Abstract
Thymulin is a thymic hormone exclusively produced by the thymic epithelial cells. It consists of a nonapeptide component coupled to the ion zinc, which confers biological activity to the molecule. After its discovery in the early 1970s, thymulin was characterized as a thymic hormone involved in several aspects of intrathymic and extrathymic T cell differentiation. Subsequently, it was demonstrated that thymulin production and secretion is strongly influenced by the neuroendocrine system. Conversely, a growing core of information, to be reviewed here, points to thymulin as a hypophysotropic peptide. In recent years, interest has arisen in the potential use of thymulin as a therapeutic agent. Thymulin was shown to possess anti-inflammatory and analgesic properties in the brain. Furthermore, an adenoviral vector harboring a synthetic gene for thymulin, stereotaxically injected in the rat brain, achieved a much longer expression than the adenovirally mediated expression in the brain of other genes, thus suggesting that an anti-inflammatory activity of thymulin prevents the immune system from destroying virus-transduced brain cells. Other studies suggest that thymulin gene therapy may also be a suitable therapeutic strategy to prevent some of the endocrine and metabolic alterations that typically appear in thymus-deficient animal models. The present article briefly reviews the literature on the physiology, molecular biology, and therapeutic potential of thymulin.
Keywords: thymulin, neuroendocrine control, hypophysiotropic activity, artificial gene, gene therapy, anti-inflammatory, ovarian dysgenesis
Relevance of the Thymus in the Immune–Neuroendocrine Homeostatic Network
The immune system is functionally linked to the nervous and endocrine systems thus constituting an integrated homeostatic network.1 Within this network, the neuroendocrine system monitors and controls the physical and chemical variables of the internal milieu. On its part, the immune system perceives, through antigenic recognition, an internal image of the macromolecular and cellular components of the body and reacts to alterations of this image, effectively participating in the “biological” homeostasis of the organism.
In mammals, the interaction of the thymus gland with the neuroendocrine system seems to be particularly important during perinatal life when the thymus and the neuroen-docrine system influence the maturation of each other. This was initially suggested by early findings showing that in species in which neonatal thymectomy does not produce any evident impairment of immune capacity,2 neuroendocrine functions are already highly developed at birth.3 In mice, the importance of the thymus for proper maturation of the neuroendocrine system is revealed by the endocrine alterations caused by neonatal thymectomy or congenital absence of the thymus. In effect, congenitally athymic (nude) female mice show significantly reduced levels of circulating and pituitary gonadotropins, a fact that seems to be causally related to a number of reproductive derangements described in these mutants.4 Thus, in homozygous (nu/nu) females the times of vaginal opening and first ovulation are delayed,5 fertility is reduced,4 and follicular atresia is increased such that premature ovarian failure results.6 Similar abnormalities result from neonatal thymectomy of normal female mice.7,8 Ovaries of athymic mice respond normally to exogenous gonadotropins, suggesting that the defect is at the level of the hypothalamic–pituitary axis.9,10 In homozygous, adult, nude CD-1, male mice, thyrotropin (TSH), prolactin (PRL), growth hormone (GH), and gonadotropin responses to immobilization and cold stress are reduced as also are serum basal levels of the same hormones compared to the heterozygous counterparts.11–13 A functional impairment of the hypothalamic–adrenal axis has been reported in nude mice, suggesting that humoral thymic factors may play a role in the maturation of this axis.14
The influence of the neuroendocrine system on thymus (and immune) function seems to continue during adult life either through a direct action of pituitary hormones or via peripheral hormones, both of which act on the thymic epithelial cells (TEC) and/or on immature thymocytes within the gland (for a review, see Ref. 15).
Thymulin
Thymulin is a thymic metallopeptide involved in several aspects of intrathymic and extrathymic T cell differentiation.16 Thymulin, which is exclusively produced by the thymic epithelial cells,17 consists of a biologically inactive nonapeptide component termed FTS (an acronym for serum thymus factor in French) coupled in an equimolecular ratio to the ion zinc,18 which confers biological activity to the molecule.19 The metallopeptide active form bears a specific molecular conformation that has been evidenced by nuclear magnetic resonance.20
Neuroendocrine Control of Thymulin Production
The control of thymulin secretion seems to be dependent on a complex network of events. Initial studies showed that the hormone itself exerts a controlling feedback effect on its own secretion both in vivo and in vitro.21,22 Additionally, thymulin production and secretion is influenced directly or indirectly by the neuroendocrine system. For instance, GH can influence thymulin synthesis and secretion. In vitro, human GH can stimulate thymulin release from TEC lines,23 which are known to possess specific receptors for GH.24 Animal studies have shown that treatment of aged dogs with bovine GH partially restored their low thymulin serum levels.25 In old mice, treatment with ovine GH increased their low circulating thymulin levels and enhanced the concanavalin A-dependent proliferative response of their thymocytes as well as interleukin-6 production.26 In old rats, combined treatment with GH and thyroxine (T4) was also able to partially restore their reduced thymulin levels.27 In clinical studies, it was reported that in congenitally GH-deficient children who consistently exhibited low plasma thymulin levels GH therapy succeeded in increasing thymic hormone levels to near normal values.28 Acromegalic middleaged patients have elevated thymulin serum levels compared to age-matched normal subjects.23,28 It is likely that these effects of GH are mediated, at least in part, by insulin-like growth factor 1 (IGF-1) as suggested by the fact that the GH-induced enhancement of thymulin production could be prevented by previous treatment with antibodies against IGF-1 or IGF-1 receptor.23
There is also evidence for a PRL–thymulin axis. Thus, it is known that TEC possess PRL receptors29 and that PRL can stimulate thymulin synthesis and secretion both in vitro and in vivo.30 Furthermore, administration of PRL to old mice elevated their reduced circulating levels of thymulin.30
The thyroid axis also influences thymulin secretion. Thus, T4 has been shown to stimulate thymulin synthesis and secretion in mice.31 In vivo treatment of mice with triiodothyronine enhanced thymulin secretion, whereas treatment of the animals with propylthiouracil, an inhibitor of thyroid hormone synthesis, decreased their circulating thymulin levels.32 In humans, hyperthyroidism brings about an increase in circulating thymulin levels, whereas hypothyroid patients show depressed levels of this thymic hormone.33 In in vitro studies, it was shown that thyroid hormones stimulate thymulin secretion by a direct action on TEC.34,35 Interestingly, it has been shown that treatment of aged animals with T4 can reverse their decreased thymulin levels.31,35
Although there are no studies documenting a direct effect of gonadotropins or adrenocorticotropic hormone (ACTH) on thymulin secretion gonadectomy or adrenalectomy in mice is known to induce a transient decrease in serum thymulin levels. This effect is potentiated by the simultaneous removal of the adrenals and gonads.34 In TEC cultures it was shown that exposure to physiological levels of glucocorticoids or gonadal steroids enhanced thymulin concentration in the cell supernatants.36,37
Although there is no rigorous evidence proving the existence of hypothalamic factors able to influence thymulin production by a direct action on TEC, there are two studies that suggest that this may be the case. Treatment of old mice with hypothalamic extracts from young mice resulted in the reappearance of detectable levels of circulating thymulin.38 Hypothalamic and pituitary extracts from young mice stimulated thymulin release from TEC cultures, but this stimulation declined when the pituitary and hypothalamic extracts were obtained from old mice.39
Hypophysiotropic Activity of Thymulin
The multilateral influence that the neuroendocrine system exerts on thymulin secretion suggests that this metallopeptide could, in turn, be part of a feedback loop acting on neuroendocrine structures. This possibility is now supported by a significant body of evidence indicating that thymulin possesses hypophysiotropic activity. Thus, thymulin has been shown to stimulate luteinizing hormone (LH) release fromperifused rat pituitaries40 and ACTH from incubated rat pituitary fragments, the latter being an effect mediated by intracellular cAMP and cGMP accumulation.41 In an in vitro study using pituitary cells obtained from female rats in different days of the estrous cycle, it was observed that thymulin modulates the stimulatory activity of gonadotropin-releasing hormone on LH and follicle-stimulating hormone (FSH) release.42 Thymulin has been found to stimulate GH, PRL, TSH, and gonadotropin release in dispersed rat pituitary cells at doses from 10−8 to 10−3 mol/L,43–45 whereas others have reported that thymulin doses of 10−11 mol/L stimulate LH, inhibit PRL release, and have no effect on GH secretion in incubated rat pituitary fragments.40 The stimulatory effect of thymulin on hormone release in rat pituitary cells declines with the age of the cell donor, which suggests that aging brings about a desensitization of the pituitary gland to thymic signals.43–45
There is in vitro and in vivo evidence suggesting that thymulin plays a role in the regulation of female spontaneous puberty, possibly through effects on pituitary gonadotropin release and ovarian steroidogenesis.40,42,44–47 Thymulin also modulates gonadotropin-induced testicular steroidogenesis.48
Recent immunoneutralization studies have strengthened the hypothesis that thymulin is a physiological mediator of the perinatal influence of the thymus on neuroendocrine maturation. Thus, neonatal immunoneutralization of circulating thymulin in otherwise normal C57BL/6 mice induced significant morphologic alterations in most anterior pituitary endocrine cell populations when the animals reached puberty.49 Thymulin immunoneutralization from birth to puberty in normal mice also induced serum gonadotropin50 and serum TSH, PRL, and GH reduction (Goya et al., unpublished results) when the animals reached puberty.
Construction of Synthetic Genes for Thymulin
The prospect of implementing thymic hormone gene therapy appears as an interesting avenue of research aimed at restoring endocrine thymic activitywhen thymus function is compromised. However, none of the genes coding for the known thymic peptides have been cloned, a situation that hinders the implementation of gene or other molecular therapies for thymic hormones. It was suggested that a possible way to overcome this problem could be to construct “artificial genes” coding for those thymic peptides whose amino acid sequences were short and required no posttranslational processing.51 This has been recently achieved for thymulin and the corresponding DNA sequence cloned in a recombinant adenoviral (RAd) vector that was subsequently used in a number of experimental gene therapy studies (see below).
In an early study aimed at upscaling thymulin production, a synthetic DNA sequence coding for FTS was inserted into a bacterial expression vector and successfully used to obtain large quantities of purified thymulin retaining full biological activity.52 More recently, a DNA sequence coding for the biologically active FTS analogue called metFTS was constructed and cloned in an adenoviral vector.53 The design of the DNA sequence for metFTS was optimized for expression in rat systems by choosing, for each amino acid of the native peptide, the codon more frequently used by rat cells (Fig. 1A). A variant of this sequence was used to construct RAd-metFTS, an adenoviral vector that harbors the synthetic gene for metFTS driven by the mouse cytomegalovirus promoter (PmCMV) (Fig. 1B and C). When intramuscularly (i.m.) administered to thymectomized (Tx) mice and rats (whose circulating levels of thymulin are nondetectable), RAd-metFTS induced sustained supraphysiological serum levels of biologically active thymulin that remained high for at least 112 days in mice53 and for over 320 days in rats. Interestingly, adenovirally mediated expression of the synthetic gene for metFTS in the substantia nigra and hypothalamus of adult Tx rats had a significantly longer duration than adenovirally mediated expression of the gene for green fluorescent protein or Escherichia coli β-galactosidase in the same brain regions.54 This phenomenon could be a result of the anti-inflammatory activity in the brain reported for thymulin and some thymulin analogues.55,56 Additionally, results from experiments using intracere-broventricular injection of thymulin in rats with experimentally induced brain inflammation suggested that this peptide has a neuroprotective role in the central nervous system and indicate a possible therapeutic use as analgesic and anti-inflammatory drug.57 The anti-inflammatory activity could prevent the immune system of the vector-injected animals from mounting a destructive response against the transduced cells. The same rationale could explain the long-term persistence of high concentrations of transgenic metFTS in the circulation of RAd-metFTS-injected Tx rodents.53 Because thymulin has no known toxic effects even at high doses, i.m. injection of RAd-metFTS could generate sustained pharmacologically effective levels of serum and brain thymulin for the amelioration of pathologies involving chronic brain inflammation. This would represent a distinct advantage over alternative anti-inflammatory approaches that use direct brain injection of viral vectors that block the production or actions of pro-inflammatory cytokines.58
Figure 1.
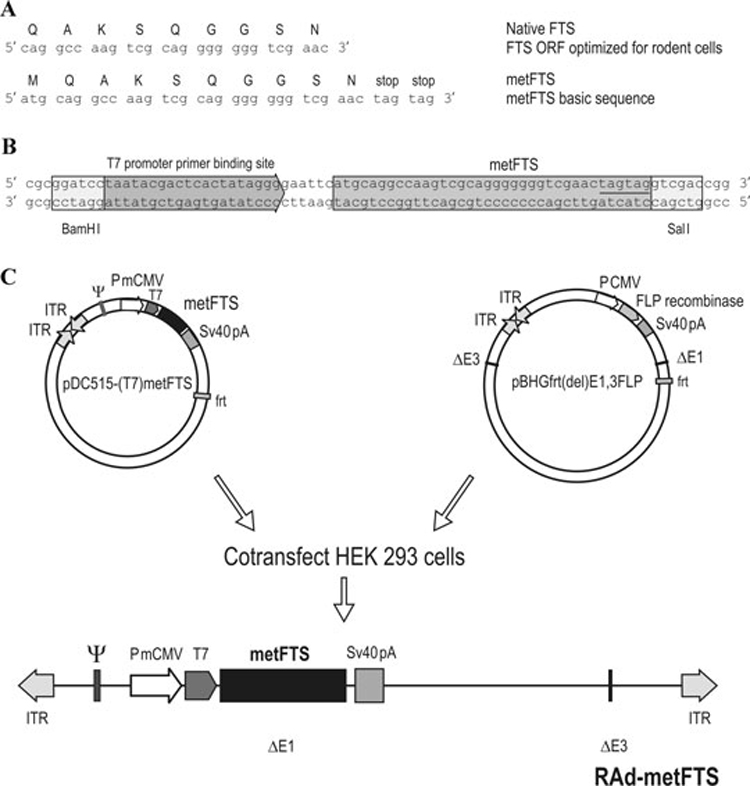
DNA constructs encoding the biologically active serum thymus factor (FTS) analogue (metFTS) and assembly of an adenoviral vector (RAd-metFTS) that harbors the synthetic gene for metFTS. A DNA sequence coding for native FTS was designed for optimal expression in rat cells. By adding an ATG starting codon upstream and two stop codons downstream of this sequence, it was converted into an open reading frame (ORF) for the analogue metFTS (A). This metFTS ORF was used to generate a construct to be cloned in the shuttle vector pDC515. The construct included the phage T7 promoter primer-binding site, which was used for sequencing purposes (B). The shuttle pDC515-metFTS was generated by inserting the T7-metFTS sequence into the BamHI and SalI sites of the multiple cloning site of the shuttle pDC515. This construct was used to generate RAd-metFTS (C). PmCMV, mouse cytomegalovirus promoter; frt, recognition element for the yeast FLP recombinase; ITR, inverted terminal repeats; ΔE1 and ΔE3, deletions in the Ad5 genome; SV40, simian virus 40 polyadenylation signal;ψ, packaging signal. From Ref. 50, used with permission.
Gene Therapy for Thymulin
Neonatal Thymulin Gene Therapy Prevents the Disruptive Impact of Athymia on the Reproductive System of Female Mice
A single i.m. injection of RAd-metFTS in newborn nude mice (nude mice have undetectable circulating levels of thymulin) elicited long-term restoration of serum thymulin in these mutants. This treatment was able to prevent the deficits in serum LH and FSH that typically appear in adult female nude mice.50 Furthermore, neonatal thymulin gene therapy in nude female mice has been found to significantly prevent the ovarian dysgenesis that usually develops in 70-day-old, female, nude mice.59
Effect of Neonatal Thymulin Gene Therapy on the Metabolic Dyshomeostasis in Nude Mice
There is evidence that the endocrine thymus may participate in glucose homeostasis. Thus, it has been reported that after 1 month of age, nude BALB/c mice develop spontaneous hyperglycemia and impaired glucose tolerance.60,61 Furthermore, these animals show peripheral insulin insensitivity with normal pancreatic β-cell reserves and normal lean body mass.62 Assessment of pancreatic islet cell populations in hyperglycemic nude mice revealed an increase in the D cell population (somatostatin producing). Also, somatostatin content in pancreatic tissue was higher in the athymic nude mice compared to heterozygous sex- and age-matched counterparts.63 Adult thymectomy in Wistar rats was reported to increase circulating insulin levels without significant changes in blood glucose.64 Other studies point to a modulatory activity of thymic factors on lipid metabolism. Thus, a thymic protein factor was reported to reduce serum cholesterol levels in rodents, increase low-density lipoprotein catabolism, and inhibit the activity of hepatic 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase, the rate-limiting enzyme in cholesterol synthesis.65–67 In adult nude mice it has been reported that the relative percentage of 16:0, 18:1 n9, and 18:1 n7 fatty acids is lower whereas that of 18:0, 20:4 n6, and 22:6 n3 fatty acids is higher in hepatic phospholipids of nu/nu animals compared to nu/+ counterparts. Some of these alterations were completely or partially prevented by neonatal thymulin gene therapy.68 Neonatal thymulin gene therapy completely prevented the adult-onset hyperglycemia of 70-day-old nude mice (Garcia-Bravo et al., unpublished results).
Concluding Remarks
Thymulin is probably the best characterized of all putative thymic hormones and seems to play a physiological role in thymus–pituitary communication, particularly during perinatal life. Interest in the therapeutic use of thymulin flourished during the 1970s and 1980s when efforts were almost exclusively focused on using thymulin (and other thymic peptides) for the treatment of autoimmune and other immunopathologies as well as cancer.69,70 Subsequent studies, most of them carried out during the last 15 years, established that thymulin is active on the hypophysis and the brain. This awareness and the recent availability of a synthetic gene for metFTS have opened new avenues for the exploration and eventual exploitation of the therapeutic potential of this metallopeptide.
Acknowledgments
Part of the work from our laboratory reviewed here was supported by National Institutes of Health Grant #R01AG029798 and Grant #PICT38214 from the National Agency for the Promotion of Science and Technology to RGG and by the Argentine Research Council (CONICET) and the Institut National de la Santé et de la Recherche Médicale (IN-SERM), France, to M.D. and R.G.G. R.G.G., O.A.B., and C.G.B. are CONICET career researchers. G.M.C. is a career researcher of the Scientific Research Commission of the Province of Buenos Aires (CIC-PBA).
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Goya RG. The immune-neuroendocrine homeostatic network and ageing. Gerontology. 1991;37:208–213. doi: 10.1159/000213262. [DOI] [PubMed] [Google Scholar]
- 2.Solomon JB. Ontogeny of defined immunity in mammals. In: Neuberger A, Tatum EL, editors. Foetal and Neonatal Immunology. Frontiers of Biology. Vol. 20. New York: American Elsevier Publishing Co.; 1971. pp. 234–306. [Google Scholar]
- 3.Jost A. The extent of foetal and endocrine autonomy. In: Wolstenholme GEW, O’Connor M, editors. Foetal Autonomy. Ciba Foundation Symposium. London: Churchill; 1969. pp. 79–94. [Google Scholar]
- 4.Rebar RW, Morandini IC, Erickson GF, et al. The hormonal basis of reproductive defects in athymic mice. Endocrinology. 1981;108:120–126. doi: 10.1210/endo-108-1-120. [DOI] [PubMed] [Google Scholar]
- 5.Besedovsky HO, Sorkin E. Thymus involvement in female sexual maturation. Nature. 1974;249:356–358. doi: 10.1038/249356a0. [DOI] [PubMed] [Google Scholar]
- 6.Lintern-Moore S, Pantelouris EM. Ovarian development in athymic nude mice. I. The size and composition of the follicle population. Mech. Age. Dev. 1975;4:385–390. doi: 10.1016/0047-6374(75)90039-1. [DOI] [PubMed] [Google Scholar]
- 7.Michael SD, Taguchi O, Nishizuka Y. Effects of neonatal thymectomy on ovarian development and plasma LH, FSH, GH and PRL in the mouse. Biol. Reprod. 1980;22:343–350. doi: 10.1093/biolreprod/22.2.343. [DOI] [PubMed] [Google Scholar]
- 8.Nishizuka Y, Sakakura T. Ovarian dysgenesis induced by neonatal thymectomy in the mouse. Endocrinology. 1971;89:889–893. doi: 10.1210/endo-89-3-886. [DOI] [PubMed] [Google Scholar]
- 9.Pierpaoli W, Besedovsky HO. Role of the thymus in programming of neuroendocrine functions. Clin. Exp. Immunol. 1975;20:323–328. [PMC free article] [PubMed] [Google Scholar]
- 10.Lintern-Moore S, Pantelouris EM. Ovarian development in athymic nude mice. III. The effect of PMSG and oestradiol upon the size and composition of the ovarian follicle population. Mech. Ageing. Dev. 1976;5:33–38. doi: 10.1016/0047-6374(76)90005-1. [DOI] [PubMed] [Google Scholar]
- 11.Goya RG, Cónsole GM, Sosa YE, et al. Altered functional responses with preserved morphology of gonadotrophic cells in congenitally athymic mice. Brain Behav. Immun. 2001;15:85–92. doi: 10.1006/brbi.2000.0595. [DOI] [PubMed] [Google Scholar]
- 12.Goya RG, Sosa YE, Cónsole GM, et al. Altered thyrotropic and somatotropic responses to environmental challenges in congenitally athymic mice. Brain Behav. Immun. 1995;9:79–86. doi: 10.1006/brbi.1995.1009. [DOI] [PubMed] [Google Scholar]
- 13.Goya RG, Sosa YE, Cónsole GM, et al. Altered regulation of serum prolactin in nude mice. Med. Sci. Res. 1996;24:279–280. [Google Scholar]
- 14.Daneva T, Spinedi E, Hadid R, et al. Impaired hypothalamo-pituitary-adrenal axis function in swiss nude athymic mice. Neuroendocrinology. 1995;62:79–86. doi: 10.1159/000126991. [DOI] [PubMed] [Google Scholar]
- 15.Savino W, Dardenne M. Neuroendocrine control of thymus physiology. Endocrine Rev. 2000;21:412–443. doi: 10.1210/edrv.21.4.0402. [DOI] [PubMed] [Google Scholar]
- 16.Bach JF. Thymulin (FTS-Zn) Clin. Immunol. Allergy. 1983;3:133–156. [Google Scholar]
- 17.Dardenne M, Papiernik M, Bach JF, et al. Studies on thymus products. III. Epithelial origin of the serum thymic factor. Immunology. 1974;27:299–304. [PMC free article] [PubMed] [Google Scholar]
- 18.Gastinel LN, Dardenne M, Pléau JM, et al. Studies on the zinc-binding site to the serum thymic factor. Biochim. Biophys. Acta. 1984;797:147–155. doi: 10.1016/0304-4165(84)90116-8. [DOI] [PubMed] [Google Scholar]
- 19.Dardenne M, Nabarra B, Lefrancier P. Contribution of zinc and other metals to the biological activity of serum thymic factor (FTS) Proc. Natl. Acad. Sci. USA. 1982;79:5370–5373. doi: 10.1073/pnas.79.17.5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cung MT, Marraud M, Lefrancier P, et al. NMR study of a lymphocyte differentiating thymic factor. J. Biol. Chem. 1988;263:5574–5580. [PubMed] [Google Scholar]
- 21.Savino W, Dardenne M, Bach JF. Thymic hormones containing cells. III. Evidence for a feedback regulation of the secretion of the serum thymic factor (FTS) by thymic epithelial cells. Clin. Exp. Immunol. 1983;52:7–12. [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen S, Berrih S, Dardenne M, et al. Feedback regulation of the secretion of a thymic hormone (thymulin) by human thymic epithelial cells in culture. Thymus. 1986;8:109–119. [PubMed] [Google Scholar]
- 23.Timsit J, Savino W, Safieh B, et al. GH and IGF-I stimulate hormonal function and proliferation of thymic epithelial cells. J. Clin. Endocrinol. Metab. 1992;75:183–188. doi: 10.1210/jcem.75.1.1619008. [DOI] [PubMed] [Google Scholar]
- 24.Ban E, Gagnerault MC, Jammes H, et al. Specific binding sites for growth hormone in cultured mouse thymic epithelial cells. Life Sci. 1991;48:2141–2148. doi: 10.1016/0024-3205(91)90147-4. [DOI] [PubMed] [Google Scholar]
- 25.Goff BL, Roth JA, Arp LH, et al. Growth hormone treatment stimulates thymulin production in aged dogs. Clin. Exp. Immunol. 1987;68:580–587. [PMC free article] [PubMed] [Google Scholar]
- 26.Goya RG, Gagnerault MC, Leite de Moraes MC, et al. In vivo effects of growth hormone on thymus function in aging mice. Brain Behav. Immun. 1992;6:341–354. doi: 10.1016/0889-1591(92)90033-k. [DOI] [PubMed] [Google Scholar]
- 27.Goya RG, Gagnerault MC, Sosa YE, et al. Effects of growth hormone and thyroxine on thymulin secretion in aging rats. Neuroendocrinology. 1993;58:338–343. doi: 10.1159/000126559. [DOI] [PubMed] [Google Scholar]
- 28.Mocchegiani E, Paolucci P, Balsamo A, et al. Influence of growth hormone on thymic endocrine activity in humans. Horm. Res. 1990;33:7–14. doi: 10.1159/000181528. [DOI] [PubMed] [Google Scholar]
- 29.Dardenne M, Kelly PA, Bach JF, et al. Identification and functional activity of Prl receptors in thymic epithelial cells. Proc. Natl. Acad. Sci. USA. 1991;88:9700–9704. doi: 10.1073/pnas.88.21.9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dardenne M, Savino W, Gagnerault MC, et al. Neuroendocrine control of thymic hormonal production. I. Prolactin stimulates in vivo and in vitro the production of thymulin by human and murine thymic epithelial cells. Endocrinology. 1989;125:3–12. doi: 10.1210/endo-125-1-3. [DOI] [PubMed] [Google Scholar]
- 31.Fabris N, Mocchegiani E. Endocrine control of thymic serum factor production in young-adult and old mice. Cell. Immunol. 1985;91:325–335. doi: 10.1016/0008-8749(85)90230-8. [DOI] [PubMed] [Google Scholar]
- 32.Savino W, Wolf B, Aratan-Spire S, et al. Thymic hormone containing cells. IV. Fluctuations in the thyroid hormone levels in vivo canmodulate the secretion of thymulin by the epithelial cells of young mouse thymus. Clin. Exp. Immunol. 1984;55:629–635. [PMC free article] [PubMed] [Google Scholar]
- 33.Fabris N, Mocchegiani E, Mariotti S, et al. Thyroid function modulates thymic endocrine activity. J. Clin. Endocrinol. Metab. 1986;62:474–478. doi: 10.1210/jcem-62-3-474. [DOI] [PubMed] [Google Scholar]
- 34.Villa-Verde DMS, Mello-Coelho V, Farias de Oliveira DA, et al. Pleiotropic influence of tri-iodothyronine on thymus physiology. Endocrinology. 1993;133:867–875. doi: 10.1210/endo.133.2.8344222. [DOI] [PubMed] [Google Scholar]
- 35.Mocchegiani E, Amadio L, Fabris N. Neuroendocrine-thymus interactions. I. In vitro modulation of thymic factor secretion by thyroid hormones. J. Endocrinol. Invest. 1990;13:139–147. doi: 10.1007/BF03349524. [DOI] [PubMed] [Google Scholar]
- 36.Dardenne M, Savino W, Duval D, et al. Thymic hormone-containing cells. VII. Adrenals and gonads control the in vivo secretion of thymulin and its plasmatic inhibitor. J. Immunol. 1986;136:1303–1308. [PubMed] [Google Scholar]
- 37.Savino W, Bartoccioni E, Homo-Delarche F, et al. Thymic hormone containing cells. IX. Steroids in vitro modulate thymulin secretion by human and murine thymic epithelial cells. J. Steroid Biochem. 1988;30:479–484. doi: 10.1016/0022-4731(88)90148-3. [DOI] [PubMed] [Google Scholar]
- 38.Folch H, Eller G, Mena M, et al. Neuroen-docrine regulation of thymus hormones: hypothalamic dependence of FTS level. Cell. Immunol. 1986;102:211–216. doi: 10.1016/0008-8749(86)90339-4. [DOI] [PubMed] [Google Scholar]
- 39.Goya RG, Gagnerault MC, Sosa YE. Reduced ability of pituitary extracts from old mice to stimulate thymulin secretion in vitro. Mech. Age. Dev. 1995;83:143–154. doi: 10.1016/0047-6374(95)01619-b. [DOI] [PubMed] [Google Scholar]
- 40.Zaidi SA, Kendall MD, Gillham B, et al. The release of LH from pituitaries perifused with thymic extracts. Thymus. 1988;12:253–264. [PubMed] [Google Scholar]
- 41.Hadley AJ, Rantle CM, Buckingham JC. Thymulin stimulates corticotrophin release and cyclic nucleotide formation in the rat anterior pituitary gland. Neuroimmunomodulation. 1997;4:62–69. doi: 10.1159/000097322. [DOI] [PubMed] [Google Scholar]
- 42.Hinojosa L, García L, Domínguez R, et al. Effects of thymulin and GnRH on the release of gonadotropins by in vitro pituitary cells obtained from rats in each day of estrous cycle. Life Sci. 2004;76:795–804. doi: 10.1016/j.lfs.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 43.Brown OA, Sosa YE, Bolognani F, et al. Thymulin stimulates prolactin and thyrotropin release in an age-related manner. Mech. Age. Dev. 1998;104:249–262. doi: 10.1016/s0047-6374(98)00072-4. [DOI] [PubMed] [Google Scholar]
- 44.Brown OA, Sosa YE, Dardenne M, et al. Studies on the gonadotropin-releasing activity of thymulin: changes with age. J. Gerontol. (Biological Sciences) 2000;55:B170–B176. doi: 10.1093/gerona/55.4.b170. [DOI] [PubMed] [Google Scholar]
- 45.Hinojosa L, Chavira R, Dominguez R, et al. Effects of thymulin on spontaneous puberty and gonadotrophin-induced ovulation in prepubertal normal and hypothymic mice. J. Endocrinol. 1999;163:255–260. doi: 10.1677/joe.0.1630255. [DOI] [PubMed] [Google Scholar]
- 46.García L, Hinojosa L, Domínguez R, et al. Effects of infantile thymectomy on ovarian functions and gonadotrophin-induced ovulation in prepubertal mice. Role of thymulin. J. Endocrinol. 2000;166:381–387. doi: 10.1677/joe.0.1660381. [DOI] [PubMed] [Google Scholar]
- 47.García L, Hinojosa L, Domínguez R, et al. Effects of injecting thymulin into the anterior or medial hypothalamus or the pituitary on induced ovulation in prepubertal mice. Neuroimmunomodulation. 2005;12:314–320. doi: 10.1159/000087111. [DOI] [PubMed] [Google Scholar]
- 48.Wise T. In vitro and in vivo effects of thymulin on rat testicular steroid synthesis. J. Steroid. Biochem. Mol. Biol. 1998;66:129–135. doi: 10.1016/s0960-0760(98)00045-4. [DOI] [PubMed] [Google Scholar]
- 49.Camihort G, Luna G, Vesenbeckh S, et al. Morphometric assessment of the impact of serum thymulin immunoneutralization on pituitary cell populations in peripubertal mice. Cells Tiss. Organs. 2006;184:23–30. doi: 10.1159/000096948. [DOI] [PubMed] [Google Scholar]
- 50.Goya RG, Reggiani PC, Vesenbeckh SM, et al. Thymulin gene therapy prevents the reduction in circulating gonadotropins induced by thymulin deficiency in mice. Am. J. Physiol.-Endocrinol. Metab. 2007;293:E182–E187. doi: 10.1152/ajpendo.00085.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goya RG, Cónsole GM, Hereñú CB, et al. Thymus and aging: Potential of gene therapy for restoration of endocrine thymic function in thymus-deficient animal models. Gerontology. 2002;48:325–328. doi: 10.1159/000065258. [DOI] [PubMed] [Google Scholar]
- 52.Calenda A, Cordonnier A, Lederer F, et al. Production of biologically active thymulin in Escherichia coli through expression of a chemically synthesized gene. Biotechnol. Lett. 1988;10:155–160. [Google Scholar]
- 53.Reggiani PC, Hereñú CB, Rimoldi OJ, et al. Gene therapy for long-term restoration of circulating thymulin in thymectomized mice and rats. Gene Ther. 2006;13:1214–1221. doi: 10.1038/sj.gt.3302775. [DOI] [PubMed] [Google Scholar]
- 54.Morel GR, Brown OA, Reggiani PC, et al. Peripheral and mesencephalic transfer of a synthetic gene for the thymic peptide thymulin. Brain Res. Bull. 2006;69:647–651. doi: 10.1016/j.brainresbull.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 55.Safieh-Garabedian B, Dardenne M, Pleau J-M, et al. Potent analgesic and anti-inflammatory actions of a novel thymulin-related peptide in the rat. Br. J. Pharmacol. 2002;136:947–955. doi: 10.1038/sj.bjp.0704793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Safieh-Garabedian B, Ochoa-Chaar CI, Poole S, et al. Thymulin reverses inflammatory hyperalgesia and modulates the increased concentration of proinflammatory cytokines induced by i.c.v. endotoxin injection. Neuroscience. 2003;121:865–873. doi: 10.1016/s0306-4522(03)00500-1. [DOI] [PubMed] [Google Scholar]
- 57.Dardenne M, Saade N, Safieh-Garabedian B. Role of thymulin or its analogue as a new analgesic molecule. Ann. N. Y. Acad. Sci. 2006;1088:153–163. doi: 10.1196/annals.1366.006. [DOI] [PubMed] [Google Scholar]
- 58.Stone D, Xiong W, Williams JC, et al. Adenovirus expression of IL-1 and NF-kappaB inhibitors does not inhibit acute adenoviral induced brain inflammation, but delays immune system-mediated elimination of transgene expression. Mol. Ther. 2003;8:400–411. doi: 10.1016/s1525-0016(03)00178-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reggiani PC, Barbeito CG, Flamini MA, et al. Neonatal thymulin gene therapy prevents the characteristic ovarian atrophy of adult nude mice (Abstract); Presented at the Seventh Meeting of the International Society for Neuroimmunomodulation; 19–23 April; Rio de Janeiro, Brazil. 2008. [Google Scholar]
- 60.Zeidler A, Tosco C, Kumar D, et al. Spontaneous hyperglycemia and impaired glucose tolerance in athymic nude BALB/c mice. Diabetes. 1982;31:821–825. doi: 10.2337/diab.31.9.821. [DOI] [PubMed] [Google Scholar]
- 61.Zeidler A, Kumar D, Johnson C, et al. Development of a diabetes-like syndrome in an athymic nude Balb/c mouse colony. Exp. Cell. Biol. 1984;52:145–149. [PubMed] [Google Scholar]
- 62.Zeidler A, Shargill NS, Chan TM. Peripheral insulin insensitivity in the hyperglycemic athymic nude mouse: similarity to noninsulin-dependent diabetes mellitus. Proc. Soc. Exp. Biol. Med. 1991;196:457–460. doi: 10.3181/00379727-196-43216. [DOI] [PubMed] [Google Scholar]
- 63.Zeidler A, Arbuckle S, Mahan E, et al. Assessment of pancreatic islet- cell population in the hyperglycemic athymic nude mouse: immunohistochemical, ultrastructural, and hormonal studies. Pancreas. 1989;4:153–160. doi: 10.1097/00006676-198904000-00003. [DOI] [PubMed] [Google Scholar]
- 64.Velkov Z, Zafirova M, Kemileva Z, et al. Time course changes in blood glucose and insulin levels of thymectomized rats. Acta Physiol. Pharmacol. Bulg. 1990;16:64–67. [PubMed] [Google Scholar]
- 65.Mondola P, Coscia Porrazzi L, Falconi C. Cholesterol and triglycerides of a liver after administration of a chromatographic fraction of thymus: variations in tissue and blood. Horm. Metab. Res. 1979;11:503–505. doi: 10.1055/s-0028-1092770. [DOI] [PubMed] [Google Scholar]
- 66.Mondola P, Santillo M, Santangelo F, et al. Effects of a new calf thymus protein on 3-hydroxy-3-methyl-glutarylCoA reductase activity in rat (rattus bubalus) hepatocyte cells (BRL-3A) Comp. Biochem. Physiol. 1992;103B:431–434. doi: 10.1016/0305-0491(92)90316-j. [DOI] [PubMed] [Google Scholar]
- 67.Mondola P, Santillo M, Tedesco I, et al. Thymus fraction (FIII) effect on cholesterol metabolism: modulation of the low density lipoprotein receptor pathway. Int. J. Biochem. 1989;21:627–630. doi: 10.1016/0020-711x(89)90381-9. [DOI] [PubMed] [Google Scholar]
- 68.García-Bravo MM, Polo MP, Reggiani PC, et al. Partial prevention of hepatic lipid alterations in nude mice by neonatal thymulin gene therapy. Lipids. 2006;41:753–757. doi: 10.1007/s11745-006-5027-4. [DOI] [PubMed] [Google Scholar]
- 69.Bach JF, Dardenne M, Goldstein AL. Clinical aspects of thymulin (FTS) In: Goldstein AL, editor. Thymic Hormones and Lymphokines. Basic Chemistry and Clinical Applications. New York: Plenum Press; 1984. pp. 593–600. [Google Scholar]
- 70.Sztein MB, Goldstein AL. Thymic hormones—a clinical update. Springer Sem. Immunopathol. 1986;9:1–18. doi: 10.1007/BF00201901. [DOI] [PubMed] [Google Scholar]


