Abstract
Family history is increasingly important in primary care as a means to detect candidates for genetic testing or tailored prevention programs. We evaluated primary care physicians’ skills in assessing family history for breast cancer risk, using unannounced standardized patient visits to 86 general internists and family medicine practitioners in King County, WA. Transcripts of clinical encounters were coded to determine ascertainment of family history, risk assessment, and clinical follow-up. Physicians in our study collected sufficient family history to assess breast cancer risk in 48% of encounters with an anxious patient at moderate risk, 100% of encounters with a patient who had a strong maternal family history of breast cancer, and 45% of encounters with a patient who had a strong paternal family history of breast and ovarian cancer. Increased risk was usually communicated in terms of recommendations for preventive action. Few physicians referred patients to genetic counseling, few associated ovarian cancer with breast cancer risk, and some incorrectly discounted paternal family history of breast cancer. We conclude that pedigree assessment of breast cancer risk is feasible in primary care, but may occur consistently only when a strong maternal family history is present. Primary care education should focus on the link between inherited breast and ovarian cancer risk and on the significance of paternal family history. Educational efforts may be most successful when they emphasize the value of genetic counseling for individuals at risk for inherited cancer and the connection between genetic risk and specific prevention measures.
Keywords: Family history, breast cancer, primary care
INTRODUCTION
Taking a family history has long been considered part of comprehensive patient care. However, surveys and chart reviews suggest that many clinicians collect only limited family history information, and lack skills for assessing it [Acheson, et al. 2000; Murff, et al. 2004; Sabatino, et al. 2007; Sweet, et al. 2002; Tyler and Snyder 2006]. This deficit is of particular concern now that family history is taking on increasing importance as a guide to prevention and management based on genetic risk [Guttmacher, et al. 2004].
Breast cancer provides an important example. The average lifetime breast cancer risk for American women is approximately 13% [National Cancer Institute web site: http:seer.cancer.gov/statistics], but a small subset of women have a substantially higher risk due to mutations in the BRCA1 and BRCA2 genes [Chen, et al. 2006]. Family history is the key to identifying women who are candidates for genetic counseling and consideration of BRCA mutation testing, and who may benefit from intensive screening, prophylactic surgery or chemoprevention [Nelson, et al. 2005].
The family history characteristics associated with high risk were summarized by the US Preventive Services Task Force in a set of family history criteria to be used in considering women for referral for counseling and consideration of BRCA testing [US Preventive Services Task Force 2005]. These criteria require primary care providers to assess the breast and ovarian cancer history of first and second degree relatives, including age of onset and presence or absence of bilateral breast cancer for each relative. However, the readiness of primary care providers to accomplish this task is uncertain [Acheson, et al. 2000; Murff, et al. 2004; Sabatino, et al. 2007; Sweet, et al. 2002; Tyler and Snyder 2006].
We utilized the analytic technique of “unannounced standardized patients” to describe primary care providers’ collection and use of family history information to assess breast cancer risk. This technique, in which individuals trained to portray a particular patient scenario present to a physician’s office for care as regular patients [Beullens, et al. 1997], provides a powerful method for evaluating aspects of care that may not be captured in the documentary record or physician surveys. Unannounced SPs have been used to evaluate many aspects of primary care practice, including physician communication skills [Fiscella, et al. 2004; Goedhuys and Rethans 2001], preventive care practices [Carney, et al. 1995; Dresselhaus, et al. 2000], diagnostic strategies [Epstein, et al. 2006], and recognition and management of depression [Geraghty, et al. 2007; Kravitz, et al. 2005]. In this study, our goal was to define family history-taking and risk assessment skills related to breast cancer, as a basis for identifying educational needs.
MATERIALS AND METHODS
Recruitment
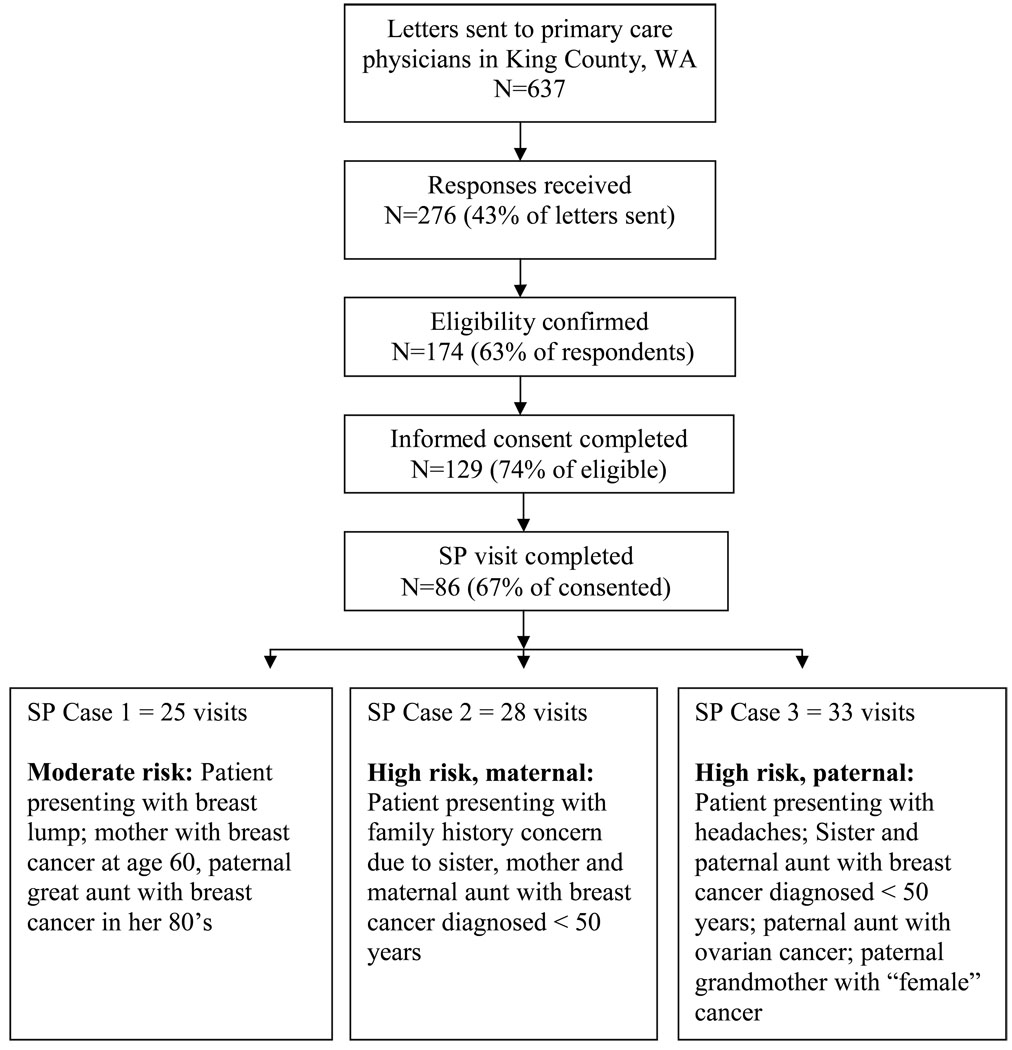
Using published Washington State physician rosters, we invited all eligible physicians practicing in the Seattle/King county area to participate. We excluded physicians employed by Group Health Cooperative of Puget Sound (GHC), a large staff model HMO, because practice guidelines for breast cancer genetics evaluation and referral had been promulgated at GHC. Using published and online directories, we identified 637 potentially eligible physicians and mailed each a recruitment packet consisting of an invitation letter, study fact sheet, eligibility questionnaire and consent form. To be eligible, the responding physician needed to confirm that he/she trained in internal medicine or family medicine, accepted new patients, saw at least 25 adult patients per week, devoted no more than 25% of clinical time to subspecialty care, and had no plans to move his/her practice within the next 12 months. Inclusion criteria other than specialty were based on self-report. A flow diagram of physician recruitment is shown in Figure 1.
Figure 1.
Flow chart showing recruitment of physicians and number of SP visits completed
Informed Consent
In the recruitment materials, the subject matter of the study was defined as “primary care practice” and did not indicate that the study intended to observe breast cancer risk assessment. The consent form specified that participating physicians would be visited by an unannounced SP, sessions would be audio-taped with a concealed microphone, and visits would be fully reimbursed for billed charges. Study procedures, including the informed consent process, were approved by the University of Washington Institutional Review Board.
Standardized Patient Procedures
We developed three SP cases for the assessment of physicians’ family history taking skills (see below), with each participating physician randomly assigned to see one of the cases. In each case, the SP presented herself as someone who had recently moved to the area. She had a presenting health concern and sought to establish care with a primary care physician. Each SP was in her 30s, European-American, and of average height and weight. The age was chosen to ensure that all SP cases were younger than the age (40 years) at which regular routine breast cancer screening is recommended. Other than the presenting health concern and family health history, lifestyle and other risk factors were standardized; all were non-smokers, with a healthy diet and regular exercise regimen. Jewish heritage was avoided because it represented a factor which might change the assessment of genetic risk for breast cancer [Tonin, et al. 1996]. Other personal characteristics and risk factors were chosen to create a neutral presentation that would differ as little as possible across SP cases, including menarche at age 13, first live birth at age 25, and no history of breast biopsies.
We trained lay people to portray each SP case based on detailed descriptions [King, et al. 1994]. A total of seven women portrayed the three cases. For each appointment with a physician participant, the woman portraying the SP was assigned a fictitious surname. A viable mailing address and a phone number (actually a voice mail box) were assigned to allow for follow-up contact by the physician or clinic. On the day of the appointment, the SP registered with the clinic, completed any medical history forms, and audio taped the session using a concealed recorder. The SPs were trained to use prompts to ask about personal breast cancer risk and the option of genetic testing, if the physician did not introduce these topics.
Standardized Patient Case Scenarios
(1) SP Case 1 (moderate risk)
A 33-year-old female seeks evaluation because she has felt a lump in her breast, although the lump is no longer present. Her mother had breast cancer at age 60 and a paternal great aunt had breast cancer in her 80s. The patient expresses anxiety about her own risk for breast cancer. Physicians were expected to evaluate the lump and take sufficient family history to determine that she was not at significantly elevated risk for breast cancer. Taking sufficient family history would include asking the SP whether there were any other affected relatives on the maternal side. SP Case 1 has a breast cancer risk estimated between 9.3% by age 79 [Claus, et al. 1994] and 19.2% by age 90 [Gail, et al. 1989].
(2) SP Case 2 (high risk – maternal)
A 36 year-old female seeks care for concerns about breast cancer risk resulting from her sister’s recently diagnosed of breast cancer at age 34. Her mother and maternal aunt also had breast cancer, both diagnosed in their 40s. Physicians were expected to take her family history, identify her significantly increased risk, and develop a management plan to include breast screening and further follow-up (a second appointment to discuss risk, or referral to genetic counseling, or other risk evaluation). SP Case 2 has a breast cancer risk estimated between 31.2% by age 90 [Gail, et al. 1989] and 37.9% by age 79 [Claus, et al. 1994]. However, identifying a potential autosomal dominant inheritance of cancer risk is the most appropriate risk assessment for SP Case 2; this assessment indicates that BRCA testing should be considered, ideally testing the affected sister first.
(3) SP Case 3: (high risk – paternal)
A 36 year-old female seeks care to renew a prescription for recurring stress-related migraine headaches. Her sister was recently diagnosed with breast cancer at age 48. A paternal aunt (one of two) had breast cancer at age 43; the other died of ovarian cancer in her early 40s, and her paternal grandmother died at a young age of a “female cancer.” If the physician fails to ask about family history, the Case 3 SP prompts this discussion by informing the physician that she feels her recent migraines are due to stress following her sister's cancer diagnosis. Physicians were expected to take her family history, identify her significantly increased risk and develop a management plan to include breast screening and further follow-up (a second appointment to discuss risk, referral to genetic counseling, or other risk evaluation). This SP’s breast cancer risk is estimated to be between 19.1% by age 90 [Gail, et al. 1989] and 31.3% by age 79 [Claus, et al. 1994]. However, identifying a potential autosomal dominant inheritance of cancer risk is the most appropriate risk assessment for SP Case 3; this assessment indicates that BRCA testing should be considered, ideally testing the affected sister first.
At the conclusion of the study, a mailing to participating physicians announced completion of the study and asked physicians to complete a brief detection questionnaire; 57 physicians (66.3%) returned the questionnaire. Two physicians suspected the identity of the SP; however, both misidentified the presenting complaint; hence they incorrectly identified which patient was an SP. Their data were included in the analysis. A second mailing identified the SP case for each physician.
Analysis of transcripts
The data reported here are derived from analysis of the audiotape transcripts of the SP visits. A coding sheet was created to capture the endpoints of interest: collection of family history information, breast cancer risk assessment, recommendations for follow-up care, and expression of an opinion regarding the patient’s potential candidacy for genetic testing to assess breast cancer risk. Coding of family history collection was determined by pedigree for the SP case, with a code established for each affected relative in the pedigree identified by the provider and, for those identified, whether or not the physician determined age of onset. In addition to identifying affected relatives for SP Case 1, we expected physicians seeing this case to elicit sufficient maternal history to confirm the lack of a high risk family history. For SP Cases 2 and 3, we assumed that identification of two affected relatives with breast cancer before age 50 was sufficient to generate concern about inherited risk [US Preventive Services Task Force 2005]. Coding categories for follow-up care included: mammography screening, follow-up visit to discuss cancer risk, referral to genetics specialist, and other referral related to cancer risk. Breast magnetic resonance imaging (MRI) was not included, because the study was completed before guidelines for this screening approach were promulgated [Saslow, et al. 2007]. Coding categories for risk assessment and candidacy for genetic testing were developed through an iterative consensus process. Initially, ten transcripts from each SP scenario were coded by two investigators. Disagreements, ambiguities, and redundancy identified in the coding definitions were discussed and resolved among the investigator team as a whole. Coding for risk was based on published estimates of risk based on family history [Claus, et al. 1994; Gail, et al. 1989]; the final coding categories were: (1) No increased risk, (2) Slightly increased risk, (3) Significantly increased risk, and (4) Increased risk but degree not specified. We considered the risk classification “Significantly increased risk”incorrect for SP Case 1; however, “No increased risk” was considered acceptable for SP Case 1 because the Claus model calculation predicts a lifetime risk of 9.3%, which is not distinguishable from average risk. We considered the risk classifications “No increased risk” and “Slightly increased risk” incorrect for SP Cases 2 and 3 because family history is compatible with autosomal dominant inheritance of cancer risk. Also noted, if present, were the expression of a numerical risk estimate and discussion of the association between a family history of ovarian cancer and breast cancer risk. The final coding categories for genetic testing candidacy were: (1) Candidate for genetic testing, (2) Not a candidate for genetic testing, and (3) No opinion.
All 86 transcripts were coded independently by two coders. Discrepancies were reviewed by the two coders; if consensus could not be readily achieved, the coding assignment was resolved by discussion with a third coder. A third coder was required for 1.0 % of coded items with SP Case 1, 0.9% with SP Case 2, and 2.1% with SP Case 3. In each case, the category that most often required a third coder was the interpretation of breast cancer risk.
The final coding data were entered into SPSS version 10.1.3 (Chicago, IL). To assess the tendency for practitioners to ascertain certain aspects of family history more than others, the equality of the ascertainment proportion between two aspects of family history within the same standardized patient scenario (e.g., mother’s breast cancer vs. maternal aunt’s breast cancer) were tested by McNemar’s Test, taking the practitioners’ ascertainments of the two aspects as paired binary responses.
RESULTS
A total of 86 physicians completed an unannounced SP session (Figure 1).
Family history obtained
Collection of family history for each of the SP cases is shown in Table 1–Table 3. For SP Case 1 (Table 1), a patient with limited family history of breast cancer, 24/25 physicians (96%) asked about the age of onset of breast cancer in the patient’s mother, but only 13/25 (52%) questioned the patient sufficiently to elicit a paternal relative with breast cancer. In addition, only 12/25 (48%) confirmed a lack of other cancer history on the mother’s side.
Table I. Family history obtained for SP Case 1.
Moderate Risk: Mother with breast cancer; paternal great-aunt with breast cancer (N=25)
| Mother | Paternal great aunt | |
|---|---|---|
| Identified relative | 25 (100%)* | 13 (52%)† P<0.001‡ |
| Identified age of onset | 24 (96%) | 5 (20%) P<0.001‡ |
Information volunteered by SP
Twelve (48%) physicians identified the aunt as paternal; 4 (16%) identified her as a great-aunt
Compared to mother
Table III. Family history obtained for SP Case 3.
High risk –paternal: Sister, paternal aunt and paternal grandmother with breast cancer; paternal aunt with ovarian cancer (N=33)
| Sister | Aunt with breast cancer** | Aunt with ovarian cancer | Grandmother with female cancer | |
|---|---|---|---|---|
| Identified relative | 33 (100%)* | 15 (46%) P<0.001† | 6 (18%) P<0.001† | 3 (9%) P<0.001† |
| Identified age of onset | 26 (79%) | 9 (27%) P<0.001† | 1 (3%) P<0.001† | 0 (0%) P<0.001† |
Information volunteered by SP
Compared to sister
For SP Case 2 (Table 2), a patient with a strong maternal family history of breast cancer, all physicians elicited information about the mother’s history of breast cancer. Other elements of family history were less reliably detected: 17/28 (61%) elicited the maternal aunt’s history, 15/28 (54%) elicited the mother’s age of onset and 9/28 (32%) elicited the aunt’s age of onset.
Table II. Family history obtained for SP Case 2.
High risk – maternal: Sister, mother and maternal aunt with breast cancer (N=28)
| Sister | Mother | Aunt | |
|---|---|---|---|
| Identified relative | 28 (100%)* | 28 (100%) P= 1.0† | 17 (61%) P= 0.001† |
| Identified age of onset | 24 (86%) | 15 (54%) P= 0.012† | 9 (32%) P<0.001† |
Information volunteered by SP
Compared to sister
For SP Case 3 (Table 3), a patient with a strong family history on the father’s side, fewer than half of physicians elicited the relevant paternal family history. More discovered the paternal aunt with breast cancer (15/33, 46%) than the paternal aunt with ovarian cancer (6/33,18%) or the grandmother with “female cancer” (3/33, 9%). Only two physicians (6%) elicited all paternal relatives with cancer.
SP errors resulted in the provision of more family history information than requested by the physician in 4/25 (16%) of SP Case 1 visits, 9/28 (32%) of SP Case 2 visits, and 11/33 (33%) of SP Case 3 visits. This unsolicited information was counted as information obtained during the visit. SP errors also resulted in the failure to disclose information requested by the physician in 1/25 (4%) of SP Case 1 visits, 0/28 (0%) of SP Case 2 visits, and 1/33 (3%) of SP Case 3 visits.
Risk assessment
The risk assessment communicated to each of the SPs is shown in Table 4. Most risk estimates were indeterminate. Incorrect risk estimates were provided in 8% of SP Case 1 encounters, 4% of SP Case 2 encounters, and 18% of SP Case 3 encounters (Table 4). Risk was usually communicated as a qualitative statement. When a numeric lifetime risk was provided, the risk was 10 to 20% for SP Case 1 (moderate risk), 31 to 35% for SP Case 2 (high risk, maternal) and 18 to 25% for SP Case 3 (high risk, paternal).
Table IV.
Breast Cancer Risk Assessment
| No increased risk | Slightly increased risk | Significantly increased risk | Increased risk, degree not specified | Range of lifetime breast cancer risk provided at visit (percent of visits at which numerical risk was provided) | |
|---|---|---|---|---|---|
| SP Case 1 (N=25) Moderate Risk: Mother with breast cancer at age 60 | 1 (4%) | 15 (60%) | 2 (8%)* | 7(32%) | 10–20% (36%) |
| SP Case 2 (N=28) High risk – maternal: Sister, mother and maternal aunt with breast cancer < 50 years | 1 (4%)* | 0 (0%)* | 7 (25%) | 20 (71%) | 31–36% (7%) |
| SP Case 3 (N=33) High risk – paternal: Sister, paternal aunt and paternal grandmother with breast cancer < 50 years, and paternal aunt with ovarian cancer | 1 (3%)* | 5 (15%)* | 2 (6%) | 25 (76%) | 18–25% (21%) |
Risk estimate considered incorrect
The majority of physicians identified SP Case 1 (moderate risk) as having only a small increase in risk:
N111A: “Your risk is probably about double the normal, yet that’s still relatively low risk…So, yeah, your risk is above normal, but not something that I would spend a lot of time worrying about. …”
V156A “Your mom having breast cancer after menopause only slightly increases your risk.”
Most physicians recognized SP Case 2 (high risk - maternal) as having increased risk, although many did not characterize the degree of risk (Table 2); 25% (7/28) included descriptors that indicated a significant risk elevation:
J109M: “…just by your family history, you are at increased risk.”
U114M “…you [have] what appears to be actually a very strong family history of breast cancer.”
Most physicians also identified SP Case 3 (high risk - paternal) as having increased risk, but few characterized the risk as significantly elevated (Table 4).
W179R: “Certainly your risk is a little bit higher but I don’t think it’s as high as you might imagine it’s going to be.”
Q531R: “…I'm sorry that I don't have the numbers in my head to quantify it, but … our antennae are up, just a little higher for you… There's a few percentage points increased risk”
In addition, some physicians who obtained SP Case 3’s paternal family history expressed erroneous conclusions about the significance of paternal versus maternal history.
O171R: “Well, the risk conferred by an aunt on the mother’s side for breast cancer is much greater than the risk conferred by an aunt on the father’s side.”
Ovarian Cancer
Of 33 SP Case 3 sessions, only 5 physicians (15%) discussed the contribution of ovarian cancer to breast cancer risk. Among those 5 physicians, 3 had identified the SP’s aunt with ovarian cancer.
Follow-up recommendations
Mammogram recommendations differed for each case (Table 5). SP Case 1 (moderate risk) presented with a history of a transient lump. In 48% of encounters, physicians recommended that a mammogram be done in follow-up; another 20% offered a mammogram as an option if the patient wished to have it. For Case 2 (high risk - maternal), physicians recommended a mammogram in 93% of encounters. Because Case 2 had already had a recent mammogram, the recommendation did not require immediate action. For SP Case 3 (high risk - paternal), physicians recommended a mammogram as an immediate follow-up test in 63% of encounters and offered it as a patient option in 9% of encounters.
Table V.
Mammography Recommendations
| SP Case 1 Moderate risk Mother with breast cancer at age 60, presenting with history of transient breast lump, N=25 |
SP Case 2 High risk-maternal Sister, mother and maternal aunt with breast cancer < 50 years; patient had mammogram within past year, N=28 |
SP Case 3 High risk-paternal Sister, paternal aunt and paternal grandmother with breast cancer < 50 years, and paternal aunt with ovarian cancer, N=33 |
|
|---|---|---|---|
| Mammogram recommended | 12 (48%) | 25/27 (93%) | 20/32 (63%) |
| Mammogram offered as an option, according to patient preference | 5 (20%) | N/A | 3 (9%) |
N/A = Not applicable because patient reports having a mammogram in the past year
Other follow-up recommendations were made as well (Table 6). For each SP case, some physicians recommended another primary care visit to discuss genetic cancer risk: 16% for SP Case 1 (moderate risk), 14% for SP Case 2 (high risk - maternal), and 9% for SP Case 3 (high risk - paternal). Follow-up with medical genetics was recommended or offered in 24% of visits for SP Case 1 (moderate risk), 29% for SP Case 2 (high risk - maternal) and 9% for SP Case 3 (high risk - paternal). No other follow-up related to genetic cancer risk was recommended or offered for SP Case 1 (moderate risk), but a little over a third of physicians recommended or offered other follow-up for SP Case 2 (high risk - maternal) (46%), including referral to an oncology specialist; other follow-up was recommended or offered in only 9% of SP Case 3 (high risk - paternal) encounters. Follow-up recommendations were often tied to risk assessment:
W108A: “[Your mother’s breast cancer history] just means you need your monthly breast exams, you need to come in for your physicals, you need to get your mammograms done at a reasonable time.” (SP Case 1 – moderate risk)
J109M: “Well, in your case, because of family history, we would probably put you on a schedule of getting mammograms every year, at an early age…And the other big question for you would be later on, when it’s time of menopause, whether you should take estrogen or not.” (SP Case 2 – high risk, maternal)
Table VI.
Other Follow-up Recommendations
| SP Case 1 Mother with breast cancer at age 60, presenting with breast lump |
SP Case 2 Sister, mother and maternal aunt with breast cancer < 50 years |
SP Case 3 Sister, paternal aunt and paternal grandmother with breast cancer < 50 years, and paternal aunt with ovarian cancer |
|
|---|---|---|---|
| Follow-up with primary care physician | 4 (16%) | 4 (14%) | 3 (9%) |
| Other follow-up related to cancer risk (such as referral to oncology) | 0 (0%) | 13 (46%) | 3 (9%) |
| Medical genetics referral | 6 (24%) | 8 (29%) | 3 (9%) |
| Genetic testing | |||
| • Candidate for genetic testing | 0 (0%) | 6 (21%) | 1 (3%) |
| • Not a candidate for genetic testing | 18 (72%) | 2 (7%) | 18 (54%) |
| • No opinion | 7 (28%) | 18 (64%) | 14 (42 %) |
Recommendations regarding genetic testing differed for the different SP cases (Table 6). No physicians considered SP Case 1 (moderate risk) to be a candidate for genetic testing, but SP Case 2 (high risk - maternal) was considered a candidate for genetic testing in 21% of encounters, and SP Case 3 (high risk - paternal) was considered a candidate for genetic testing in one encounter (3%). Conversely, in response to a scripted SP probe, patients were told they were not candidates for genetic testing in 72% of SP Case 1 encounters, 7% of SP Case 2 encounters, and 54% of SP Case 3 encounters. Many physicians were skeptical about genetic testing:
H115R “And you know, what if you had the gene? Would you get a prophylactic mastectomy? Certainly not. Would take Tamoxifen? I doubt it. I think, I don’t think you would do anything different than you already are doing if you knew that your risk was higher.” (SP Case 3 – high risk, paternal)
O157R “The gene markers and other things would actually be more indicated in people who have a strong family history like if your mother or aunts or grandmother had breast cancer.” (SP Case 3 – high risk, paternal)
However, some physicians expressed support for genetic testing:
U102M: “Your family would be a candidate for genetic testing. You can make better decisions with more information.” (SP Case 2 – high risk, maternal)
DISCUSSION
In our study, family history collection was most complete for the patient whose sister, mother and maternal aunt had breast cancer (SP Case 2). Conversely, physicians did least well with a patient whose strong family history of cancer was on the paternal side (SP Case 3): fewer than half elicited relevant cancer history in paternal relatives, with the resulting loss of significant risk information. Some physician comments incorrectly ascribed less significance to paternal than to maternal family history of breast cancer, an error also evident in a physician survey [Yong, et al. 2003] and among women reporting a family history of breast cancer [Quillin, et al. 2006]. Most physicians provided an appropriate risk assessment for SP Case 1 – reassuring the patient that her risk was only moderately increased – but half did so on the basis of incomplete family history information, and would presumably have missed relevant maternal family history if it had been present.
Most physician statements about breast cancer risk were general rather than specific. This approach differs from the usual practice in medical genetics, which utilizes risk models to generate quantitative estimates for cancer risk and likelihood of a BRCA mutation [Antoniou, et al. 2004; Claus, et al. 1993; Gail, et al. 1989; Parmigiani, et al. 1998; Tyrer, et al. 2004], but may reflect an appropriately cautious approach to estimating risk based on a single primary care visit. It could also reflect a style of practice that is focused less on defining risk than on determining appropriate preventive strategies. The primary care physicians in our study appeared to interpret risk as an action guide, and often linked a general statement about risk to a specific statement about breast cancer screening or referral care. This finding is consistent with a qualitative study of primary care physicians’ attitudes toward genetic testing, which found that the value of genetic information was judged by its potential to improve clinical management [Robins and Metcalfe 2004]. The focus on follow up action may also explain why some physicians expressed skepticism about genetic testing. Many primary care providers appeared to consider positive family history a sufficient basis for early mammography screening. The few who discussed prophylactic mastectomy perceived it as an undesirable option, and oophorectomy was not considered, apparently because few providers in our study were aware of the link between inherited risk of breast and ovarian cancer or the breast-cancer risk reducing effect of pre-menopausal oophorectomy. As a result, physicians in our study did not appear to view genetic testing data as necessary for determining follow-up.
The main limitation of our methodology was the use of SPs instead of actual patients. We cannot rule out the possibility that an actual patient would have provided a physician with more information about her family history than was requested, and primary care providers may assume that family history will be volunteered. However, SP methodology has been standardized [King, et al. 1994], and has provided a reliable evaluation tool in medical education [Anderson, et al. 1994] and, with the use of unannounced SPs, in primary care practice [Beullens, et al. 1997; Carney, et al. 1995; Dresselhaus, et al. 2000; Epstein, et al. 2006; Fiscella, et al. 2004; Geraghty, et al. 2007; Goedhuys and Rethans 2001; Kravitz, et al. 2005].
Our study did not allow us to determine whether primary care providers viewed candidacy for breast MRI screening as a rationale for genetic testing, because our data were collected prior to the promulgation of national guidelines [Saslow, et al. 2007]. In addition, our study evaluated physician performance from a single visit rather than over the course of several visits. Finally, SP errors in our study resulted in either the provision of unsolicited family history or the failure to provide family history when asked. Because too much information was provided more often than too little, we think it unlikely that our data underestimate physician collection of family history.
Our study confirms findings from other studies based on chart review or survey [Acheson, et al. 2000; Murff, et al. 2004; Sabatino, et al. 2007; Sweet, et al. 2002; Tyler and Snyder 2006] regarding incomplete collection of family history collection in primary care. However, in each of our scenarios a substantial proportion of physicians elicited sufficient information for an appropriate risk assessment. Nearly half of physicians seeing SP Case 1 elicited sufficient maternal history to confirm the lack of a high risk family history. All SP Case 2 encounters and 45% of SP Case 3 encounters collected adequate information, if we assume that identification of two affected relatives with breast cancer before age 50 is sufficient to generate concern about inherited risk [US Preventive Services Task Force 2005], Although primary care providers have limited time for the collection of family history [Rich, et al. 2004], our data suggest that a directed assessment of family history is feasible in primary care practice.
Nevertheless, our study also suggests that primary care providers need to be better informed about the link between inherited breast and ovarian cancer risk, and about the significance of a history of these cancers in paternal relatives. Further, primary care providers’ appropriate focus on actions to reduce risk suggests that educational efforts will be most successful when they link collection of family history and referral to genetic counseling for consideration of BRCA testing to specific risk interventions. The growing evidence of effectiveness for such interventions, including early initiation of mammography, breast MRI screening, chemoprevention, and prophylactic oophorectomy [Nelson, et al. 2005; US Preventive Services Task Force 2005], provides a strong basis for educational efforts.
Acknowledgments
Supported in part by grant RO1 HG02263 from the National Human Genome Research Institute.
REFERENCES
- Acheson LS, Wiesner GL, Zyzanski SJ, Goodwin MA, Stange KC. Family history-taking in community family practice: implications for genetic screening. Genet Med. 2000;2(3):180–185. doi: 10.1097/00125817-200005000-00004. [DOI] [PubMed] [Google Scholar]
- Anderson M, Stillman P, Wang Y. Growing use of standardized patients in teaching and evaluation in medical education. Teach Learn Med. 1994;6:15–22. [Google Scholar]
- Antoniou AC, Pharoah PP, Smith P, Easton DF. The BOADICEA model of genetic susceptibility to breast and ovarian cancer. Br J Cancer. 2004;91(8):1580–1590. doi: 10.1038/sj.bjc.6602175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beullens J, Rethans JJ, Goedhuys J, Buntinx F. The use of standardized patients in research in general practice. Fam Pract. 1997;14(1):58–62. doi: 10.1093/fampra/14.1.58. [DOI] [PubMed] [Google Scholar]
- Carney PA, Dietrich AJ, Freeman DH, Jr, Mott LA. A standardized-patient assessment of a continuing medical education program to improve physicians' cancer-control clinical skills. Acad Med. 1995;70(1):52–58. doi: 10.1097/00001888-199501000-00014. [DOI] [PubMed] [Google Scholar]
- Chen S, Iversen ES, Friebel T, Finkelstein D, Weber BL, Eisen A, Peterson LE, Schildkraut JM, Isaacs C, Peshkin BN, Corio C, Leondaridis L, Tomlinson G, Dutson D, Kerber R, Amos CI, Strong LC, Berry DA, Euhus DM, Parmigiani G. Characterization of BRCA1 and BRCA2 mutations in a large United States sample. J Clin Oncol. 2006;24(6):863–871. doi: 10.1200/JCO.2005.03.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus EB, Risch N, Thompson WD. The calculation of breast cancer risk for women with a first degree family history of ovarian cancer. Breast Cancer Res Treat. 1993;28:115–120. doi: 10.1007/BF00666424. [DOI] [PubMed] [Google Scholar]
- Claus EB, Risch N, Thompson WD. Autosomal dominant inheritance of early-onset breast cancer. Cancer. 1994;73(3):643–651. doi: 10.1002/1097-0142(19940201)73:3<643::aid-cncr2820730323>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Dresselhaus TR, Peabody JW, Lee M, Wang MM, Luck J. Measuring compliance with preventive care guidelines: standardized patients, clinical vignettes, and the medical record. J Gen Intern Med. 2000;15(11):782–788. doi: 10.1046/j.1525-1497.2000.91007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein RM, Shields CG, Meldrum SC, Fiscella K, Carroll J, Carney PA, Duberstein PR. Physicians' responses to patients' medically unexplained symptoms. Psychosom Med. 2006;68(2):269–276. doi: 10.1097/01.psy.0000204652.27246.5b. [DOI] [PubMed] [Google Scholar]
- Fiscella K, Meldrum S, Franks P, Shields CG, Duberstein P, McDaniel SH, Epstein RM. Patient trust: is it related to patient-centered behavior of primary care physicians? Med Care. 2004;42(11):1049–1055. doi: 10.1097/00005650-200411000-00003. [DOI] [PubMed] [Google Scholar]
- Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, Mulvihill JJ. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81(24):1879–1886. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- Geraghty EM, Franks P, Kravitz RL. Primary care visit length, quality, and satisfaction for standardized patients with depression. J Gen Intern Med. 2007;22(12):1641–1647. doi: 10.1007/s11606-007-0371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedhuys J, Rethans JJ. On the relationship between the efficiency and the quality of the consultation. A validity study. Fam Pract. 2001;18(6):592–596. doi: 10.1093/fampra/18.6.592. [DOI] [PubMed] [Google Scholar]
- Guttmacher AE, Collins FS, Carmona RH. The family history--more important than ever. N Engl J Med. 2004;351(22):2333–2336. doi: 10.1056/NEJMsb042979. [DOI] [PubMed] [Google Scholar]
- King A, Perowski-Rogers L, Pohl H. Planning standardized patient programs: case development, patient training, and costs. Teach Learn Med. 1994;6:6–14. [Google Scholar]
- Kravitz RL, Epstein RM, Feldman MD, Franz CE, Azari R, Wilkes MS, Hinton L, Franks P. Influence of patients' requests for direct-to-consumer advertised antidepressants: a randomized controlled trial. Jama. 2005;293(16):1995–2002. doi: 10.1001/jama.293.16.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murff HJ, Byrne D, Syngal S. Cancer risk assessment: quality and impact of the family history interview. Am J Prev Med. 2004;27(3):239–245. doi: 10.1016/j.amepre.2004.05.003. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. web site: http:seer.cancer.gov/statistics.
- Nelson HD, Huffman LH, Fu R, Harris EL. Genetic risk assessment and BRCA mutation testing for breast and ovarian cancer susceptibility: systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2005;143(5):362–379. doi: 10.7326/0003-4819-143-5-200509060-00012. [DOI] [PubMed] [Google Scholar]
- Parmigiani G, Berry D, Aguilar O. Determining carrier probabilities for breast cancer-susceptibility genes BRCA1 and BRCA2. Am J Hum Genet. 1998;62(1):145–158. doi: 10.1086/301670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quillin JM, Ramakrishnan V, Borzelleca J, Bodurtha J, Bowen D, Baer Wilson D. Paternal relatives and family history of breast cancer. Am J Prev Med. 2006;31(3):265–268. doi: 10.1016/j.amepre.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Rich EC, Burke W, Heaton CJ, Haga S, Pinsky L, Short MP, Acheson L. Reconsidering the family history in primary care. J Gen Intern Med. 2004;19(3):273–280. doi: 10.1111/j.1525-1497.2004.30401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins R, Metcalfe S. Integrating genetics as practices of primary care. Soc Sci Med. 2004;59(2):223–233. doi: 10.1016/j.socscimed.2003.10.025. [DOI] [PubMed] [Google Scholar]
- Sabatino SA, McCarthy EP, Phillips RS, Burns RB. Breast cancer risk assessment and management in primary care: provider attitudes, practices, and barriers. Cancer Detect Prev. 2007;31(5):375–383. doi: 10.1016/j.cdp.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Saslow D, Boetes C, Burke W, Harms S, Leach MO, Lehman CD, Morris E, Pisano E, Schnall M, Sener S, Smith RA, Warner E, Yaffe M, Andrews KS, Russell CA. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- Sweet KM, Bradley TL, Westman JA. Identification and referral of families at high risk for cancer susceptibility. J Clin Oncol. 2002;20(2):528–537. doi: 10.1200/JCO.2002.20.2.528. [DOI] [PubMed] [Google Scholar]
- Tonin P, Weber B, Offit K, Couch F, Rebbeck TR, Neuhausen S, Godwin AK, Daly M, Wagner-Costalos J, Berman D, Grana G, Fox E, Kane MF, Kolodner RD, Krainer M, Haber DA, Struewing JP, Warner E, Rosen B, Lerman C, Peshkin B, Norton L, Serova O, Foulkes WD, Lynch HT, Lenoir GM, Narod SA, Garber JE. Frequency of recurrent BRCA1 and BRCA2 mutations in Ashkenazi Jewish breast cancer families. Nat Med. 1996;2(11):1179–1183. doi: 10.1038/nm1196-1179. [DOI] [PubMed] [Google Scholar]
- Tyler CV, Jr, Snyder CW. Cancer risk assessment: examining the family physician's role. J Am Board Fam Med. 2006;19(5):468–477. doi: 10.3122/jabfm.19.5.468. [DOI] [PubMed] [Google Scholar]
- Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23(7):1111–1130. doi: 10.1002/sim.1668. [DOI] [PubMed] [Google Scholar]
- US Preventive Services Task Force. Genetic risk assessment and BRCA mutation testing for breast and ovarian cancer susceptibility: recommendation statement. Ann Intern Med. 2005;143:355–361. doi: 10.7326/0003-4819-143-5-200509060-00011. [DOI] [PubMed] [Google Scholar]
- Yong MC, Zhou XJ, Lee SC. The importance of paternal family history in hereditary breast cancer is underappreciated by health care professionals. Oncology. 2003;64(3):220–226. doi: 10.1159/000069309. [DOI] [PubMed] [Google Scholar]