmHg) | 4.3 ± 0.3 | 4.6 ± 0.8 | 5.4 ± 0.7 | 0.608 |
| Mean heart rate (b.p.m.) | 90 ± 3 | 83 ± 7 | 81 ± 7 | 0.659 |
| Mean ventricular rate (b.p.m.) | 84 ± 3 | 110 ± 5 | 114 ± 4 | 0.003 |
| Molecular markers | ||||
| Nox1 (OD) | 0.140 ± 0.021 | 0.248 ± 0.064 | 0.151 ± 0.045 | 0.246 |
| Nox2 (OD) | 0.344 ± 0.049 | 0.597 ± 0.096 | 0.326 ± 0.053 | 0.039 |
| p67phox (OD) | 0.380 ± 0.081 | 0.358 ± 0.034 | 0.501 ± 0.073 | 0.305 |
| F2-isoprostane (pg/mg) | 303.67 ± 23.48 | 445.00 ± 36.25 | 300.00 ± 17.19 | 0.008 |
| LOX-1 (%) | 100.04 ± 28.59 | 527.10 ± 140.78 | 65.10 ± 35.86 | 0.004 |
| eNOS (%) | 100.00 ± 36.35 | 130.04 ± 33.28 | 98.52 ± 17.39 | 0.691 |
| eNOS mRNA (%) | 100.00 ± 19.99 | 20.07 ± 8.56 | 13.02 ± 2.41 | 0.002 |
| Flow parameters | ||||
| FFR baseline (%) | 100.00 ± 0.00 | 99.96 ± 2.58 | 101.36 ± 1.37 | |
| 7 h | 93.40 ± 4.60 | 96.53 ± 6.64 | 0.333* | |
| Post | 94.27 ± 0.68 | 96.54 ± 6.18 | 100.60 ± 3.28 | 0.327** |
| CFR baseline (%) | 100.00 ± 0.00 | 100.00 ± 5.97 | 104.36 ± 10.39 | |
| 7 h | 62.70 ± 3.68 | 93.28 ± 5.92 | 0.004* | |
| Post | 97.23 ± 2.77 | 67.73 ± 7.24 | 93.16 ± 3.29 | 0.001** |
Abbreviations are as explained in the text. OD, optical density.
P-values <0.05 are marked bold.
*P-value for factor ‘Group’ in ANCOVA to compare RAP vs. RAP+Irb after 7 h pacing with ‘baseline’ as covariable.
**P-value for factor ‘Group’ in ANCOVA to compare the three groups at the end of the experiment with ‘baseline’ as covariable.
Table 2.
Group comparisons (post-hoc analysis with Tukey test and derived confidence intervals, CI)
| Groups | Sham–RAP |
Sham–RAP+Irb |
RAP–RAP+Irb |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Difference of mean | CI | P-value | Difference of mean | CI | P-value | Difference of mean | CI | P-value |
| Mean ventricular rate | 25.5 | 7.6 to 43.3 | 0.007 | 29.3 | 11.4 to 47.1 | 0.003 | 3.8 | −11.6 to 19.2 | 0.783 |
| Nox2 (OD) | 0.252 | −0.021 to 0.526 | 0.070 | 0.018 | −0.255 to 0.291 | 0.982 | 0.271 | 0.003 to 0.544 | 0.052 |
| F2-isoprostane (pg/mg) | 141.3 | 25.3 to 257.4 | 0.020 | 3.7 | −112.4 to 119.7 | 0.996 | 145.0 | 37.6 to 252.5 | 0.012 |
| LOX-1 (%) | 427.1 | 106.6 to 747.6 | 0.011 | 35.0 | −269.1 to 339.0 | 0.947 | 462.0 | 158.0 to 766.0 | 0.005 |
| eNOS mRNA (%) | 79.93 | 30.06 to 129.80 | 0.004 | 86.94 | 37.11 to 136.85 | 0.002 | 7.05 | −42.82 to 56.92 | 0.918 |
| CFR (7 h) | 34.78 | 19.84 to 49.71 | 0.002 | ||||||
| CFR (post) | 29.51 | 8.28 to 50.73 | 0.009 | 4.073 | −16.22 to 24.37 | 1.000 | 25.43 | 6.79 to 44.08 | 0.022 |
Abbreviations are as explained in the text. OD, optical density.
P-values <0.05 are marked bold.
Measurements of FFR and CFR were performed during normal sinus rhythm before and 15 min after 7 h pacing (end of experiment). To compare these parameters, differences between ‘baseline’ and this time point are of special interest. We performed an ANOVA with ‘group’ as main effect and ‘baseline value’ as covariate. In these models, the group effect gives information for differences between baseline and the examined time point. All statistical decisions were made two-tailed with a critical probability of α = 5% without α-adjustment except the Tukey test for pairwise group comparisons. Statistical analyses were performed using SAS® software, version 9.1.3 (SAS Institute Inc., Cary, NC, USA).
Results
Haemodynamic parameters
Haemodynamic parameters were comparable in the three groups throughout the experiment (Table 1). Mean ventricular rates were similar at baseline (sham: 90 ± 3 b.p.m.; RAP: 83 ± 7 b.p.m.; RAP+Irb: 81 ± 7 b.p.m.; P = 0.659), but increased significantly in the two pacing groups compared with baseline (RAP: 134 ± 9 b.p.m., P = 0.013; RAP+Irb:143 ± 3 b.p.m., P = 0.002) and sham animals (P = 0.001).
Impact of rapid atrial pacing on NADPH oxidase subunit expression
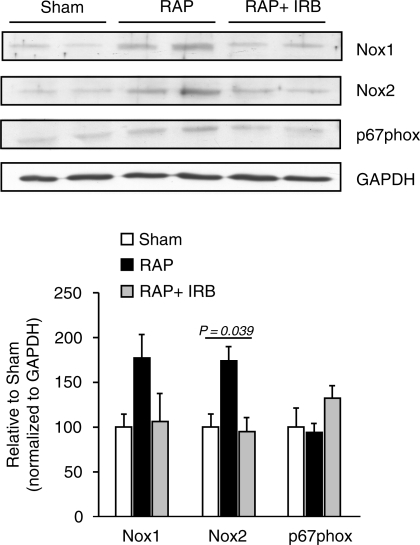
Next we tested whether RAP increases the protein levels of LV NADPH oxidase subunit isoforms (Tables 1 and 2). Although NOX-1 tended to be up-regulated with RAP (177 ± 26% of sham; Tables 1 and 2), no between-group differences were detectable with ANOVA analysis. Nox2 proteins were increased by RAP compared with sham (173 ± 16% of sham; P = 0.070) and irbesartan prevented the RAP-related Nox2 increase (95 ± 16% of sham; P = 0.982; Figure 2). Although there was a trend towards increased protein amounts of p67phox in the RAP+Irb group, the between-group differences did not reach the level of statistical significance with ANOVA analysis (Figure 2).
Figure 2.
Expression of NADPH oxidase subunits in response to rapid atrial pacing (RAP). Protein expression of NADPH oxidase subunits Nox1, Nox2, and p67phox was determined in ventricular myocardium of pigs without RAP (sham), with 7 h of RAP, or RAP with irbesartan (RAP+IRB). The protein expression was subsequently normalized on the same gel to GAPDH as internal control (*P = 0.039, one-factor ANOVA).
Impact of rapid atrial pacing on expression of LOX-1, eNOS, and F2-isoprostanes
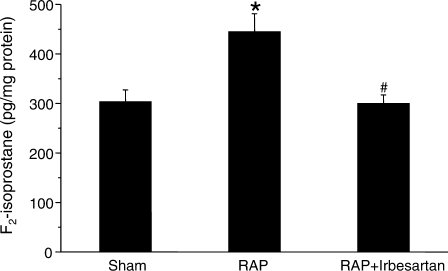
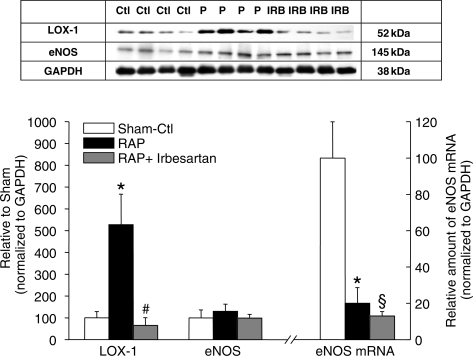
Besides increasing NADPH subunit abundance, RAP enhanced the levels of two additional markers of oxidative stress, i.e. ventricular F2-isoprostanes and LOX-1 protein (Tables 1 and 2). F2-isoprostane concentrations (sham: 303.7 ± 23.5 pg/mg protein) were significantly higher with RAP (445 ± 36.3 pg/mg protein; P = 0.02), but remained stable with irbesartan treatment (300 ± 17.2 pg/mg protein; P = 0.996 vs. sham) (Figure 3). Similarly, LOX-1 protein increased by 527.1 ± 140.8% with RAP compared to sham (P = 0.011) (Figure 4). This increase was prevented by irbesartan (65.1 ± 35.9% of sham; P = 0.005 vs. sham).
Figure 3.
Impact of rapid atrial pacing (RAP) on left ventricular F2-isoprostane concentrations (P = 0.008, ANOVA). There was a significant increase after 7 h of RAP compared with the sham group (*P = 0.02; Tukey test). The increment in F2-isoprostane levels was blunted in the presence of irbesartan (P = 0.996: sham vs. RAP+Irb; #P = 0.012 RAP vs. RAP-Irb; Tukey test).
Figure 4.
Effect of rapid atrial pacing (RAP) on LOX-1 and eNOS expression in the left ventricle. (A) Western blot showing expression of LOX-1 and eNOS in the three groups. GAPDH was used as internal control. (B) Quantitative analyses of immunoblots shown in (A). Increased amounts of LOX-1 protein were observed in response to 7 h of RAP (*P = 0.011 vs. sham, Tukey test). The pacing-induced increase of LOX-1 expression is prevented by irbesartan (#P = 0.005 RAP vs. RAP-Irb, Tukey test). The amounts of eNOS proteins were similar in all groups (P = 0.691, ANOVA). However, the levels of eNOS mRNA were reduced in response to RAP, independent of RAP being performed in the absence (RAP vs. sham: *P = 0.004, Tukey test) or presence of irbesartan (RAP+Irb vs. sham: §P = 0.002, Tukey test).
Levels of eNOS mRNA were reduced in the RAP group to 20.1 ± 8.6% of sham (P = 0.004) without being influenced significantly by irbesartan treatment (13.0 ± 2.4% of sham; Figure 4 and Table 2). This indicates ANG-II-independent mechanisms of transcriptional eNOS regulation during RAP. Nevertheless, protein levels of eNOS (Table 1) were similar in all groups (P = 0.691; Figure 4).
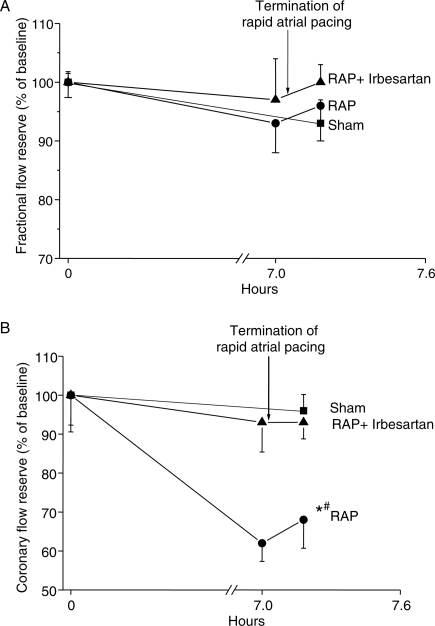
Coronary flow reserve and fractional flow reserve measurements
We performed the analyses of CFR and FFR in two steps. First, we compared the values at the end of the experiment (Figure 1) with respect to the baseline using all three groups. In the second step, we compared the values after 7 h pacing between RAP and RAP+Irb. In these models, the group effect compares differences to baseline.
Fractional flow reserve values (marker for epicardial flow) measured before, during, and after 7 h RAP (Figure 5A) were comparable in all groups (P = 0.327; Table 1). In contrast, CFR (index of microvascular abnormalities if FFR is normal) differed significantly between the three groups (P = 0.0013, Table 1). Coronary flow reserve decreased to 62.7 ± 3.7% of baseline during RAP (Figure 5B) and was at the end significantly lower compared with sham animals (P = 0.009, Table 2). In addition, comparison of CFR showed significant differences between the two pacing groups after 7 h pacing (P = 0.002, Table 2) as well as to the end of the experiment (P = 0.022, Table 2). However, CFR was not different in sham animals compared with RAP+Irb (P = 1.0; Tables 1 and 2) suggesting involvement of ANG-II-dependent mechanisms in microvascular abnormalities. The impact of irbesartan therapy in the present pacing model is underlined by the confidence interval of the mean difference in each case of two pacing groups. Irbesartan therapy reduced significantly the decline of CRF (Figure 5B). The difference of the mean between RAP and RAP+Irb was 34.78 with a confidence interval from 19.8 to 49.7. At the end of our experiment, the confidence interval for the difference of the mean between Sham and RAP+Irb is nearly symmetric to zero (Table 2). Nevertheless, baseline values affected the decline with more pronounced changes in case of high baseline values (P < 0.05). We found this effect in all calculations for FFR as well as for CFR.
Figure 5.
(A) Fractional flow reserve during 7 h of rapid atrial pacing (RAP) with and without administration of irbesartan. RAP has no effect on fractional flow reserve compared with sham animals suggesting that RAP does not affect blood flow in the epicardial coronary arteries (mean ± SEM). (B) Coronary flow reserve (CFR) in the left anterior descending artery at baseline (normalized to sham) and after 7 h of RAP. RAP led to a reduction of CRF compared with sham (*P = 0.009, Tukey test) and RAP+Irb (#P = 0.022, Tukey test). In contrast, CFR was comparable in sham and RAP+Irb animals (P = 1.0). A similar effect could not be observed when RAP was performed in the presence of irbesartan. Sham-operated animals served as control.
Plasma concentrations of NT-pro-BNP and cTn-I
To prove the consequences of increased oxidative stress for ventricular function,21–24 we measured the plasma concentrations of NT-Pro-BNP and cTn-I. Plasma concentrations of NT-Pro-BNP in pigs (n = 10) were 0.786 ± 0.085 pmol/L with no change during 7 h of RAP or sham operation (0.750 ± 0.053 pmol/L). There were no significant differences in NT-Pro-BNP levels between the experimental groups. In contrast, plasma levels of cTn-I increased with RAP from 82.5 ± 12.5 to 613.3 ± 125.8 pmol/L (n = 3; P = 0.013) and this increase was partly prevented by irbesartan (baseline: 82.3 ± 59.0 vs. 390.0 ± 90.7 pmol/L after RAP; P = 0.02; n = 3). Sham-operated pigs showed no change in plasma concentrations of cTn-I (56.7 ± 29.1 vs. 70.0 ± 50 pmol/L after 7 h).
Discussion
This study shows that acute RAP induces ANG-II-mediated oxidative stress in the LV myocardium that involves NADPH oxidase activation, increased LOX-1 expression, and elevated F2-isoprostane concentrations. This is associated with compromised microvascular blood flow and increased systemic release of cTn-I. Blockade of AT1-receptors with irbesartan prevents the maladaptive alterations in the LV suggesting ANG-II signalling as a major underlying mechanism. Our findings provide a plausible mechanism for the frequently observed cTn-I elevation accompanied with typical angina pectoris symptoms in patients with paroxysmal AF and normal coronary arteries on angiography.
Atrial fibrillation, oxidative stress, and microvascular blood flow
Patients without previously documented coronary artery disease sometimes develop chest discomfort with the onset of AF.25 Recent studies report that patients with AF have ventricular-flow abnormalities and higher incidence of cardiac events.7,26 Consistent with this notion, coronary artery resistance is markedly elevated (by 62%), whereas myocardial blood flow is substantially reduced in AF patients.7 These findings are supported by a 67% increase of the Doppler-derived coronary vascular resistance index observed in an experimental AF model.6 Furthermore, vasodilation in response to exercise is compromised during AF.27 One potential link between AF and LV malperfusion is ANG-II. Recent studies have clearly shown that AF increases systemic ANG-II levels.8,9 In a previous study using the same experimental approach, we have demonstrated that 7 h of RAP almost doubles systemic ANG-II levels.8 ANG-II activates NADPH oxidase thereby generating reactive oxygen species (ROS) that can rapidly react with nitric oxide (NO), leading to peroxynitrite formation, reduced NO availability, and endothelial dysfunction.10,28 Major downstream effectors of oxidative stress are LOX-1 and F2-isoprostane (8-iso-prostaglandin F2α)29,30 with the latter being a specific and chemically stable marker of oxidative stress with established involvement in ventricular dilatation and progression to heart failure.29,31
Here, we clearly showed that RAP induces ANG-II-dependent activation of NADPH oxidase (Nox2 subunit) and increases LOX-1 expression and F2-isoprostane generation. The subsequent increase in oxidative stress was associated with microvascular flow disturbances. Specifically, we observed an up-regulation of Nox2 (gp91phox) protein expression without concomitant changes in the cytosolic subunit p67phox with RAP. The changes in NADPH oxidase expression were prevented by blockade of AT1-receptors with irbesartan. In endothelial cells, ANG-II causes a rapid (after 7 h) up-regulation of Nox2-protein expression that is also prevented by AT1-receptor blockade.12 The Nox2-containing NADPH oxidase complex has been established in cardiomyocytes and endothelial cells32,33 suggesting this complex as a major source of cardiac ROS generation in response to RAP.
The increase of LOX-1 receptor in the present model and its prevention by blockade of AT1-receptors resembled the ANG-II-mediated up-regulation of LOX-1 in human endothelial cells.30 The up-regulation of LOX-1 could be a response to increased oxLDL formation during pacing-induced oxidative stress. LOX-1 acts as an adhesion molecule in inflammatory processes34 and is involved in a variety of cardiovascular diseases associated with increased ANG-II levels (e.g. heart failure).35 These findings indicate LOX-1 as an important mediator in the ANG-II-related promotion of ventricular oxidative stress induced by RAP. Taken together, the blockade of AT1-receptors and the reduction of Nox2, LOX-1, and F2-isoprostane (oxidative stress) effectively prevent the microvascular flow abnormalities and may contribute to the effectiveness of AT1-inhibitors to halt the progression of ventricular remodelling.
In addition to oxidative stress, we show that a relatively brief episode of AF with a moderate increase in ventricular response may enhance the systemic cTn-I levels demonstrating discrete cardiac ischaemia. This can be explained by the induction of oxidative stress-induced microvascular flow abnormalities in the LV. Accordingly, we detected reduced CFR in the presence of stable FFR. Most importantly, the CFR abnormalities remained present for at least 15 min after termination of RAP. Reduced NO availability in the coronary circulation is one potential mechanism to explain the CFR abnormalities. Besides consequences for the vascular tree, NO deficiency increases chamber stiffness and impairs diastolic performance, which may contribute to reduced circulation in the microvasculature. In the present study, we demonstrate stable amounts of eNOS protein in the LV excluding significant down-regulation of eNOS within 7 h of RAP. However, eNOS mRNA levels strongly declined with RAP, by ∼80%, suggesting that reduced eNOS proteins may play a role if AF episodes persist for longer periods of time. Since eNOS activity was not determined in the present study, we cannot exclude the possibility that reduced eNOS activity contributes to the abnormalities in this setting.
ARBs and microvascular blood flow
ANG-II has been reported to be abundant in fibrillating atria36 and AF is associated with a down-regulation of atrial AT1-receptor and an up-regulation of AT2-receptor proteins.37 Cardiac AT2-receptor expression is upregulated during myocardial ischaemia and in contrast to AT1-receptor activation, the AT2-receptor promotes NO-dependent vasodilatation and inhibits growth and remodelling processes.38 At the molecular level, ANG-II-receptor blockers (ARBs) have been shown to increase NO availability and it is possible that this effect is mediated by a stronger ANG-II-dependent activation of AT2-receptors. ARBs also attenuate aortic intimal proliferation and markedly decrease the enhanced LOX-1 expression in the aorta of hypercholesterolemic animals.39 Irbesartan reduces the formation of vascular superoxide (O2−) in a rat model of congestive heart failure.40 Here we show that oxidative stress and microvascular flow abnormalities occur immediately after new-onset AF likely representing key initiator mechanisms of AF-related ventricular remodelling. Of note, ARB therapy could attenuate most of the functional and molecular changes in the LV during RAP. However, further clinical studies will be needed to validate the present findings and to determine whether ARB therapy can also reduce ANG-II-dependent ventricular abnormalities if they are already established.
Atrial fibrillation and left ventricular dysfunction
Atrial fibrillation is known to be associated with an increased risk of LV dysfunction, especially in patients with ischaemic cardiomyopathy.41,42 Here we show that brief episodes of AF do not significantly change ventricular function because systemic levels of NT-Pro-BNP and diastolic LV pressures are unchanged. Nevertheless, it is quite possible that repetitive episodes of AF might cause ventricular dysfunction providing a plausible explanation for the higher incidence of heart failure deteriorations with increasing number of AF recurrences.4,5,41,42
Clinical implications
To the best of our knowledge, the present study is the first to provide a potential underlying mechanism for the typical cTn-I elevation accompanying the angina pectoris symptoms in patients with paroxysmal AF and normal (non-stenotic) coronary arteries. With RAP, we showed oxidative stress and microvascular flow disturbances that are consistent with previous reports showing that AF can induce ischaemic ST-segment depression in the surface ECG of patients with normal coronary arteries.43 Importantly, we can show that treatment with ARBs prevents both the molecular basis of oxidative stress and the related ventricular ischaemia induced by RAP. Thus, ARB therapy may be an effective therapeutic option for this clinical situation.
Several studies have identified AF as an independent risk factor for cardiovascular events and death, especially in patients with ischaemic heart disease.44,45 The present study suggests that AF can aggravate ventricular ischaemia, which might further compromise ventricular function potentially explaining the worse outcome of heart failure patients developing AF. Our results also support recent findings that asymptomatic (occult) coronary artery disease might become manifest during an episode of AF. Of note, the actual ventricular rate accompanying AF is not related to the ischaemic ventricular changes on the surface ECG suggesting that an AF-related, but ventricular rate-independent, mechanism induces myocardial perfusion abnormalities.46 This notion is supported by the moderate increase in ventricular rates during RAP we observed.
Atrial tachyarrhythmias and AF are recognized risk factors for the increased mortality of patients with acute myocardial infarction,47,48 and large myocardial infarctions frequently promote the development of AF.49 Conversely, our study suggests that AF per se may increase infarct expansion by impairing microcirculation. Thus, future studies are warranted to determine whether immediate antiarrhythmic interventions in patients with myocardial infarction and new-onset AF may improve patient's prognosis.
Study limitations
The present study has several limitations. The number of included animals was limited. However, the observed functional changes have consistent molecular correlates at several components of the cellular signal transduction suggesting validity of our findings. We did not measure activity of NADPH oxidase, but the protein level of Nox2 (gp91phox) highly correlates with enzymatic activity of NADPH oxidase.11 Nevertheless, we cannot exclude that additional significant differences between the groups exist and contribute to the clinical phenotype.
We have not studied all potential mechanisms, which may influence microvascular flow during RAP. Activation of inflammatory pathways, endothelial damage, increased catecholamine levels, and/or enhanced activation of the sympathetic nervous system may also produce ventricular flow abnormalities. In addition, the impact of chronic persistent AF on LV perfusion has to be established. However, patients with persistent or permanent AF rarely present with acute angina pectoris symptoms, whereas the latter are characteristic for patients with paroxysmal AF.1 Finally, we did not assess the effects of repetitive episodes of RAP on LV function. Thus, further studies are needed to determine the additive effects of repetitive AF episodes on LV perfusion and performance.
Conclusions
The data obtained from these acute pacing experiments clearly demonstrate that RAP induces ANG-II-dependent NADPH oxidase-mediated oxidative stress in the LV myocardium, which may contribute to the impaired microvascular blood flow and increased cTn-I release. These findings provide a plausible underlying mechanism for the occurrence of acute chest pain and cTn-I releases in patients with paroxysmal AF and normal (non-stenotic) coronary arteries.
Funding
Supported by the German Federal Ministry of Education and Research (BMBF) through the Atrial Fibrillation Competence Network (01GI0204), BMBF program NBL3 of Dresden University of Technology (Dr H.M., Professorship of Vascular Endothelium and Microcirculation), and by a grant from Fondation Leducq (07 CVD 03). We thank sanofi-aventis Deutschland GmbH for providing irbesartan. Funding to pay the Open Access publication charges for this article was provided by sanofi-aventis.
Conflict of interest: A.G. and D.D. have received speaker fees from sanofi-aventis. A.G. was supported by a research grant from sanofi-aventis.
Acknowledgements
We are grateful to Doris Trzeczak, Rita Murau, Katja Mook, Annika Frenzel, Anett Opitz, Heidemarie Faber, Elke Wölfel, and Sabine Kirsch for excellent technical assistance.
References
- 1.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257–e354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 2.Brown AM, Sease KL, Robey JL, Shofer FS, Hollander JE. The risk for acute coronary syndrome associated with atrial fibrillation among ED patients with chest pain syndromes. Am J Emerg Med. 2007;25:523–528. doi: 10.1016/j.ajem.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 3.Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE, Corley SD. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 4.Van Gelder IC, Hagens VE, Bosker HA, Kingma JH, Kamp O, Kingma T, Said SA, Darmanata JI, Timmermans AJ, Tijssen JG, Crijns HJ. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834–1840. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- 5.Shinbane JS, Wood MA, Jensen DN, Ellenbogen KA, Fitzpatrick AP, Scheinman MM. Tachycardia-induced cardiomyopathy: a review of animal models and clinical studies. J Am Coll Cardiol. 1997;29:709–715. doi: 10.1016/s0735-1097(96)00592-x. [DOI] [PubMed] [Google Scholar]
- 6.Kochiadakis GE, Skalidis EI, Kalebubas MD, Igoumenidis NE, Chrysostomakis SI, Kanoupakis EM, Simantirakis EN, Vardas PE. Effect of acute atrial fibrillation on phasic coronary blood flow pattern and flow reserve in humans. Eur Heart J. 2002;23:734–741. doi: 10.1053/euhj.2001.2894. [DOI] [PubMed] [Google Scholar]
- 7.Range FT, Schafers M, Acil T, Schafers KP, Kies P, Paul M, Hermann S, Brisse B, Breithardt G, Schober O, Wichter T. Impaired myocardial perfusion and perfusion reserve associated with increased coronary resistance in persistent idiopathic atrial fibrillation. Eur Heart J. 2007;28:2223–2230. doi: 10.1093/eurheartj/ehm246. [DOI] [PubMed] [Google Scholar]
- 8.Goette A, Bukowska A, Lendeckel U, Erxleben M, Hammwohner M, Strugala D, Pfeiffenberger J, Rohl FW, Huth C, Ebert MP, Klein HU, Rocken C. Angiotensin II Receptor Blockade Reduces Tachycardia-Induced Atrial Adhesion Molecule Expression. Circulation. 2008;117:732–742. doi: 10.1161/CIRCULATIONAHA.107.730101. [DOI] [PubMed] [Google Scholar]
- 9.Cardin S, Li D, Thorin-Trescases N, Leung TK, Thorin E, Nattel S. Evolution of the atrial fibrillation substrate in experimental congestive heart failure: angiotensin-dependent and -independent pathways. Cardiovasc Res. 2003;60:315–325. doi: 10.1016/j.cardiores.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 10.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II mediated mitochondrial dysfunction. Linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 11.Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–840. doi: 10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 12.Kern MJ, Lerman A, Bech JW, De Bruyne B, Eeckhout E, Fearon WF, Higano ST, Lim MJ, Meuwissen M, Piek JJ, Pijls NH, Siebes M, Spaan JA. Physiological assessment of coronary artery disease in the cardiac catheterization laboratory: a scientific statement from the American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology. Circulation. 2006;114:1321–1341. doi: 10.1161/CIRCULATIONAHA.106.177276. [DOI] [PubMed] [Google Scholar]
- 13.Fearon WF, Farouque HM, Balsam LB, Caffarelli AD, Cooke DT, Robbins RC, Fitzgerald PJ, Yeung AC, Yock PG. Comparison of coronary thermodilution and Doppler velocity for assessing coronary flow reserve. Circulation. 2003;108:2198–2200. doi: 10.1161/01.CIR.0000099521.31396.9D. [DOI] [PubMed] [Google Scholar]
- 14.Popov N, Schmitt M, Schulzeck S, Matthies H. Reliable micromethod for determination of the protein content in tissue homogenates. Acta biologica et medica Germanica. 1975;34:1441–1446. [PubMed] [Google Scholar]
- 15.El-Armouche A, Boknik P, Eschenhagen T, Carrier L, Knaut M, Ravens U, Dobrev D. Molecular determinants of altered Ca2+ handling in human chronic atrial fibrillation. Circulation. 2006;114:670–680. doi: 10.1161/CIRCULATIONAHA.106.636845. [DOI] [PubMed] [Google Scholar]
- 16.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 17.Bukowska A, Lendeckel U, Hirte D, Wolke C, Striggow F, Rohnert P, Huth C, Klein HU, Goette A. Activation of the calcineurin signaling pathway induces atrial hypertrophy during atrial fibrillation. Cell Mol Life Sci. 2006;63:333–342. doi: 10.1007/s00018-005-5353-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiswedel I, Hirsch D, Kropf S, Gruening M, Pfister E, Schewe T, Sies H. Flavanol-rich cocoa drink lowers plasma F(2)-isoprostane concentrations in humans. Free Radic Biol Med. 2004;37:411–421. doi: 10.1016/j.freeradbiomed.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 19.Morrow DM, Zackert WE, Van der Ende, Reich EE, Terry ES, Cox B, Sanchez SC, Montine TJ, Roberts LJ. Quantification of isoprostanes as indicators of oxidant stress in vivo. In: Cadenas E, Packer L, editors. Handbook of Antioxidants. Second ed. New York, Basel: Marcel Dekker Inc.; 2002. pp. 57–74. [Google Scholar]
- 20.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 21.MacLennan DH, Kranias EG. Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol. 2003;4:566–577. doi: 10.1038/nrm1151. [DOI] [PubMed] [Google Scholar]
- 22.Bers DM. Altered cardiac myocyte Ca regulation in heart failure. Physiology (Bethesda) 2006;21:380–387. doi: 10.1152/physiol.00019.2006. [DOI] [PubMed] [Google Scholar]
- 23.Stelzer JE, Patel JR, Moss RL. Protein kinase A-mediated acceleration of the stretch activation response in murine skinned myocardium is eliminated by ablation of cMyBP-C. Circ Res. 2006;99:884–890. doi: 10.1161/01.RES.0000245191.34690.66. [DOI] [PubMed] [Google Scholar]
- 24.El-Armouche A, Pohlmann L, Schlossarek S, Starbatty J, Yeh YH, Nattel S, Dobrev D, Eschenhagen T, Carrier L. Decreased phosphorylation levels of cardiac myosin-binding protein-C in human and experimental heart failure. J Mol Cell Cardiol. 2007;43:223–229. doi: 10.1016/j.yjmcc.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Fineschi M, Bravi A, Gori T. The ‘slow coronary flow’ phenomenon: Evidence of preserved coronary flow reserve despite increased resting microvascular resistances. Int J Cardiol. 2007 doi: 10.1016/j.ijcard.2007.06.010. doi:10.1016/j.ijcard.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Abidov A, Hachamovitch R, Rozanski A, Hayes SW, Santos MM, Sciammarella MG, Cohen I, Gerlach J, Friedman JD, Germano G, Berman DS. Prognostic implications of atrial fibrillation in patients undergoing myocardial perfusion single-photon emission computed tomography. J Am Coll Cardiol. 2004;44:1062–1070. doi: 10.1016/j.jacc.2004.05.076. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi N, Ishibashi Y, Shimada T, Sakane T, Ohata S, Sugamori T, Ohta Y, Inoue S, Nakamura K, Shimizu H, Katoh H, Murakami Y. Impaired exercise-induced vasodilatation in chronic atrial fibrillation–role of endothelium-derived nitric oxide. Circ J. 2002;66:583–588. doi: 10.1253/circj.66.583. [DOI] [PubMed] [Google Scholar]
- 28.Oudot A, Vergely C, Ecarnot-Laubriet A, Rochette L. Angiotensin II activates NADPH oxidase in isolated rat hearts subjected to ischaemia-reperfusion. Eur J Pharmacol. 2003;462:145–154. doi: 10.1016/s0014-2999(03)01315-3. [DOI] [PubMed] [Google Scholar]
- 29.Elesber AA, Best PJ, Lennon RJ, Mathew V, Rihal CS, Lerman LO, Lerman A. Plasma 8-iso-prostaglandin F2alpha, a marker of oxidative stress, is increased in patients with acute myocardial infarction. Free Radic Res. 2006;40:385–391. doi: 10.1080/10715760500539154. [DOI] [PubMed] [Google Scholar]
- 30.Morawietz H, Rueckschloss U, Niemann B, Duerrschmidt N, Galle J, Hakim K, Zerkowski HR, Sawamura T, Holtz J. Angiotensin II induces LOX-1, the human endothelial receptor for oxidized low-density lipoprotein. Circulation. 1999;100:899–902. doi: 10.1161/01.cir.100.9.899. [DOI] [PubMed] [Google Scholar]
- 31.Mallat Z, Philip I, Lebret M, Chatel D, Maclouf J, Tedgui A. Elevated levels of 8-iso-prostaglandin F2alpha in pericardial fluid of patients with heart failure: a potential role for in vivo oxidant stress in ventricular dilatation and progression to heart failure. Circulation. 1998;97:1536–1539. doi: 10.1161/01.cir.97.16.1536. [DOI] [PubMed] [Google Scholar]
- 32.MacCarthy PA, Grieve DJ, Li JM, Dunster C, Kelly FJ, Shah AM. Impaired endothelial regulation of ventricular relaxation in cardiac hypertrophy: role of reactive oxygen species and NADPH oxidase. Circulation. 2001;104:2967–2974. doi: 10.1161/hc4901.100382. [DOI] [PubMed] [Google Scholar]
- 33.Rueckschloss U, Galle J, Holtz J, Zerkowski HR, Morawietz H. Induction of NAD(P)H oxidase by oxidized low-density lipoprotein in human endothelial cells: antioxidative potential of hydroxymethylglutaryl coenzyme A reductase inhibitor therapy. Circulation. 2001;104:1767–1772. doi: 10.1161/hc4001.097056. [DOI] [PubMed] [Google Scholar]
- 34.Chen M, Masaki T, Sawamura T. LOX-1, the receptor for oxidized low-density lipoprotein identified from endothelial cells: implications in endothelial dysfunction and atherosclerosis. Pharmacol Ther. 2002;95:89–100. doi: 10.1016/s0163-7258(02)00236-x. [DOI] [PubMed] [Google Scholar]
- 35.Morawietz H. LOX-1 and atherosclerosis: proof of concept in LOX-1-knockout mice. Circ Res. 2007;100:1534–1536. doi: 10.1161/CIRCRESAHA.107.101105. [DOI] [PubMed] [Google Scholar]
- 36.Goette A, Staack T, Rocken C, Arndt M, Geller JC, Huth C, Ansorge S, Klein HU, Lendeckel U. Increased expression of extracellular signal-regulated kinase and angiotensin-converting enzyme in human atria during atrial fibrillation. J Am Coll Cardiol. 2000;35:1669–1677. doi: 10.1016/s0735-1097(00)00611-2. [DOI] [PubMed] [Google Scholar]
- 37.Goette A, Arndt M, Rocken C, Spiess A, Staack T, Geller JC, Huth C, Ansorge S, Klein HU, Lendeckel U. Regulation of angiotensin II receptor subtypes during atrial fibrillation in humans. Circulation. 2000;101:2678–2681. doi: 10.1161/01.cir.101.23.2678. [DOI] [PubMed] [Google Scholar]
- 38.Sadoshima J, Izumo S. Molecular characterization of angiotensin II-induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ Res. 1993;73:413–423. doi: 10.1161/01.res.73.3.413. [DOI] [PubMed] [Google Scholar]
- 39.Chen H, Li D, Sawamura T, Inoue K, Mehta JL. Upregulation of LOX-1 expression in aorta of hypercholesterolemic rabbits: modulation by losartan. Biochem Biophys Res Commun. 2000;276:1100–1104. doi: 10.1006/bbrc.2000.3532. [DOI] [PubMed] [Google Scholar]
- 40.Schafer A, Fraccarollo D, Tas P, Schmidt I, Ertl G, Bauersachs J. Endothelial dysfunction in congestive heart failure: ACE inhibition vs. angiotensin II antagonism. Eur J Heart Fail. 2004;6:151–159. doi: 10.1016/j.ejheart.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 41.Akoum N, Hamdan MH. Atrial fibrillation and congestive heart failure: a two-way street. Curr Heart Fail Rep. 2007;4:78–83. doi: 10.1007/s11897-007-0004-7. [DOI] [PubMed] [Google Scholar]
- 42.Cleland JG, Shelton R, Nikitin N, Ford S, Frison L, Grind M. Prevalence of markers of heart failure in patients with atrial fibrillation and the effects of ximelagatran compared to warfarin on the incidence of morbid and fatal events: a report from the SPORTIF III and V trials. Eur J Heart Fail. 2007;9:730–739. doi: 10.1016/j.ejheart.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 43.Androulakis A, Aznaouridis KA, Aggeli CJ, Roussakis GN, Michaelides AP, Kartalis AN, Stougiannos PN, Dilaveris PE, Misovoulos PI, Stefanadis CI, Kallikazaros IE. Transient ST-segment depression during paroxysms of atrial fibrillation in otherwise normal individuals: relation with underlying coronary artery disease. J Am Coll Cardiol. 2007;50:1909–1911. doi: 10.1016/j.jacc.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 44.Siu CW, Jim MH, Ho HH, Miu R, Lee SW, Lau CP, Tse HF. Transient atrial fibrillation complicating acute inferior myocardial infarction: implications for future risk of ischemic stroke. Chest. 2007;132:44–49. doi: 10.1378/chest.06-2733. [DOI] [PubMed] [Google Scholar]
- 45.Pedersen OD, Sondergaard P, Nielsen T, Nielsen SJ, Nielsen ES, Falstie-Jensen N, Nielsen I, Kober L, Burchardt H, Seibaek M, Torp-Pedersen C. Atrial fibrillation, ischaemic heart disease, and the risk of death in patients with heart failure. Eur Heart J. 2006;27:2866–2870. doi: 10.1093/eurheartj/ehl359. [DOI] [PubMed] [Google Scholar]
- 46.Gulec S, Ertas F, Karaoouz R, Guldal M, Alpman A, Oral D. Value of ST-segment depression during paroxysmal supraventricular tachycardia in the diagnosis of coronary artery disease. Am J Cardiol. 1999;83:458–460. doi: 10.1016/s0002-9149(98)00888-1. A410. [DOI] [PubMed] [Google Scholar]
- 47.Asanin M, Vasiljevic Z, Matic M, Vujisic-Tesic B, Arandjelovic A, Marinkovic J, Ostojic M. Outcome of patients in relation to duration of new-onset atrial fibrillation following acute myocardial infarction. Cardiology. 2007;107:197–202. doi: 10.1159/000095417. [DOI] [PubMed] [Google Scholar]
- 48.Kober L, Swedberg K, McMurray JJ, Pfeffer MA, Velazquez EJ, Diaz R, Maggioni AP, Mareev V, Opolski G, Van de Werf F, Zannad F, Ertl G, Solomon SD, Zelenkofske S, Rouleau JL, Leimberger JD, Califf RM. Previously known and newly diagnosed atrial fibrillation: a major risk indicator after a myocardial infarction complicated by heart failure or left ventricular dysfunction. Eur J Heart Fail. 2006;8:591–598. doi: 10.1016/j.ejheart.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 49.Lehto M, Snapinn S, Dickstein K, Swedberg K, Nieminen MS. Prognostic risk of atrial fibrillation in acute myocardial infarction complicated by left ventricular dysfunction: the OPTIMAAL experience. Eur Heart J. 2005;26:350–356. doi: 10.1093/eurheartj/ehi064. [DOI] [PubMed] [Google Scholar]