Abstract
NO2Tyr (3-Nitrotyrosine) is a modified amino acid that is formed by nitric oxide-derived species and has been implicated in the pathology of diverse human diseases. Nitration of active-site tyrosine residues is known to compromise protein structure and function. Although free NO2Tyr is produced in abundant concentrations under pathological conditions, its capacity to alter protein structure and function at the translational or posttranslational level is unknown. Here, we report that free NO2Tyr is transported into mammalian cells and selectively incorporated into the extreme carboxyl terminus of α-tubulin via a posttranslational mechanism catalyzed by the enzyme tubulin–tyrosine ligase. In contrast to the enzymatically regulated carboxyl-terminal tyrosination/detyrosination cycle of α-tubulin, incorporation of NO2Tyr shows apparent irreversibility. Nitrotyrosination of α-tubulin induces alterations in cell morphology, changes in microtubule organization, loss of epithelial-barrier function, and intracellular redistribution of the motor protein cytoplasmic dynein. These observations imply that posttranslational nitrotyrosination of α-tubulin invokes conformational changes, either directly or via allosteric interactions, in the surface-exposed carboxyl terminus of α-tubulin that compromises the function of this critical domain in regulating microtubule organization and binding of motor- and microtubule-associated proteins. Collectively, these observations illustrate a mechanism whereby free NO2Tyr can impact deleteriously on cell function under pathological conditions encompassing reactive nitrogen species production. The data also yield further insight into the role that the α-tubulin tyrosination/detyrosination cycle plays in microtubule function.
Nitric oxide (•NO) is a pervasive signaling molecule generated from l-arginine via the catalytic action of both constitutive and inducible forms of •NO synthases (1). A large body of evidence has amassed in the last decade, establishing the operative role of inducible •NO synthase in the pathogenesis of inflammatory, infectious, and degenerative human diseases (2). The detrimental effects ascribed to •NO often arise from its conversion to more reactive species through reactions with partially reduced oxygen species (3).
The pathophysiological actions of •NO congeners are primarily rooted in their capacity to alter the function of biological macromolecules through covalent modifications. A metabolite generally reflecting in vivo production of reactive nitrogen intermediates is the amino acid derivative 3-nitrotyrosine (NO2Tyr). Evidence for NO2Tyr formation in vivo was found when the free amino acid and its deaminated/decarboxylated metabolite 3-nitro-4-hydroxyphenylacetic acid were detected as excretory products in human urine (4). The significance of NO2Tyr in vivo is highlighted further by observations that protein-linked NO2Tyr is markedly elevated in a broad range of human diseases and clinical disorders (5). In vitro studies have identified a number of distinct, yet redundant, mechanisms for tyrosine nitration that underscore the general significance of this process under pathologic conditions. These mechanisms include the reaction of •NO with superoxide (O2⨪), forming peroxynitrite (ONOO−) (reviewed in ref. 3), peroxidase-dependent nitrite oxidation (6–9), •NO reaction with protein tyrosyl radicals (10), and nitrous acid (HNO2) formed in acidic environments (i.e., gastric compartment and phagolysosomes; ref. 11).
Recent studies have established that in addition to serving as a “marker” of reactive-nitrogen-species formation, nitration of active-site tyrosine residues can compromise protein/enzyme function in vitro (12–16) and in vivo (17, 18). The free amino acid form of NO2Tyr also has been shown to alter hemodynamic responses in vivo (19), is cleared from the circulation (t1/2 ≈ 60 min; ref. 20), and induces cytotoxicity, growth inhibition, and morphological changes in cultured cells (21, 22). However, the mechanisms underlying these effects are not known. Based on the observed biological activities of free NO2Tyr, combined with its abundant production [1–120 μM under pathological conditions such as rheumatoid arthritis (23), liver transplantation (24), septic shock (25), and amyotrophic lateral sclerosis (26)], we hypothesized that free NO2Tyr could mediate cellular dysfunction by its translational or posttranslational incorporation into proteins. We show here that free NO2Tyr is taken up by mammalian cells and irreversibly incorporated into α-tubulin—but no other protein—via a posttranslational mechanism catalyzed by tubulin–tyrosine ligase (TTL), a process that alters microtubule function.
METHODS
Cell Culture.
A549 cells, an alveolar type II epithelial cell line derived from a lung carcinoma, were obtained from the American Type Culture Collection (Rockville, MD) and cultured in Ham’s F-12 medium containing 10% FBS (≈40 μM Tyr). Primary cultures of fetal rat lung fibroblasts (FRLFs) and type-II epithelial cells (FRLE-TIIs) were isolated and cultured in MEM containing 10% heat-inactivated FBS (≈210 μM Tyr) as previously described (27). Human umbilical vein endothelial cells (HUVECs) were isolated and cultured in M199 medium containing 10% FBS (≈230 μM Tyr) as described (28). Cells were initially plated at 50% confluence in 100-mm tissue-culture dishes and grown to confluence in basal medium or in medium supplemented with NO2Tyr (Sigma).
Cell-Permeability Measurements.
A549 cells were plated in 6-well Falcon transwell cell culture inserts (3090; 0.4-μm pore size; polyethylene teraphthalate track-etched membranes) and grown to confluence in the absence or presence of NO2Tyr. At confluence (≈2 days), cells were rinsed with Hanks’ balanced salt solution, and the medium in the upper and lower wells was replaced with fresh medium. To the basolateral side was added 4.64 μCi [125I]BSA (Amersham Pharmacia; 1.0916 mCi/ml). After 4 h, aliquots of medium (10 μl) were taken from the apical compartment, and [125I]BSA was quantitated by liquid scintillation counting.
Western Blotting and Immunoprecipitation.
Confluent cells were rinsed briefly with Hanks’ balanced salt solution and solubilized in lysis buffer (20 mM Tris•HCl, pH 8.0/137 mM NaCl/1 mM MgCl2/1 mM CaCl2/10% (vol/vol) glycerol/1% NP-40/1 mM DTT/1 mM PMSF/1 μg/ml leupeptin/1 μg/ml pepstatin/1 μg/ml aprotinin). Proteins were separated by SDS/PAGE [12%; 6% for cytoplasmic dynein heavy chain (DHC)] and then transferred to nitrocellulose membranes. After electrotransfer, nitrocellulose membranes were incubated overnight at 4°C in blocking buffer (PBS containing 0.05% Tween 20) containing 3% nonfat milk. For Western blotting, rabbit polyclonal antibody to NO2Tyr (1:3,000; ref. 29), mouse mAb against α-tubulin (clone B-5-1-2; Sigma; 1:3,000), mAb against tyrosinated tubulin (Tyr-Tub; clone TUB-1A2; Sigma; 1:3,000), mAb against α-tubulin (clone DM1A; Amersham Pharmacia; 1:2,500), mAb against DHC (clone 440.4; Sigma; 1:2,000), or mAb against dynein intermediate chain (DIC; clone 70.1; Sigma; 1:2,500) were used. Horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (Bio-Rad; 1:2,500), HRP-conjugated goat anti-mouse IgG (Sigma; 1:3,000), or HRP-conjugated protein A (Sigma; 1:3,000) was used for detection. Proteins were visualized by enhanced chemiluminescence (Amersham Pharmacia). For immunoprecipitation, cell lysates were precleared by incubation with protein-G Sepharose (Amersham Pharmacia) for 30 min at 4°C. The supernatants were then incubated with primary antibody and protein-G Sepharose for 6–8 h at 4°C. The immunoprecipitates then were washed three times in lysis buffer, and proteins were detected as described above.
Differential Detergent Extraction of Dynein.
Differential detergent extraction of control and NO2Tyr-treated cells was performed by using saponin and Triton X-100 as described (30). The quantity of DIC in each fraction was determined by laser scanning densitometry of Western blots by using National Institutes of Health image 1.61, a public domain program (rsb.info.nih.gov/nih-image).
Limited Proteolysis of Tubulin.
Immunoprecipitated α-tubulin from A549 cells was resuspended in K+Mes (50 mM; pH 6.8) and treated with either pancreatic carboxypeptidase A (CPA; 0.25 μg/ml) or subtilisin (SUB; 0.3 μg/ml) at 27°C for 30 min or 15 min, respectively. Reactions were terminated by addition of DTT (20 mM) or PMSF (2 mM), respectively.
Immunofluorescence Microscopy.
A549 cells grown in Lab-Tek II multi-chamber slides (Nunc) were fixed in methanol for 10 min at −20°C, rinsed with PBS, blocked in PBS containing 10% goat serum (1 h at 25°C), and labeled with primary antibodies (polyclonal anti-NO2Tyr, 1:1,000; monoclonal anti-α-tubulin, 1:400; monoclonal anti-Tyr-Tub, 1:800; monoclonal anti-DHC clone 440.4, 1:100; all from Sigma) overnight at 4°C. Secondary antibodies were Cy3-conjugated goat anti-rabbit IgG and FITC-conjugated goat anti-mouse IgG (1:200, Jackson ImmunoResearch). In some cases, cells were double-labeled with anti-NO2Tyr and anti-α-tubulin. Nuclear counterstaining was performed by using 4′,6-diamidine-2′-phenylindole dihydrochloride (1 μg/ml). An Olympus IX-70 epifluorescence microscope, an OlymPix Digitally Cooled Camera (resolution of 1,328 × 1,024 pixels), and esprit software were used for imaging.
RESULTS AND DISCUSSION
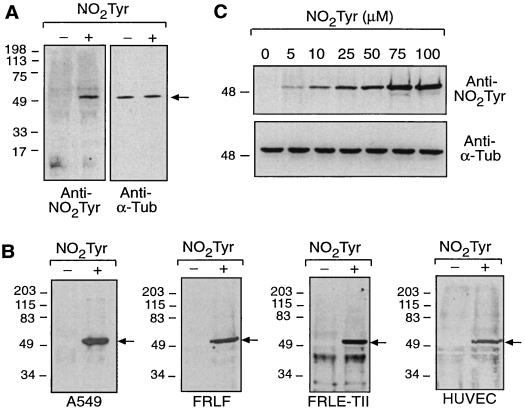
To determine whether NO2Tyr could be incorporated into cellular proteins at the translational or posttranslational level, A549 cells were cultured in medium supplemented with free NO2Tyr (50 μM) for 24 h. As shown in Fig. 1A, NO2Tyr was incorporated selectively into a single protein that comigrated with α-tubulin. Inhibition of protein synthesis with cycloheximide (5 μg/ml; 8–10 h) did not affect the extent of NO2Tyr incorporation (not shown), supporting a posttranslational mechanism. Immunoprecipitation and subsequent Western blot analysis confirmed that NO2Tyr was incorporated into α-tubulin (Fig. 1B). Similar results were obtained in primary cultures of FRLFs, FRLE-TIIs, and HUVECs (Fig. 1B), indicating a common pathway of α-tubulin modification by NO2Tyr in different species and cell types. Covalent incorporation of NO2Tyr into α-tubulin was dose-dependent (Fig. 1C) and occurred over a wide range of NO2Tyr concentrations. Nitrotyrosination of α-tubulin was detectable when NO2Tyr was 1–2 mol % of tyrosine, levels easily attained in vivo (23–26). The deaminated/decarboxylated form of NO2Tyr (3-nitro-4-hydroxyphenylacetic acid, NO2HPA) was not incorporated into α-tubulin.
Figure 1.
Cellular uptake and selective incorporation of NO2Tyr into the carboxyl terminus of α-tubulin. (A) Total cell lysates of A549 cells cultured in the absence (−) or presence (+) of NO2Tyr (50 μM; 125% of Tyr) were analyzed by immunoblotting with anti-NO2Tyr (Left) or anti-α-tubulin (Right). (B) Detection of NO2Tyr in α-tubulin immunoprecipitated from A549 cells, FRLFs, FRLE-TIIs, and HUVECs cultured in the absence (−) or presence (+) of NO2Tyr (100 μM; 250%, 47%, 47%, and 43% of Tyr, respectively). (A and B) Arrows indicate the mobility of α-tubulin. (C) Immunoblot illustrating dose-dependent incorporation of NO2Tyr into α-tubulin immunoprecipitated from A549 cells. The level of NO2Tyr relative to Tyr for A549 cells in Ham’s F-12 medium was 12%, 25%, 62%, 125%, 187%, and 250%, respectively, for the dose-response.
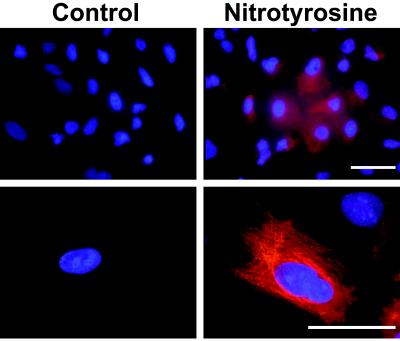
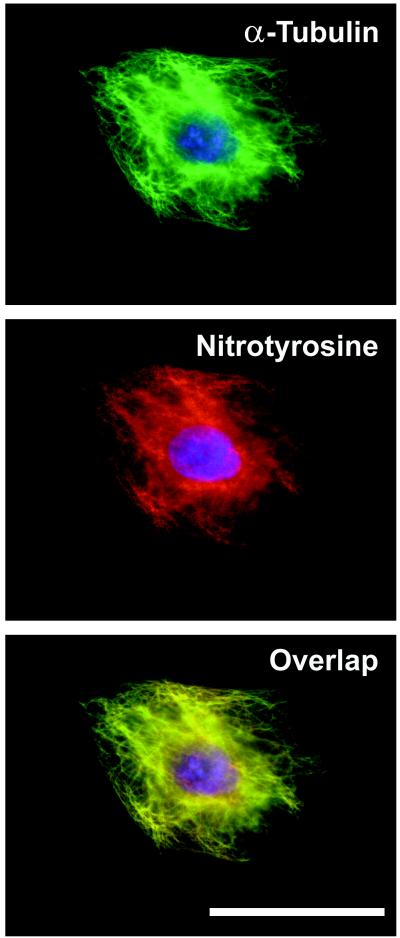
To support the biochemical evidence of NO2Tyr incorporation into α-tubulin, immunofluorescence microscopy studies were conducted. Whereas control cells displayed minimal NO2Tyr immunoreactivity, the immunofluorescence of NO2Tyr-treated cells was highly elevated (Fig. 2, Upper), having staining patterns consistent with microtubule distribution (Fig. 2, Lower). To define the localization of NO2Tyr with α-tubulin, cells treated with NO2Tyr were double-labeled with anti-NO2Tyr and anti-α-tubulin. As shown in Fig. 3, concordant fluorescence patterns were observed for α-tubulin (green staining) and NO2Tyr (red staining), with the extent of colocalization indicated by overlaid images (yellow staining).
Figure 2.
NO2Tyr immunoreactivity in A549 cells grown in basal medium (control) or medium supplemented with NO2Tyr (100 μM). Shown are cells stained with anti-NO2Tyr and viewed at low (×20, Upper) or high (×100, Lower) magnification. In all cases, NO2Tyr immunoreactivity was blocked by preabsorbing polyclonal anti-NO2Tyr with free NO2Tyr (5 mM). (Bars = 50 μm.)
Figure 3.
Protein-associated NO2Tyr immunoreactivity colocalizes with α-tubulin. Double-label immunofluorescence microscopy was performed on A549 cells cultured with NO2Tyr (100 μM). Cells were stained with both anti-α-tubulin (green staining; Top) and anti-NO2Tyr (red staining; Middle). Immunolocalization studies of the same cells detect nearly identical localization of NO2Tyr and α-tubulin when the images are overlaid (yellow staining; Bottom). (Bar = 50 μm.)
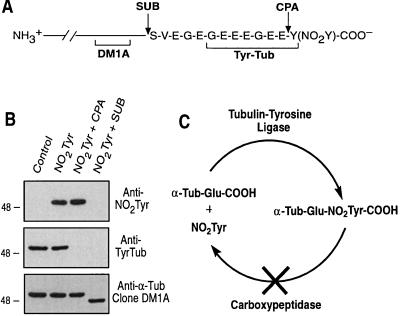
A unique property of α-tubulin in all higher eukaryotes is the reversible posttranslational removal and reincorporation of tyrosine at the extreme carboxyl terminus (31). These posttranslational modifications are mediated by a tubulin-specific carboxypeptidase that removes the genetically encoded carboxyl-terminal tyrosine (32) and a TTL that catalyzes readdition of tyrosine to the penultimate glutamate of α-tubulin (33). This cycle of posttranslational modification yields at least two distinct subsets of α-tubulin, Tyr-Tub and detyrosinated forms, that have differential cellular localization (34) and distinct functions (35). To examine whether NO2Tyr was incorporated selectively into the extreme carboxyl terminus of α-tubulin, limited proteolysis was performed with CPA, an enzyme that removes carboxyl-terminal tyrosines without digesting tubulin further (36), or with SUB, a protease that removes a ≈2-kDa peptide, including residues 439–451, from the carboxyl terminus of α-tubulin (ref. 37; Fig. 4A). A549 cells treated with NO2Tyr contained both NO2Tyr-Tub and Tyr-Tub (Fig. 4B). Treatment of immunoprecipitated α-tubulin with CPA resulted in the complete loss of Tyr-Tub immunoreactivity (Fig. 4B, anti-Tyr-Tub), as shown previously (38), but had no effect on NO2Tyr immunoreactivity (Fig. 4B, anti-NO2Tyr), indicating that α-tubulin–NO2Tyr is resistant to CPA cleavage. Proteolytic removal of the α-tubulin carboxyl-terminal peptide with SUB resulted in complete loss of NO2Tyr and Tyr-Tub immunoreactivity. SUB-cleaved α-tubulin was immunoreactive with the α-tubulin antibody clone DM1A, which recognizes an epitope located outside of the SUB fragment (primarily residues 426–430, ref. 39), but had increased electrophoretic mobility (Fig. 4B), consistent with the loss of the SUB fragment. Because the sequence of the SUB fragment from porcine and human brain α-tubulin contains only one tyrosine residue located at the extreme carboxyl terminus (439SVEGEGEEEGEEY451; ref. 37), these data confirm that incorporation of NO2Tyr occurs exclusively at the carboxyl terminus of α-tubulin presumably via the action of TTL. In contrast to the enzymatic cleavage of the carboxyl-terminal tyrosine of α-tubulin, the data suggest that posttranslational nitrotyrosination of α-tubulin is irreversible. The significance of this apparently irreversible modification is underscored by evidence implicating the α-tubulin detyrosination pathway as an important event in cellular growth, differentiation, and motility (40–42).
Figure 4.
Limited proteolysis indicates that NO2Tyr is incorporated irreversibly into the extreme carboxyl terminus of α-tubulin in A549 cells. (A) Schematic representation of antibody epitope locations and proteolytic cleavage sites of SUB and CPA within the carboxyl terminus of α-tubulin. (B) α-Tubulin from A549 cells grown in basal medium (Control) or medium supplemented with NO2Tyr that was untreated (NO2Tyr), treated with CPA (NO2Tyr + CPA), or treated with SUB (NO2Tyr + SUB). Membranes were probed with anti-NO2Tyr, anti-Tyr-Tub, or anti-α-tubulin (clone DM1A). (C) Schematic illustrating the irreversible incorporation of NO2Tyr into α-tubulin.
The extent of NO2Tyr incorporation into α-tubulin is decreased with lower ratios of NO2Tyr to tyrosine (Fig. 1C), suggesting competing physiologic processes. Preliminary studies have suggested that at least two independent mechanisms may be accountable. First, NO2Tyr (50 μM) partially inhibits the uptake of 14C-labeled tyrosine into A549 cells by ≈25%, suggesting that transport may occur through a mechanism similar to tyrosine. It is also possible that NO2Tyr is transported differently than tyrosine, as suggested for the tryptophan-specific permease of Salmonella (43). Second, incorporation of NO2Tyr into α-tubulin in cell lysates, which precludes amino acid uptake, is competitive with tyrosine (not shown). These data suggest that α-tubulin nitrotyrosination may be competitive with tyrosine at both the level of amino acid uptake and use by TTL.
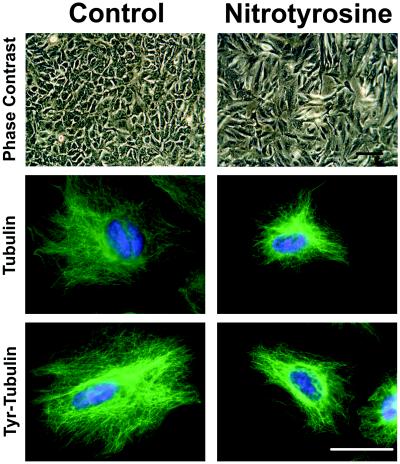
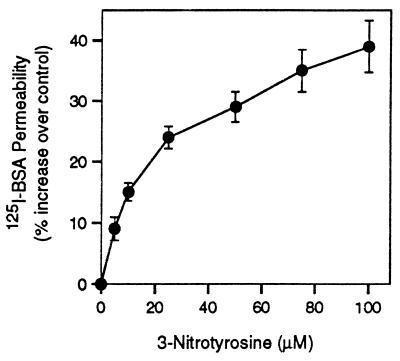
Because microtubules, in concert with other cytoskeletal proteins, play important roles in determining cell shape, the effect of α-tubulin nitrotyrosination on cell morphology and microtubule structure was investigated. A549 cells grown in basal medium displayed characteristic epithelial cell morphology, forming a uniform monolayer on reaching confluence (Fig. 5, Control), whereas cells cultured in medium containing NO2Tyr had distinctly altered morphologies (Fig. 5, NO2Tyr). Prolonged culture of cells with NO2Tyr beyond confluence led to apoptosis, cellular retraction, and detachment (not shown). Alterations in cell morphology were paralleled by a change in microtubule structure and organization (Fig. 5). Whereas immunoreactivity for α-tubulin in control cells displayed a characteristic and uniform microtubule distribution, microtubules in NO2Tyr-treated cells displayed apparent decreased length, as well as increased perinuclear localization and aggregation. Similar observations were obtained with the Tyr-Tub-specific antibody (Fig. 5). Consistent with alterations in cell morphology and altered microtubule structure, A549 cells treated with NO2Tyr displayed increased permeability to [125I]BSA, an indication of epithelial barrier dysfunction (Fig. 6).
Figure 5.
Incorporation of NO2Tyr into α-tubulin induces alterations in cellular morphology and microtubule organization. A549 cells were cultured in basal medium (Control) or in medium supplemented with NO2Tyr (100 μM). Phase-contrast microscopy of A549 cells showed altered cellular morphology. Cells from both Control and NO2Tyr treatments were stained with either anti-α-tubulin (clone B-5-1-2) or anti-Tyr-Tub. (Bar = 50 μm.)
Figure 6.
NO2Tyr induces barrier dysfunction in A549 epithelial cells. Barrier function was assessed by the permeability of confluent cells to [125I]BSA as described in Methods. The percentage of NO2Tyr relative to tyrosine in the medium was 0%, 12%, 25%, 62%, 125%, 187%, and 250%. Values are expressed as the percentage of increase over control cells (basal medium) and represent the mean ± SD of four separate experiments.
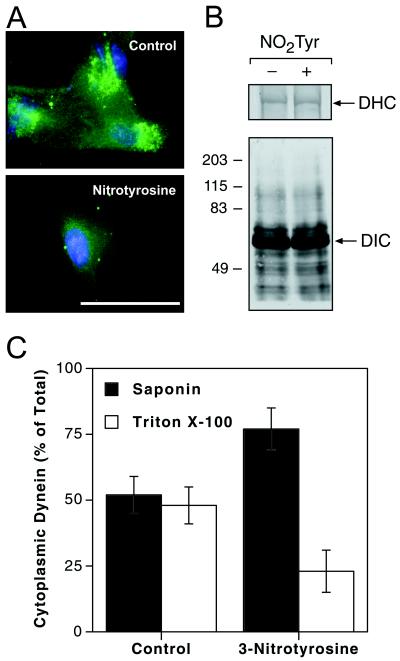
The short, surface-exposed carboxyl-terminal domain of α-tubulin functions as the site for regulatory interactions of microtubules with motor proteins (i.e., cytoplasmic dynein) and microtubule-associated proteins (MAP2, MAP4, and tau; refs. 44 and 45), as well as with molecular chaperones (i.e., heat-shock proteins 70 and 90; ref. 46). We therefore studied whether nitrotyrosination influenced the interaction of α-tubulin with the retrograde motor protein cytoplasmic dynein. The immunofluorescence staining of dynein in untreated A549 epithelial cells localized in a distinctly punctate pattern throughout the cytosol, with the greatest fluorescence intensity located in the perinuclear region (Fig. 7A, Control). In contrast, cells treated with NO2Tyr displayed a nearly complete loss of DHC immunoreactivity (Fig. 7A, NO2Tyr), with remaining immunofluorescence localized primarily to the perinuclear region. The loss of dynein immunoreactivity was not caused by protein degradation or decreased protein synthesis, as Western blots of immunoprecipitated DHC or cytoplasmic DIC showed equal quantities of protein after either treatment (Fig. 7B). A differential-detergent-extraction method was employed to determine whether intracellular redistribution of dynein was responsible for the loss of immunofluorescence. As previously reported (30), the quantity of dynein in untreated cells was equally distributed between saponin- and Triton X-100-extractable fractions. A significantly greater proportion of DIC was present in the initial saponin extract of NO2Tyr-treated cells, with the remainder residing in the Triton X-100 extract (Fig. 7C), suggesting that dynein is redistributed from a membrane-bound compartment to a more readily extractable cytoplasmic pool. These data suggest that intracellular redistribution of cytoplasmic dynein is a consequence of decreased affinity of the microtubule-interacting domain of DHC (47) for the structurally altered nitrotyrosinated carboxyl terminus of α-tubulin (44, 45). Alterations in microtubule association of cytoplasmic dynein that lead to intracellular redistribution may severely compromise dynein function in organelle transport (48).
Figure 7.
Cytoplasmic dynein undergoes intracellular redistribution and loss of immunoreactivity concomitant with nitrotyrosination of α-tubulin. (A) A549 cells cultured in basal medium (Control) or in medium supplemented with NO2Tyr (100 μM) were stained with anti-DHC. (Bar = 50 μm.) (B) A549 cells cultured in the absence (−) or presence (+) of NO2Tyr (100 μM) were immunoprecipitated and probed with anti-DHC (Upper) or anti-DIC (Lower). (C) Quantitation of cytoplasmic dynein pools by using differential detergent extraction. Data are expressed as mean ± SD for four (Control) and five (NO2Tyr) experiments.
Several lines of evidence suggest that posttranslational nitrotyrosination compromises the function of the surface-exposed carboxyl-terminal domain of α-tubulin. First, in contrast to the enzymatically regulated carboxyl-terminal tyrosination/detyrosination cycle of α-tubulin, incorporation of NO2Tyr is resistant to cleavage by CPA. Therefore, cellular processes such as growth, differentiation, and motility that require α-tubulin detyrosination (34, 40–42) may be compromised by nitrotyrosination. Second, nitrotyrosination of α-tubulin disrupts binding of microtubule-associated proteins (i.e., dynein; Fig. 7) and induces alterations in microtubule organization (Fig. 5). These observations may be attributed to the significant changes in the ionization state and steric restrictions imposed by the NO2 group in NO2Tyr. Moreover, observations indicating that the antibody specific for Tyr-Tub does not bind nitrotyrosinated α-tubulin (Fig. 4B) suggest that incorporation of NO2Tyr invokes conformational changes, either directly or via allosteric interactions, in the carboxyl-terminal domain of α-tubulin. Nitrotyrosination of α-tubulin thus seems to alter binding of microtubule-associated proteins, which can result in altered microtubule assembly and organization (49).
In eukaryotic cells, tubulin heterogeneity is generated at the genetic level and is broadened considerably by posttranslational modifications, including tyrosination, phosphorylation, polyglycylation, polyglutamylation, and acetylation (31). All of these posttranslational modifications are mediated enzymatically and are reversible, and all but acetylation occur in the short carboxyl-terminal domain that extends from the microtubule filament into the cytoplasm (31). Previous in vitro studies showing TTL-catalyzed incorporation of structurally altered tyrosine derivatives such as 3-iodotyrosine (50), 3-fluorotyrosine (51), and 3,4-dihydroxyphenylalanine (52) into α-tubulin illustrates the promiscuous substrate specificity of this unique enzyme system. Our observations illustrating nitrotyrosination of α-tubulin in cellular systems reinforces this tenet and advances this concept into one with significant pathophysiological implications. The consequences of α-tubulin modification by tyrosine derivatives also may be extended to include 3-chlorotyrosine and 3-bromotyrosine, characteristic products of the myeloperoxidase and eosinophil peroxidase systems of phagocytes that are formed both in vitro and in vivo (8, 53–55). Collectively, the enzymatic incorporation of nitrated and halogenated tyrosine derivatives into α-tubulin thus illustrates a distinct mechanism of tissue injury during inflammation.
These observations illustrate the role for NO2Tyr in altering microtubule structure and function via posttranslational incorporation into α-tubulin and suggest a distinct mechanism to explain the previously observed biological effects of free NO2Tyr (19–22). The irreversibility of this process underscores the potent influence that nitrotyrosination of α-tubulin can have on microtubule functions in disease processes that encompass pathologic roles for nitric oxide and oxidative stress. Cytoskeletal reorganization and disruption, as well as nitration of cytoskeletal proteins, are associated with oxidative stress and inflammatory injury (56), with the present results providing one explanation for these observations. Further investigation into the impact of α-tubulin modification on cell and organ function by tyrosine derivatives will yield insight into both mechanisms of inflammatory injury and the role that the α-tubulin tyrosination cycle plays in microtubule function.
Acknowledgments
We thank G. Johnson, A. van der Vliet, C. E. Cross, V. O’Donnell, and W. R. Berrington for helpful discussions, J. Hurt for technical assistance, and R. White for providing HUVECs. This work was supported by grants from the National Institutes of Health (to B.A.F. and J.S.B.).
ABBREVIATIONS
- NO2Tyr
3-nitrotyrosine
- TTL
tubulin–tyrosine ligase
- FRLF
fetal rat lung fibroblast
- FRLE-TII
fetal rat lung type-II epithelial cell
- HUVEC
human umbilical vein endothelial cell
- DHC
dynein heavy chain
- DIC
dynein intermediate chain
- Tyr-Tub
tyrosinated tubulin
- CPA
carboxypeptidase A
- SUB
subtilisin
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Michel T, Feron O. J Clin Invest. 1997;100:2146–2152. doi: 10.1172/JCI119750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kröncke K-D, Fehsel K, Kolb-Bachofen V. Clin Exp Immunol. 1998;113:147–156. doi: 10.1046/j.1365-2249.1998.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beckman J S. Chem Res Toxicol. 1996;9:836–844. doi: 10.1021/tx9501445. [DOI] [PubMed] [Google Scholar]
- 4.Ohshima H, Friesen M, Brouet I, Bartsch H. Food Chem Toxicol. 1990;28:647–652. doi: 10.1016/0278-6915(90)90173-k. [DOI] [PubMed] [Google Scholar]
- 5.Ischiropoulos H. Arch Biochem Biophys. 1998;356:1–11. doi: 10.1006/abbi.1998.0755. [DOI] [PubMed] [Google Scholar]
- 6.Eiserich J P, Cross C E, Jones A D, Halliwell B, van der Vliet A. J Biol Chem. 1996;271:19199–19208. doi: 10.1074/jbc.271.32.19199. [DOI] [PubMed] [Google Scholar]
- 7.van der Vliet A, Eiserich J P, Halliwell B, Cross C E. J Biol Chem. 1997;272:7617–7625. doi: 10.1074/jbc.272.12.7617. [DOI] [PubMed] [Google Scholar]
- 8.Eiserich J P, Hristova M, Cross C E, Jones A D, Freeman B A, Halliwell B, van der Vliet A. Nature (London) 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- 9.Sampson J B, Ye Y Z, Rosen H, Beckman J S. Arch Biochem Biophys. 1998;356:207–213. doi: 10.1006/abbi.1998.0772. [DOI] [PubMed] [Google Scholar]
- 10.Gunther M R, Hsi L C, Curtis J F, Gierse J K, Marnett L J, Eling T E, Mason R P. J Biol Chem. 1997;272:17086–17090. doi: 10.1074/jbc.272.27.17086. [DOI] [PubMed] [Google Scholar]
- 11.Knowles M E, McWeeny D J, Couchman L, Thorogood M. Nature (London) 1974;247:288–289. doi: 10.1038/247288a0. [DOI] [PubMed] [Google Scholar]
- 12.MacMillan-Crow L A, Crow J P, Thompson J A. Biochemistry. 1998;37:1613–1622. doi: 10.1021/bi971894b. [DOI] [PubMed] [Google Scholar]
- 13.Haddad I Y, Zhu S, Ischiropoulos H, Matalon S. Am J Physiol. 1996;270:L281–L288. doi: 10.1152/ajplung.1996.270.2.L281. [DOI] [PubMed] [Google Scholar]
- 14.Zhou M, Martin C, Ullrich V. Biol Chem. 1997;378:707–713. doi: 10.1515/bchm.1997.378.7.707. [DOI] [PubMed] [Google Scholar]
- 15.Crow J P, Ye Y Z, Strong M, Kirk M, Barnes S, Beckman J S. J Neurochem. 1997;69:1945–1953. doi: 10.1046/j.1471-4159.1997.69051945.x. [DOI] [PubMed] [Google Scholar]
- 16.Kong S K, Yim M B, Stadtman E R, Chock P B. Proc Natl Acad Sci USA. 1996;93:3377–3382. doi: 10.1073/pnas.93.8.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ara J, Przedborski S, Naini A B, Jackson-Lewis V, Trifiletti R R, Horwitz J, Ischiropoulos H. Proc Natl Acad Sci USA. 1998;95:7659–7663. doi: 10.1073/pnas.95.13.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacMillan-Crow L A, Crow J P, Kerby J D, Beckman J S, Thompson J A. Proc Natl Acad Sci USA. 1996;93:11853–11858. doi: 10.1073/pnas.93.21.11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kooy N W, Lewis S J. Eur J Pharmacol. 1996;310:155–161. doi: 10.1016/0014-2999(96)00376-7. [DOI] [PubMed] [Google Scholar]
- 20.Kamisaki Y, Wada K, Ataka M, Yamada Y, Nakamoto K, Ashida K, Kishimoto Y. Biochim Biophys Acta. 1997;1362:24–28. doi: 10.1016/s0925-4439(97)00052-5. [DOI] [PubMed] [Google Scholar]
- 21.Riccardi V M, Maragos V A. In Vitro. 1980;16:706–714. doi: 10.1007/BF02619200. [DOI] [PubMed] [Google Scholar]
- 22.MacLean S J, Huber R E. Cancer Res. 1971;31:1669–1672. [PubMed] [Google Scholar]
- 23.Kaur H, Halliwell B. FEBS Lett. 1994;350:9–12. doi: 10.1016/0014-5793(94)00722-5. [DOI] [PubMed] [Google Scholar]
- 24.Skinner K A, Crow J P, Skinner H B, Chandler R T, Thompson J A, Parks D A. Arch Biochem Biophys. 1997;342:282–288. doi: 10.1006/abbi.1997.0114. [DOI] [PubMed] [Google Scholar]
- 25.Fukuyama N, Takebayashi Y, Hida M, Ishida H, Ichimori K, Nakazawa H. Free Radical Biol Med. 1997;22:771–774. doi: 10.1016/s0891-5849(96)00401-7. [DOI] [PubMed] [Google Scholar]
- 26.Beal M F, Ferrante R J, Browne S E, Matthews R T, Kowall N W, Brown R H., Jr Ann Neurol. 1997;42:644–654. doi: 10.1002/ana.410420416. [DOI] [PubMed] [Google Scholar]
- 27.Caniggia I, Tseu I, Han R N N, Smith B T, Tanswell K, Post M. Am J Physiol. 1991;261:L424–L433. doi: 10.1152/ajplung.1991.261.6.L424. [DOI] [PubMed] [Google Scholar]
- 28.Brock T A, Capasso E A. J Cell Physiol. 1988;136:54–62. doi: 10.1002/jcp.1041360107. [DOI] [PubMed] [Google Scholar]
- 29.Ye Y Z, Strong M, Huang Z O, Beckman J S. Methods Enzymol. 1996;269:201–209. doi: 10.1016/s0076-6879(96)69022-3. [DOI] [PubMed] [Google Scholar]
- 30.Lin S X H, Ferro K L, Collins C A. J Cell Biol. 1994;127:1009–1019. doi: 10.1083/jcb.127.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacRae T H. Eur J Biochem. 1997;244:265–278. doi: 10.1111/j.1432-1033.1997.00265.x. [DOI] [PubMed] [Google Scholar]
- 32.Argaraña C E, Barra H S, Caputto R. Mol Cell Biochem. 1978;19:17–21. doi: 10.1007/BF00231230. [DOI] [PubMed] [Google Scholar]
- 33.Ersfeld K, Wehland J, Plessmann U, Dodemont H, Gerke V, Weber K. J Cell Biol. 1993;120:725–732. doi: 10.1083/jcb.120.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gundersen G, Kalnoski M H, Bulinski J C. Cell. 1984;38:779–789. doi: 10.1016/0092-8674(84)90273-3. [DOI] [PubMed] [Google Scholar]
- 35.Liao G, Gundersen G G. J Biol Chem. 1998;273:9797–9803. doi: 10.1074/jbc.273.16.9797. [DOI] [PubMed] [Google Scholar]
- 36.Raybin D, Flavin M. J Cell Biol. 1977;73:492–504. doi: 10.1083/jcb.73.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rüdiger A, Rüdiger M, Weber K, Schomburg D. Anal Biochem. 1995;224:532–537. doi: 10.1006/abio.1995.1083. [DOI] [PubMed] [Google Scholar]
- 38.Webster D R, Gundersen G G, Bulinski J C, Borisy G G. J Cell Biol. 1987;105:265–276. doi: 10.1083/jcb.105.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Breitling F, Little M. J Mol Biol. 1986;189:367–370. doi: 10.1016/0022-2836(86)90517-6. [DOI] [PubMed] [Google Scholar]
- 40.Webster D R, Modesti N M, Bulinski J C. Biochemistry. 1992;31:5849–5856. doi: 10.1021/bi00140a021. [DOI] [PubMed] [Google Scholar]
- 41.Gundersen G G, Bulinski J C. Proc Natl Acad Sci USA. 1988;85:5946–5950. doi: 10.1073/pnas.85.16.5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arregui C, Barra H S. J Neurochem. 1989;52:1708–1713. doi: 10.1111/j.1471-4159.1989.tb07248.x. [DOI] [PubMed] [Google Scholar]
- 43.Li J N, Bjork G R. J Bacteriol. 1995;177:6593–6600. doi: 10.1128/jb.177.22.6593-6600.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hagiwara H, Yorifuji H, Sato-Yoshitake R, Hirokawa N. J Biol Chem. 1994;269:3581–3589. [PubMed] [Google Scholar]
- 45.Paschal B M, Obar R A, Vallee R B. Nature (London) 1989;342:569–572. doi: 10.1038/342569a0. [DOI] [PubMed] [Google Scholar]
- 46.Liang P, McRae T H. J Cell Sci. 1997;110:1431–1440. doi: 10.1242/jcs.110.13.1431. [DOI] [PubMed] [Google Scholar]
- 47.Gee M A, Heuser J E, Vallee R B. Nature (London) 1997;390:636–639. doi: 10.1038/37663. [DOI] [PubMed] [Google Scholar]
- 48.Hirokawa N. Science. 1998;279:519–526. doi: 10.1126/science.279.5350.519. [DOI] [PubMed] [Google Scholar]
- 49.Kumar N, Flavin M. Eur J Biochem. 1982;128:215–222. doi: 10.1111/j.1432-1033.1982.tb06954.x. [DOI] [PubMed] [Google Scholar]
- 50.Joniau M, Coudijzer K, De Cuyper M. Anal Biochem. 1990;184:325–329. doi: 10.1016/0003-2697(90)90689-7. [DOI] [PubMed] [Google Scholar]
- 51.Monasterio O, Nova E, López-Brauet A, Lagos R. FEBS Lett. 1995;374:165–168. doi: 10.1016/0014-5793(95)01099-z. [DOI] [PubMed] [Google Scholar]
- 52.Arce C A, Rodriguez J A, Barra H S, Caputto R. Eur J Biochem. 1975;59:145–149. doi: 10.1111/j.1432-1033.1975.tb02435.x. [DOI] [PubMed] [Google Scholar]
- 53.Domigan N M, Charlton T S, Duncan M W, Winterbourn C C, Kettle A J. J Biol Chem. 1995;270:16542–16548. doi: 10.1074/jbc.270.28.16542. [DOI] [PubMed] [Google Scholar]
- 54.Hazen S L, Heinecke J W. J Clin Invest. 1997;99:2075–2081. doi: 10.1172/JCI119379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu W, Chen Y, d’Avignon A, Hazen S L. Biochemistry. 1999;38:3538–3548. doi: 10.1021/bi982401l. [DOI] [PubMed] [Google Scholar]
- 56.Boota A, Zar H, Kim Y-M, Johnson B, Pitt B, Davies P. Am J Physiol. 1996;271:L932–L938. doi: 10.1152/ajplung.1996.271.6.L932. [DOI] [PubMed] [Google Scholar]