Abstract
We provide our detailed, standardized in vitro protocol for culture and differentiation of human retinal pigment epithelial (RPE) cells into a highly polarized, functional monolayer. Disruption of polarized RPE function plays an important role in the pathogenesis of common blinding disorders of the retina. The availability of this polarized RPE monolayer allows for reproducible evaluation of RPE function, modeling of RPE dysfunction in retinal disease, and in vitro evaluation of novel therapies. The protocol, which takes approximately 6 weeks, describes the culture of RPE from human fetal donor eyes, and the differentiation of these cells into a polarized monolayer with high transepithelial resistance, and morphologic characteristics that mimic the RPE monolayer in vivo. By modifying the procedure for initial isolation of pure RPE cells, and culture conditions used in existing protocols, we have established a standardized protocol that provides highly reproducible RPE monolayers from the same donor eye.
INTRODUCTION
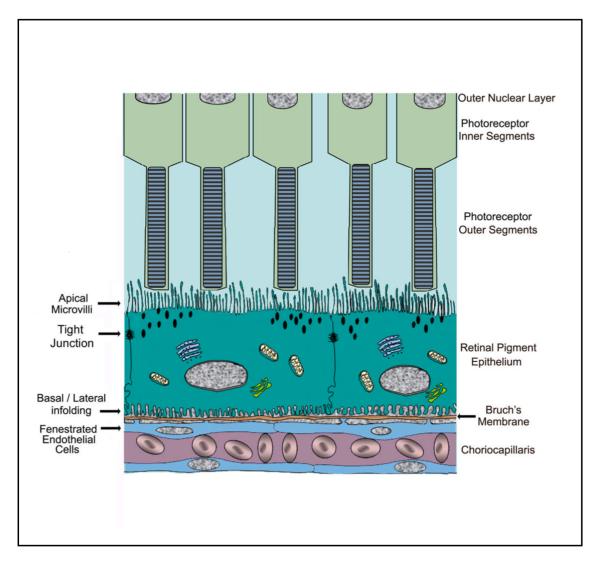
The retinal pigment epithelium (RPE) represents a monolayer of highly polarized pigmented cells located between the neural retina and the choroid that plays a critical role in the maintenance of visual function 1. The RPE monolayer regulates the volume and chemical composition of subretinal space and mediates bidirectional transport between the neural retina and the choroid 2-6. Thus, the RPE serves as a blood-retinal barrier that selectively transports biomolecules between the neural retina and choriocapillaris and releases various factors that protect the health and integrity of the outer retina, the choriocapillaris, and the intervening Bruch’s membrane (Fig. 1) 7,8.
Figure 1.
Diagram of the outer retina illustrating the polarized nature of the RPE monolayer and its relationship to the photoreceptor inner and outer segments, Bruch’s membrane and the choriocapillaris. The RPE shows apical microvilli, tight junctions and basal/lateral infolding, The endothelial cells of the choroid are fenestrated.
Cell culture models of RPE can play an important role in gaining knowledge about native tissue. Our protocol uses fetal donor eyes obtained within a 24 h post-mortem time period and processed to produce RPE cells that closely mimic cells in native tissue. The dissection process will yield cells that will eventually reach confluence within 2 weeks. At this time, the cells are passaged and seeded onto coated Transwell membranes with apical and basal compartments. High TER resembling that in vivo is usually obtained within 4 weeks post-passage. RPE isolated by this procedure are well suited to study the transport of substrates and their localization across membranes, secretory patterns of growth factors as well as drug toxicity. Several groups have established a polarized human RPE cell culture model using Transwell filters that produces confluent RPE with morphological and physiological characteristics of the intact RPE monolayer, such as apical microvilli, well-defined tight junctions, membrane transport capability and melanocytic pigmentation9-12 (Table 1). The Transwell filter functions to separate the culture wells into two compartments, the apical (upper) domain corresponding to the retinal facing side of the RPE monolayer and basolateral (lower) domain corresponding to the choroidal facing side of the RPE monolayer9,10,12-15. The polarized separation of RPE to two domains is generally characterized by distinct localization of marker proteins. Na/K ATPase, which is necessary for providing a Na+ rich environment required for photoreceptor function is used as an apical marker 16. The barrier function of the tight junctions enables the RPE monolayer to establish and maintain concentration gradients between the apical and basal environment. The junction-specific protein, ZO-1 is a peripheral membrane protein bound on the cytoplasmic surface of junctional complexes 5. Well developed, functional tight junctions are identified by ultrastructural evaluation of the complexes by electron microscopy and by the presence of a high TER 17. In some cases, the distribution of specific transporters has been described on both apical and basolateral membrane domains, while in other cases, there is selective expression on either apical or basolateral domains 6,18. For example, GLUT1 is present in both the apical and basolateral membranes of the RPE facilitating the transepithelial movement of glucose from choroid to the retina. On the other hand, the presence of monocarboxylate transporter1 (MCT1) on the apical surface and MCT3 on the basolateral surface of the RPE points to the coordinated action of the two proteins in mediating the transepithelial transfer of H+-lactate between the subretinal space and the systemic circulation 19. The polarized human RPE model has also been successfully utilized to demonstrate asymmetrical, polarized secretion of cytokines9,12,20,21. Our group recently found that highly polarized RPE cells secreted significantly higher amounts of pigment epithelium-derived factor (PEDF) and vascular endothelial growth factor A (VEGF-A) compared to non-polarized RPE monolayers isolated from the same donors22.
Table 1.
Development of polarized culture RPE
| Author | REP cell type |
Chamber | Medium | TER (Ω·cm2) | Features of differentiation |
|---|---|---|---|---|---|
| Geisen et al.15 | ARPE-19 | Transwell | DMEM/F12 (10%FBS) | <30 | Na-K ATPase, ZO-1, stress fiber |
| Geisen et al.15 | D407 | Transwell | DMEM-high glucose (5%FBS) DMEM-high glucose (20%FBS) |
<10 | ZO-1(-) |
| Geisen et al.15 | mRPE | Transwell | (3days)→DMEM-high glucose (10%FBS) |
<30 | ZO-1, stress fiber |
| Geisen et al.15 | hRPE | Transwell (human extracellular matrix coat) |
MEM (15 5%FBS) with 5%FBS) withN1supplement,nonessential amino acids, hydrocortisone, taurine and triiodo-thyronin |
>500 | ZO-1 |
| Hu et al.11 | hRPE | Millicell-PCF or Millicell-HA (both mouse laminin coat) |
Chee’s essential medium | >500 | Na-K ATPase, bestrophin |
| >700 | |||||
| Blaauwgeers et al.10 |
hRPE | Transwell (Matrigel coat) |
Iscove’s modified Dulbecco’s medium (1% normal human serum) |
<100 (3week) | ZO-1, TJ complexes , microvilli |
| Holtkamp et al.9 |
hRPE, ARPE-19 |
Transwell (Matrigel coat) |
Iscove’s modified Dulbecco’s medium (1% fetal calf serum) |
<20 (about 19day) |
ZO-1, cytokeratin 8/18, a glucose transporter protein, TJ complexes, microvilli |
| Dunn et al.13 | ARPE-19 | Ttranswell or Transwell-COL filters or Millicell CM or HA insert (Matrigel coat) |
DMEM/F12 (10%FBS) or specialized medium developed for tissue culture of human RPE.28 |
<40 DMEM/F12 <100(special medium) |
CRALBP, RPE65, pigment,TJ complexes, microvilli, polygonal shape |
| Maminishkis et al.12 |
hRPE | Transwell (human extracellular matrix coat) |
MEM (15-5%FBS) with N1 supplement, Glutamine-Penicillin-Streptomycin, Non essential amino acids, Taurine, Hydrocortisone, Triiodo-thyronin |
>500 | ZO-1, occludin, claudin, ezrin, CRALBP, RPE65, Na-K ATPase, pigmentation, TJ complexes, microvilli, basal infold |
| Kannan et al.14 | ARPE-19 | Transwell (laminin coat) |
MEM (10-1%FBS) with N1 supplement, Glutamine-Penicillin-Streptomycin, Non essential amino acids, Taurine, Hydrocortisone, Triiodo-thyronin |
<50 | ZO-1, occludin, NaK-ATPase (apical) |
| Ohno et al.20 | hRPE | T75 flask (laminin coat) |
DMEM (10%FBS), 10ng/ml bFGF | N/A | CRALBP |
| Ban et al.29 | Chick RPE | 12mm Transwell (laminin coat) |
SF2, culturing E14 neural retina in SF2 | <100 | ZO-1 |
| Sonoda et al.22 Sreekumar et al.24 |
hRPE | Transwell (fibronectin coat) |
MEM (15-1%FBS) with N1 supplement, Glutamine-Penicillin-Streptomycin, Non essential amino acids, Taurine, Hydrocortisone, Triiodo-thyronin |
>500 | ZO-1, occludin, Na-K ATPase, pigmentation, TJ complexes, microvilli, basal infold |
D-MEM; Dulbecco’s Modified Eagle Medium, ZO-1; Zonula occludens-1, CRALBP; cellular retinaldehyde-binding protein, N/A; not applicable
Additional applications for the polarized RPE culture system include the in vitro evaluation of drug toxicity23,24. For example, we recently found that a retinoic acid derivative fenretinide, under phase I clinical trial for dry AMD, while non toxic under highly differentiated polarized conditions, was found to induce cell death in nonpolarized human RPE24. The polarized cultures may also be valuable for the study of retinal development; in one study RPE cells were shown to promote spatial reorganization and differentiation of retinal photoreceptors25. As well, RPE monolayer cultures may be valuable for modeling retinal disease; for example, disruption of the equilibrium of secretion from apical and basolateral surfaces may result in a pathologic microenvironment that is found in several retinal diseases9,10,20. In this report, we present a detailed procedure for the culture and characterization of a highly differentiated and highly polarized human RPE monolayer. Limitations include availability of donor tissue of specified gestational age, length of time to produce polarized RPE with high TER, and detailed analysis required to validate the extent of the polarized phenotype for further experimentation.
The existing methods to generate polarized RPE (Table 1) include utilization of immortalized human RPE cell lines, such as ARPE19 and D407 9,13-15 ;however, the cell lines result in monolayers with low TER (<50Ω·cm2). Methods using fetal human RPE 11,12 give similar high TER as found in this study; however, differences exist in the procedures for isolation of RPE. Hu and Bok described a complex culture medium containing bovine brain extract and floating RPE cells were used for isolation 11. Maminishkis et al. 12 used a younger gestation age (16-18weeks) as compared to ours (18-20weeks) and their culture medium contained 15% FBS as opposed to 1% FBS used in our study. Further, to attain higher purity of RPE and prevent inclusion of contaminating cells, we adopted additional filtration steps before seeding RPE on Transwell filters.
EXPERIMENTAL DESIGN
Human Tissue
Human fetal eyes are obtained from Advanced Bioscience Resources, (ABR) Inc. (Alameda, CA). Typically, tissues with a gestational age of 18-20 weeks are used. The tissue is harvested by ABR within 2-4 hours from death to preservation (DTP) and kept at 4°C in RPMI media containing antibiotics. The eye is shipped the same day via a courier and is processed the same day of arrival. The time from DTP to tissue processing is within 24-30 hours. Although a special preparation medium for transportation has been recommended12, we did not find a significant difference in the outcome of the tissue preparation when that medium was compared to RPMI media that we use routinely.
Approximately 80% of eyes will result in culture of highly polarized RPE monolayers; however predicting those eyes whose culture will be unsuccessful is generally not possible. Thus, the determination of whether the cells will attain the high TER and in vivo-like morphological characteristics will be known only at a later stage, at least two weeks after RPE culture on Transwells (see below).
Dissection of the eye
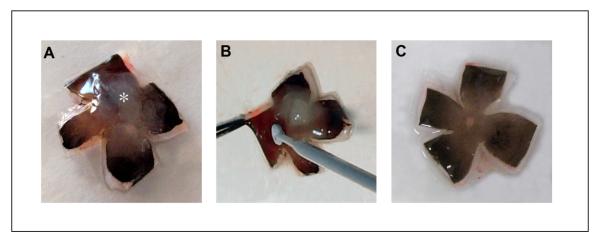
An initial incision is made in cornea of the eye (Fig. 2), the cornea/iris complex is carefully cut off 1-2 mm posterior to the corneal limbus and the vitreous is removed (Fig. 3). The posterior eye cup is dissected into 4 quadrants with a razor blade and eye cup is placed flat (Fig. 4a). The sensory retina is removed with an Inoculating loop (Fig. 4b,c) and the RPE/choroid layer from the sclera is gently peeled (Fig. 5) and placed in a holding buffer. The RPE-choroid sheets are incubated in 2% dispase solution for 30 min at 37°C, 5% CO2 and are placed back in holding buffer to stop the dispase activity. Rinsing off the dispase is important in order to prevent excess digestion of the tissue and release of contaminating cells from the choroid. The RPE layer is peeled off in large sheets from the choroid with fine forceps (Fig. 6) and the crude RPE containing preparation is filtered to eliminate the other contaminating cells. The dissection step prior to the the dispase incubation step usually takes 10-15 minutes. All the steps should be performed under clean conditions at room temperature (RT, 22-24°C), utilizing universal precautions for working with human tissue and completed as fast as possible.
Figure 2.

Human fetal eye with the first incision. A radial incision was made into the cornea (arrow) using a sterile Teflon coated razor blade without high pressure. Bar equals 5mm.
Figure 3.
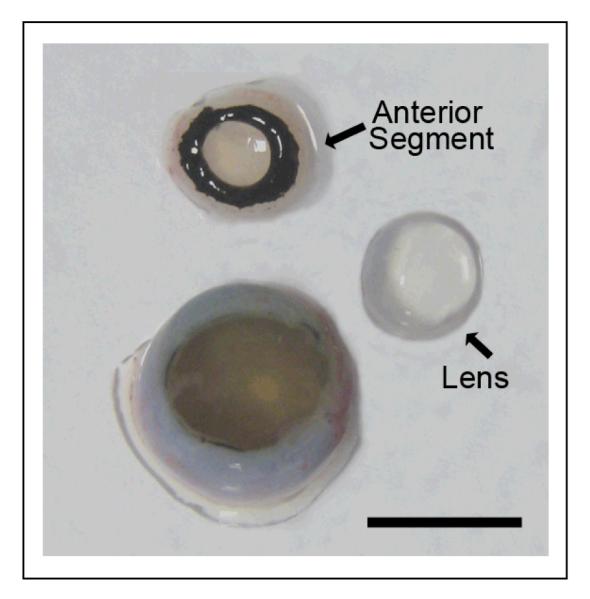
The fetal eye is split into cornea/iris (anterior segment), lens, and posterior eye cup. When the cornea/ iris complex is removed, the lens easily comes off with the anterior segment. Bar equals 5 mm.
Figure 4.
The human fetal eye is dissected into 4 quadrants. An incision should be made as close to the optic disc with a razor blade in order for the eye cup to lay flat (a). The sensory retina (asterisk) is firmly attached to the optic disc. The sensory retina is gently removed from the RPE layer with a sterile disposable inoculating loop, care being taken not to make scratches into the RPE layer (b). After removal of retina, there is no obvious damage to the RPE layer (c).
Figure 5.
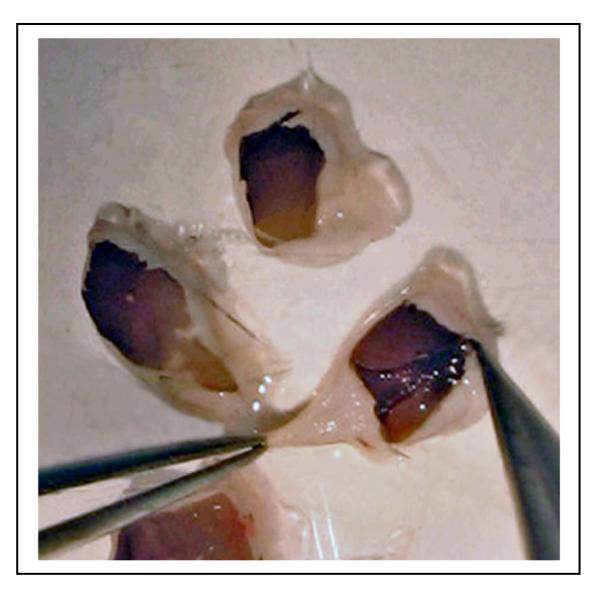
The RPE/choroid layer is peeled off from the sclera using forceps. Usually the layer can be isolated as an intact sheet.
Figure 6.
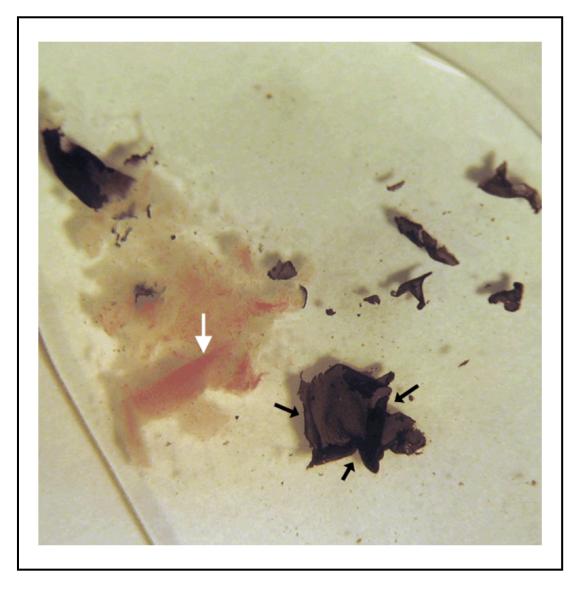
Intact RPE sheets (black arrows) are peeled from the choroid with fine forceps under a dissecting microscope. Vessels (white arrow) in choroid are clearly visible after removing the RPE layer.
Optimal conditions for polarized RPE cell culture
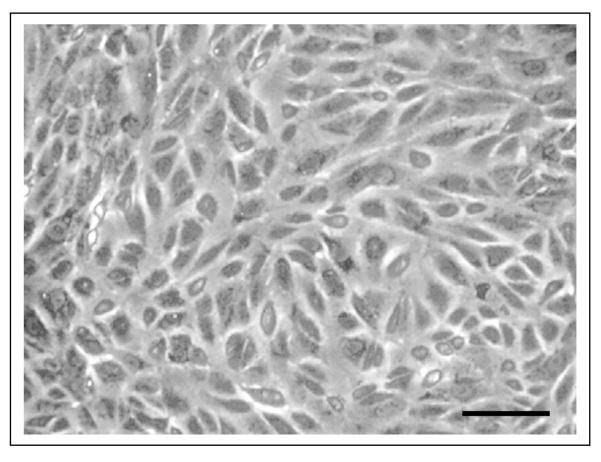
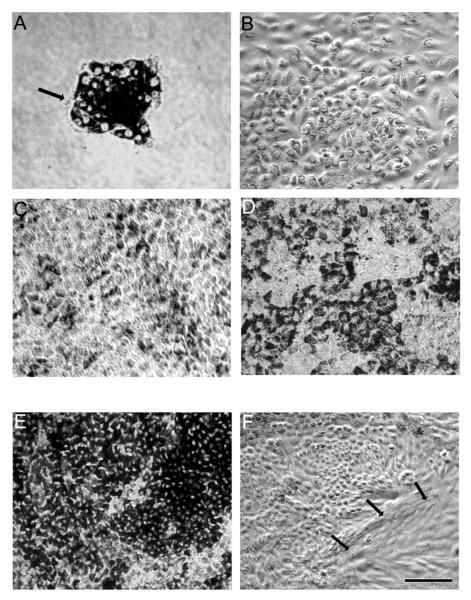
Primary cultures of human fetal RPE grow as sheets of hexagonal cells, however they lose their pigmentation and do not attain a highly polarized phenotype. Experiments are typically performed using RPE cells that have been passaged 2-4 times. Early passage human fetal RPE grown on plastic grow as non-pigmented, epithelioid, non-polarized cells (Fig. 7). In contrast, using the method presented here, RPE cultures form sheets of pigmented, hexagonal cells with a polarized phenotype (Fig. 8d). In the initial step of culturing the RPE cells in a T75 flask, RPE medium containing 10%FBS is used to protect the survival of the cells. Next day, the medium is replaced with 5%FBS containing medium to prevent overgrowth of RPE cells; if this is not done, the RPE cells become overcrowded and do not form a single monolayer. Typically, most of the live cells attach within first 24h. The initial growth of the culture occurs in the form of small islands, consisting of 15-25 RPE cells (Fig. 8a). Over the next few days, the cells proliferate rapidly, while pigment density decreases (Fig. 8b). Approximately 14days later, the cells show increased cell density with hexagonal shape, and repigmentation occurs with increased density of melanin pigment granules (Fig. 8c,d). In some cultures, the RPE cells show degenerative changes, develop hyperpigmentation, or show overgrowth or contamination with other cell types (Fig. 8e,f); typically, these cultures will not develop a high TER. In the next step, seeding on the Transwell inserts, we use RPE medium containing 10% FBS in first 24h as before, and then replace the medium with RPE medium containing 1%FBS to avoid overcrowding of RPE cells and to attain consistent monolayer culture.
Figure 7.
Light microscopic images of early passage confluent fetal RPE cells grown on a plastic tissue culture plate. Cells do not show hexagonal shape and lack pigmentation under these culture conditions. Bar equals 40 μm.
Figure 8.
Light microscopic images showing pattern of time dependent growth of normal RPE in a T75 flask (a-d), and highly pigmented (e) and overgrown multi-layers of RPE (f) for comparison. (a) Day1; Cells are attached and initial growth of the culture occurred in the form of small islands, consisting of 15-25 RPE cells. (b) Day4; Rapid growth of cells with a decrease in pigment is seen. (c) Day14; Cells show evidence of repigmentation. (d) Day21; Cells showing hexagonal shape and increase in pigment density. (e) Hyperpigmented RPE on day 21. These cell are not suitable for establishing polarity. (f) RPE cells with abnormal morphology on day 21. Arrows show cells with fibroblastic morphology. Bar equals 60 μm.
Transwell culture system
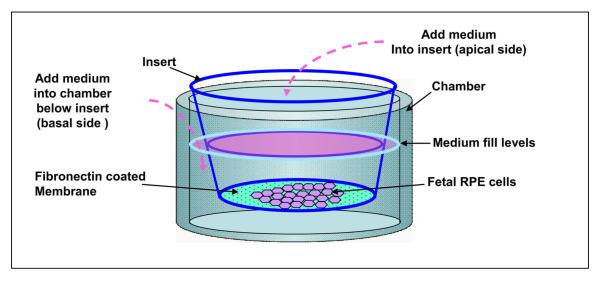
For epithelial cells, the use of permeable supports in vitro allows cells to be grown in a polarized state under more natural conditions. Thus, they permit cells to take up and secrete molecules on both their basal and apical surfaces and thereby enable cells to carry out metabolic activities in a more physiological fashion9,11,12,14. Cellular differentiation can also proceed to higher levels resulting in cells that morphologically and functionally better represent their in vivo counterparts. Coating of the membrane with extra cellular matrix accelerates cell growth9-13,15. In this model, fibronectin coating is most suitable, other extracellular matrices such as laminin and gelatin were tested but did not promote uniform cell attachment. Permeable membranes generate two compartments, in this model, the apical (upper compartment) domain corresponds to the retinal facing side of the RPE monolayer and the basolateral (lower compartment) domain corresponds to the choroidal facing side of the RPE monolayer (Fig. 9).
Figure 9.
Schematic diagram showing culture of highly differentiated, polarized human RPE in culture wells with a Transwell insert.
Transepithelial resistance (TER)
Polarization of the RPE monolayer, characterized by a high TER, requires establishment of functional tight junctions between the cells. Chambers containing no cells or subconfluent RPE have no resistance to passage of current between electrodes placed in the inner and outer chambers26. The electrodes of the epithelial voltohmmeter (EVOM) are at first rinsed with 70% ethanol. All TER measurements are made in a cell culture hood since TER fluctuates with temperature, measurments are made within 3 min of removal of Transwells from the incubator when the average temperature is 32.2 ±1.85°C (see ref. 14). Net TERs are calculated by subtracting the value of a blank, fibronectin-coated filter without cells from the experimental value. Final resistance-area products (Ω·cm2) are obtained by multiplication with the effective growth area.
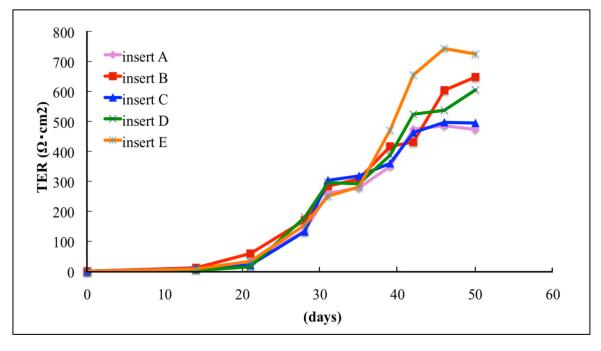
To determine if the cells attain functional polarization, TER is measured starting from two weeks of Transwell culture. Monolayer cultures have been used in experiments when they have a TER of at least 200Ω·cm2 12,18. based on studies showing that TER of the human RPE monolayer in vivo is 150Ω·cm2. Because the TER continues to increase with further culture, and typically reaches a consistent plateau at TER > 500Ω·cm2 ,we recommend utilizing cultures with a resistance > 500Ω·cm2 . Multiple Tranwells prepared from single donor eye cultures develop high TER in a consistent, reproducible and time dependent manner (Fig. 10). RPE cells from an occasional donor do not develop high resistance. In some instances the monolayer became intensely pigmented with very dark patches and the TER never rose above 50 Ω·cm2. Such donors are excluded.
Figure 10.
Time dependent increase in TER from multiple Transwells containing RPE from a single donor. Note the time-dependent increase in TER beginning after 15 days in culture, and that RPE from multiple Transwells (inserts A-E) develop TER in a reproducible manner.
Characterization of the polarized RPE cells
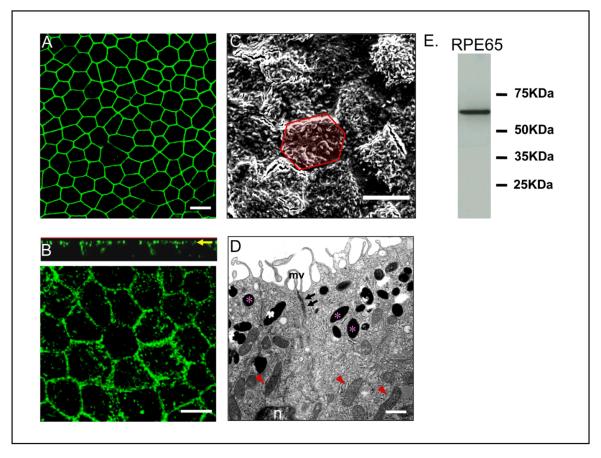
As in native tissue, human RPE cells on Transwell filters form a single monolayer, are well pigmented, and are arranged in a regular hexagonal mosaic. The development of a polarized human RPE monolayer is characterized by apical localization of tight junction protein, ZO-1 and occluding, however, this does not prove the presence of functional tight junctions (Fig. 11a). A better indicator of monolayer polarization is the apical expression of Na/K ATPase which generally takes at least 1 month of Transwell culture. Na/K ATPase is localized to the apical plasma membrane of the human RPE cells as shown in the confocal vertical (X-Z) section (Fig. 11b). The polarized morphology of the cells can also be evaluated using electron microscopy. Scanning electron micrograph (SEM) images provides support that the cells are polarized with the demonstration of well-developed apical microvilli (Fig. 11c). Transmission electron micrographs (TEM) show that RPE has basally located nuclei, contains pigment granules that are present in the apical side of the cytoplasm and exhibit well-developed tight-junctional complexes and apical microvilli (Fig. 11D). RPE65, a protein preferentially and abundantly expressed in the RPE, usually is expressed after several weeks in Transwell culture27, so that RPE65 is a suitable marker for polarized RPE cells (Fig. 11E). Such marker proteins as those described here have also been used by other investigators to provide evidence for polarization of RPE cells 9,11-15. Our immunohistochemistry protocol uses optimal dilutions of antibodies that were determined experimentally from the range recommended by the manufacturer.
Figure 11.
Evidence for differentiation and polarization in human fetal RPE cells cultured on Transwell filters for 4 weeks. (a) Immunofluorescence pattern of tight junction protein ZO-1 visualized by confocal microscopy. (b) Localization of Na/K ATPase to the apical plasma membrane as lower panel and vertical (X-Z) section (upper panel). Na/K ATPase is preferentially located at the apical surface. (c) Well differentiated apical microvilli observed by scanning electron microscopy. This image contains 17 cells; the yellow hexagonal area in the figure outlines a single RPE cell. (d) Localization of pigment in the apical cytoplasm (asterisk), nucleus (n) in the basal side of the cell, apical tight-junctional complexes (arrows) and mitochondria (arrowheads) by transmission electron microscopy. (e) The expression of the RPE-specific protein, RPE65 was confirmed by western blot analysis in lysate from 4week old polarized RPE cells. a,b,c; Bar equals 10 μm. d; Bar equals 1μm.
MATERIALS
REAGENTS
-
Human fetal eyes (Advanced Bioscience Resources, (ABR) Inc. (Alameda, CA).
CAUTION All experiments involving human subjects should conform to National and Institutional guidelines. Approval should be obtained from the appropriate Institutional Review Board (IRB). It is the policy of ABR to obtain written consent from the mother of the eye tissue donor.
CAUTION All studies using human specimens should be considered as infectious and the appropriate biosafety precautions taken including the wearing of personal protective equipment.
Fibronectin, (Fisher Scientific, ATL Suwanee, GA, cat. no. 07-200-161)
RPMI Media (VWR International Inc., PA, cat. no. 45000-404)
Phosphate Buffered Saline (PBS) (VWR International Inc., PA, cat. no. 45000-434)
Penicillin-streptomycin (100U ml-1 penicillin, 100 U ml-1 streptomycin) (VWR International Inc., PA, cat. no. 30-001-CI).
Dispase (Gibco, Grand Island, NY, cat. no. 17105-041) (see REAGENT SETUP)
Bovine Serum Albumin (BSA), (Sigma Aldrich US, MO, cat. no. A4503-100g),
RPE culture medium (see REAGENT SETUP)
Fetal bovine serum (Omega Scientific, Tarzana, CA, cat. no. FB-01)
N1 supplement (50mg ml-1 transferrin, 5 mg ml-1 insulin, 100mM putrescine, 20 nM progesterone, 20 nM selenium, and 10 ng ml-1 biotin) (Sigma Aldrich US, MO, cat. no. N-6530).
Glutamine-Penicillin-Streptomycin (Omega Scientific, Tarzana, CA, cat. no. PG-30)
Non essential amino acids (Sigma Aldrich US, MO, cat. no. M-7145)
Taurine (Sigma Aldrich US, MO, cat. no. T-0625)
Hydrocortisone (Sigma Aldrich US, MO, cat. no. H-0396)
Triiodo-thyronin (Sigma Aldrich US, MO, cat. no. T-5516)
MEM alpha modification (Sigma Aldrich US, MO, cat. no. M-4526)
Paraformaldehyde (Ted Pella, Redding, CA, cat. no. 18501 and 18505)
1X EDTA trypsin (VWR International Inc., PA, cat. no. 25-052-CI)
Rabbit anti-ZO-1 (Zymed Laboratories, San Francisco, CA, cat. no. 61-7300)
Rabbit anti-Occludin (Zymed Laboratories, San Francisco, CA, cat. no. 71-1500)
Anti-Na/K ATPase α-1 (mouse monoclonal), (Upstate Biotechnology Inc., Lake Placid, NY, cat. no. 05-369)
Fluorescein anti-rabbit IgG (Vector Laboratories, Burlingame, CA, cat. no. FI-1000)
Fluorescein anti-mouse IgG (Vector Laboratories, Burlingame, CA, cat. no. FI-2000)
Fluorescent Mounting Media (Vector Laboratories, Burlingame, CA, cat. no. H-1000)
Mouse monoclonal anti-RPE65 (1:10000 dilution), (Novus biologicals, Littleton, CO, cat. no. NB100-355SS)
Triton X(VWR International Inc., PA, cat. no. BDH3929-2)
4′,6-diamino-2-phenylindol (DAPI ) containing Mount (Vector Laboratories, Burlingame, CA, cat. no. H-1200)
Sodium cacodylate (Ted Pella, Redding, CA, cat. no. 18851)
Glutaraldehyde (Ted Pella, Redding, CA, cat. no. 18432) !Caution Glutaraldehyde is toxic and muse be kept and used within a fume hood.
Calcium chloride (VWR International Inc., PA, cat. no. 25-052-CI)
Osmium tetroxide (Ted Pella, Redding, CA, cat. no. 18459) !Caution Osmium tetroxide is toxic and must be kept and used within a fume hood and disposed of appropriately once used.
Uranyl acetate (Ted Pella, Redding, CA, cat. no. 19481) !Caution Uranyl acetate muse be kept and used within a fume hood.
Ethanol (Gold Shield, Hayward, CA cat. no. DSP-CA-151)
HMDS (Ted Pella, Redding, CA, cat. no. 18605)
Colloidal Silver liquid (Ted Pella, Redding, CA, cat. no. 16034)
Lead citrate (Ted Pella, Redding, CA, cat. no. 19312) !Caution This solution should be made fresh each time.
EQUIPMENT
15-ml centrifuge tube (VWR International Inc., PA, cat. no. 21008-103 )
50-ml centrifuge tube (VWR International Inc., PA, cat. no. 21008-951)
Scissors (Storz Ophthalmics, St. Louis, MO, cat. no. E3408)
Vannas curved iris scissors (Storz Ophthalmics, St. Louis, MO, cat. no. E3347)
Teflon coated razor blade (Ted Pella, Redding, CA, cat. no. 121-3)
No. 5 Dumont forceps (Ted Pella, Redding, CA, cat. no. 5622)
Sterile disposable 1-ml pipette (VWR International Inc., PA, cat. no. 53300-283)
Tissue culture treated 6-well plates coated with laminin (VWR International, cat. no. 62405-434)
Tissue culture plastic flasks (VWR International Inc., PA, cat. no. BD353136)
Disposable inoculating loop (VWR International)
Sterile Bard-Parker no. 11 scalpel blade and handle (BD Medical, Oceanside, CA, cat. no. 372611)
Operating microscope ( Carl Zeiss, Thornwood, NY)
10cm sterile petri-dish (VWR International Inc., PA, cat. no. 25382-166)
35mm sterile dish, (VWR International Inc., PA, cat. no. 25382-064)
T75 flask (Fisher Scientific, ATL Suwanee, GA, cat. no. 08-772-46)
12 well plastic culture plates (VWR International Inc., PA, cat. no. 62406-165)
Transwell (Corning Costar, 3460-Clear, 0.4 μm pores, 12mm inner diameter, polyester membranes, Fisher Scientific, ATL Suwanee, GA, cat. no. 07-200-161)
Inoculating Loops, Sterile, Disposable, 1.0 μL Tip (VWR International Inc., PA, cat. no. 60872-410)
70-μm nylon mesh filter, (VWR International Inc., PA, cat. no. 21008-952)
40-μm nylon mesh filter (VWR International Inc., PA, cat. no. 21008-949)
0.2 um sterile syringe filter (VWR International Inc., PA, cat. no. 194-2520)
500 ml filter unit (VWR International Inc., PA, cat. no. 28199-155)
9 inch glass pipette (VWR International Inc., PA, cat. no. 53499-632)
Sterile transfer pipette (VWR International Inc., PA, cat. no. 357524)
5ml Sterile pipette (VWR International Inc., PA, cat. no. 53300-421)
10ml Sterile pipette (VWR International Inc., PA, cat. no. 53300-523)
25ml Sterile pipette (VWR International Inc., PA, cat. no. 53106-195)
Cover slip (VWR International Inc., PA, cat. no. 48380-046)
Glass scintillation vial (VWR International Inc., PA, cat. no. 66026-453)
Eponate 12TM (Ted Pella, Redding, CA, cat. no. 18012)
Specimen mount (Ted Pella, Redding, CA, cat. no. 16221)
EVOM epithelial tissue voltohmmeter (World Precision Instruments, Sarasota. FL)
Accuspin centrifuge (Beckman Coulter, Holbrook, NY)
Dissecting microscope (Carl Zeiss, Thornwood, NY)
Inverted microscope (Carl Zeiss, Thornwood, NY)
LSM 510 laser-scanning microscope (Carl Zeiss, Thornwood, NY)
JEOL JSM 6390 LV Scanning Electron Microscope (Japan Electron Optics Laboratory Corporation, JEOL, Tokyo, Japan)
JEOL JEM 2100 electron microscope (JEOL, Tokyo, Japan)
Digital camera, SC1000B Gatan (Orius, Pleasanton, CA)
REAGENT SETUP
2%(wt/vol) Dispase
Add 0.2 grams of dispase to 10 ml of 1M PBS and filter through a 0.2 um sterile syringe filter. Prepare dispase solution at RT just before use.
Holding buffer (1%BSA/PBS)
Add 0.5 grams of BSA to 500 ml of 1M PBS and filter through a 500 ml filter unit. This solution can be stored at 4°C for about 2 months.
2% (wt/vol) paraformaldehyde
A total volume of 10 ml of 16% formaldehyde is added to 70ml of 1M PBS. This solution can be stored at RT for about 1 week.
RPE culture medium
500 ml of MEM Alpha is used as a base medium to prepare RPE culture medium. 5ml of N1 Supplement, 5ml of Glutamine-Penicillin-Streptomycin, 5ml of Non essential amino acids, 125 mg of Taurine, 10ug of Hydrocortisone and 0.0065ug of Triiodo-thyronin are added. Add heat inactivated FBS (1%, 5%, 10%, or 15% (wt/vol)), This solution can be stored at 4°C for about 1 month.
0.2% Sodium Cacodylate Buffer
Dissolve 42.8g of sodium cacodylate in 1 liter of double distilled water. Stir until dissolved and pH to 7.2.
1/2 strength Karnovsky’s fixative in cacodylate buffer 10% stock
Mix with 20 ml of 10% paraformaldehyde, 5 ml of 50% glutaraldehyde, 50 ml of 0.1M cacodylate buffer, 12.5 ml of 200mg/ml Calcium Chloride and 12.5 ml of distilled water. Adjust pH to 7.2. This solution can be stored at 4°C for about 1 month.
Ethanol
30, 50, 70, 95%, solution in dH20 and 100%.
EQUIPMENT SETUP
All instruments are sterilized using a steam autoclave. Autoclave setting (56min, cycle attaining 250°F). !CAUTION Alcoholic sterilization is not recommended.
PROCEDURE
Procurment of donor eyes.
1. One donor eye is placed in a 15 ml centrifuge tube containing 13ml RPMI media with antibiotics (1% vol/vol penicillin and streptomycin) and should be shipped on wet ice the same day via a courier.
Isolation of primary human fetal RPE cells
2. Sterilize all instruments using a steam autoclave.
3. Once the fetal eyes have been received, place the globes into a 50 ml tube containing 25ml sterile PBS containing 5% vol/vol penicillin-streptomycin for a minimum of 20 min at RT.
4. Place the eye in a new 50ml tube and rinse the globes 2 times with 10ml sterile PBS to wash away the antibiotics.
5. Place one fetal donor eye into a 10cm sterile petri-dish. Using a dissecting microscope make an incision into the cornea using a sterile teflon coated razor blade. Since the blade is very sharp, incision can be made without causeing damaging compression to the globe (Fig. 2).
6. Place the point of one blade of an iris scissor into the opening and carefully cut the cornea/iris complex 1-2 mm posterior to the corneal limbus and remove the cornea/iris tissue (Fig. 3).
?TROUBLESHOOTING
7. Remove the vitreous using a sterile inoculating loop. Gently hold one edge of the eye cup with a pair of tweezers (#5 Dumont forceps) and use the loop to gently tease the vitreous out. The vitreous is typically removed intact.
8. Holding one edge of the eye, dissect the globe into 4 quadrants with a razor blade in order for the eye cup to lay flat. The cut is made as close to the optic disc as possible (Fig. 4a).
9. Remove the sensory retina using an inoculating loop (Fig. 4b,c).
▲CRITICAL STEP Take care not to scratch the RPE layer.
10. Using one pair of tweezers (#5 Dumont forceps) hold the edge of the dissected eye, with another pair of tweezers gently peel off the RPE/choroid layer from the sclera and place into 10ml of holding buffer (RT), in a 35mm sterile dish (Fig. 5). RPE sheet when large is placed into the holding buffer and when small is picked up with a sterile transfer pipette and place into the buffer.
11. Using transfer pipette gently pick up the RPE-choroid sheets and transfer to 5ml of RT 2% wt/vol dispase solution (dispase solution dose not require to be pre-warmed).Incubate for 30 min at 37 °C, with 5% CO2.
12. After dispase incubation of the sheets, gently transfer the RPE sheets with a sterile transfer pipette to 5ml of holding buffer (RT) to stop the activity of the dispase. Keep the RPE sheet in holding buffer until dissection is complete.
▲CRITICAL STEP: Rinsing off the dispase is important in order to prevent excess digestion of the tissue and release of contaminating cells from the choroid.
13. Transfer the RPE sheet to a 10cm sterile petri dish containing 1ml of holding buffer. Peel the RPE layer from the choroid with fine forceps in the holding buffer. Place the sheets again in the 5ml of holding buffer until all the RPE sheets are collected (Fig. 6).
?TROUBLESHOOTING
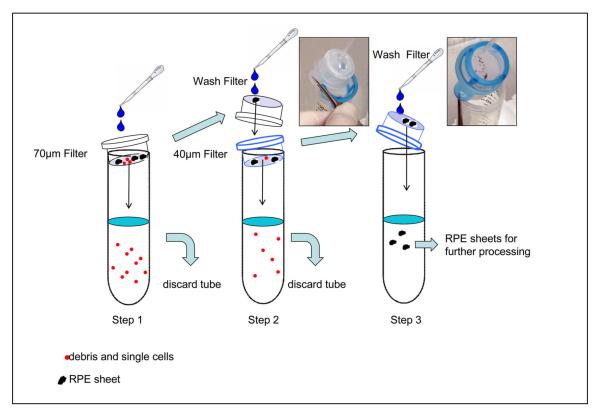
14. Filter the solution containing RPE sheets (from previous step) through a 70-μm nylon mesh filter (Fig. 12 step1).
Figure 12.
Steps involved in the isolation of pure RPE sheets. Please note two different filters (70 and 40 μm) are used. Holding buffer (25 ml) was used for filtration in each step. Red dots indicate debris and single cells, whereas black dots represent RPE sheets.
15. Wash the sheets remaining in the top of the filter with 25ml holding buffer and filter again using a 40-μm nylon mesh filter (Fig. 12 step2). Discard the single cells and contaminating cells that pass through the filter.
16. Collect the sheets retained in the 40-μm filter and place into a 50 ml centrifuge tube in 25ml holding buffer (Fig. 12 step3). By gently pipetting slowly up and down, break apart the RPE sheets using a sterile 9 inch glass pipette.
▲CRITICAL STEP: This step is critical to ensure purity of the RPE cells.
17. Pellet the cells and partial sheets by centrifugation for 5 min at 400 g and re-suspend in 1 ml of RPE medium containing 15% (vol/vol) serum. Place the cells into a T75 flask with an additional 20 ml of media and incubate at 37 °C, with 5% CO2. Typically one fetal donor eye is used for one T75 flask.
18. Culture overnight and change the media to media containing 10% serum. At this point, typically cells are attached to the flask. PBS wash is not necessary.
19. Culture the cells for a further 24 h and then change to media containing 5% serum.
20. Change the culture medium twice per week until the cells attain confluency. After 3-4 weeks the cells become 70-90% confluent, retain their hexagonal shape and are highly pigmented (Fig. 8d).
▲CRITICAL STEP Only cultures that show prominent pigmentation and 70-90% confluence should be used further for growth on Transwells.
?TROUBLESHOOTING
Generation of polarized RPE monolayers
21. Prepare the Transwell filters by coating the upper compartment membrane with 100μl of fibronectin (5ug ml-1). Air dry the coated filters overnight at RT in the laminar flow hood (Fig. 9).
22. Wash cells with 15ml of PBS. Remove PBS and add 5ml of 1X EDTA trypsin. Trypsinization is complete when the cells are detached from the plastic flask. Flasks are gently tapped and visualization of cell detachment is confirmed using an inverted microscope. Trypsinization is usually complete within 10 minutes.
23. Resuspend the cells in RPE media containing 10% serum. Mix the cells well by pipetting up and down using sterile glass pipette and pass through a 40-um filter to form a single cell suspension.
▲CRITICAL STEP When seeding the cells, it is important to ascertain single cell suspension and absence of cell clumps.
24. Seed onto the Transwells, at a density of 1 × 105 cells per Transwell (500μl) and introduce 1ml of 10% FBS containing medium to the lower compartment. (Fig. 9).
▲CRITICAL STEP: Do not freeze the primary cultures or passage 1 cells for later use; only fresh passage 1 cells are used for the Transwells.
25. After overnight incubation with RPE media containing 10% FBS, change the media to 1%FBS RPE media. Change the medium every 3-4 days with 1% FBS RPE medium. Add 0.5ml of medium first to the top chamber of the Transwell followed by 1.5 ml of medium to the bottom chamber.
Measuring transepithelial resistance (TER)
26. Following 2 weeks of culture measure the TER of the cells. Rinse the electrodes of EVOM epithelial tissue voltohmmeter with 70% ethanol. Measurement is made in the hood with 2-3 min after the cells are taken from the incubator. Ideally TER is measured around at 32° C14,18 (Fig. 13) .
Figure 13.
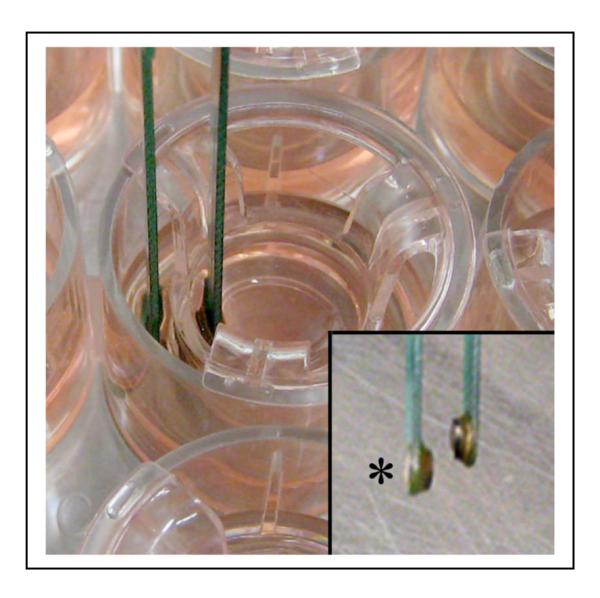
Measurment of TER of polarized human RPE using a voltohmmeter. All TER measurements are made in a cell culture hood within 3 min of removal of Transwells from the incubator. The lower end of the longer electrode (inset asterisk) is inserted into the basolateral medium and shorter electrode into the apical medium.
27. Net TERs are calculated by subtracting the value of a blank, fibronectin-coated filter without cells from the experimental value. Final resistance-area products (Ω·cm2) are obtained by multiplication with the effective growth area. Once the TER of the cells has reached >200 Ω·cm2 the cells are ready for use (3–4 weeks)12,18.
?TROUBLESHOOTING
Characterizing polarized RPE monolayers
28. Characterization of polarized epithelial cells can be carried out using Option (A) Immunohistochemistry, Option (B) SEM or Option (C) TEM.
(A) Characterization of RPE monolayers using immunohistochemistry
Fix human RPE monolayers grown on Transwell filters in 2% cold paraformaldehyde for 30 min at 4°C. Add 0.5ml of the solution to the top and 1.5ml to the bottom chamber 30.
Remove the fixative and permeabilize the RPE cells. Incubate the monolayer with 0.2% (vol/vol) Triton X-100 for 30 min at RT. Add 0.5ml of the solution to the top and 1.5ml to the bottom chamber. After permeabilization this solution is discarded.
Wash the Transwell with 5ml of PBS (RT) for two times and block specimens in 5ml of 1% (wt/vol) BSA/PBS solution for 1h at RT.
- Incubate with antibodies at 4 °C diluted in 1% (wt/vol) BSA/PBS overnight. Antibodies should be diluted as described in the table below.
Antibody Rabbit anti-ZO-1 Dilution 1 : 100 Antibody Rabbit anti-Occludin Dilution 1 : 100 Antibody Anti-Na+/K+ ATPase α-1 Dilution 1 : 100 Remove the antibody and wash three times (each 5min) with 5ml of PBS and incubate with FITC conjugated secondary antibodies (use fluorescein anti-rabbit IgG for ZO-1 and occludin, fluorescein anti-mouse IgG for Na/K ATPase, both antibodies; dilution 1:100) for 30 min at RT in the dark.
Discard the secondary antibodies and wash the membranes again with PBS.
Remove the membranes from the inserts with a sterile razor and then gently place onto a glass microscope slide cell side facing up.
Cut the membrane circles into squares to allow them to lie flat on the slide and mount with 100μl DAPI containing fluorescent medium and coverslip and view on an LSM 510 laser-scanning microscope at 488nm.
(B) Characterization of RPE monolayers using SEM
Fix the membrane on Transwell filters for 24h with half strength Karnovsky’s fixative at RT. Add 0.5ml of the fixative to the top and 1.5ml to the bottom chamber.
Rinse in 0.1M cacodylate buffer pH 7.4 (sodium cacodylate and HCl).
Remove the membranes containing the cells from the insert using a sharp razor blade and place in a glass scintillation vial.
Post fix with 5ml of 2% (vol/vol) osmium tetroxide in 0.1M cacodylate buffer for 2 h at RT.
Rinse in 0.1M cacodylate buffer pH 7.4 and dehydrate cells in graded series of 5ml ethanol from 30% to 100% and transfer from 100% ethanol to 100% hexamethyldisilasane (HMDS) (15min each wash) .
Air dry the tissues on membrane for 24 h.
Mount on to stubs and coat with gold and palladium on a sputter-coater using colloidal silver liquid.
The cells are imaged with a JEOL JSM 6390 LV Scanning Electron Microscope (filament voltage at 15 KV).
(C) Characterization of RPE monolayers using TEM
Fix the membrane on Transwell filters for 24h with half strength Karnovsky’s fixative at RT. Add 0.5ml of the fixative to the top and 1.5ml to the bottom chamber.
Rinse in 5 ml of 0.1M cacodylate buffer pH 7.4 (sodium cacodylate and HCl).
Remove the Transwell membranes using a Teflon coated razor blade from the insert to glass.
Post fix with 5ml of 2% (vol/vol) osmium tetroxide in 0.1M cacodylate buffer for 2 h at RT.
Rinse in 0.1M cacodylate buffer pH 7.4 and dehydrate cells in graded series of 5ml ethanol from 30% to 100% (15min wash each).
Infiltrate in Eponate overnight prior to embedding (infiltrate with Eponate/100% ethanol (2:1) for 2h followed by 100% Eponate for 2h. Then, place under vacuum at 65° C for 12h).
Cut ultrathin sections at a thickness of 70nm and stain with UranylAcetate (5-10min) and Lead Citrate (30sec) 31.
Examine sections on a JEOL JEM 2100 electron microscope and photograph with the Orius SC1000B Gatan digital camera.
?TROUBLESHOOTING
Troubleshooting advice can be found in Table2.
Table2.
Troubleshooting table.
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 6 | Imprecise removal of anterior segment of the eye |
Damaging tissue by separately removing cornea and lens using dull scissors |
Do not attempt separate removal of cornea and lens. Typically the lens is attached to the anterior segment and it is removed with the anterior segment in one piece. Use sharp scissors. |
| 13 | Incomplete removal of choroidal tissues from RPE sheet |
Insufficient dispase digestion |
Treat with dispase for longer duration. Residual choroidal tissues on the RPE sheet increase the chance of contamination that may lead to abnormal cell growth. Make sure to exclude the choroidal tissues by viewing under a dissection microscope. |
| 20 | Abnormal pigmentation of cells |
donor related problem |
Cell cultures that do not show pigmentation or show hyperpigmentation should be discarded. Use a differernt donor eye. |
| Low yield of RPE (<70% confluency) |
donor related problem long postmortem interval |
Obtain new donor eye with postmortem interval < 24h. |
|
| 27 | Low TER after 1month of culture |
contamination overgrowth abnormal pigment |
Discard the cells and obtain a new donor eye. |
| 28 a-c |
Damage to membrane during removal from Transwell |
Mechanical damage from pressure of razor blade |
Use fine forceps and new razor blade; apply minimal pressure. |
| 28a | Weak immunopositivity for tight junction proteins |
Antibody expired or degraded |
Obtain new antibodies. |
| 28a | Poor apical localization of Na/K ATPase |
Delay in fixation |
Rapid fixation of membrane in paraformaldehyde. |
| 28c | The Transwell support does not section well |
Difference in density between Transwell support and plastic embedded membrane |
Take special care when ultrathin sectioning to obtain intact sections. |
ANTICIPATED RESULTS
The whole process from procurement of the eye tissue to obtaining a polarized RPE monolayer with high resistance takes approximately 6 weeks. By modifying the method for initial isolation of pure RPE cells and culture conditions from available method protocols, we have established a method that yields highly reproducible RPE monolayers from the same donor eye. From one donor eye, 4 culture plates of 12 wells each can be seeded.
In our experience, approximately 80% of the eyes will result in culture of highly polarized RPE monolayers. A good preparation consist of cells with hexagonal shape forming a monolayer and moderate pigmentation which begins to show gradual increase in TER starting at 3 weeks. A non-usable preparation will show either no pigmentation or hyperpigmentation in about 2 weeks and the TER remains <50 Ω·cm2 and does not increase. Generally, the cells undergo change from an island of RPE on day1, lose pigmentation on day 4 and recover on day 14 with an increase in confluency (Fig. 8a-d). Highly polarized RPE cells exhibit well developed tight junctions (Fig. 11d), apical Na/K ATPase by confocal microscopy (Fig. 11b), well defined apical microvilli (Fig. 11c), and expression of RPE specific marker RPE65 (Fig. 11e). Cultures in which the RPE cells show degenerative changes, or hyperpigmentation, or cultures that show overgrowth, contamination with other cell types (Fig. 8e,f), or low TER (after 1 month of Transwell culture) should be discarded.
ACKNOWLEDGMENTS
Supported by funds from The Arnold and Mabel Beckman Foundation, National Institutes of Health Grants (EY01545, EY03040), and the Research to Prevent Blindness Inc. The authors thank P. G. Sreekumar, for his early contributions to the development of these methods.
Footnotes
COMPETING INTERESTS STATEMENT The authors declare that they have no competing financial interests.
REFERENCES
- 1.Thumann G, Hoffmann S, Hinton DR. Cell biology of the retinal pigment epithelium. In: Ryan SJ, editor. Retina. 4rd ed Vol. 1. Elsevier Mosby; Philadelphia, USA: 2006. pp. 137–152. [Google Scholar]
- 2.Rodriguez-Boulan E, Nelson WJ. Morphogenesis of the polarized epithelial cell phenotype. Science. 1989;245:718–725. doi: 10.1126/science.2672330. [DOI] [PubMed] [Google Scholar]
- 3.Marmor MF, Abdul-Rahim AS, Cohen DS. The effect of metabolic inhibitors on retinal adhesion and subretinal fluid resorption. Invest Ophthalmol Vis Sci. 1980;19:893–903. [PubMed] [Google Scholar]
- 4.Miller SS, Hughes BA, Machen TE. Fluid transport across retinal pigment epithelium is inhibited by cyclic AMP. Proc Natl Acad Sci U S A. 1982;79:2111–2115. doi: 10.1073/pnas.79.6.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson JM, Van Itallie CM. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol. 1995;269:G467–475. doi: 10.1152/ajpgi.1995.269.4.G467. [DOI] [PubMed] [Google Scholar]
- 6.Rizzolo LJ. Polarity and the development of the outer blood-retinal barrier. Histol Histopathol. 1997;12:1057–1067. [PubMed] [Google Scholar]
- 7.Hogan MJ. Role of the retinal pigment epithelium in macular disease. Trans Am Acad Ophthalmol Otolaryngol. 1972;76:64–80. [PubMed] [Google Scholar]
- 8.Sheedlo HJ, Li L, Turner JE. Effects of RPE-cell factors secreted from permselective fibers on retinal cells in vitro. Brain Res. 1992;587:327–337. doi: 10.1016/0006-8993(92)91015-7. [DOI] [PubMed] [Google Scholar]
- 9.Holtkamp GM, et al. Polarized secretion of IL-6 and IL-8 by human retinal pigment epithelial cells. Clin Exp Immunol. 1998;112:34–43. doi: 10.1046/j.1365-2249.1998.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blaauwgeers HG, et al. Polarized vascular endothelial growth factor secretion by human retinal pigment epithelium and localization of vascular endothelial growth factor receptors on the inner choriocapillaris. Evidence for a trophic paracrine relation. Am J Pathol. 1999;155:421–428. doi: 10.1016/S0002-9440(10)65138-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu J, Bok D. A cell culture medium that supports the differentiation of human retinal pigment epithelium into functionally polarized monolayers. Mol Vis. 2001;7:14–19. [PubMed] [Google Scholar]
- 12.Maminishkis A, et al. Confluent monolayers of cultured human fetal retinal pigment epithelium exhibit morphology and physiology of native tissue. Invest Ophthalmol Vis Sci. 2006;47:3612–3624. doi: 10.1167/iovs.05-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996;62:155–169. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- 14.Kannan R, et al. Stimulation of apical and basolateral VEGF-A and VEGF-C secretion by oxidative stress in polarized retinal pigment epithelial cells. Mol Vis. 2006;12:1649–1659. [PubMed] [Google Scholar]
- 15.Geisen P, McColm JR, King BM, Hartnett ME. Characterization of barrier properties and inducible VEGF expression of several types of retinal pigment epithelium in medium-term culture. Curr Eye Res. 2006;31:739–748. doi: 10.1080/02713680600837408. [DOI] [PubMed] [Google Scholar]
- 16.Okami T, et al. Immunocytochemical localization of Na+,K(+)-ATPase in rat retinal pigment epithelial cells. J Histochem Cytochem. 1990;38:1267–1275. doi: 10.1177/38.9.2167328. [DOI] [PubMed] [Google Scholar]
- 17.Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quinn RH, Miller SS. Ion transport mechanisms in native human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1992;33:3513–3527. [PubMed] [Google Scholar]
- 19.Philp NJ, Wang D, Yoon H, Hjelmeland LM. Polarized expression of monocarboxylate transporters in human retinal pigment epithelium and ARPE-19 cells. Invest Ophthalmol Vis Sci. 2003;44:1716–1721. doi: 10.1167/iovs.02-0287. [DOI] [PubMed] [Google Scholar]
- 20.Ohno-Matsui K, et al. Novel mechanism for age-related macular degeneration: an equilibrium shift between the angiogenesis factors VEGF and PEDF. J Cell Physiol. 2001;189:323–333. doi: 10.1002/jcp.10026. [DOI] [PubMed] [Google Scholar]
- 21.Shi G, et al. Control of chemokine gradients by the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2008;49:4620–4630. doi: 10.1167/iovs.08-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sonoda S, 1, A., Sreekumar PG, 1,2A, Spee C, 1,2A, Ryan SJ, 1,2A, Kannan R, 1,2A, Hinton DR., 1,2B Selective Increase in Basolateral Secretion of VEGF Upon BMP-4 Treatment of Highly Polarized Human RPE Cells. Invest. Ophthalmol. Vis. Sci, 2008. 2008;49 ARVO E-Abstract 855. [Google Scholar]
- 23.Zhang N, et al. Characterization of brimonidine transport in retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2006;47:287–294. doi: 10.1167/iovs.05-0189. [DOI] [PubMed] [Google Scholar]
- 24.Sreekumar PG, et al. N-(4-hydroxyphenyl) retinamide augments laser-induced choroidal neovascularization in mice. Invest Ophthalmol Vis Sci. 2008;49:1210–1220. doi: 10.1167/iovs.07-0667. [DOI] [PubMed] [Google Scholar]
- 25.German OL, Buzzi E, Rotstein NP, Rodriguez-Boulan E, Politi LE. Retinal pigment epithelial cells promote spatial reorganization and differentiation of retina photoreceptors. J Neurosci Res. 2008 doi: 10.1002/jnr.21813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Senanoyake P, et al. Glycosaminoglycan synthesis and secretion by the retinal pigment epithelium: polarized delivery of hyaluronan from the apical surface. J Cell Sci. 2001;114:199–205. doi: 10.1242/jcs.114.1.199. [DOI] [PubMed] [Google Scholar]
- 27.Hamel CP, et al. A developmentally regulated microsomal protein specific for the pigment epithelium of the vertebrate retina. J Neurosci Res. 1993;34:414–425. doi: 10.1002/jnr.490340406. [DOI] [PubMed] [Google Scholar]
- 28.Ban Y, Rizzolo LJ. A culture model of development reveals multiple properties of RPE tight junctions. Mol Vis. 1997;3:18. [PubMed] [Google Scholar]
- 29.Frambach DA, Fain GL, Farber DB, Bok D. Beta adrenergic receptors on cultured human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1990;31:1767–1772. [PubMed] [Google Scholar]
- 30.Jin M, Barron E, He S, Ryan SJ, Hinton DR. Regulation of RPE intercellular junction integrity and function by hepatocyte growth factor. Invest Ophthalmol Vis Sci. 2002;43:2782–2790. [PubMed] [Google Scholar]
- 31.Tandler B. Improved uranyl acetate staining for electron microscopy. J Electron Microsc Tech. 1990;16:81–82. doi: 10.1002/jemt.1060160110. [DOI] [PubMed] [Google Scholar]