Abstract
We and others have reported significant expression of the Ang II Type 1 receptor (AT1R) on renal nuclei; thus, the present study assessed the functional pathways and distribution of the intracellular AT1R on isolated nuclei. Ang II (1 nM) stimulated DCF fluorescence, an intranuclear indicator of reactive oxygen species (ROS), while the AT1R antagonist losartan or the NADPH oxidase (NOX) inhibitor DPI abolished the increase in ROS. Dual labeling of nuclei with antibodies against nucleoporin 62 (Nup62) and AT1R or the NADHPH oxidase isoform NOX4 revealed complete overlap of the Nup62 and AT1R (99%) by flow cytometry, while NOX4 was present on 65% nuclei. Treatment of nuclei with a PKC agonist stimulated increased ROS while the PKC inhibitor GF109203X or PI3 kinase inhibitor LY294002 abolished Ang II stimulation of ROS. We conclude that the Ang II-AT1R-PKC axis may influence nuclear function within the kidney through a redox sensitive pathway.
Keywords: Angiotensin II, nuclei, kidney, angiotensin type 1 receptor, NOX4, reactive oxygen species
Introduction
The well-accepted model of G-protein coupled receptors (GPCRs) entails their cellular orientation on the plasma membrane to facilitate binding to extracellular or circulating peptides and the subsequent conformational changes to induce cell signaling. An intricate system of receptor-associated intracellular proteins is requisite for the regulation and integration of GPCR -activated signaling that encompasses an array of kinases, phosphatases and nuclear transcription factors. The angiotensin type 1 (AT1) receptor is one prototypic GPCR whereby alterations in either receptor levels or its downstream signaling pathways are associated with the development and progression of cardiovascular pathologies. Indeed, AT1 receptor antagonists have emerged as one of the leading therapies for the treatment of hypertension and tissue injury.
Increasing evidence now supports the intracellular expression of various peptide GPCRs in tissues and cells [1–3]. Our laboratory has reported a significant density of AT1 receptors on nuclei isolated from both rat and sheep kidney [4; 5]. Importantly, Li and Zhou [6] demonstrated that angiotensin II (Ang II) stimulates nuclear AT1 receptors of the renal cortex to induce mRNA transcripts for the sodium hydrogen exchanger (NHE-3), the chemokine moncyte chemoattractant protien (MCP-1) and the pro-fibrotic peptide tumor growth factor beta (TGF-β). Their findings are consistent with the long-term actions of Ang II - AT1 receptor activation to increase sodium retention and stimulate inflammatory pathways within the kidney. Although the nature of the signaling pathways for the AT1 receptor within the nucleus is not known, the cell surface receptor mediates multiple intracellular signals including the release of PI3 kinase-dependent phospholipids, diacylglycerol (DAG), alterations in cell calcium, activation of protein kinase C (PKC) and the generation of reactive oxygen species (ROS) through NADPH oxidase (NOX) and associated protein components [7]. ROS may activate signaling pathways in the nucleus to influence gene expression [8] or promote oxidative damage to DNA that may enhance cell senescence [9]. Moreover, NOX4 localizes to the nucleus or perinuclear region and contributes to superoxide (SO−) and/or hydrogen peroxide (H2O2) generation [8; 10]. To elucidate the functional properties of the nuclear AT1 receptor, we determined whether Ang II stimulates ROS in freshly isolated nuclei from the rat renal cortex, as well as evaluated the signaling pathways downstream from activation of the AT1 receptor.
Methods
Animals
Experiments were performed in 12 – 15 week old normotensive male Lewis rats. The rats were purchased from Charles River Laboratories (Raleigh, NC) and housed in an AALAC-approved facility in a temperature-controlled room (22 ± 2°C) with a 12 hour light: dark cycle and free access to food and water. These procedures were approved by the Wake Forest University School of Medicine Institutional Animal Care and Use Committee.
ROS measurement
Cortical nuclei were freshly isolated [4] and incubated in 100 mM KH2PO4, 1 mM NaN3, 1 mM EGTA, 100 μM FAD, and 100 μM NADH [8]. Losartan, an AT1 receptor antagonist (10 μM), LY294002, a PI3 kinase inhibitor (10 μM), bisindolylmaleimide I (GF 109203X), a protein kinase C inhibitor (500 nM), and diphenyliodonium (DPI), a NOX inhibitor (10 μM) were pre-incubated with nuclei for 10 min at 25°C – all given at their final concentrations in the assay. The reaction was initiated by addition of Ang II [1 nM or 1μM, final concentration] or buffer alone to renal nuclei for 5 min at 37°C and the nuclei subsequently centrifuged at 1,200 × g for 3 min. The fluorescent dye, 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate-acetyl ester (DCF, C6827, Molecular Probes, Eugene OR) was added to the nuclei at a final concentration of 20 μM and incubated for 30 minutes at 37°C. DCF incubation was terminated by the addition of phosphate buffered saline (pH 7.0) and the nuclei centrifuged twice at 1,500 × g for 3 min. Nuclei were acquired (~25,000 events) on a FACSCalibur (BD, Franklin Lakes, NJ). Data were analyzed with FlowJo software (Ashland, OR) and expressed as the percent change in mean fluorescence intensity (%MFI).
Antibody labeling
Antibody labeling of nuclei was performed using a method adapted from Michalek et al. [11]. Nuclei were washed with 2% fetal calf serum (FCS), centrifuged at 2,000 x g for 3 min and fixed with 2% paraformaldehyde (PFA) for 20 min at 4°C. After fixation, the nuclei were washed with 2% FCS, centrifuged at 2,000 × g for 2 min thrice and incubated with primary antibodies to NOX4 (Lot# 487762, 1:250, Abcam, Cambridge, MA) or the AT1 receptor (Lot# 3329S1, 1:100, Alpha Diagnostics, San Antonio, TX), and nucleoporin 62 labeled with fluorosceinisothyocyanate (Nup62-FITC, 1:25, BD Transduction, San Diego, Ca) for 30 min at 4°C in the dark. To control for nonspecific antibody labeling, the primary antibodies were substituted with either rabbit serum (AT1 receptor), rabbit IgG (NOX4), or mouse IgG-FITC (Nup62). Nuclei were then washed with 2% FCS, centrifuged at 3,500 × g for 2 min thrice and incubated with the secondary antibody donkey anti-rabbit IgG labeled phycoerythrin (PE, Jackson ImmunoResearch Laboratories, West Grove, PA) for 30 min at 4° C. After completion of the secondary antibody incubation, nuclei were washed thrice with 2% FCS at 3,500 × g and finally resuspended in 2% PFA. Approximately 25,000 nuclei were acquired on a FACSCalibur and the data analyzed by FlowJo software.
Immunoblot Analysis
Isolated renal nuclei were suspended in PBS and added to Laemmli buffer containing mercaptoethanol as described [4]. Immunodetection was performed on blots blocked for 1 h with 5% Dry Milk (Bio-Rad) and Tris-buffered saline containing 0.05% Tween, then probed with the AT1 receptor and NOX4 antibodies.
Statistical Analysis
Data represented as means ± standard error (SE). The data were analyzed with student’s t-test or One-Way ANOVA with Dunnet’s Post hoc test and significance was achieved at p<0.05. All statistics were computed with Graphpad 4.0 Prism software (San Diego, CA).
Results and Discussion
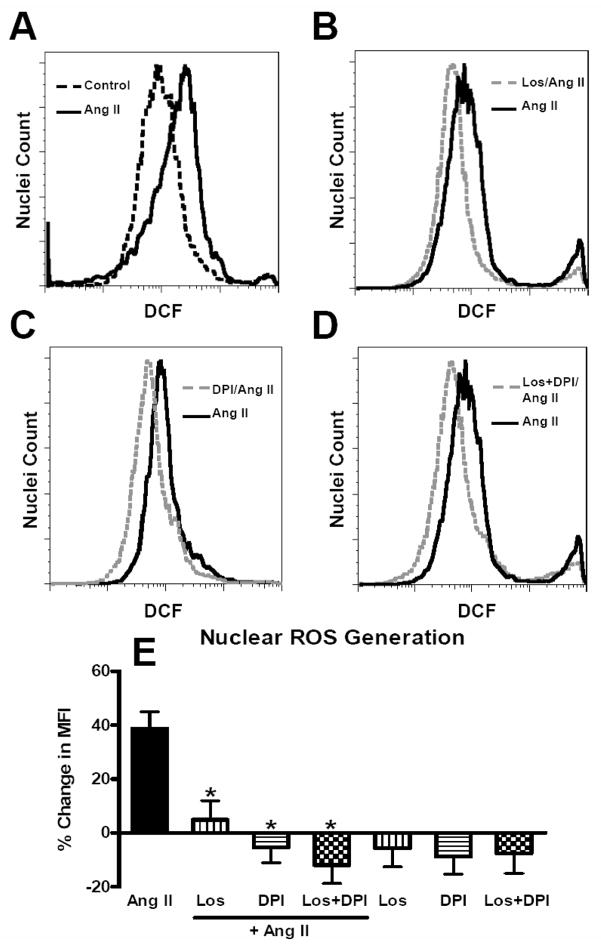
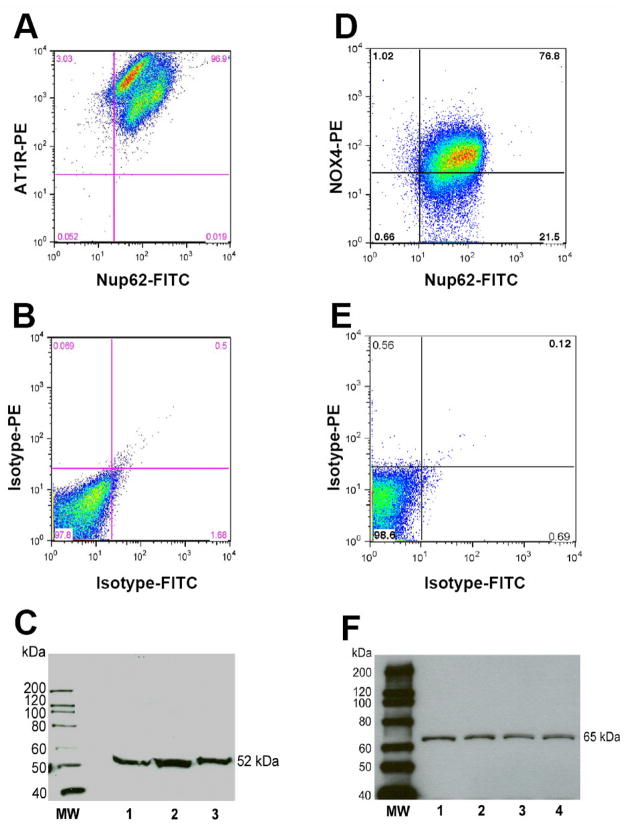
Using flow cytometry of DCF-labeled intact nuclei isolated from the renal cortex, addition of Ang II (1 nM) for 5 minutes resulted in a 55% shift in mean fluorescence intensity (MFI) as compared to non-stimulated (control) nuclei loaded with DCF in the fluorescence graph (Figure 1A). Pre-treatment of nuclei with the AT1 receptor antagonist losartan (10 μM), the NOX inhibitor DPI (10 μM) or their combination reduced the shift in DCF fluorescence with 1 nM Ang II (Figure 1B, 1C and 1D, respectively). The increased in the mean fluorescence response to Ang II (1 nM) was 39 ± 6% (p < 0.01; N=8). A higher concentration of Ang II (1 μM) did not result in a greater production of ROS as compared to the lower dose of the hormone (28 ± 9%, N=4, p > 0.05 vs. 1 nM). Treatment of nuclei with either losartan, DPI or their combination essentially abolished Ang II-dependent stimulation of ROS (Figure 1E). Addition of losartan, DPI or losartan/DPI in the absence of Ang II did not significantly reduce DCF fluorescence in the control nuclei (Figure 1E). The blockade of the Ang II response by losartan in isolated nuclei is consistent with previous studies demonstrating a high density of AT1 receptors on renal cortical nuclei by radioligand binding and immunoblot methods [4; 6]. Since the renal cortex contains multiple cell types and the whole cortex was utilized in these studies, it is not known to what extent the AT1 receptor is expressed on all nuclei. Therefore, we applied flow cytometry to probe the renal cortical nuclei with antibodies against the unique nuclear protein nucleoporin (Nup62) and the AT1 receptor. As shown in the upper right panel of Figure 2A, the majority of nuclei identified by the Nup62 antibody were also positive for the AT1 receptor antibody. In three separate preparations of nuclei, the AT1 receptor was positive for 99 ± 1%, (N=3) of isolated cortical nuclei. The flow analysis also revealed two distinct densities for the AT1 receptor (3145 ± 317 vs. 928.0 ± 84.0 fluorescence intensity units, p < 0.01, N=3) that differed approximately three-fold on the cortical nuclei. It is unclear whether the different densities in the nuclear AT1 receptor arise from distinct cell types of the renal cortex (i.e., mesangial versus tubular epithelial cells) or whether there are subpopulations of nuclei within the same cell type that express different receptor densities. Substitution of the Nup62 and AT1 primary antibodies with their isotype controls (labeled with FITC and PE, respectively) did not reveal evidence for significant co-localization (Figure 2B). As shown in Figure 2C, the full-length immunoblot using the same AT1 receptor antibody as for the flow analysis reveals a single band at 52 kDa, a molecular weight identical to that found in isolated nuclei from the rat and sheep kidney [4; 5].
Figure 1.
Angiotensin (Ang) II stimulates reactive oxygen species (ROS) in renal cortical nuclei. ROS was visualized with dichlorofluroscein (DCF, 20 μM) in isolated renal cortical nuclei by flow cytometry. Nuclei were incubated with Ang II (1 nM) for 5 min at 37°C. Inhibitors were added 10 min prior to Ang II-stimulated or control nuclei. Panel A: Representative fluorescence tracings of Ang II (1 nM) as compared to control. Panel B: Ang II and the AT1 receptor antagonist losartan (Los, 10 μM); Panel C: Ang II and the NADPH oxidase inhibitor DPI (10 μM). Panel D: Ang II and the combination of LOS and DPI. Panel E: Changes in mean fluorescence intensity (% MFI) as compared to control nuclei. Data are means + SE from 4 – 6 experiments, *P<0.01 vs. Ang II.
Figure 2.
AT1 receptor and NOX4 localization in renal cortical nuclei by flow cytometry. Panel A: Nuclear labeling with the AT1 receptor and nucleoporin 62 (Nup62) antibodies. Panel B: Nuclear labeling with the isotype antibody controls for AT1 receptor (rabbit IgG-labeled phycoerythrin, PE) and Nup62 (mouse IgG-labeled fluorosceinisothyocyanate, FITC). Panel C: Immunoblot of 3 separate renal nuclear preparations with the AT1 receptor antibody. Panel D: NOX4 antibody labeling with Nup62. Panel E: Nuclear labeling with the isotype antibody controls for NOX4 receptor (rabbit IgG-PE) and Nup62 (mouse IgG-FITC). Panel F: Immunoblot of 4 separate renal nuclear preparations with the NOX4 antibody. Molecular weight (MW) expressed in kilodaltons (kDa).
Studies in intact cells or isolated tissues clearly demonstrate the activation of NADPH oxidases by Ang II in the production of ROS [16]. NOX4 is considered the predominant NADPH isozyme in the kidney with prevalent expression in the proximal tubules [9; 13]. Although we are not aware of subcellular studies on NOX4 expression in the kidney, nuclear forms of NOX4 were identified in endothelial and vascular smooth muscle cells (VSMC) in culture [8; 10; 14–16]. The nuclear localization of NOX4 is consistent with the protein’s two canonical nuclear localization signals (NLS) on residues 90–97 and 451–458. Moreover, NOX4 may either directly generate H2O2 or, similar to other NADPH oxidases, form SO− that rapidly dismutates to H2O2, a more stable product and readily detectable with DCF [16–18]. Therefore, we probed the nuclei isolated from the renal cortex with fluorescently tagged antibodies against Nup62 and NOX4 to assess the distribution of the oxidase. As shown in the upper right panel of Figure 1D, flow cytometry revealed that NOX4 was colocalized on 77% of renal nuclei with a mean value of 65 ± 4% (N=3). The isotype control antibodies for NOX4 and Nup62 did not reveal evidence of colocalization (Figure 1E). The immunoblot reveals a single band of 65 kDa using the same affinity-purified NOX4 antibody as for flow cytometry (Figure 1F). The molecular size of nuclear NOX4 in the renal cortex demonstrated in the gel is identical to the predicted size of the enzyme [9; 19] and to the nuclear form in 3T3 adipocytes and endothelial cells [15; 16; 18]. Thus, the flow analysis indicated a substantial overlap of the AT1 receptor and NOX4 on nuclei isolated from the renal cortex. Originally thought to be constitutively active [9], recent evidence demonstrates NOX4-dependent stimulation of ROS by insulin, TGF-β and Ang II [14; 15; 18; 20; 21]. It remains to be determined, however, whether NOX4 or other oxidases that are expressed in the kidney (NOX 1 and NOX 2) participate in the Ang II-dependent formation of ROS in renal nuclei
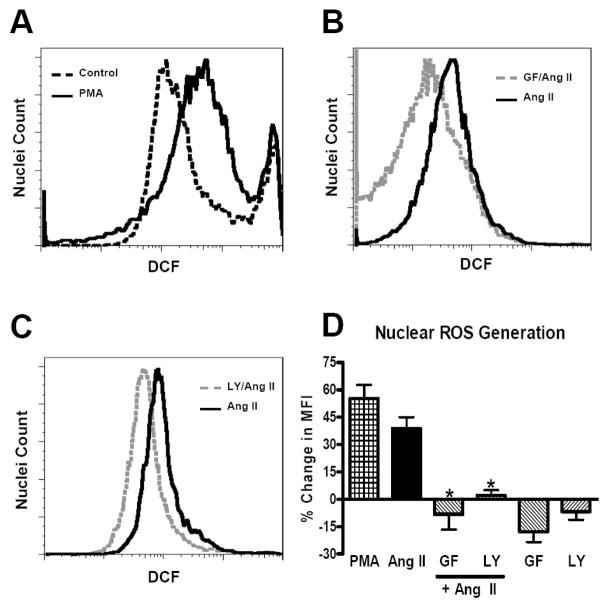
The downstream signaling for Ang II-dependent activation of NAPDH oxidases and ROS generation is complex, which may reflect tissue and/or cell specific pathways [7; 22]; however, there are no data regarding the pathways within the nucleus that contribute to Ang II stimulation of ROS. Addition of the protein kinase C (PKC) activator phorbol myristate acetate (PMA, 1 μM), a known stimulator of ROS in intact cells [17], resulted in a shift in MIF of 72% as compared to control (Figure 3A). Since the PKC agonist PMA increased DCF fluorescence (58 ± 1%, N=4) to a similar extent as Ang II in nuclei (Figure 3D), we determined whether PKC inhibition would block the stimulation of ROS by Ang II. As shown in Figure 3B, GF109203X (GF, 500 nM), an inhibitor of both conventional and novel PKCs, shifted the nuclear Ang II-dependent ROS production by 62%. The PKC inhibitor essentially abolished Ang II stimulation of ROS in the isolated nuclei that was not different than the NOX inhibitor DPI (Figures 3D and 1E, respectively). PKC activation typically depends on the increased availability of calcium and phospholipids such as diacylglycerol [23; 24]. There is compelling evidence for a nuclear phosphoinositol system that includes several PI3 kinase isoforms [25; 26]. As the Ang II-AT1 receptor stimulation of ROS is linked to PI3 kinase and PKC activation in intact cells [27], we then assessed whether PI3 kinase inhibition would abrogate the nuclear Ang II response. Pre-treatment of isolated renal nuclei with the selective PI3 kinase inhibitor LY294002 (LY, 10 μM) shifted the Ang II fluorescence trace by 31% (Figure 3C) and attenuated the increase in ROS generation to Ang II to the same extent as the PKC or NOX inhibitors (Figures 3D and 1E, respectively). It should be noted that pretreatment of the nuclei with the PKC or PI3 kinase inhibitors alone did not significantly alter DCF fluorescence as compared to the control nuclei (Figure 3D). These data support findings by Griendling and colleagues that PI3 kinase and PKC are essential in Ang II-dependent formation of ROS in intact VSMC [28]. To our knowledge, the current studies are the first to provide evidence for a functional role of PI3 kinase and PKC in Ang II-dependent actions within isolated renal nuclei; however, we cannot distinguish whether PKC is upstream or downstream from PI3 kinase. Moreover, further studies are required to define the identity of the nuclear PKC and PI3 kinase isoforms required for Ang II generation of ROS.
Figure 3.
Angiotensin (Ang) II-dependent reactive oxygen species (ROS) production is sensitive to PKC and PI3 kinase inhibitors. ROS was visualized with dichlorofluroscein (DCF, 20 μM) in isolated renal cortical nuclei by flow cytometry. Nuclei were incubated with Ang II (1 nM) for 5 min at 37°C. Inhibitors were added 10 min prior to Ang II-stimulated or control nuclei. Panel A: Representative fluorescence tracing of the PKC activator PMA (1 μM) compared to control. Panel B: Ang II (1 nM) and the PKC inhibitor GF 109203X (GF, 500 nM). Panel C: Ang II and the PI3 kinase inhibitor LY 294002 (LY, 10 μM). Panel D: Changes in mean fluorescence intensity (% MFI) as compared to control nuclei. Data are means + SE from 3 – 6 experiments, *P<0.01 vs. Ang II.
Intracellular or nuclear Ang II receptors have been demonstrated in various tissues or cells [4; 5; 29–32]. Indeed, the AT1 receptor shares a canonical localization sequence (KKFKR) along with other G-protein coupled receptors (GPRC) that traffic to the nucleus [32]. The demonstration of a nuclear Ang II – AT1 receptor-mediated pathway to rapidly generate ROS is in keeping with an intracrine RAS for functional intracellular hormones [33; 34]. In isolated nuclei from rat hepatocytes, Ang II stimulated transcripts for renin and angiotensinogen [31]. Moreover, Baker and colleagues found that intracellular expression of Ang II significantly increased cardiac hypertrophy in the absence of alterations in blood pressure or circulating levels of Ang II [35]. In contrast, expression of secreted Ang II in the heart did not influence cardiac hypertrophy except when sufficient levels were achieved that raised both blood pressure and plasma Ang II [36]. Finally, the Ang II-AT1 receptor stimulates cytokines and sodium transporter mRNA in cortical nuclei that would clearly contribute to the actions of a renal RAS [6].
We conclude that the rapid but low-level stimulation of ROS by Ang II would support a redox sensitive signaling pathway within the nucleus, perhaps to influence gene expression [8]. The use of AT1 receptor blockers (ARBs) to prevent the binding of extracellular Ang II has become a widely accepted therapy to lower blood pressure. Although patients treated with ARBs may achieve adequate control of their blood pressure, they may still exhibit indices of renal injury including proteinuria and an impaired filtration fraction. Moreover, higher doses of ARBs may have added benefit within the kidney which may reflect greater tissue penetration of these agents to intracellular targets [37]. In lieu of the present data for functional AT1 receptors within the nucleus coupled with the existing evidence that Ang II directly influences gene regulation, the issue of adequate intracellular access of ARBs becomes important. Indeed, the nuclear Ang II receptor, if evident in the human kidney and other tissues, should be considered a new treatment target.
Acknowledgments
Sources of Funding
This work was supported by grants from the National Heart, Lung, and Blood Institute, NIH (HL-56973, HL-51952).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Gobeil F, Fortier A, Zhu T, Bossolasco M, Leduc M, Grandbois M, Heveker N, Bkaily G, Chemtob S, Barbaz D. G-protein-coupled receptors signaling at the cell nucleus: an emerging paradigm. Can J Physiol Pharmacol. 2006;84:287–297. doi: 10.1139/y05-127. [DOI] [PubMed] [Google Scholar]
- 2.Goetzl EJ. Diverse pathways for nuclear signaling by G protein-coupled receptors and their ligands. FASEB J. 2007;21:638–642. doi: 10.1096/fj.06-6624hyp. [DOI] [PubMed] [Google Scholar]
- 3.Boivin B, Vaniotis G, Allen BG, Hebert TE. G protein-coupled receptors in and on the cell nucleus: a new signaling paradigm? J Recept Signal Transduct Res. 2008;28:15–28. doi: 10.1080/10799890801941889. [DOI] [PubMed] [Google Scholar]
- 4.Pendergrass KD, Averill DB, Ferrario CM, Diz DI, Chappell MC. Differential expression of nuclear AT1 receptors and angiotensin II within the kidney of the male congenic mRen2. Lewis rat. Am J Physiol Renal Physiol. 2006;290:F1497–F1506. doi: 10.1152/ajprenal.00317.2005. [DOI] [PubMed] [Google Scholar]
- 5.Gwathmey TM, Shaltout HA, Pendergrass KD, Pirro NT, Figueroa JP, Rose JC, Diz DI, Chappell MC. Nuclear Angiotensin II-Type 2 (AT2) receptors are functionally liked to nitric oxide production. Am J Physiol Renal Physiol. 2009 doi: 10.1152/ajprenal.90766.2008. Epub Feb 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li XC, Zhuo JL. Intracellular angiotensin II induces in vitro transcription of TGF-β, MCP-1 and NHE3 mRNAs in rat renal cortical nuclei via activation of nuclear AT1 receptors. Am J Physiol Cell Physiol. 2008;294:C1034–1045. doi: 10.1152/ajpcell.00432.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garrido AM, Griendling KK. NADPH oxidases and angiotensin II receptor signaling. Mol Cell Endocrinol. 2008;302:148–158. doi: 10.1016/j.mce.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuroda J, Nakagawa K, Yamasaki T, Nakamura K, Takeya R, Kuribayashi F, Imajoh-Ohmi S, Igarashi K, Shibata Y, Sueishi K, Sumimoto H. The superoxide-producing NAD(P)H oxidase Nox4 in the nucleus of human vascular endothelial cells. Genes Cells. 2005;10:1139–1151. doi: 10.1111/j.1365-2443.2005.00907.x. [DOI] [PubMed] [Google Scholar]
- 9.Geiszt M, Kopp JB, Varnal P, Leto TL. Identification of Renox, an NAD(P)H oxidase in kidney. PNAS. 2000;97:8010–8014. doi: 10.1073/pnas.130135897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:677–683. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- 11.Michalek RD, Nelson KJ, Holbrook BC, Yi JS, Stridiron D, Daniel LW, Fetrow JS, King SB, Poole LB, Grayson JM. The requirement of reversible cysteine sulfenic acid formation for T cell activation and function. J Immunol. 2007;179:6456–6467. doi: 10.4049/jimmunol.179.10.6456. [DOI] [PubMed] [Google Scholar]
- 12.Sachse A, Wolf G. Angiotensin II-induced reactive oxygen species and the kidney. J Am Soc Neph. 2007;18:2439–2436. doi: 10.1681/ASN.2007020149. [DOI] [PubMed] [Google Scholar]
- 13.Shiose A, Kuroda J, Tsuruya K, Hirai M, Hirakata H, Naito S, Hattori M, Sakaki Y, Sumimoto H. A novel superoxide-producing NAD(P)H oxidase in kidney. J Biol Chem. 2001;276:1417–1423. doi: 10.1074/jbc.M007597200. [DOI] [PubMed] [Google Scholar]
- 14.Sturrock A, Huecksteadt TP, Norman K, Sanders K, Murphy TM, Chitano P, Wilson K, Hoidal JR, Kennedy TP. Nox4 mediates TGF-beta1-induced retinoblastoma protein phosphorylation, proliferation, and hypertrophy in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1543–L1555. doi: 10.1152/ajplung.00430.2006. [DOI] [PubMed] [Google Scholar]
- 15.Hu T, Ramachandrarao SP, Siva S, Valancius C, Zhu Y, Mahadev K, Toh I, Goldstein BJ, Woolkalis M, Sharma K. Reactive oxygen species production via NADPH oxidase mediates TGF-beta-induced cytoskeletal alterations in endothelial cells. Am J Physiol Renal Physiol. 2005;289:F816–F825. doi: 10.1152/ajprenal.00024.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Natarajan V, Pendyala S, Gorshkova IA, Usatyuk P, He D, Pennathur A, Lambeth JD, Thannickal VJ. Role of NOX4 and NOX2 in hyperoxia-induced reactive oxygen species generation and migration of human lung endothelial cells. Antioxid Redox Signal. 2008 doi: 10.1089/ars.2008.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martyn KD, Frederick LM, von LK, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 18.Mahadev K, Motoshima H, Wu X, Ruddy JM, Arnold RS, Cheng G, Lambeth JD, Goldstein BJ. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol. 2004;24:1844–1854. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–140. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 20.Gorin Y, Ricono JM, Kim NH, Bhandari B, Choudhury GG, Abboud HE. Nox4 mediates angiotensin II-induced activation of Akt/protein kinase B in mesangial cells. Am J Physiol Renal Physiol. 2003;285:F219–F229. doi: 10.1152/ajprenal.00414.2002. [DOI] [PubMed] [Google Scholar]
- 21.Block K, Eid A, Griendling KK, Lee DY, Wittrant Y, Gorin Y. Nox4 NAD(P)H oxidase mediates Src-dependent tyrosine phosphorylation of PDK-1 in response to angiotensin II: role in mesangial cell hypertrophy and fibronectin expression. J Biol Chem. 2008;283:24061–24076. doi: 10.1074/jbc.M803964200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perisic O, Wilson MI, Karathanassis D, Bravo J, Pacold ME, Ellson CD, Hawkins PT, Stephens L, Williams RL. The role of phosphoinositides and phosphorylation in regulation of NADPH oxidase. Adv Enzyme Regul. 2004;44:279–298. doi: 10.1016/j.advenzreg.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Oliva JL, Griner EM, Kazanietz MG. PKC isozymes and diacylglycerol-regulated proteins as effectors of growth factor receptors. Growth Factors. 2005;23:245–252. doi: 10.1080/08977190500366043. [DOI] [PubMed] [Google Scholar]
- 24.Parekh DB, Ziegler W, Parker PJ. Multiple pathways control protein kinase C phosphorylation. EMBO J. 2000;19:496–503. doi: 10.1093/emboj/19.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deleris P, Gayral S, Breton-Douillon M. Nuclear Ptdlns(3,4,5)P3 signaling: an ongoing story. J Cell Biochem. 2006;98:469–485. doi: 10.1002/jcb.20695. [DOI] [PubMed] [Google Scholar]
- 26.Irvine RF. Nuclear lipid signaling. Nat Rev Mol Cell Biol. 2003;4:349–360. doi: 10.1038/nrm1100. [DOI] [PubMed] [Google Scholar]
- 27.Choi H, Leto TL, Hunyady L, Catt KJ, Bae YS, Rhee SG. Mechanism of angiotensin II-induced superoxide production in cells reconstituted with angiotensin type 1 receptor and the components of NADPH oxidase. J Biol Chem. 2008;283:255–267. doi: 10.1074/jbc.M708000200. [DOI] [PubMed] [Google Scholar]
- 28.Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res. 2002;91:406–413. doi: 10.1161/01.res.0000033523.08033.16. [DOI] [PubMed] [Google Scholar]
- 29.Robertson AL, Jr, Khairallah PA. Angiotensin II: rapid localization in nuclei of smooth and cardiac muscle. Science. 1971;172:1138–1139. doi: 10.1126/science.172.3988.1138. [DOI] [PubMed] [Google Scholar]
- 30.Lu D, Yang H, Shaw G, Raizada MK. Angiotensin II-induced nuclear targeting of the angiotensin type 1 (AT1) receptor in brain neurons. Endocrinology. 1998;139:365–375. doi: 10.1210/endo.139.1.5679. [DOI] [PubMed] [Google Scholar]
- 31.Booz GW, Conrad KM, Hess AL, Singer HA, Baker KM. Angiotensin-II-binding sites on hepatocyte nuclei. Endocrinology. 1992;130:3641–3649. doi: 10.1210/endo.130.6.1597161. [DOI] [PubMed] [Google Scholar]
- 32.Lee DK, Lanca AJ, Cheng R, Nguyen T, Ji XD, Gobeil F, Jr, Chemtob S, George SR, O’Dowd BF. Agonist-independent nuclear localization of the apelin, angiotensin AT1, and bradykinin B2 receptors. J Biol Chem. 2004;279:7901–7908. doi: 10.1074/jbc.M306377200. [DOI] [PubMed] [Google Scholar]
- 33.Re R. Implications of intracrine hormone action for physiology and medicine. Am J Physiol Heart Circ Physiol. 2003;284:H757–H757. doi: 10.1152/ajpheart.00935.2002. [DOI] [PubMed] [Google Scholar]
- 34.Kumar R, Singh VP, Baker KM. The intracellular renin-angiotensin system: implications in cardiovascular remodeling. Curr Opin Nephrol Hypertens. 2008;17:168–173. doi: 10.1097/MNH.0b013e3282f521a8. [DOI] [PubMed] [Google Scholar]
- 35.Baker KM, Chernin MI, Schreiber T, Sanghi S, Haiderzaidi S, Booz GW, Dostal DE, Kumar R. Evidence of a novel intracrine mechanism in angiotensin II-induced cardiac hypertrophy. RegulPept. 2004;120:5–13. doi: 10.1016/j.regpep.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 36.van Kats JP, Methot D, Paradis P, Silversides DW, Reudelhuber TL. Use of a biological peptide pump to study chronic peptide hormone action in transgenic mice: Direct and indirect effects of angiotensin II on the heart. J Biol Chem. 2001;276:44012–44017. doi: 10.1074/jbc.M106132200. [DOI] [PubMed] [Google Scholar]
- 37.Yu C, Gong R, Tolbert EM, Dworkin LD. Long-term, high dosage candesartan suppresses inflammation and injury in chronic kidney disease: non-hemodynamic renal protection. J Am Soc Nephrol. 2007;18:750–759. doi: 10.1681/ASN.2006070770. [DOI] [PubMed] [Google Scholar]