Abstract
Aging is associated with declines in episodic memory. In this study, the authors used a path analysis framework to explore the mediating role of differences in brain structure, executive functions, and processing speed in age-related differences in episodic memory. Measures of regional brain volume (prefrontal gray and white matter, caudate, hippocampus, visual cortex), executive functions (working memory, inhibitory control, task switching, temporal processing), processing speed, and episodic memory were obtained in a sample of young and older adults. As expected, age was linked to reduction in regional brain volumes and cognitive performance. Moreover, neural and cognitive factors completely mediated age differences in episodic memory. Whereas hippocampal shrinkage directly affected episodic memory, prefrontal volumetric reductions influenced episodic memory via limitations in working memory and inhibitory control. Age-related slowing predicted reduced efficiency in temporal processing, working memory, and inhibitory control. Lastly, poorer temporal processing directly affected episodic memory. No direct effects of age on episodic memory remained once these factors were taken into account. These analyses highlight the value of a multivariate approach with the understanding of complex relationships in cognitive and brain aging.
Keywords: MRI, brain volumetry, brain aging, hippocampus, prefrontal
Advanced age is associated with significantly reduced performance on episodic memory tasks, such as list recall, paired-associate learning, and prose recall. Age differences in recall and recognition are observed in laboratory and naturalistic settings, and in cross-sectional and longitudinal studies (see Balota, Dolan, & Duchek, 2000; Prull, Gabrieli, & Bunge, 2000; Verhaeghen, Marcoen, & Goosens, 1993 for reviews). However, the mechanisms that mediate age-related declines in episodic memory are not well understood.
As aging is associated with significant deterioration of the brain (see Raz & Rodrigue, 2006 for a review), age-related weakening of putative neural substrates of memory may underlie the observed differences in cognitive performance. Although multiple regions of the brain may be involved in memory-related operations, frontostriatal and medial–temporal circuits are viewed as especially important in encoding, maintenance, and retrieval of information (Gabrieli, 1998; Moscovitch, Nadel, Winocur, Gilboa, & Rosenbaum, 2006; Squire, 1987, 1992; see Milner, 2005; Squire, Stark, & Clark, 2004; Blumenfeld & Ranganath, 2007 for reviews). As it happens, the prefrontal cortex, the caudate nucleus, and the hippocampus are particularly vulnerable to advancing age (Raz et al., 2005; Raz & Rodrigue, 2006; but see Sullivan, Marsh, & Pfefferbaum, 2005 for discussion of possible hippocampal stability in aging). Furthermore, age-related differences and longitudinal declines in white matter volume and microstructural integrity tend to be greater in anterior than posterior regions (Bartzokis et al., 2003; Head, Buckner, et al., 2004, Head, Synder, et al., 2005; Jernigan et al., 2001; Kochunov et al., 2007; O'Sullivan et al., 2001; Pfefferbaum, Sullivan, Hedehus, Lim, & Adalsteinsson, 2000, Pfefferbaum, Adalsteinsson, & Sullivan, 2005; Raz et al., 2005; Yoon et al., 2007). Thus, it is plausible that deterioration of the frontal–striatal and hippocampal circuits contribute to age-induced memory difficulties.
Establishing the links between brain structure and memory performance is complicated by the fact that memory is not a unitary construct and mnemonic performance relies on multiple cognitive processes, such as working memory and other executive control functions (Blumenfeld & Ranganath, 2007; Knowlton & Squire, 1995; Milner & Petrides, 1984; Moscovitch & Winocur, 2002; Schacter, 1987; Shimamura, Janowskey, & Squire, 1987; Squire et al., 1993; Tremont, Halpert, Javorsky, & Stern, 2000; Vanderploeg, Schinka, & Retzlaff, 1994). Executive control functions, which are believed to rely on the prefrontal circuits (Baddeley, 1986; Burgess & Shallice, 1996; Duncan & Owen, 2000; Fuster, 2001; Norman & Shallice, 1986; Stuss & Benson, 1986), are commonly conceptualized as domain-general control processes that monitor and regulate other cognitive processes to guide the attainment of future goals. A broad range of cognitive processes that fall under the rubric of “executive functions” include working memory, inhibition, temporal processing, attention switching, and planning (Miyake, Friedman, Emerson, Witzki, & Howerter, 2000; Monsell, 1996; Pennington, Bennetto, McAleer, & Roberts, 1996; see Eslinger, 1996; Heyder, Suchan, & Daum, 2004; Kane & Engle, 2002 for reviews). Advancing age is also associated with declines in inhibitory mechanisms (Dempster, 1992; Hasher & Zacks, 1988; Persad, Abeles, Zacks, & Denburg, 2002; but see McDowd, 1997; Verhaeghen & DeMeersman, 1998), working memory (Salthouse, 1990, 1994; Verhaeghen et al., 1993; see Park & Payer, 2006; Reuter-Lorenz & Sylvester, 2005 for reviews), temporal processing (Cabeza, Anderson, Houle, Mangels, & Nyberg, 2000; Dumas & Hartman, 2003; Fabiani & Friedman, 1997; Kausler & Wiley, 1990; Vakil et al., 1997), and task switching (Kramer, Hahn, & Gopher, 1999; Kray, 2006; Mayr, 2001; Salthouse, Fristoe, McGuthry, & Hambrick, 1998; see Verhaeghen & Cerella, 2002 for a review).
Age-related decline in executive control functions can have a broad impact on behavior and cognition, and specifically on episodic memory (Bryan, Luszcz, & Pointer, 1999; Buckner, 2004; Crawford, Bryan, Luszcz, Obonsawin, & Stewart, 2000; Ferrer-Caja, Crawford, & Bryan, 2002; Moscovitch & Winocur, 1995; Parkin & Java, 2000; Perfect, 1997; Salthouse, Atkinson, Berish, 2003; Troyer, Graves, & Cullum 1994; West, 1996). The literature on age-related differences in memory suggests several candidate executive functions as mediators of age-related differences in memory. These include working memory (Salthouse, 1990, 1994), inhibition (Dempster, 1992; Earles et al., 1997; Hasher & Zacks, 1988; Zacks & Hasher, 1994), temporal processing (Fabiani & Friedman, 1997; Dumas & Hartman, 2003), and the ability to switch between two or more tasks (Hogan, Kelly, & Craik, 2006; Salthouse et al., 1998). As an alternative to multifactorial executive control mechanisms of age-related reductions in memory performance, a general factor view holds that a pervasive reduction in processing speed may provide a sufficient explanation (Finkel, Reynolds, McArde, & Pedersen, 2007; Fisk & Warr, 1996; Salthouse, 1985, 1996, 2000).
Converging evidence indicates that prefrontal lesions that impair executive functions result in adverse effects on the strategic components of encoding and retrieval of memories (Alexander, Stuss, Picton, Shallice, & Gillingham, 2007; Butters, Kaszniak, Glisky, Eslinger, & Schacter, 1994; Dimitrov et al., 1999; Stuss, Alexander, & Palumbo, 1994; Wheeler, Stuss, & Tulving, 1995; see Blumenfeld & Ranganath, 2007 for a review). Furthermore, hippocampal damage is associated with impairments in memory (e.g., Zola-Morgan, Squire, & Amaral, 1986; see Milner, 2005 for a review). Thus, it is highly plausible that age-related declines in the prefrontal cortex and the hippocampus would predict declines in executive and mnemonic functions. Nonetheless, to date, the attempts to link age-related declines in executive functions and memory to regional brain shrinkage yield mixed results. Some studies revealed associations between structural integrity and cognitive performance (e.g., Head & Raz, 2002), whereas others produced contradicting results (Salat, Kaye, & Janowsky, 2002; Van Petten et al., 2004; see Van Petten, 2004 for review of episodic memory and hippocampal relationships). Although age-related declines in processing speed have been associated with deterioration of the prefrontal cortex (Raz, Briggs, Marks, & Acker, 1999) and cerebral white matter (Gunning-Dixon & Raz, 2000; Madden et al., 2004; Rabbitt et al., 2007a, Rabbit et al., 2007b), the investigations of the relationships between brain structure, memory, and executive functions tend to ignore the indices of speed (but see Bucur et al., 2007; Rabbitt et al., 2007b).
The primary goal of this study was to establish whether age-related differences in episodic memory could be explained by age-related deterioration of the relevant brain structures and declines in cognitive operations that are considered more basic than episodic memory, that is, speed of processing and various executive functions. To that end, we measured regional brain volumes and multiple indicators of cognitive performance. We fit a series of competing models to the data and examined the roles of hypothesized mediators of age-related differences in episodic memory. The competing models were evaluated hierarchically within a path analysis framework.
Construction of classic statistical models at any level of complexity is driven by theoretical reasoning, and we present our reasons for model construction below. In the models employed in this study, the main objective was to partition and explain the age-related variance in episodic memory performance. Therefore, calendar age was presumed the origin of the path diagram, a variable measured without error and not influenced by any other variable in the model. The effects of age on episodic memory were presumed to be mediated by differences in processing speed and four executive function processes—working memory, inhibition, temporal processing, and task switching—which in turn were mediated by age-related differences in regional brain volumes of the hippocampus, the caudate nucleus, the prefrontal cortex, and prefrontal white matter. To test the specificity of the hypothesized influences of brain variables on cognition, we selected the primary visual cortex as a control region that was not expected to be associated with age differences in the cognitive processes examined in the study. The hypothesized flow of variance in the models was predicated on the assumptions that (1) age affects brain and cognitive variables without being affected by them; (2) regional brain volumes affect cognitive performance without reciprocal influence; (3) processing speed, working memory, and inhibitory control are more “basic” indices than selected executive functions such as task-switching and temporal processing measures; and, (4) performance on the episodic memory tasks is affected by all of the preceding variables without influencing them.
Although it is theoretically possible to reduce the components of any cognitive activity to progressively restricted cognitive (and neural) “atoms,” practicality requires settling on a reasonably molar level of conceptualization. Processing speed, working memory, and inhibitory control were considered “cognitive primitives” or fundamental processes that have a broad influence on cognitive function, but are, at the selected level of discourse, not divisible into component processes (Salthouse, 1985, 1996; Verhaeghen & Salthouse, 1997; Zacks & Hasher, 1994). While task switching has been proposed as a cognitive primitive and has been found to account for some age-related differences in episodic memory, much of its variance is shared with other variables such as processing speed (Salthouse et al., 1998). In addition, temporal processing has the potential of influencing a wide range of cognitive processes (Butters et al., 1994; Milner & Petrides, 1984; Milner, Corsi, & Leonard, 1991), but its role as a cognitive primitive is perhaps less well established than that of processing speed, working memory, or inhibitory control. Processing speed and inhibitory control are hypothesized to control the flow of information through the working memory system (Salthouse, 1985, 1996; Zacks & Hasher, 1994). In addition, processing speed has previously been found to mediate age differences in inhibition (Earles et al., 1997). Thus, we hypothesized that processing speed and inhibition would mediate age differences in working memory and that processing speed would limit inhibitory control. While it is conceivable that some aspects of episodic memory, such as consolidation and maintenance of information across time, may influence other cognitive variables in the models, our primary goal was to examine factors that contribute to age-related variation in scores on episodic memory tests, that is, measures of memory performance that reflect all mnemonic processes. As the classic path analysis approach does not permit reciprocal causation (Pedhazur, 1997), we limited our analyses to examining unidirectional flow of variance.
Materials and Method
Participants
Healthy young and older adults were recruited from two major U. S. metropolitan areas, Memphis, Tennessee and Detroit, Michigan, through advertisement in the local media. Young adults in Detroit also included undergraduates from Wayne State University. All participants were screened by telephone interview and a health questionnaire, for history of head trauma with unconsciousness of more than 10 minutes, neurological and cardiovascular conditions, history of drug or alcohol abuse, hypertension, and significant vision or hearing problems, as well as for medications (stimulant or depressive medications) that could affect reaction time. Participants were also screened for gross cognitive status and depression using the Mini-Mental Status Exam (MMSE, Folstein et al., 1975) and the Center for Epidemiological Studies-Depression Scale (Radloff, 1977) with cut-offs of 26 and 15, respectively. Participants were right-handed (75% and above on the Edinburgh Handedness Questionnaire; Oldfield, 1971) and native English speakers. All participants provided written, informed consent.
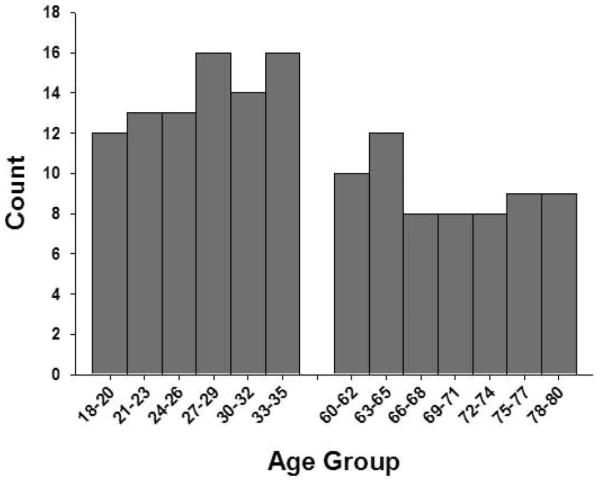
The Memphis sample consisted of 19 young (19–33 years of age) adults and 15 older (61–77 years) adults. The Detroit sample was comprised of 51 young (18–35 years) adults and 32 older (60–80 years) adults. The young adult sample from Memphis had more years of formal education (t = 3.54, p < .01) and higher vocabulary scores (t = 3.00, p < .01) than the young adult sample from Detroit. There were no significant differences between the older adult Memphis and Detroit samples. Thus, a total of 117 healthy adults participated in the study. The demographic characteristics of the sample are presented in Table 1 and the age distribution is presented in Figure 1.
Table 1.
Sample Description
| Memphis |
Detroit |
Sample comparison (t-test) |
||||||
|---|---|---|---|---|---|---|---|---|
| YNG | OLD | t -test | YNG | OLD | t -test | YNG | OLD | |
| N | 19 | 15 | 51 | 32 | ||||
| % Female | 68 | 60 | 76 | 69 | ||||
| Age - mean (std) | 27 (4) | 68 (4) | 25 (5) | 67 (6) | 1.40 | .24 | ||
| Age - skew | −.25 | .63 | .52 | .37 | ||||
| Age - kurtosis | .02 | .06 | −.89 | −.73 | ||||
| Education - mean (std) | 17.2 (2.3) | 14.3 (2.3) | 3.55** | 15.3 (2.0) | 15.2 (2.9) | .15 | 3.25** | 1.08 |
| MMSE - mean (std) | 29.4 (.83) | 28.9 (1.5) | 1.27 | 28.7 (1.0) | 28.3 (1.3) | 1.62 | 2.46* | 1.32 |
| CFIT | 29.6 (3.7) | 21.0 (3.6) | 6.85*** | 28.7 (4.4) | 22.8 (4.1) | 6.17*** | .85 | 1.37 |
| ETS vocab - mean (std) | 24.4 (7.8) | 24.7 (9.9) | .10 | 19.0 (6.2) | 28.1 (6.3) | 6.47*** | 3.00** | 1.45 |
Note. MMSE = Mini-Mental Status Exam; CES-D = Center for Epidemiological Studies-Depression Scale; CFIT = Cattell Fluid Intelligence Test; ETS Vocab = Educational Testing Service-Vocabulary Subtest.
p < .05;
p < .01;
p < .001
Figure 1.
Age distribution of the sample.
Cognitive Testing
Verbal working memory tasks
In Computation Span (Salthouse, Mitchell, Skovronek, & Babcock, 1989), the participant is required to solve simple arithmetic problems while simultaneously remembering the last digit of each problem with the number of items progressing from one to seven across trials. The index of performance was the sum of the number of correct items across blocks of trials on which the participant answered all items correctly. The estimated test-retest reliability of this measure is .90 (Salthouse et al., 1989). For the N-back task (Dobbs & Rule, 1989; Hultsch, Hertzog, & Dixon, 1990) participants were sequentially presented with a randomly ordered string of digits (0 to 9), with the length varying from five to nine digits, and were required to report the digit in the nth position after the full string was presented. There were three blocks of trials and for each block, participants reported either the digit just seen (0-back), the digit that was 1-back in the sequence or the digit 2-back in the sequence. The index of performance was proportion correct in the 2-back condition, as the other conditions evidenced ceiling effects. The estimated test-retest reliability (using the Spearman-Brown formula) for this task is .91 (Salthouse, Hancock, Meinz, & Hambrick, 1996).
Nonverbal working memory tasks
In the modified Size Judgment Span task (Cherry & Park, 1993) participants listened to a list of objects or animals orally presented and were asked to reorder them according to their size from smallest to largest with the number of items progressing from two to seven. The sum of the number of correct trials was the index of performance. The estimated test-retest reliability of this task is .79 (Cherry & Park, 1993). The procedure for the nonverbal N-back task (Dobbs & Rule, 1989; Hultsch et al., 1990) was the same as for the verbal version except the stimuli were from Kroll and Potter (Kroll & Potter, 1984). In addition, the test phase consisted of a screen displaying all nine objects paired with digits and participants indicated the object in the nth position. The index of performance was proportion-correct in the 1-back condition, as performance in the 0-back condition evidenced ceiling effects and performance in the 2-back condition evidenced floor effects. The estimated test-rest reliability (using the Spearman-Brown formula) for this task is .88 (Salthouse et al., 1996).
Inhibitory control tasks
Three tasks were used to measure inhibitory processes. The stop-signal task was taken from the work of Kramer, Humphrey, Larish, Logan, & Strayer (1994). The first phase was 50 trials of simple reaction time (SRT) to a tone. The second phase was a visual choice reaction time (CRT) that required discriminating between F and E and consisted of 70 trials. In the stopping task, participants performed the CRT task except when a tone occurred (30% of the trials) indicating that the response should be inhibited. There were three blocks of 120 experimental trials of the stopping task. The score from the stop-signal task is the stop-signal reaction time, which provides an estimate of the ability to inhibit an overt response. Stop-signal reaction time is estimated from the distribution of the observed go signal RT and the probability of responding given a stop signal (see Logan, 1994 for a detailed description). The estimated internal consistency (Cronbach's alpha) for this task is .88 (Nigg, 1999).
Two versions of the Stroop test (Salthouse & Meinz, 1995) were also administered. For the color version, participants read color words presented in compatible ink (compatible condition), named the color of the ink in which X's were printed (neutral condition), and named the color of the ink in which color words were printed in an incompatible color (incompatible condition). For the position version, participants read the position of position words (e.g., above, below) that were in a compatible position relative to a line (compatible condition), reported the position of X's relative to a line (neutral condition), and reported the position of position words that were in an incompatible position (incompatible condition). The interference score for both of these tasks was the residual performance on the incompatible condition after regressing out performance on the neutral condition. The estimated split-half reliability is .72 for the color task and .70 for the position task (Salthouse & Meinz, 1995).
Switching tasks
Two measures of task switching efficiency were obtained. These tasks were modified from Salthouse et al. (Salthouse et al., 1998). For one task, participants were presented with two digits simultaneously and required to switch between indicating the digit appearing on the right and the digit appearing on the left. For the second switch task, one digit appeared in the center of the screen and the participants were required to switch between reporting whether the digit was more or less than five and whether the digit was odd or even. There was one block with 50 trials of each component task. Next, two blocks of switch trials were administered with each containing 124 trials with 24 switches (every 5th trial) in each block. The estimate of global switch costs for both of these tasks was the residual performance on the switch block after regressing out performance on the nonswitch blocks. The estimated split-half reliability (Spearman-Brown formula) was .81 for the right-left task and .72 for the even-odd task.
Temporal processing tasks
Participants were administered a verbal and nonverbal version of a temporal order memory task. The nonverbal stimuli were selected from the Vanderplas figures normed set (Vanderplas & Garvin, 1959). We selected figures with low associations with identifiable objects (association values were ≤28; Vanderplas & Garvin, 1959). The word stimuli were selected from Kucera and Frances (Kucera & Frances, 1967) with word frequencies ranging from 10–140 per million. During the study phases of the verbal and nonverbal temporal order memory tasks, participants were presented with 16 words or 12 figures, respectively. The stimuli were sequentially presented two consecutive times (Vakil, Blachstein, & Hoofien, 1991). During the test phase, which immediately followed the study phase, the stimuli were presented on the screen simultaneously and participants used the mouse to place the stimuli in the same order as presented in the study phase. The score on these tasks was the average Spearman rank-order correlation between the correct order and the participant's reconstruction of the order across four study-test trials. The internal consistency (Cronbach's alpha) of this task was .76 for the verbal version and .70 for the nonverbal version.
Participants were also administered a verbal and nonverbal version of a recency judgment task (Fabiani & Friedman, 1997; Milner, McAndrews, & Leonard, 1990). The word stimuli for the verbal task were selected from Kucera and Frances (1967) with word frequencies ranging from 10–140 per million. The nonverbal stimuli were 300 line patterns modified from Musen and Triesman (1990) and rated on how closely the pattern resembled any familiar object (Gunning-Dixon, 1998). Participants were presented with a series of 63 study trials, consisting of a word (or a line pattern), one at a time on a computer screen. On Trials 64 to 387, test trials were intermixed with the study trials. On test trials, two stimuli were presented and the participant was required to indicate which stimulus was presented most recently. On recency test trials, the two stimuli were both previously presented at different lags (e.g., 4 vs. 8 items back, 32 vs. 64, etc.). For recognition test trials, one of the stimuli was new and one was previously presented. In the nonverbal version, there were 64 recency test trials and 30 recognition trials. In the verbal version, there were 70 recency test trials and 40 recognition trials. Proportion correct was the index of performance for both the verbal and nonverbal versions of the task. The estimated reliabilities (using the Kuder-Richardson formula 20) for the verbal and nonverbal recency judgment tasks were .93 and .87, respectively.
Processing speed tasks
Paper-and-pencil tasks requiring participants to make rapid judgments about whether or not two sets of stimuli are the same or different served as measures of processing speed (Salthouse & Meinz, 1995). The letter comparison task consisted of pairs of letter strings and the pattern comparison task consisted of pairs of line patterns. Estimated test-retest reliability (using Spearman-Brown formula) for letter comparison is .80 and .82 for pattern comparison (Salthouse et al., 1996). The third processing speed measure was the simple reaction time component of the stop-signal task with an estimated split-half reliability of .91.
Verbal episodic memory tasks
On the California Verbal Learning Test (CLVT, The Psychological Corporation, San Antonio, Texas) participants were orally presented with a list of 16 categorizable grocery items, five times. Proportion of the 16 words correctly recalled on the delayed free recall of the list was the index of performance, which has an estimated test-retest reliability of .92. The Woodcock-Johanson Revised (WJ-R) Memory for Names subtest is a visual-auditory paired associates task. During the acquisition phase, participants learned to associate a picture of an imaginary “space creature” with the creature's name. In the delayed test phase participants viewed pages with several space creatures and were asked to point to creatures named by the examiner. The index of performance was the proportion of correct responses at delayed testing. The estimated split-half reliability (using the Spearman-Brown formula) of this measure is .91 (Woodcock & Johnson, 1989).
Nonverbal episodic memory tasks
In each trial of the spatial recall task (Salthouse, 1994) participants first viewed 5 × 5 matrices with seven target square locations for 3 seconds. For the test phase, participants were provided with test sheets containing a series of 5 × 5 matrices and asked to place an “X” in the location of the target squares. The average number of correct items across trials was the index of performance for this test. The estimated test-retest reliability of this task is .67 (Salthouse, 1994). In the Buildings Memory test (Ekstrom, French, Harman, & Dermen, 1976), the participant first studies a map of a fictitious urban location with landmark buildings on it and during the study phase participants were shown a blank map and tested on their memory for the location of the landmarks in a multiple-choice format. The estimated reliability is .80 (Ekstrom et al., 1976).
MRI Protocol - Memphis Sample
Imaging was performed on a 1.5T Signa scanner (General Electric Co., Milwaukee, Wisconsin) at Baptist Memorial Hospital-East, in Memphis, TN. The protocol is described in detail in our previous publications (Head & Raz, 2002). All volumetric measures were performed on the reformatted images acquired using a T1-weighted 3-D spoiled gradient recalled acquisition sequence (SPGR) with 124 contiguous axial slices, time for echo (TE) = 5 ms, and time for repetition (TR) = 24 ms, field of view (FOV) = 22 cm, acquisition matrix 256 × 192, slice thickness = 1.3 mm, and flip angle = 30°. In addition, a set of interleaved proton-density/T2-weighted axial images was acquired with a Fast Spin Echo (FSE) sequence with 18 to 20 contiguous axial slices, TE = 90 ms, TR = 3300, FOV = 20 × 20 cm, matrix 256 × 256, and slice thickness = 5.0 mm. These images were used to identify potential cerebrovascular pathology.
MRI Protocol – Detroit Sample
Imaging was performed on a 1.5T Signa scanner (General Electric Co, Milwaukee, Wisconsin) at Children's Hospital of Michigan in Detroit, Michigan. The acquisition sequence was identical to the one used in Memphis, that is, a T1-weighted 3-D SPGR with 124 contiguous axial slices, TE = 5 ms, TR = 24 ms, FOV = 22 cm, acquisition matrix 256 × 192, slice thickness = 1.3 mm, and flip angle = 30°.
MRI Image Processing
All images were processed on the same workstation by the same operators. They were reformatted to correct for undesirable effects of head tilt, pitch, and rotation, with BrainImage 2.3.3 software. The procedure is described in detail elsewhere (Head & Raz, 2002). The images acquired at each site (Memphis vs. Detroit) were coded and the operators who traced the regions of interest (ROIs) were blind to the site of acquisition of the specific images and the demographic characteristics of the participants. Reliability of ROI measures (intraclass correlation for random raters) (Shrout & Fleiss, 1979) exceeded .90.
Volumetry: Region Selection, Demarcation, and Tracing
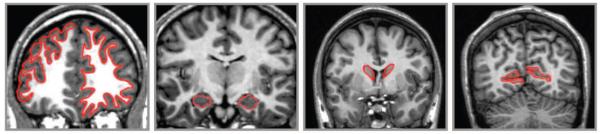
The rules of demarcation and tracing of brain regions obtained with National Institutes of Health Image software (Version 1.62) are described in detail in previous publications (Head & Raz, 2002) and will be presented in condensed form. Illustrations of the traced ROIs are presented in Figure 2. We use the sum of the left and right hemisphere in statistical analyses.
Figure 2.
Examples of region demarcations. (A) Lateral prefrontal cortex in the left hemisphere and white matter in the right hemisphere. (B) Hippocampus. (C) Caudate nucleus. (D) Visual cortex.
Lateral prefrontal cortex
Lateral prefrontal cortex was measured on the 8–12 coronal slices located within the posterior 40% of the distance between the genu of the corpus callosum and the frontal pole.
Prefrontal cortex white matter
The range is identical to lateral prefrontal cortex above and also includes orbital frontal white. The volume includes all white matter on the coronal slice, excluding ventricles and other cerebrospinal fluid spaces.
Caudate nucleus
The volume of the head and the body of the caudate were estimated from 15–20 coronal slices. The most rostral slice was the one on which the caudate first appeared; usually lateral to the lateral ventricles. On the one to three sections on which the nucleus accumbens was visible, a diagonal line was drawn from the most inferior tip of the internal capsule to the lateral ventricle. The caudate was traced on every other slice (interslice distance 3 mm) until no longer visible.
Hippocampus
Hippocampal volume was measured on continuous slices aligned perpendicular to the long axis of the hippocampus between the mammillary bodies and the slice showing the fornices rising from the fimbria. The hippocampus included sectors CA1-CA4, the dentate gyrus, and the subiculum.
Visual cortex
The visual cortex was estimated as the volume of the cortical ribbon lining the calcarine sulcus. The inferior and superior boundaries of this ROI were defined as the point at which the opening of the sulcus occurred. At this point a line was drawn horizontally so that no cortex (dorsal or ventral) outside of the calcarine sulcus was included, confining this ROI to area 17.
Intracranial volume (ICV)
The ICV was estimated from the coronal sections and consisted of all contents within the cranial cavity, including the brain stem and cerebellum. The operator traced ICV on every eight slice (a total of 11–12 slices; 12-mm interslice distance) between the first slice following the orbits and last slice on which brain tissue was visible. Previous work indicates that there is minimal reduction in accuracy and reliability at this sampling density compared to tracing every slice (Eritaia et al., 2000).
Data Conditioning
MRI Data
Intracranial volume differed between the sexes with men having larger crania (t = 8.64, p < .0001). No age differences were detected between (t<1) or within age groups (young, correlation with age r = .05, ns; old, r =−.14, ns). Therefore, ICV was used to adjust the regional volumes for body size differences. The adjustment was performed on each ROI volume in each hemisphere via a formula based on the analyses of covariance approach: Adjusted volume = raw volume − b × (ICV−mean ICV), where b is the slope of the regression of an ROI volume on ICV. The adjusted volumes were used as dependent variables in the analyses presented below.
Outliers and data transformation
In accord with usual practice with response time (RT) data, all RT trials from the Stop-Signal task, the two task switching tasks and the two Stroop tasks that resulted in incorrect responses were eliminated from analyses (Pachella, 1974). Next, each individual's RT data were examined for outliers. Response times that were more than three standard deviations from the mean were replaced with the value that was three standard deviations from the mean.
A multistep process was used to identify univariate, bivariate, and multivariate outliers at the group level. A Monte Carlo simulation was run to establish cut-off values of an outlier statistic. The statistic is an adjustment to the Mahalanobis distance that reduces the possibility that an outlier hides itself by inflating the means and variances. Values that were beyond the 99th percentile of the statistic were considered outliers. Outlier values were considered missing data.
Missing data were handled by a multiple imputation regression method, which involves replacing each missing value with the combined results of multiple simulated versions of the data thus representing the uncertainty about the right value. The simulated versions are derived from fitting regression models to each variable that has missing values with other variables as covariates. 1.4% of the data was considered missing and included outliers considered as missing and tasks that were not administered due to administration error or participant refusal.
The statistical procedures to be applied to the data assume normally distributed data. A significant violation of the assumption of normality can seriously increase the chances of committing either a Type I or Type II error. The distribution of several measures was skewed and/or kurtotic. Thus, data transformations were performed to normalize the distribution of the data. The specific mathematical modification for each variable was selected based on the nature of the non-normality and the scale of the variable (e.g., count, proportion, etc.). For example, the index of performance for the N-back verbal task was proportion correct so an arcsine transformation was selected. A common logarithmic transformation was applied to the Stroop position data, letter comparison data, frontal white matter volume, and caudate nucleus volume. An arcsine transformation was applied to the N-back verbal and spatial location memory data. A reciprocal of the square root transformation was applied to the right-left task switching data. A reciprocal of the fourth power transformation was applied to the simple reaction time data. Finally, a square transformation was applied to the verbal temporal order memory data. After these transformations the data for most tasks approached normality and evidenced only mild heterogeneity of variance.
Floor effects
For the recency judgment tasks, mean performance for both the younger and older adult groups was relatively low in comparison to chance levels (.50). For the nonverbal version of the task, performance was barely above chance levels (M = .53 for the older and .57 for the younger participants). Thus, the nonverbal recency judgment task was excluded from further analyses.
Data reduction
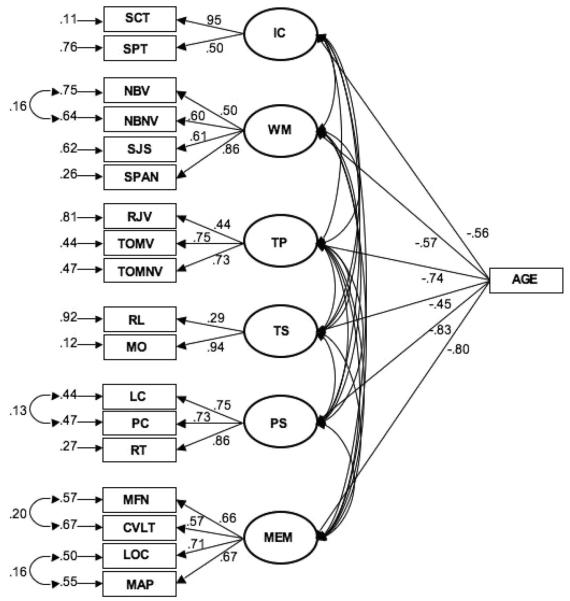
Considering the relatively small sample size in relation to the number of variables, and that we did not have the recommended minimum of three tasks per hypothesized construct, we elected to forgo structural equation modeling. However, to validate that the selected tasks represented the hypothesized constructs, we tested a measurement model with the hypothesized constructs of processing speed, working memory, inhibitory control, temporal processing, task switching, and episodic memory. Each variable was standardized to the entire sample. We then fit a Multiple Indicators, Multiple Indicator Causes (MIMIC) model to the cognitive data, with age as a continuous variable (within each age group). A MIMIC model can be considered an extension of confirmatory factor analysis in a structural equations modeling framework (Jöreskog & Goldberg, 1975; Muthén, 1989). A MIMIC model incorporates both observable indicator variables (i.e., the individual cognitive tasks) and exogenous covariates (i.e., age) to define latent variables (i.e., the cognitive constructs). In our initial model, the factor loading for the stop-signal task was low (.09). Thus, in a hierarchically reduced MIMIC model, the stop-signal task was excluded. The model (see Figure 3 and Table 2) provided a fair fit to the data (χ2 = 148.725, df = 124, p = .07; χ2/df = 1.20; the comparative fit index (CFI) = .975; standardized root-mean-square residual (SRMR) = .048). Next, composites for each construct were derived by summing the scores from each of the component tasks.
Figure 3.
Measurement model for cognitive data with age as a covariate. The circles represent latent variables and the rectangles on the left are the endogenous indicators. Age is a measured covariate with the paths to latent variables representing regression coefficients. Paths from latent variables to individual tasks represent the factor loadings. Arrows to the left of the individual tasks represent error terms. Connected arrows between error terms represent correlated errors between the tasks. See Table 3 for correlations between latent variables. SCT = Stroop color task; SPT = Stroop position task; NBV = N-back verbal; NBNV = N-back nonverbal; SJS = size judgment span; SPAN = computation span; RJV = recency judgment-verbal; TOMV = temporal order memory-verbal; TOMNV = temporal order memory-nonverbal; RL = right-left task switching; MO = more-odd task switching; LC = letter comparison; PC = pattern comparison; RT = simple reaction time; MFN = WJ-R memory for names; CVLT = California Verbal Learning Test; LOC = spatial location recall; MAP = ETS building memory.
Table 2.
Correlations Among Latent Variables in Measurement Model
| Inhibitory control |
Working memory |
Temporal processing |
Task switching |
Processing speed |
|
|---|---|---|---|---|---|
| Working memory | .24 | ||||
| Temporal processing | .23 | .44 | |||
| Task switching | .11 | .28 | .21 | ||
| Processing speed | .00 | .24 | .13 | .00 | |
| Episodic memory | .23 | .53 | .53 | .32 | .20 |
Site comparisons
The differences between the sites (Memphis vs. Detroit) in regional brain volumes and cognitive performance were examined using a series of independent t tests. The results of these analyses revealed no systematic differences in measured brain volumes, in any of the 18 individual tasks, nor in any of the cognitive composites. Although no significant site differences were observed for the cognitive composites, there was still the possibility that the observed measurement model differed by site. The current sample size was not sufficient for a formal multiple groups analysis, especially considering the size of the Memphis sample (N = 34). Thus, to provide an estimate of the stability of the model, we examined the fit of the measurement model in the Detroit sample separately. This model was a fair fit to the data given the reduced sample size: χ2 = 147.182, df = 124, p = .08; χ2/df = 1.19; CFI = .966; SRMR = .061. Considering the lack of systematic differences across sites, analyses were conducted on the data aggregated across sites.
Data Analyses
Our goal was to test mediation hypotheses, and our approach for assessing mediation is consistent with the method outlined by Baron and Kenny (1986) and Judd and Kenny (1981). Thus, we sought to establish that the relationship between age (predictor variable) and episodic memory (outcome variable) was substantially reduced or effectively zero when controlling for the hypothesized mediators (regional brain volumes, processing speed, and executive functions). Using this analytic approach, we can only infer the above-chance possibility of mediation and not its actual nature. To examine the relative contributions of regional brain volumes, executive functions, and processing speed to age differences in episodic memory, we conducted a series of path analyses using Mplus (Muthén & Muthén, 2004) with maximum likelihood estimator. In these analyses, systems of hierarchically nested linear models with an increasing number of restrictions on association between variables were constructed. A classic path analysis approach (see Pedhazur, 1997, for a concise tutorial) was used in constructing the path analyses with an emphasis on parsimony and theoretical interpretability. Thus, the objective was to find the smallest number of variables and effects to account for the observed data. Using a 2-index presentation strategy (Hu & Bentler, 1999), the primary indices of model fit were the SRMR and CFI with cut-offs of .06 and .95, respectively. The SRMR provides an estimate of model misspecification and the CFI provides an estimate of whether the model fits better than a null model. Following the recommendations of Jöreskog and Sörbom (1993), we calculated the ratio of χ2 to the degrees of freedom (df) to obtain a more informative estimate of goodness of fit than the χ2 statistic itself. In this instance, a relatively small χ2 in comparison to the associated degrees of freedom is suggestive of a reasonable fit (Mueller, 1996). Although there is no definitive cut-off for determining that a ratio is “high” or “low,” we adhered to a relatively more conservative cut-off ratio of ≤2.00 (Mueller, 1996).
For the basic structure of the models, the first tier consisted of age as a continuous variable (within each age group); the second tier consisted of regional brain volumes adjusted for the cranium size (lateral prefrontal cortex, prefrontal white matter caudate, hippocampus, visual cortex); the third tier consisted of processing speed, working memory, and inhibitory control; the fourth tier consisted of temporal processing and task switching, that were deemed less basic skills than those included in the previous tier; and the fifth tier consisted of episodic memory, the target construct of the analysis. The residual of age was set to zero. To maintain the recommended 1:10 ratio of variables to observations, we did not test any laterality effects or verbal versus nonverbal effects. One assumption of the classic path analysis approach implemented here is that there is no reciprocal causation in the models (Pedhazur, 1997). Thus, only unidirectional effects were postulated.
Results
Age Differences on Cognitive Tasks
Younger adults outperformed older adults on 16 of the 18 individual cognitive tasks. There were no significant age differences on the stop-signal task and right-left task switching. Significant age differences were also observed for each of the composites (see Table 3). To assess the differential effects of age, we computed the effect sizes (Cohen's d; Cohen, 1988) for each cognitive composite and examined 95% confidence intervals for overlap. As indicated by overlapping confidence limits, there were no significant differences in the magnitude of the effect of age on the four processes of executive functions and all showed substantially better performance in younger participants (inhibitory control: d = 1.06, 95%CI = .66–1.44; working memory: d = 1.22, CI = .81–1.61; task switching: d = .72, CI = .34–1.10; temporal processing: d = 1.35, CI = .93–1.75). The magnitude of the effect of age on task switching was significantly smaller than the effects of age on memory (d = 1.80, CI = 1.36–2.23) and processing speed (d = 1.70, CI = 1.26–2.12).
Table 3.
Descriptive Statistics for Cognitive Composite and Brain Volumes for Young and Older Participants
| Young |
Old |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Mean | SD | CV | Skew | Kurtosis | Mean | SD | CV | Skew | Kurtosis | t -test |
| Working memory | 1.43 | 2.84 | 1.99 | −.18 | −.39 | −1.33 | 2.50 | −1.89 | .04 | −.51 | 5.39*** |
| Inhibitory control | .85 | 1.40 | 1.64 | .33 | .45 | −.64 | 1.43 | −2.24 | −.49 | −.13 | 5.60*** |
| Temporal processing | 1.36 | 1.91 | 1.40 | −.34 | −.14 | −1.10 | 1.69 | −1.53 | −.07 | −.62 | 7.17*** |
| Task switching | .59 | 1.28 | 2.16 | −.29 | −.24 | −.39 | 1.46 | −3.73 | −.04 | −.09 | 3.85** |
| Processing speed | 1.25 | 1.35 | 1.07 | −.38 | −.25 | −1.05 | 1.37 | −1.30 | .22 | −.64 | 9.03*** |
| Episodic memory | 2.16 | 2.08 | .96 | −1.12 | 2.32 | −1.83 | 2.42 | −1.32 | −.56 | −.18 | 9.53*** |
| Lateral prefrontal | .70 | .79 | 1.12 | −.08 | −.69 | −.53 | .67 | −1.26 | −.05 | .14 | 8.81*** |
| Frontal white matter | .49 | .77 | 1.56 | −.36 | .62 | −.33 | 1.02 | 3.09 | .01 | −.46 | 4.97*** |
| Caudate nucleus | .53 | .73 | 1.39 | −.24 | −.38 | −.46 | .99 | −2.14 | .09 | .08 | 7.20*** |
| Hippocampus | .55 | .78 | 1.41 | .45 | .03 | −.35 | .69 | −1.99 | −.30 | .84 | 6.32*** |
| Visual cortex | .10 | 1.07 | .15 | .16 | −.49 | −.15 | .88 | .10 | .10 | −.56 | 1.29 |
p < .01;
p < .001
Age Differences in Regional Brain Volumes
Younger adults had larger brain volumes (adjusted for ICV) across all regions measured as compared with older adults, with a notable exception of the visual cortex (see Table 3). There were no significant differences in the magnitude of the effect of age on the lateral prefrontal cortex (d = 1.65, 95% CI = 1.22–2.07), prefrontal white (d = .93, CI = .54–1.32), caudate (d = 1.16, CI = .76–1.55), or the hippocampus (d = 1.21, CI = .80–1.60). The effect of age on the visual cortex was not significantly different from zero and lower than for all other regions (d = .25, CI =−.12–.62).
Mediational Analyses
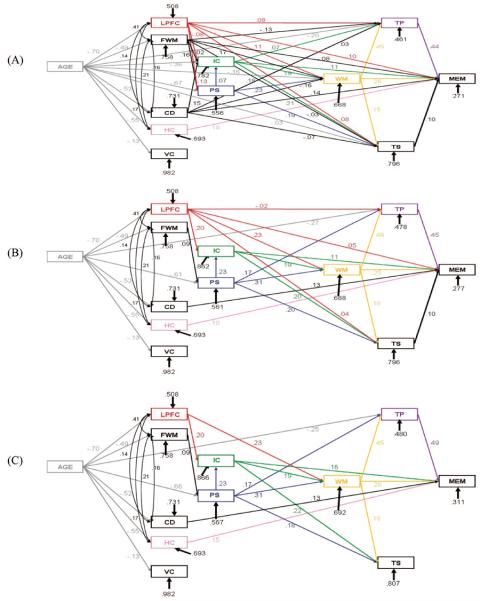
The first reduced model was created by imposing restrictions on the baseline model by turning respective paths to zero. The construction of Reduced Model I (see Figure 4) was based on theoretical considerations. The assumptions based on previous research cited in the introduction were that (1) prefrontal–caudate circuits would affect memory via their effects on executive functions; (2) prefrontal white matter volume would affect memory via its affect on processing speed; (3) the hippocampus would have a direct effect on memory; and, (4) the volume of the visual cortex would not affect any cognitive variables. The relationships among the constructs of processing speed and executive functions were based on the idea that processing speed, working memory, and inhibitory control were more fundamental cognitive primitives than temporal processing and task switching. We further hypothesized that processing speed and inhibitory control would exert influence on the flow of information through working memory (Salthouse, 1985, 1996; Zacks & Hasher, 1994) and that processing speed would mediate age differences in inhibition (Earles et al., 1997). In Reduced Model I the following paths were included: (1) age directly affected the regional brain volumes and processing speed, inhibitory control, working memory, temporal processing, and task switching; (2) lateral prefrontal cortex affected processing speed, inhibitory control, working memory, temporal processing, task switching, and episodic memory; (3) prefrontal white matter affected processing speed, inhibitory control, working memory, temporal processing, task switching, and episodic memory; (4) caudate affected inhibitory control, working memory, task switching, temporal processing, and episodic memory; (5) hippocampus affected episodic memory; (6) inhibitory control affected temporal processing, working memory, task switching, and episodic memory; (7) processing speed affected inhibitory control, working memory, temporal processing, and task switching; (8) working memory affected temporal processing, task switching, and episodic memory; (9) temporal processing affected episodic memory; and (10) task switching affected episodic memory. This model provided a good fit for the data χ2 = 22.064, df = 20, p = .34; χ2/df = 1.11; CFI = .997; SRMR = .039. Although the model as a whole was a good fit, several paths were not significantly different from zero, including those between prefrontal–caudate volumes and executive control processes.
Figure 4.
Path models of relationships among age, regional brain volumes, and cognitive performance. Numbers at the paths are path coefficients; three-digit numbers at the variable boxes are residuals. (A) Reduced Model I. (B) Reduced Model II. (C) Reduced Model III. See text for model fit indices. LPFC = lateral prefrontal cortex; FWM = prefrontal white matter; Cd = caudate nucleus; HC = hippocampus; VC = visual cortex; PS = processing speed; IC = inhibitory control; WM = working memory; TP = temporal processing; TS = task switching; MEM = episodic memory.
To ensure the most parsimonious and theoretically relevant fit to the data, further restrictions were imposed on Reduced Model I to create Reduced Model II (see Figure 4). In Reduced Model II we tested whether the effects of age on inhibitory control, working memory, and task switching were mediated by prefrontal volume and other cognitive processes; and whether lateral prefrontal cortex would emerge as a significant predictor of executive control process when other nonsignificant paths were removed. In addition, other paths that were nonsignificant were set to zero. In Reduced Model II, which was nested within Reduced Model I, the following paths were set to zero: age to inhibitory control, age to working memory, age to task switching, lateral prefrontal cortex to processing speed, caudate to inhibitory control, caudate to working memory, caudate to task switching, caudate to temporal processing, prefrontal white matter to inhibitory control, prefrontal white matter to working memory, prefrontal white matter to task switching, prefrontal white matter to temporal processing, and prefrontal white matter to episodic memory. Reduced Model II provided a good fit to the data (χ2 = 44.141, df = 34, p = .11; χ2/df = 1.30; CFI = .985; SRMR = .053). As Reduced Model II was hierarchically nested within Reduced Model I, we were able to directly examine whether there was a significant difference between the fit of Reduced Models I and II using χ2 difference testing (Loehlin, 2004). There was no significant difference between the model fits: χ2 difference = 22.077, df = 14, ns.
We constructed the final model to test whether prefrontal–caudate circuits exerted their effects on memory via executive functions or directly. The model was based on considerations of parsimony such that nonsignificant paths that were approximately zero in Reduced Model II were set to zero in Reduced Model III (see Figure 4). Specifically, the following paths were set to zero: lateral prefrontal cortex to temporal processing, lateral prefrontal cortex to task switching, lateral prefrontal cortex to episodic memory, caudate to episodic memory, prefrontal white matter to processing speed, and task switching to episodic memory. Reduced Model III also provided an adequate fit for the data (χ2 = 52.504, df = 38, p = .06; χ2/df = 1.38; CFI = .978; SRMR = .059). We next directly examined whether there was a significant difference between the fit of Reduced Model II and III using the χ2 difference testing. There was no significant difference between the model fits: χ2 difference = 8.363, df = 4, ns. Thus, Reduced Model II was retained as the most parsimonious model consistent with theoretical considerations.
Although no significant site differences were observed for either the cognitive composites or the regional brain volumes, there was still the possibility that the relationships among the variables, and hence best fitting models, differed by site. Thus, we wanted to directly test the for site differences in the mediational models in addition to the previously described examination of site differences in cognitive composites and brain volume. The current sample size was not sufficient for a formal multiple groups analysis, especially considering that the Memphis sample consisted of only 34 individuals. Thus, we examined the fit of Reduced Model III in the Detroit only sample to provide an estimate of the stability of the model. The model fit for the Detroit data was fair given the reduced sample size: χ2 = 51.428, df = 38, p = .07; χ2/df = 1.35; CFI = .970; SRMR = .061.
Relationships Among Processing Speed, Working Memory, and Inhibitory Control
Processing speed, working memory, and inhibitory control have been hypothesized as cognitive primitives that explain age-related variation in episodic memory (Salthouse, 1985, 1996; Verhaeghen & Salthouse, 1997; Zacks & Hasher, 1994). In Reduced Model III, age-related slowing reduces inhibitory control, and both inhibitory control and processing speed limit the efficiency of the working memory system. However, the relationships among these three processes are poorly understood. Thus, it is conceivable that impairments in inhibition lead to slowing, working memory limitations constrain inhibition, or working memory limitations induce slowing. We constructed three additional parallel models to test alternative hypotheses about the relationships among processing speed, inhibition, and working memory. As these models were not hierarchically nested versions of Reduced Model III or of each other we could not directly compare their goodness of fit.
In Reduced Model IIIa, the path between processing speed and inhibition was reversed (e.g., inhibitory control to processing speed instead of processing speed to inhibitory control) to test the alternative hypothesis that limitations in inhibition produce slowing. This model did not fit the data: χ2 = 57.883, df = 38, p = .02, χ2/df = 1.52, CFI = .970, SRMR = .072.
In Reduced Model IIIb, the path between working memory and inhibition was reversed (e.g., working memory to inhibitory control instead of inhibitory control to working memory) to test the alternative hypothesis that working memory limitations constrain inhibition. This model produced an adequate fit to the data (χ2 = 52.504, df = 38, p = .06, χ2/df = 1.38, CFI = .978, SRMR = .59).
In Reduced Model IIIc, the path between processing speed and working memory was reversed (e.g., working memory to processing speed instead of processing speed to working memory) to test the alternative hypothesis that working memory limitations produce slowing. This model did not fit the data: χ2 = 58.854 df = 38, p = .02, χ2/df = 1.55, CFI = .968 SRMR = .071.
Discussion
Age Differences in Cognition and Regional Brain Volumes
Age differences in cognitive performance were observed across all the measured cognitive domains. Each of the tasks assessed, with the notable exception of the stop-signal and right-left task switching tasks, evidenced significant differences favoring younger participants. When the tasks were combined to create composite measures, each of the composites of processing speed, working memory, inhibition, task switching, temporal processing, and episodic memory was negatively influenced by age. The effect of age on the cognitive composites was relatively uniform, except there was a smaller effect on task switching than for processing speed or memory.
Consistent with existing literature, significant age differences observed in prefrontal gray and white matter and the caudate nucleus were contrasted with lack of age-related variance in the visual cortex volume (Raz & Rodrigue, 2006). Age-related differences in hippocampal volume were also observed. Although there is debate in the literature regarding the existence and extent of hippocampal volumetric reductions in nondemented samples (see Sullivan et al., 2005 for a discussion), meta-analytic reviews of the literature reveal at least mild age-related effects (Raz et al., 2004), which may be nonlinear, that is, age-accelerated in nature (Allen, Bruss, Brown, & Damaio, 2005; Raz et al., 2005) and may be exacerbated by such factors as vascular risk (Raz et al., 2003). Although the magnitude of the effect of age on prefrontal gray matter was numerically higher than other regions, there were no significant differences in age effects across the brain regions (with the exception of the visual cortex).
Mediators of Age Differences in Memory
The fact that both brain and cognition are negatively affected by age is not new and by itself does not imply the connection between the two. Thus, we examined specific theoretically and statistically plausible models of connections between brain and cognition in the context of aging. We found that age-related differences in episodic memory were mediated by multiple factors, neural and cognitive (see Reduced Model III). Notably, no direct effects of calendar age remained once the influence of those factors was taken into account. It is important to note, however, that most of the neuroanatomical variables exerted no direct effect on memory.
Of all regional volumes, only the hippocampal volume exerted a direct effect on memory. This seemingly contradicts the findings of a recent meta-analysis (Van Petten, 2004) in which correlational studies of hippocampal volume and memory were combined regardless of the participant's age. However, as noted previously (Raz & Rodrigue, 2006), examination of the effect size scatterplot by age, presented in that analysis, suggests that increase in the mean sample age may be associated with positive and linearly increasing effect. Subsequent cross-sectional studies support an association between hippocampal volume and episodic memory in adult samples (Fjell et al., 2005; Lye et al., 2004, 2006; Walhovd et al., 2004). Longitudinal investigations also suggest that the hippocampus contributes to decline in episodic memory in nondemented samples (Golomb et al., 1996; Persson et al., 2006, but see Rodrigue & Raz, 2004 for contradicting findings). It is also conceivable, as it always is in a cross-sectional study, that individuals with a subclinical dementia were included among the older participants. Gross screening with a measure such as the MMSE could have been insensitive to subtle preclinical forms of cognitive deterioration. As Alzheimer's disease is associated with significant shrinkage of the hippocampus, the inclusion of individuals with subclinical or very early stage Alzheimer's disease might have contributed in part to the observed relationship between hippocampus and episodic memory (see Buckner, 2004 for discussion of this issue). Thus, a possibility of an admixture of very early dementia cases should be taken into account in interpretation of the findings and only longitudinal follow-up can resolve that issue.
The finding of the hippocampal volume predicting episodic memory performance without mediation by working memory or speed of processing is consistent with the Multiple Trace Theory (MTT) view of separate roles of hippocampal and cortical networks in mnemonic functions (Moscovitch et al., 2006). It does not imply, however, lack of cognitive mediation. According to MTT, the hippocampus is necessary for retrieval of all memories all the time, whereas the prefrontal and other cortical modules are crucial for acquisition of memories through facilitation of contextually rich encoding of specific material. In our models, the cognitive “primitives” describing such cortically driven processes were specified, whereas the cognitive functions inherent to the hippocampus were not. Thus, the latter were subsumed under the hippocampal volume variance and created an impression of direct effect.
The prefrontal cortex volume affected memory indirectly through a multistep path via its effect on working memory and inhibitory control. The observed relationship between prefrontal volume and executive control functions is in accord with previously reported associations in multiple independent samples (Head & Raz, 2002). The remaining age-related variance in mnemonic performance was mediated by variables unrelated to the brain regions examined in this study. Specifically, age-related slowing was directly linked to reduced efficiency in temporal processing, working memory, and inhibitory control. Limitations in temporal processing directly affected memory performance.
The findings pertaining to the role of working memory and processing speed in episodic memory are in accord with models that indicate that these constructs are important for accounting for age-related differences in cognition (e.g., Park et al., 1996, 2002; see Park & Payer, 2006; Reuter-Lorenz & Sylvester, 2005; Verhaeghen & Salthouse, 1997 for reviews). The observed association between inhibitory control and memory is consistent with a view that successful retrieval depends on the ability to ignore irrelevant information and focus on the target item. Inhibitory control is closely linked to working memory and is proposed to control the flow of information within working memory, both serving to prevent irrelevant information from entering working memory and to suppress information that is no longer relevant (Hasher & Zacks, 1988; Zacks & Hasher, 1994). Previous mediational models of cognitive aging have also revealed a significant contribution of inhibition, though to varying degrees (Kwong See & Ryan, 1995; Persad et al., 2002; Salthouse & Meinz, 1995; Salthouse et al., 2003). Our findings that a model linking working memory to inhibitory control and a separate model with a significant path from inhibitory control to working memory were both adequate fits to the data suggests interactions between the two constructs may contribute to age differences in memory.
Current results, while revealing multiple paths of influence mediating age effects on memory, are consistent with the view that ascribes processing speed a central role in cognitive aging (Salthouse, 1985; Fisk & Warr, 1996; Salthouse, 1996, 2000; Finkel et al., 2007). Slower cognitive processing was significantly associated with poorer temporal processing and task switching. Again, a model with the path from processing speed to working memory reversed was only a marginally “poor” fit to the data, as was a model with the path from inhibitory control reversed. One implication is that rather than processing speed, working memory, or inhibitory control being more fundamental than the others, the three constructs work interactively to contribute to age-related declines in memory.
We also demonstrated that working memory not only affected episodic memory directly, but also via its effect on temporal processing. Theoretically, temporal processing and working memory are closely linked. Temporal information may be important in forming contextual support for memory encoding and retrieval. Just as with spatial and verbal information, temporal information may be maintained within a working memory system, at least at the time of encoding. Strategic processes such as working memory and inhibition may provide support for various aspects of temporal processing (Brown, Preece & Hulme, 2000; Mangels, 1997; Mangels, Ivry, & Shimizu, 1998; Meck, 1996; Naveh-Benjamin, 1990; Olton, Wenk, Church, & Meck, 1988; see Lustig, Mattell & Meck, 2005 for a review). Serial order reconstruction and recency discrimination may in particular place high demands on strategic processing and planning. The direct association between episodic memory and the aspects of temporal processing examined here is consistent with the notion that prefrontally mediated impairments in monitoring the temporal order of events during encoding can lead to deficient episodic memory (Butters et al., 1994; Milner & Petrides, 1984; Milner, Corsi, & Leonard, 1991; Shimamura et al., 1990). Episodic retrieval is enhanced by contextual information bound to the target items during encoding (Tulving & Thompson, 1973), and temporal context is important in organizing information. The role of temporal tagging, that is, keeping track of the items' order as they were presented, can be seen intuitively in the episodic memory tasks in the current study. For example, in the CVLT items may be encoded using semantic or serial clustering. Notably, as strategy application may draw upon significant resources that may be deficient (Craik & Byrd, 1982; Rabinowitz, Craik, & Ackerman, 1982) older adults tend to not engage in the strategy of semantic clustering of words and may instead rely on serial clustering (Sanders et al., 1980; Wegesin, Jacobs, Zubin, Ventura, & Stern, 2000; Witte, Freund, & Brown-Whistler, 1993). In light of the observed deficits in temporal processing, reliance on serial clustering by older adults may actually be problematic, and the apparent relevance of temporal processing to age differences in episodic memory is one important point of the current study.
The ability to manage multiple tasks efficiently may be important for encoding context surrounding the target items of the episodic memory tasks. However, despite age differences evident in task switching that are consistent with previous literature (Kramer et al., 1999; Kray, 2006; Mayr, 2001; Salthouse et al., 1998; see Verhaeghen & Cerella, 2002 for a review), it explained no significant variance in age-related differences in episodic memory. This is consistent with previous reports of age differences in switching efficiency during encoding, but no subsequent impact on recognition (Hogan et al., 2006) and with findings that when measures of reaction time are controlled, switching is no longer related to higher-order cognitive processes such as episodic memory (Salthouse et al., 1998). Thus, there is currently no strong support for a significant mediating role of switching efficiency in age differences in episodic memory.
An interesting finding was, no direct effects of age on episodic memory remained after the specific effects of brain and cognitive mediators were taken into account. That is, the effects of age on episodic memory were completely mediated by the variables in each of models examined. In Verhaeghen and Salthouse's (Verhaeghen & Salthouse, 1997) meta-analytic review of the predictive power of processing speed and working memory, direct paths from age to cognitive variables were still observed in the models. The inclusion of regional brain volumes and additional components of executive functions in the current models provided sufficient explanatory power to account for the residual direct effects observed by others when only speed and working memory are assessed. Past research examining multiple aspects of executive control have also observed complete mediation of age-related variance in memory (Ferrer-Caja et al., 2002; Troyer et al., 1994). As Verhaeghen and Salthouse (1997) note, the supplementation of mediational models with multiple indices of hypothesized predictors of age-cognition relations should be an essential objective of future research. The lack of a direct effect of age in the current models should not, however, be taken as an indication that variables absent from the models are not critical determinants of age-related declines in episodic memory performance.
Methodological Limitations
It is important to note that a different selection and/or combination of tasks may have yielded a different pattern of results. The selection of tasks and cognitive processes may be a critical determinant of which executive factors are found to be relevant to aging of episodic memory. The classification of tasks into composite measures of processes of executive functions was based on theory and the results of factor analyses in studies with larger samples (e.g., Miyake et al., 2000). In addition, the formation of the composites was substantiated by our measurement model. It is nonetheless feasible that separation of the tasks into other composites would have yielded a different pattern of results. However, the current findings are fairly consistent with theoretical accounts and previous research. A conspicuous exception to this is the mediational role of temporal processing in episodic memory.
Neither prefrontal white matter nor the caudate nucleus volumes mediated age-cognition relations in the current model. The shared influence of these variables with prefrontal cortex may partially account for this. Alternatively, integrity of white matter tracts as indexed by white matter hyperintensities or microstructure (e.g., Madden et al., 2004; Rabbitt et al., 2007a, 2007b) may be more relevant for accounting for age influences on cognition rather than their volume. It is important to note that the inclusion of other brain regions and other markers of neural functioning (e.g., neurochemical, metabolic) may provide a better account of age-related disparities in cognitive performance. However, increase in the number of explanatory constructs will require a substantial enlargement of the sample.
Finally, because the results were obtained in a cross-sectional study, we are only able to examine statistical age-related variability without claiming that the differences actually represent age-related declines. The variance associated with calendar age may include variance from other sources and the error variance is most likely to include individual variation attributable to factors not specified in the model. Our pattern of results can be considered consistent with the mediational hypotheses postulated, rather than conclusive. A cross-sectional design allows testing hypotheses about associations among the sources of differences (in this case, age-related) but does not permit assessment of true change or the relative time course of change among the variables. Thus, it is unclear whether the observed associations between brain and cognition are causal or coincidental. For example, whether age-related decline in hippocampal volume leads to decline in episodic memory, or whether shrinkage of the prefrontal cortex precedes changes in executive functions that in turn predict memory declines, can be addressed only in the framework of a longitudinal study. Nonetheless, the results reported here show that under reasonable theoretical constraints and after testing models with opposite flow of variance, we can infer that the observed age-related variance in performance on episodic memory tests is not related to calendar age but explained by multiple variables associated with aging.
Conclusions
We have demonstrated that age-related differences in episodic memory can be explained to a large extent by a complex interplay between shrinkage of selected brain regions, processing speed, and executive functions. Notably, not all executive processes (e.g., task switching) contribute significantly to age differences in episodic memory. Regional brain volume effects on episodic memory are selective. The volumes of the hippocampal and the prefrontal but not primary visual cortex mediated effects of age on episodic memory, either directly or via their influence on executive functions. These results support the view of specific brain structures as direct substrates of specific cognitive operations, and are in line with reports of positive associations between regional brain volumes and cognitive functions. However, these findings contradict reports of lack or even negative association between structure and cognition. There may be multiple reasons for such discrepancies that cannot be examined except in a framework of longitudinal multivariate analyses that take into account complex relationships among the cognitive and neuroanatomical variables and differential contributions of genetic, metabolic, and physiological factors to age-related changes in brain and cognition.
Acknowledgments
This study was supported in part by National Institute of Health Grant AG-11230 to NR.
References
- Alexander MP, Stuss DT, Picton T, Shallice T, Gillingham S. Regional frontal injuries cause distinct impairments in cognitive control. Neurology. 2007;68:1450–1453. doi: 10.1212/01.wnl.0000261482.99569.fb. [DOI] [PubMed] [Google Scholar]
- Allen JS, Bruss J, Brown CK, Damaio H. Normal neuroanatomical variation due to age: The major lobes and a parcellation of the temporal region. Neurobiology of Aging. 2005;26:1245–1260. doi: 10.1016/j.neurobiolaging.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Working memory. Clarendon; Oxford: 1986. [Google Scholar]
- Balota DA, Dolan PO, Duchek JM. Memory changes in healthy older adults. In: Tulving E, Craik FIM, editors. The oxford handbook of memory. Oxford University Press; New York: 2000. pp. 395–409. [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychology research: Conceptual, strategic, and statistical consideration. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Cummings JL, Sultzer D, Henderson VW, Nuechterlein KH, Mintz J. White matter structural integrity in healthy aging adults and patients with Alzheimer disease: A magnetic resonance imaging study. Archives of Neurology. 2003;60:393–398. doi: 10.1001/archneur.60.3.393. [DOI] [PubMed] [Google Scholar]
- Blumenfeld RS, Ranganath C. Prefrontal cortex and long-term memory encoding: An integrative review of findings from neuropsychology and neuroimaging. The Neuroscientist. 2007;13:280–291. doi: 10.1177/1073858407299290. [DOI] [PubMed] [Google Scholar]
- Brickman AM, Zimmerman ME, Paul RH, Grieve SM, Tate DF, Cohen RA, et al. Regional white matter and neuropsychological functioning across the adult lifespan. Biological Psychiatry. 2006;60:444–453. doi: 10.1016/j.biopsych.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Brown GDA, Preece T, Hulme C. Oscillator-based memory for serial order. Psychological Review. 2000;107:127–181. doi: 10.1037/0033-295x.107.1.127. [DOI] [PubMed] [Google Scholar]
- Bryan, Luszcz MA, Pointer S. Executive function and processing resources as predictors of adult age differences in the implementation of encoding strategies. Aging, Neuropsychology, and Cognition. 1999;6:273–287. [Google Scholar]
- Buckner RL. Memory and executive function in aging and AD, multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Bucur B, Madden DJ, Spaniol J, Provenzale JM, Cabeza R, White LE, et al. Age-related slowing of memory retrieval: Contributions of perceptual speed and cerebral white matter integrity. Neurobiology of Aging. 2007;23:1–10. doi: 10.1016/j.neurobiolaging.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess PW, Shallice T. Response suppression, initiation and strategy use following frontal lobe lesions. Neuropsychologia. 1996;34:263–272. doi: 10.1016/0028-3932(95)00104-2. [DOI] [PubMed] [Google Scholar]
- Butters MA, Kaszniak AW, Glisky EL, Eslinger PJ, Schacter DL. Recency discrimination deficits in frontal lobe patients. Neuropsychology. 1994;8:343–353. [Google Scholar]
- Cabeza R, Anderson ND, Houle S, Mangels JA, Nyberg L. Age-related differences in neural activity during item and temporal-order memory retrieval: A positron emission tomography study. Journal of Cognitive Neuroscience. 2000;12:197–206. doi: 10.1162/089892900561832. [DOI] [PubMed] [Google Scholar]
- Charlton RA, Barrick TR, McIntyre DJ, Shen Y, O'Sullivan M, Howe FA, et al. White matter damage on diffusion tensor imaging correlates with age-related cognitive decline. Neurology. 2006;66:217–222. doi: 10.1212/01.wnl.0000194256.15247.83. [DOI] [PubMed] [Google Scholar]
- Cherry K, Park D. Individual differences and contextual variables influence spatial memory in younger and older adults. Psychology of Aging. 1993;8:517–526. doi: 10.1037//0882-7974.8.4.517. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd Ed. Erlbaum; Hillsdale: 1988. [Google Scholar]
- Craik FIM, Byrd M. Aging and cognitive deficits, the role of attentional response. In: Craik FIM, Trehub S, editors. Aging and cognitive processes. Plenum Press Publishing Company; 1982. pp. 191–211. [Google Scholar]
- Crawford JR, Bryan J, Luszcz MA, Obonsawin MC, Stewart L. The executive decline hypothesis of cognitive aging: Do executive deficits qualify as differential deficits and do they mediate age-related memory decline? Aging, Neuropsychology, and Cognition. 2000;7:9–31. [Google Scholar]
- Dempster FN. The rise and fall of the inhibitory mechanism: Toward a unified theory of cognitive development and aging. Developmental Review. 1992;12:45–75. [Google Scholar]
- Dimitrov M, Granetz J, Peterson M, Hollnagel C, Alexander G, Grafman J. Associative learning impairments in patients with frontal lobe damage. Brain and Cognition. 1999;41:213–230. doi: 10.1006/brcg.1999.1121. [DOI] [PubMed] [Google Scholar]
- Dobbs AR, Rule BG. Adult age differences in working memory. Psychology and Aging. 1989;4:500–503. doi: 10.1037//0882-7974.4.4.500. [DOI] [PubMed] [Google Scholar]
- Dumas JA, Hartman M. Adult age differences in temporal and item memory. Psychology and Aging. 2003;18:573–586. doi: 10.1037/0882-7974.18.3.573. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neuroscience. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Earles JL, Connor LT, Frieske D, Park DC, Smith AD, Zwahr M. Age differences in inhibition: Possible causes and consequences. Aging, Neuropsychology, and Cognition. 1997;4:45–57. [Google Scholar]
- Ekstrom RB, French JW, Harman HH, Dermen D. Manual for kit of factor-referenced cognitive tests. Educational Testing Service; Princeton: 1976. [Google Scholar]
- Eritaia J, Wood SJ, Stuart GW, Bridle N, Dudgeon P, Maruff P, et al. An optimized method for estimating intracranial volume from magnetic resonance images. Magnetic Resonance Medicine. 2000;44:973–977. doi: 10.1002/1522-2594(200012)44:6<973::aid-mrm21>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ. Conceptualizing, describing, and measuring components of executive function, A summary. In: Lyon GR, Krasnegor NA, editors. Attention, memory, and executive function. Brookes; Baltimore: 1996. pp. 367–395. [Google Scholar]
- Fabiani M, Friedman D. Dissociations between memory for temporal order and recognition memory in aging. Neuropsychologia. 1997;35:129–141. doi: 10.1016/s0028-3932(96)00073-5. [DOI] [PubMed] [Google Scholar]
- Ferrer-Caja E, Crawford JR, Bryan J. A structural modeling examination of the executive decline hypothesis of cognitive aging through reanalysis of Crawford et al. (2000). data. Aging, Neuropsychology, and Cognition. 2002;9:231–249. [Google Scholar]
- Finkel D, Reynolds CA, McArde JJ, Pedersen NL. Age changes in processing speed as a leading indicator of cognitive aging. Psychology and Aging. 2007;22:558–568. doi: 10.1037/0882-7974.22.3.558. [DOI] [PubMed] [Google Scholar]
- Fisk JE, Warr P. Age and working memory: The role of perceptual speed, the central executive, and the phonological loop. Psychology of Aging. 1996;11:316–323. doi: 10.1037//0882-7974.11.2.316. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Reinvang I, Lundervold A, Dale AM, Quinn BT, et al. Age does not increase rate of forgetting over weeks: Neuroanatomical volumes and visual memory across the adult life-span. Journal of the International Neuropsychological Society. 2005;11:2–15. doi: 10.1017/S1355617705050046. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Reinvang I, Lundervold A, Salat D, Quinn BT, Fischl B, Dale AM. Selective increase of cortical thickness in high-performing elderly-structural indices of optimal cognitive aging. Neuroimage. 2006;29:984–994. doi: 10.1016/j.neuroimage.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex – an update, time is of the essence. Neuron. 2001;30:319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD. Cognitive neuroscience of human memory. Annual Review of Psychology. 1998;49:87–115. doi: 10.1146/annurev.psych.49.1.87. [DOI] [PubMed] [Google Scholar]
- Golomb J, Kluger A, de Leon MJ, Ferris SH. Hippocampal formation size predicts declining memory performance in normal aging. Neurology. 1996;47:810–813. doi: 10.1212/wnl.47.3.810. [DOI] [PubMed] [Google Scholar]
- Golomb J, Kluger A, de Leon MJ, Ferris SH, Convit A, Mittelman MS, et al. Hippocampal formation size in normal human aging, a correlated of delayed secondary memory performance. Learning and Memory. 1994;1:45–54. [PubMed] [Google Scholar]
- Gunning-Dixon F. The influence of age, familiarity, and levels of processing on implicit and episodic memory [master's thesis] University of Memphis; Memphis: 1998. [Google Scholar]
- Gunning-Dixon F, Raz N. The cognitive correlates of white matter abnormalities in normal aging, a quantitative review. Neuropsychology. 2000;14:224–232. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon F, Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults, a prospective MRI study. Neuropsychologia. 2003;41:1929–1941. doi: 10.1016/s0028-3932(03)00129-5. [DOI] [PubMed] [Google Scholar]
- Hasher L, Zacks R. Working memory, comprehension, and aging: A review and a new view. In: Bower GH, editor. The psychology of learning and motivation. Vol. 22. Academic Press; New York: 1988. pp. 193–225. [Google Scholar]
- Head D, Buckner RL, Shimony JS, Girton LE, Akbudak E, Conturo TE, et al. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type, Evidence from diffusion tensor imaging. Cerebral Cortex. 2004;14:410–423. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- Head D, Raz N. Age-related differences in the course of cognitive skill acquisition: The role of regional cortical shrinkage and cognitive resources. Psychology and Aging. 2002;17:72–84. doi: 10.1037//0882-7974.17.1.72. [DOI] [PubMed] [Google Scholar]
- Heyder K, Suchan B, Daum I. Cortico-subcortical contributions to executive control. Acta Psychologica. 2004;115:271–289. doi: 10.1016/j.actpsy.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Hogan MJ, Kelly CA, Craik FIM. The effects of attention switching on encoding and retrieval of words in younger and older adults. Experimental Aging Research. 2006;32:153–183. doi: 10.1080/03610730600553935. [DOI] [PubMed] [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis, conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- Hultsch DF, Hertzog C, Dixon RA. Ability correlates of memory performance in adulthood and aging. Psychology and Aging. 1990;5:356–368. doi: 10.1037//0882-7974.5.3.356. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, et al. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiology and Aging. 2001;22:581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Joreskog KG, Goldberg AS. Estimation of a model with multiple indicators and multiple causes of a single latent variable. Journal of the American Statistical Association. 1975;70:631–639. [Google Scholar]
- Jöreskog KG, Sörbom D. LISREL 8: Structural equation modeling with the SIMPLIS command language. Erlbaum; Mahweh: 1993. [Google Scholar]
- Judd CM, Kenny DA. Process analysis: Estimating mediation in evaluation research. Evaluation Research. 1981;5:602–619. [Google Scholar]
- Kane MJ, Engle RW. The role of prefrontal cortex in working memory capacity, executive attention, and general fluid intelligence: An individual-differences perspective. Psychonomic Bulletin & Review. 2002;9:637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- Kausler DH, Wiley JG. Temporal memory and content memory for actions, Adult age differences in acquisition and retention. Experimental Aging Research. 1990;16:147–150. doi: 10.1080/07340669008251542. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Squire LR. Remembering and knowing: Two different expressions of declarative memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1995;21:699–710. doi: 10.1037//0278-7393.21.3.699. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Thompson PM, Lancaster JL, Bartzokis G, Smith S, Coyle T, et al. Relationship between fractional anisotropy and other indices of cerebral health in normal aging, tract-based spatial statistics study of aging. Neuroimage. 2007;35:478–487. doi: 10.1016/j.neuroimage.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Gopher D. Task coordination and aging: Explorations of executive control processes in the task switching paradigm. Acta Psychological. 1999;101:339–378. doi: 10.1016/s0001-6918(99)00011-6. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Humphrey DG, Larish JF, Logan GD, Strayer DL. Aging and inhibition, beyond a unitary view of inhibitory processing in attention. Psychology and Aging. 1994;9:491–512. [PubMed] [Google Scholar]
- Kray J. Task-set switching under cue-based versus memory-based switching conditions in younger and older adults. Brain Research. 2006;1105:83–92. doi: 10.1016/j.brainres.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Kroll JF, Potter MC. Recognizing words, pictures, and concepts: A comparison of lexical, object and reality decisions. Journal of Verbal Learn and Verbal Behavior. 1984;23:39–66. [Google Scholar]
- Kucera M, Francis W. Computational analysis of present-day American English. Brown University Press; Providence: 1967. [Google Scholar]
- Kwong See ST, Ryan EB. Cognitive mediation of adult age differences in language performance. Psychology and Aging. 1995;10:458–468. doi: 10.1037//0882-7974.10.3.458. [DOI] [PubMed] [Google Scholar]
- Loehlin JC. Latent variable models: An introduction to factor, path, and structural equation analysis. 4th Ed. Erlbaum; Mahweh: 2004. [Google Scholar]
- Logan GD. On the ability to inhibit thought and action: a users' guide to the stop signal paradigm. In: Dagenbach D, Car TH, editors. Inhibitory processes in attention, memory and language. Academic Press; San Diego, CA: 1994. pp. 189–239. [Google Scholar]
- Lustig C, Matell MS, Meck WH. Not “just” a coincidence: Frontal-striatal interactions in working memory and interval timing. Memory. 2005;13:441–448. doi: 10.1080/09658210344000404. [DOI] [PubMed] [Google Scholar]
- Lye TC, Grayson DA, Creasey H, Piguet O, Bennett HP, Ridley LJ, et al. Predicting memory performance in normal ageing using different measures of hippocampal size. Neuroradiology. 2006;48:90–99. doi: 10.1007/s00234-005-0032-5. [DOI] [PubMed] [Google Scholar]
- Lye TC, Piguet O, Grayson DA, Creasey H, Ridley LJ, Bennett HP, et al. Hippocampal size and memory function in the ninth and tenth decades of life: The Sydney Older Persons Study. Journal of Neurology, Neurosurgery and Psychiatry. 2004;75:548–554. doi: 10.1136/jnnp.2003.010223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Whiting W, Huette SA, White LE, MacFall JR, Provenzale JM. Diffusion tension imaging of adult age differences in cerebral white matter: Relation to response time. Neuroimage. 2004;21:1174–1181. doi: 10.1016/j.neuroimage.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Mangels JA. Strategic processing and memory for temporal order in patients with frontal lobe lesions. Neuropsychology. 1997;11:207–221. doi: 10.1037//0894-4105.11.2.207. [DOI] [PubMed] [Google Scholar]
- Mangels JA, Ivry RB, Shimizu N. Dissociable contributions of the prefrontal and neocerebellar cortex to time perception. Cognitive Brain Research. 1998;7:15–39. doi: 10.1016/s0926-6410(98)00005-6. [DOI] [PubMed] [Google Scholar]
- Mayr U. Age differences in the selection of mental sets: The roe of inhibition, stimulus ambiguity, and response-set overlap. Psychology and Aging. 2001;16:96–109. doi: 10.1037/0882-7974.16.1.96. [DOI] [PubMed] [Google Scholar]
- Meck WH. Neuropharmacology of timing and time perception. Cognitive Brain Research. 1996;3:227–242. doi: 10.1016/0926-6410(96)00009-2. [DOI] [PubMed] [Google Scholar]
- McDowd JM. Inhibition in attention and aging. Journals of Gerontology. 1997;52B:P265–P273. doi: 10.1093/geronb/52b.6.p265. [DOI] [PubMed] [Google Scholar]
- Milner B. The medial temporal-lobe amnesic syndrome. Psychiatry Clinics of North America. 2005;28:599–611. doi: 10.1016/j.psc.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Milner B, Corsi P, Leonard G. Frontal-lobe contribution to recency judgment. Neuropsychologia. 1991;29:601–618. doi: 10.1016/0028-3932(91)90013-x. [DOI] [PubMed] [Google Scholar]
- Milner B, McAndrews MP, Leonard G. Frontal lobes and memory for the temporal order of recent events. Cold Spring Harbor Symposium on Quantitative Biology. 1990;55:987–994. doi: 10.1101/sqb.1990.055.01.094. [DOI] [PubMed] [Google Scholar]
- Milner B, Petrides M. Behavioural effects of frontal-lobe lesions in man. Trends in Neuroscience. 1984;7:403–407. [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Monsell S. Control of mental processes. In: Bruce V, editor. Unsolved mysteries of the mind: Tutorial essays in cognition. Erlbaum, Taylor, & Francis; Hove: 1996. pp. 93–148. [Google Scholar]
- Moscovitch M, Nadel L, Winocur G, Gilboa A, Rosenbaum RS. The cognitive neuroscience of remote episodic, semantic and spatial memory. Current Opinions in Neurobiology. 2006;16:179–190. doi: 10.1016/j.conb.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Winocur G. Frontal lobes, memory, and aging. In: Grafman J, Holyoak KJ, Bollor F, editors. Structure and function in human prefrontal cortex. NY Academy of Sciences; New York, NY: 1995. pp. 119–150. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Winocur G. The frontal cortex and working with memory. In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. Oxford University Press; New York: 2002. pp. 188–209. [Google Scholar]
- Mueller R. Basic principles of structural equation modeling. Springer-Verlag; New York: 1996. [Google Scholar]
- Musen G, Triesman A. Implicit and episodic memory for visual patterns. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1990;16:127–137. doi: 10.1037//0278-7393.16.1.127. [DOI] [PubMed] [Google Scholar]
- Muthén B. Latent variable modeling in heterogeneous populations. Psychometrika. 1989;54:557–585. [Google Scholar]
- Muthén L, Muthén B. Mplus User's Guide. 3rd Edition Muthén and Muthén; Los Angeles: 2004. [Google Scholar]
- Naveh-Benjamin M. Coding of temporal order information: An automatic coding process? Journal of Experimental Psychology: Learning, Memory & Cognition. 1990;17:702–709. doi: 10.1037//0278-7393.13.4.595. [DOI] [PubMed] [Google Scholar]
- Nigg JT. The ADHD response-inhibition deficit as measured by the stop task: Replication with DSM-IV combined type, extension, and qualification. Journal of Abnormal Child Psychology. 1999;27:393–402. doi: 10.1023/a:1021980002473. [DOI] [PubMed] [Google Scholar]
- Norman DA, Shallice T. Attention to action: Willed and automatic control of behaviour. In: Davidson RJ, Schwartz GE, Shapiro D, editors. Consciousness and Self-Regulation: Advances in Research and Theory. Plenum Press; 1986. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Olton DS, Wenk GL, Church RM, Meck WH. Attention and the frontal cortex as examined by simultaneous temporal processing. Neuropsychologia. 1988;26:307–318. doi: 10.1016/0028-3932(88)90083-8. [DOI] [PubMed] [Google Scholar]
- O'Sullivan M, Jones DK, Summers PE, Morris RG, Williams SC, Markus HS. Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology. 2001;57:632–638. doi: 10.1212/wnl.57.4.632. [DOI] [PubMed] [Google Scholar]
- Pachella RG. The interpretation of reaction time in information-processing research. In: Kantowitz BH, editor. Human information processing: Tutorials in performance and cognition. Erlbaum; Hillsdale: 1974. [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult lifespan. Psychology of Aging. 2002;17:299–320. [PubMed] [Google Scholar]
- Park DC, Payer D. Working memory across the adult lifespan. In: Bialystok E, Craik FIM, editors. Lifespan cognition: Mechanisms of change. Oxford University Press; New York: 2006. pp. 128–142. [Google Scholar]
- Park DC, Smith AD, Lautenschlager G, Earles JL, Frieske D, Zwahr M, Gaines CL. Mediators of long-term memory performance across the life span. Psychology of Aging. 1996;11:621–637. doi: 10.1037//0882-7974.11.4.621. [DOI] [PubMed] [Google Scholar]
- Parkin J, Java RI. Determinants of age-related memory loss. In: Perfect TJ, Maylor EA, editors. Models of cognitive aging. Oxford University Press; New York, NY: 2000. pp. 188–203. [Google Scholar]
- Paul R, Grieve SM, Chaudary B, Gordon N, Lawrence J, Cooper N, et al. Relative contributions of the cerebellar vermis and prefrontal lobe volumes on cognitive performance across the adult lifespan. Neurobiology of Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Paul R, Grieve SM, Chaudary B, Gordon N, Lawrence J, Cooper N, Clark CR, Kukla M, Mulligan R, Gordon E. Relative contributions of cerebellar vermis and prefrontal lobe volumes on cognitive function across the adult lifespan. Neurobiology of Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.07.017. doi; 10.1016/j.neurobiolaging.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Pedhazur EJ. Multiple regression in behavioral research: Explanation and prediction. 3rd Edition Harcourt Brace College Publishers; Forth Worth, TX: 1997. [Google Scholar]
- Pennington BF, Bennetto L, McAleer O, Roberts RJ., Jr. Executive function and working memory, theoretical and measurement issues. In: Lyon GR, Krasnegor NA, et al., editors. Attention, memory, and executive function. Brookes; Baltimore: 1996. pp. 327–347. [Google Scholar]
- Perfect TJ. Memory aging as frontal lobe dysfunction. In: Conway MA, editor. Cognitive models of memory. US: The MIT Press; Cambridge, MA: 1997. pp. 315–339. [Google Scholar]
- Persad CC, Abeles N, Zacks RT, Denburg NL. Inhibitory changes after age 60 and their relationship to measures of attention and memory. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2002;57:P223–232. doi: 10.1093/geronb/57.3.p223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Nyberg L, Lind J, Larsson A, Nilsson LG, Ingvar M, et al. Structure-function correlates of cognitive decline in aging. Cerebral Cortex. 2006;16:907–915. doi: 10.1093/cercor/bhj036. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Frontal circuityr degradation marks healthy adult aging: Evidence from diffusion tensor imaging. Neuroimage. 2005;26:891–899. doi: 10.1016/j.neuroimage.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E. Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magnetic Resonance Medicine. 2000;44:259–268. doi: 10.1002/1522-2594(200008)44:2<259::aid-mrm13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Prull MW, Gabrieli JDE, Bunge SM. Age-related changes in memory, A cognitive neuroscience perspective. In: Craik FIM, Salthouse TA, editors. Handbook of aging and cognition. Vol. 2. Erlbaum; Mahweh: 2000. pp. 91–153. [Google Scholar]
- Rabbitt P, Mogapi O, Scott M, Thacker N, Lowe C, Horan M, et al. Effects of global atrophy, white matter lesions, and cerebral blood flow on age-related changes in speed, memory, intelligence, vocabulary, and frontal function. Neuropsychology. 2007b;21:684–695. doi: 10.1037/0894-4105.21.6.684. [DOI] [PubMed] [Google Scholar]
- Rabbitt P, Scott M, Lunn M, Thacker N, Lowe C, Pendleton N, et al. White matter lesions account for all age-related declines in speed but not in intelligence. Neuropsychology. 2007a;21:363–370. doi: 10.1037/0894-4105.21.3.363. [DOI] [PubMed] [Google Scholar]
- Rabinowitz JC, Craik FI, Ackerman BP. A processing resource account of age differences in recall. Canadian Journal of Psychology. 1982;36:325–344. [Google Scholar]
- Radloff LS. The CES-D scale, A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Raz N, Briggs SD, Marks W, Acker JD. Age-related deficits in generation and manipulation of mental images, II. The role of dorsolateral prefrontal cortex. Psychology and Aging. 1999;14:436–444. doi: 10.1037/0882-7974.14.3.436. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging, evidence from structural magnetic resonance imaging. Neuropsychology. 1998;12:95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Dupuis JH, McQuain J, Briggs SD, et al. Selective aging of the human cerebral cortex observed in vivo, differential vulnerability of the prefrontal gray matter. Cerebral Cortex. 1997;7:268–282. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Rodrigue KM, Williamson A, Acker JD. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex, replicability of regional differences in volume. Neurobiology of Aging. 2004;25:377–396. doi: 10.1016/S0197-4580(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, et al. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cerebral Cortex. 2005;15:1679–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KR. Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neuroscience and Biobehavioural Reviews. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KR, Acker JD. Hypertension and the brain: Vulnerability of the prefrontal regions and executive functions. Behavioral Neuroscience. 2003;117:1169–1180. doi: 10.1037/0735-7044.117.6.1169. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Sylvester CY. The cognitive neuroscience of aging and working memory. In: Cabeza R, Nyberg L, Park D, editors. The cognitive neuroscience of aging. Oxford University Press; 2005. pp. 186–217. [Google Scholar]
- Rodrigue KM, Raz N. Shrinkage of the entorhinal cortex over five years predicts memory performance in healthy adults. The Journal of Neuroscience. 2004;24:956–963. doi: 10.1523/JNEUROSCI.4166-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Kaye JA, Janowsky JS. Greater orbital prefrontal volume selectively predicts worse working memory performance in older adults. Cerebra Cortex. 2002;12:494–505. doi: 10.1093/cercor/12.5.494. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Speed of behavior and its implications for cognition. In: Birren JE, Schaie KW, editors. Handbook of the psychology of aging. Van Nostrand Reinhold; New York: 1985. pp. 400–426. [Google Scholar]
- Salthouse TA. Working memory as a processing resource in cognitive aging. Developmental Review. 1990;10:101–124. [Google Scholar]
- Salthouse TA. The aging of working memory. Neuropsychology. 1994;8:535–543. [Google Scholar]
- Salthouse TA. The processing speed theory of adult age differences in cognition. Psychological Review. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Aging and measures of processing speed. Biological Psychology. 2000;54:35–54. doi: 10.1016/s0301-0511(00)00052-1. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Atkinson TM, Berish DE. Executive functioning as a potential mediator of age-related cognitive decline in normal adults. Journal of Experimental Psychology: General. 2003;132:566–594. doi: 10.1037/0096-3445.132.4.566. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Fristoe N, McGuthry KE, Hambrick DZ. Relation of task switching to speed, age, and fluid intelligence. Psychology and Aging. 1998;13:445–461. doi: 10.1037/0882-7974.13.3.445. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Hancock HE, Meinz EJ, Hambrick DZ. Interrelations of age, visual acuity, and cognitive functioning. Journals of Gerontology Series B: Psychological Sciences. 1996;51B:317–330. doi: 10.1093/geronb/51b.6.p317. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Meinz EJ. Aging, inhibition, working memory, and speed. Journals of Gerontology Series B: Psychological Sciences. 1995;50B:297–306. doi: 10.1093/geronb/50b.6.p297. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Mitchell D, Skovronek E, Babcock R. Effects of adult age and working memory on reasoning and spatial abilities. Journal of Experimental Psychology: Learnining, Memory & Cognition. 1989;1:507–516. doi: 10.1037//0278-7393.15.3.507. [DOI] [PubMed] [Google Scholar]
- Sanders RE, Murphy MD, Schmitt FA, Walsh KK. Age differences in free recall rehearsal strategies. Journal of Gerontology. 1980;35:550–558. doi: 10.1093/geronj/35.4.550. [DOI] [PubMed] [Google Scholar]
- Schacter DL. Memory, amnesia, and frontal lobe dysfunction. Psychobiology. 1987;15:21–36. [Google Scholar]
- Schretlen D, Pearlson GD, Anthony JC, Aylward EH, Augustine AM, Davis A, et al. Elucidating the contributions of processing speed, executive ability, and frontal lobe volume to normal age-related differences in fluid intelligence. Journal of the International Neuropsychological Society. 2000;6:52–61. doi: 10.1017/s1355617700611062. [DOI] [PubMed] [Google Scholar]
- Shimamura AP, Janowsky JS, Squire LR. Memory for the temporal order of events in patients with frontal lobe lesions and amnesic patients. Neuropsychologia. 1990;28:803–813. doi: 10.1016/0028-3932(90)90004-8. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychological Bulletin. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Squire LR. The organization and neural substrates of human memory. International Journal of Neurology. 1987;21:218–222. [PubMed] [Google Scholar]
- Squire LR. Declarative and nondeclarative memory: Multiple brain systems supporting learning and memory. Journal of Cognitive Neuroscience. 1992;4:232–243. doi: 10.1162/jocn.1992.4.3.232. [DOI] [PubMed] [Google Scholar]
- Squire LR, Knowlton B, Musen G. The structure and organization of memory. Annual Review of Psychology. 1993;44:453–495. doi: 10.1146/annurev.ps.44.020193.002321. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CEL, Clark RE. The medial temporal lobe. Annual Reviews of Neuroscience. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP, Palumbo CL. Organizational strategies with unilateral or bilateral frontal lobe injury in word list learning tasks. Neuropsychology. 1994;8:355–373. [Google Scholar]
- Stuss DT, Benson DF. The Frontal Lobes. Raven Press; New York: 1986. [Google Scholar]
- Sullivan EV, Marsh L, Pfefferbaum A. Preservation of hippocampal volume throughout adulthood in healthy men and women. Neurobiology of Aging. 2005;26:1093–1908. doi: 10.1016/j.neurobiolaging.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Tremont G, Halpert S, Javorsky DJ, Stern Differential impact of executive dysfunction on verbal list learning and story recall. Clinical Neuropsychologist. 2000;14:295–302. doi: 10.1076/1385-4046(200008)14:3;1-P;FT295. R. A. [DOI] [PubMed] [Google Scholar]
- Troyer AK, Graves RE, Cullum CM. Executive functioning as a mediator of the relationship between age and episodic memory in healthy aging. Aging, Neuropsychology and Cognition. 1994;1:45–53. [Google Scholar]
- Tulving E, Thompson DM. Encoding specificity and retrieval processes in episodic memory. Psychological Review. 1973;80:352–373. [Google Scholar]
- Vakil E, Blachstein H, Hoofien D. Automatic temporal order judgment, the effect of intentionality of retrieval on closed head injury. Journal of Clinical and Experimental Neuropsychology. 1991;13:291–298. doi: 10.1080/01688639108401044. [DOI] [PubMed] [Google Scholar]
- Vakil E, Weise M, Enbar S. Direct and indirect memory measures of temporal order, younger versus older adults. International Journal of Aging Human Development. 1997;5:195–206. doi: 10.2190/N54R-9Q1M-27F3-GTRY. [DOI] [PubMed] [Google Scholar]
- Vanderplas JM, Garvin EA. The association value of random shapes. Journal of Experimental Psychology. 1959;57:147–154. doi: 10.1037/h0048723. [DOI] [PubMed] [Google Scholar]
- Vanderploeg RD, Schinka JA, Retzlaff P. Relationship between measures of auditory verbal learning and executive functioning. Journal of Clinical and Experimental Neuropsychology. 1994;16:243–252. doi: 10.1080/01688639408402635. [DOI] [PubMed] [Google Scholar]
- Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan, review and meta-analysis. Neuropsychologia. 2004;42:1394–1413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Van Petten C, Plante E, Davidson PS, Kuo TY, Bajuscak L, Glisky EL. Memory and executive functions in older adults, relationship with temporal and prefrontal gray matter volumes and white matter hyperintensities. Neuropsychologia. 2004;42:1313–1335. doi: 10.1016/j.neuropsychologia.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Cerella J. Aging, executive control, and attention, a review of meta-analyses. Neuroscience and Biobehavioral Review. 2002;26:849–857. doi: 10.1016/s0149-7634(02)00071-4. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, DeMeersman LD. Aging and the Stroop effect, A meta-analysis. Psychology and Aging. 1998;13:120–126. doi: 10.1037//0882-7974.13.1.120. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Marcoen A, Goosens L. Facts and fiction about memory aging, A quantitative integration of research findings. Journals of Gerontology Series B: Psychological Sciences. 1993;48:157–171. doi: 10.1093/geronj/48.4.p157. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Salthouse TA. Meta-analyses of age-cognition relations in adulthood, estimates of linear and nonlinear age effects and structural models. Psychological Bulletin. 1997;122:231–249. doi: 10.1037/0033-2909.122.3.231. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Dale AM, Fischl B, Quinn BT, Makris N, et al. Regional cortical thickness matters in recal after months more than minutes. Neuroimage. 2006;31:1343–1351. doi: 10.1016/j.neuroimage.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Fischl B, Quinn BT, et al. Size does matter in the long run: Hippocampal and cortical volume predict recall across weeks. Neurology. 2004;63:1193–1197. doi: 10.1212/01.wnl.0000140489.33249.95. [DOI] [PubMed] [Google Scholar]
- Wegesin DJ, Jacobs DM, Zubin NR, Ventura PR, Stern Y. Source memory and encoding strategy in normal aging. Journal of Clinical and Experimental Neuropsychology. 2000;22:455–464. doi: 10.1076/1380-3395(200008)22:4;1-0;FT455. [DOI] [PubMed] [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychological Bulletin. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Wheeler MA, Stuss DT, Tulving E. Frontal lobe damage produces episodic memory impairment. Journal of the International Neuropsychological Society. 1995;1:525–536. doi: 10.1017/s1355617700000655. [DOI] [PubMed] [Google Scholar]
- Witte KL, Freund JS, Brown-Whistler S. Adult age differences in free recall and category clustering. Experimental Aging Research. 1993;19:15–28. doi: 10.1080/03610739308253920. [DOI] [PubMed] [Google Scholar]
- Woodcock RW, Johnson MB. Manual for Woodcock-Johnson Psychoeducational Battery-Revised. DLM Teaching Resources; Allen, TX: 1989. [Google Scholar]
- Yoon B, Shim YS, Lee KS, Shon YM, Yang DW. Region-specific changes of cerebral white matter during normal aging: A diffusion-tensor imaging analysis. Archives of Gerontology and Geriatrics. 2007 doi: 10.1016/j.archger.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Zacks RT, Hasher L. Directed ignoring, inhibitory regulation of working memory. In: Dagenbach D, Carr TH, editors. Inhibitory processes in attention, memory, and language. Academic Press; San Diego: 1994. pp. 241–264. [Google Scholar]
- Zimmerman ME, Brickman AM, Paul RH, Grieve SM, Tate DF, Gunstad J, et al. The relationship between frontal gray matter volume and cognition varies across the healthy adult lifespan. American Journal of Geriatric Psychiatry. 2006;14:823–833. doi: 10.1097/01.JGP.0000238502.40963.ac. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Amaral DG. Human amnesia and the medial temporal region, enduring memory impairment following a bilateral lesion to field CA1 of the hippocampus. Journal of Neuro-science. 1986;6:2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]