Abstract
Methamphetamine (METH) and many other abused substances interact with σ receptors. σ Receptors are found on dopaminergic neurons and can modulate dopaminergic neurotransmission. Antisense knock down of σ receptors also mitigates METH-induced stimulant effects, suggesting that these proteins are viable medication development targets for treating pscyhostimulant abuse. In the present study, AC927, a σ receptor antagonist, was evaluated for its ability to attenuate METH-induced effects in vivo and in vitro. Radioligand binding studies showed that AC927 had preferential affinity for σ receptors compared to 29 other receptors, transporters and ion channels. Pretreatment of male, Swiss Webster mice with AC927 significantly attenuated METH-induced locomotor stimulation, striatal dopamine depletions, striatal dopamine transporter reductions, and hyperthermia. When the neurotoxicity of METH was further examined in vitro under temperature-controlled conditions, co-incubation with AC927 mitigated METH-induced cytotoxicity. Together, the results demonstrate that AC927 protects against METH-induced effects, and suggests a new strategy for treating psychostimulant abuse.
Keywords: Dopamine, Dopamine transporter, Hyperthermia, Locomotor activity, Methamphetamine, NG108-15 cells, Sigma receptors, Striatum
1. Introduction
Methamphetamine is a central nervous system stimulant that enhances synaptic dopamine levels through numerous mechanisms, including inhibition of dopamine uptake and facilitation of dopamine release (Barr et al., 2006). Methamphetamine can also act as a neurotoxin to dopamine neurons when administered at high doses or repeatedly (Davidson et al., 2001; Volz et al., 2007). The toxic effects of methamphetamine are often accompanied by hyperthermia which can exacerbate damage to the nervous system (Bowyer et al., 1994; Cadet et al., 2007; Davidson et al., 2001). However, the mechanisms underlying these effects are not yet fully understood.
In addition to its effects on dopamine systems, methamphetamine interacts with σ receptors (Itzhak, 1993; Nguyen et al., 2005). σ Receptors represent a unique structural class of proteins with a drug selectivity pattern, amino acid sequence, and anatomical distribution that is distinct from other mammalian proteins (Guitart et al., 2004; Matsumoto et al., 2003; Walker et al., 1990). These receptors are found in organ systems that mediate the actions of methamphetamine, including brain regions containing dopaminergic nerve terminals and cell bodies (Gundlach et al., 1986). The activation of σ receptors can stimulate dopamine synthesis and release in the brain (Bastianetto et al., 1995; Booth and Baldessarini, 1991; Weiser et al., 1995), thereby modulating a key neurochemical system that is implicated in the effects of methamphetamine.
Biochemical and pharmacological studies indicate the existence of multiple σ receptor subtypes, of which σ1 and σ2 receptors are the best characterized (Guitart et al., 2004; Matsumoto et al., 2003; Walker et al., 1990). The σ1 receptor, which has been cloned from several species, is a highly conserved 223 amino acid protein (Mei and Pasternak, 2001; Prasad et al., 1998). The σ1 receptor participates in protein-protein interactions and modulates the activity of G protein coupled receptors, ion channels, and signaling molecules such as inositol phosphates, protein kinases, and calcium (Aydar et al., 2002; Hayashi and Su, 2007). These interactions are thought to be exerted through chaperone-like characteristics of the protein which allow translocation between different cellular compartments (Hayashi and Su, 2007). In contrast, the σ2 receptor is an 18–22 kDa protein that has not yet been cloned (Hellewell et al., 1994). The σ2 receptor is enriched in lipid rafts, and has been implicated in calcium signaling and cell cycle functions (Crawford et al., 2002; Gebreselassie and Bowen, 2004).
Previous studies have demonstrated that σ receptors are involved in the stimulant actions of methamphetamine. It has been known for many years that putative σ receptor antagonists, such as MS-377 and BMY 14802, block the development and expression of methamphetamine-induced sensitization (Takahashi et al., 2000; Ujike et al., 1992). In addition, recent studies have shown that antagonism of σ receptors using either well established σ receptor antagonists, such as BD1063 and BD1047, or an antisense oligonucleotide attenuates the acute locomotor stimulatory effects of methamphetamine (Nguyen et al., 2005). Rats self-administering methamphetamine also exhibit an up-regulation of σ1 receptors in the midbrain, as compared to saline-treated or yoked controls (Stefanski et al., 2004), providing further support for an involvement of σ receptors in the actions of methamphetamine.
In contrast to accumulating evidence that σ receptors are involved in the stimulant effects of methamphetamine, the role of these proteins in the neurotoxic effects of methamphetamine remains unclear. Earlier studies provided equivocal results because they were performed while the field was still in its infancy (Terleckyj and Sonsalla, 1994), and involved σ receptor ligands which are now known to be non-selective or to act as agonists. Numerous studies, however, demonstrate cytotoxic effects of σ2 receptor agonists (Crawford and Bowen, 2002; Crawford et al., 2002; Ostenfeld et al., 2005) providing compelling evidence for a potential role of σ receptors in cell death processes, including methamphetamine-induced neurotoxicity.
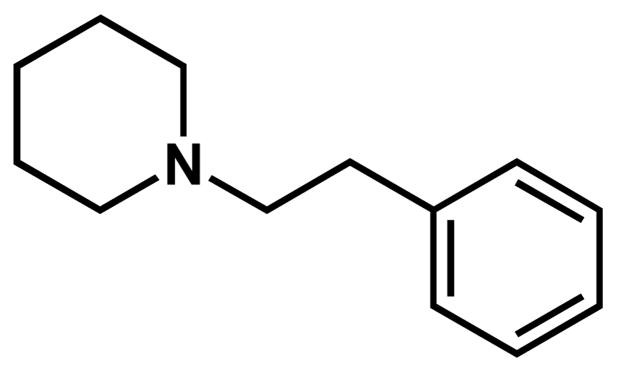
Therefore, in the present study, the involvement of σ receptors in the actions of methamphetamine was further evaluated using the putative σ receptor antagonist AC927 (N-phenethylpiperidine oxalate; Figure 1). AC927 was selected for this investigation because it can attenuate apoptosis in tumor cells through a σ receptor-mediated mechanism (Crawford et al., 2002). In addition to cytoprotective effects, AC927 has the potential to mitigate the locomotor stimulant effects of methamphetamine, in a manner similar to other well established σ receptor antagonists (Nguyen et al., 2005).
Figure 1.
Chemical structure of AC927 (N-phenethylpiperidine oxalate).
Therefore, in the present study, radioligand binding assays were conducted to evaluate the selectivity of AC927 for σ receptors. The capability of AC927 to attenuate methamphetamine-induced locomotor activity, hyperthermia, and neurotoxicity in the brains of mice were also determined. Finally, to assess whether the neuroprotective effects of AC927 depended on its ability to reduce methamphetamine-induced hyperthermia, the cytotoxicity of methamphetamine was determined in NG108-15 cells in the presence and absence of AC927 under temperature-controlled conditions. NG108-15 cells were used in this part of the investigation because they are neuronally-derived cells that have been employed by others to study σ receptor-mediated effects (Hayashi and Su, 2003 a, b). Together, the studies evaluate whether selective antagonism of σ receptors, using the prototypic ligand AC927, can mitigate the stimulant and toxic effects of methamphetamine.
2. Experimental procedures
2.1. Drugs and reagents
Methamphetamine hydrochloride was obtained from Research Biochemicals International (Natick, MA). AC927 was purchased from Sigma-Aldrich, Inc. (St. Louis, MO) as the free base, and converted to the oxalate salt (Maeda et al., 2002). Dopamine Enzyme Immunoassay kits were purchased from Rocky Mountain Diagnostics (Colorado Springs, CO).
2.2. Animals
Male, Sprague Dawley rats were used for the receptor binding studies (150–200 g, Harlan, Indianapolis, IN). Male, Swiss Webster mice (21–30 g, Harlan, Indianapolis, IN) were used for the behavioral experiments. The rodents were housed in groups of 2–6 with a 12:12-h light/dark cycle and ad libitum food and water. The animals were acclimated for one week before being used in experiments and they were randomly assigned to their treatment groups. All procedures were performed as approved by the Institutional Animal Care and Use Committees at the University of Mississippi.
2.3. Radioligand binding assays
The affinities of AC927 for σ receptor subtypes, monoamine transporters, and a select group of receptors and ion channels were determined either by us using procedures previously described (Matsumoto et al., 2002), or by the NIDA Treatment Discovery Program (TDP, Division of Treatment Research & Development) or NOVASCREEN (Hanover, MD). The assay conditions are briefly summarized in Table 1. For the assays conducted by us, twelve concentrations of AC927 (0.05–10,000 nM) were incubated for 120 min at 25° C for the σ receptor assays, 60 min at 25° C for the dopamine, 5-HT1A, α1-adrenergic and opioid receptor assays, 30 min at 37° C for the 5-HT2 receptor assays, 120 min at 4° C for the dopamine transporter assays, 90 min at 25° C for the serotonin transporter assays, and 60 min at 4° C for the N-methyl-D-aspartate (NMDA) receptor and norepinephrine transporter assays. All of the assays were terminated with the addition of ice-cold buffer and vacuum filtration through glass fiber filters. Additional details of the assays conducted by NOVASCREEN (Hanover, MD) are available through their website: www.novascreen.com/allassay.asp.
Table 1.
Binding affinities of AC927
| Radioligand | Nonspecific binding | Tissue | Ki | |
|---|---|---|---|---|
| Sigma receptors: | ||||
| σ1 | 5 nM [3H](+)-pentazocine | 10 μM haloperidol | Rat brain | 30 ± 2 |
| σ2 | 3 nM [3H]di-o-tolylguanidine | 10 μM haloperidol | Rat brain | 138 ± 18 |
| Monoamine transporters: | ||||
| Dopamine | 0.5 nM [3H]WIN 35,428 | 50 μM cocaine | Rat striatum | 2939 ± 452 |
| Serotonin | 0.2 nM [3H]paroxetine | 1.5 μM imipramine | Rat brainstem | >10,000 |
| Norepinephrine | 0.5 nM [3H]nisoxetine | 4 μM desipramine | Rat cerebral cortex | 8828 ± 1197 |
| Other receptors: | ||||
| Adenosine | 4.0 nM [3H]NECA | 1 μM NECA | Bovine striatum | >10,000b |
| Adrenergic, alpha-1 | 3 nM [3H]prazosin | 10 μM phentolamine | Rat brain | >10,000 |
| Adrenergic, alpha-2 | 1 nM [3H]RX 821002 | 1 μM phentolamine | Rat cerebral cortex | >10,000b |
| Adrenergic, beta | 2 nM [3H]DHA | 1 μM alprenolol | Rat cerebral cortex | >10,000b |
| Dopamine, non-selective | 0.3 nM [3H]spiperone | 1 μM spiperone | Bovine striatum | >10,000b |
| Dopamine D1 | 0.18 nM [3H]SCH 23390 | 1 μM SCH 23390 | LHD1 cells | >10,000a |
| Dopamine D2 | 5 nM [3H](-)-sulpiride | 1 μM haloperidol | Rat brain | >10,000 |
| 0.21 nM [3H]YM-09151-2 | 1 μM chlorpromazine | CHOp cells | >10,000a | |
| Dopamine D3 | 0.21 nM [3H]YM-09151-2 | 1 μM chlorpromazine | CHOp cells | >10,000a |
| GABA | 5 nM [3H]GABA | 5 μM GABA | Rat brain | >10,000 b |
| Melatonin | 70 pM [125I]2-Iodomelatonin | 0.1 μM 2-iodomelatonin | Chicken brain | >10,000 b |
| Muscarinic, central | 0.15 nM [3H]QNB | 0.1 μM atropine | Rat cerebral cortex | >10,000 b |
| Muscarinic, peripheral | 0.3 nM [3H]QNB | 0.1 μM atropine | Guinea pig bladder | >10,000 b |
| NMDA | 5 nM [3H]TCP | 10 μM cyclazocine | Rat brain | >10,000 |
| Opioid | 0.5 nM [3H]bremazocine | 10 μM levallorphan | Rat brain | >10,000 |
| 1 nM [3H]naloxone | 1 μM naloxone | Rat forebrain | >10,000b | |
| Serotonin, non-selective | 5 nM [3H]LSD | 10 μM methysergide | Rat cerebral cortex | >10,000b |
| 5-HT1A | 0.50 nM [3H]8-OH-DPAT | 1 μM dihydroergotamine | HA7 cells | 4985 ± 330a |
| 5-HT2 | 2 nM [3H]ketanserin | 1 μM mianserin | Rat brain | >10,000 |
| 5-HT2A | 0.40 nM [3H]ketanserin | 1 μM ketanserin | NIH-3T3-GF6 cells | 1129 ± 49a |
| 5-HT2C | 0.40 nM [3H]mesulergine | 10 μM mesulergine | NIH-3T3-Po cells | >10,000a |
| Ion channels: | ||||
| Calcium, type L (BTZ) | 5 nM [3H]diltiazem | 10 μM diltiazem | Rat cerebral cortex | >10,000 b |
| Calcium, type L (DHP) | 0.2 nM [3H]nitrendipine | 1 μM nifedipine | Rat cerebral cortex | >10,000 b |
| Calcium, type N | 0.01 nM [125I]ω-conotoxin GVIA | 0.1 μM ω-conotoxin GVIA | Rat cerebral cortex | >10,000 b |
| Chloride, GABA (TBOB) | 20 nM [3H]TBOB | 10 μM TBPS | Rat cerebral cortex | >10,000 b |
| Potassium, ATP-sensitive | 0.2 nM [3H]glibenclamide | 0.1 μM glibenclamide | Rat cerebral cortex | >10,000 b |
| Potassium, hERG | 40 nM [3H]astemizole | 100 μM terfenadine | CHO cells | >10,000 b |
| Sodium, site 2 | 2 nM [3H]batrachotoxin | 1 mM aconitine | Rat forebrain | >10,000 b |
Affinities (Ki in nM) were determined in tissue or cell homogenates. The values in this table represent the mean ± SEM from replicate assays. Values of >10,000 signify that there was less than 30% displacement of the radioligand at that concentration.
These values were determined by the NIDA Cocaine Treatment Discovery Program.
These values were determined by NOVASCREEN. All other values were determined in-house using the assay conditions described in the methods section. BTZ=benzothiazepine; DHA=dihydroalprenolol; DHP=dihydropyridine; GABA=γ-aminobutyric acid; LSD=lysergic acid diethylamide; NECA=5′-N-ethylcarboxamidoadenosine; QNB=quinuclidinyl benzilate; TBOB=t-butylbicycloorthobenzoate; TBPS=t-butylbicyclophosphorothionate; TCP=1-[1-)2-thienyl)cyclohexyl]piperidine
2.4. Locomotor activity measurements
Locomotor activity was measured as an index of the stimulant actions of methamphetamine using an automated activity monitoring system (San Diego Instruments, San Diego, CA). The mice were acclimated to the testing room for 30–60 min, and then habituated to the testing chambers for an additional 30 min. Each testing chamber was surrounded by two 16 × 16 photobeam arrays to detect the movements of the animals. Ambulatory, fine, and rearing movements were quantified to give an overall activity score. There were three parts to the study.
In the first part, the dose response curve for methamphetamine was determined. The mice (N=38) were injected with different doses of methamphetamine (0–1 mg/kg, i.p.), placed into the testing chambers, and activity quantified for 30 min.
In the second part, the dose response curve for AC927 was determined to identify a suitable dose to use in the subsequent antagonism study. The mice (N=26) were injected with different doses of AC927 (0–20 mg/kg, i.p.), placed into the testing chambers, and activity quantified for 30 min.
In the third part, the ability of AC927 to attenuate methamphetamine-induced locomotor activity was evaluated. The mice (N=70) were pretreated with either saline or AC927 (10 mg/kg, i.p.), followed 15 min later with a dose of methamphetamine (0–1 mg/kg, i.p.). The mice were returned to the testing chambers, and activity was quantified for the next 30 min.
2.5. Dopamine assays
The mice (N=64) were randomly assigned to one of the following treatment groups: (1) Saline + Saline; (2) Saline + Methamphetamine (1.25, 2.5 or 5 mg/kg); (3) AC927 (5, 10 or 20 mg/kg) + Methamphetamine (5 mg/kg); (4) AC927 (5, 10 or 20 mg/kg) + Saline. The first compound in each treatment combination (saline or AC927) was administered as a 15 min pretreatment to the second (saline or methamphetamine). All of the combinations of treatments were given (i.p.) at 2 h intervals, a total of four times.
The animals were sacrificed by decapitation and their brains removed one week after treatment to allow ample time for degeneration of dopamine nerve terminals to occur (Cappon et al., 2000). In addition, tissues from naïve mice (N=6) were collected as an additional control. The striatum and cerebellum were dissected from each of the mice and frozen in liquid nitrogen. The tissues were stored at −80° C for later analysis of dopamine content.
Brain striatal and cerebellar dopamine was quantified using a Dopamine Research Enzyme Immunoassay kit and protocols supplied by the manufacturer (Rocky Mountain Diagnostics, Colorado Springs, CO). Briefly, brain tissues were homogenized in 0.01 N HCl. Dopamine was first extracted, then acylated to N-acyldopamine using the buffer and reagents provided with the kit. Acylated dopamine from the tissue samples was then incubated with solid phase bound dopamine, dopamine antiserum, and antiserum buffer to compete for a fixed number of antiserum binding sites. Free antigen and free antigen-antiserum complexes were removed by washing. The antibody bound to the solid phase dopamine was detected using an anti-rabbit IgG-peroxidase conjugate with TMB as the substrate. The amount of antibody bound to the solid phase dopamine was measured by monitoring the reaction at 450 nm. The solid phase dopamine so measured was inversely proportional to the dopamine concentration of the tissue sample and was quantified relative to a standard curve of known concentrations.
2.6. Immunohistochemistry
To corroborate that the dopamine depletions reflected neurotoxic damage, striatal sections were evaluated for dopamine transporter expression, a well established marker of methamphetamine-induced neurotoxicity (Cadet et al., 2007; Davidson et al., 2001; Volz et al., 2007). The mice were randomly assigned to one of the following treatment groups (N=4/group): (1) Saline + Saline; (2) Saline + Methamphetamine (5 mg/kg); (3) AC927 (10 mg/kg) + Methamphetamine (5 mg/kg); (4) AC927 (10 mg/kg) + Saline. The treatments were administered as described for the dopamine assays at 2 h intervals, a total of four times.
One week later, the mice were perfused transcardially with 0.1 M phosphate buffered saline (pH 7.4), followed by 4% paraformaldehyde. The brains were further fixed overnight in 4% paraformaldehyde. Coronal sections (50 μm) of the fixed tissue were made throughout the rostral-caudal extent of the striatum using a cryostat, and processed in a free-floating state in 0.1 M Tris-HCl buffered saline (TBS, pH 7.5). The sections were treated with 0.3% H2O2 in TBS for 30 min at room temperature. The sections were then treated with TBS containing 0.2% Triton X-100 and 1.5% normal goat serum for 30 min at room temperature. Incubation of the sections with anti-rat DAT antibody (Chemicon International, Temecula, CA; MAB369, dilution 1:10,000) was performed for 36 h at 4° C. The labeled sections were then washed twice in TBS, and processed using Vectastain Elite ABC (Vector Laboratories, Burlingame, CA). Briefly, the sections were incubated with biotinylated secondary anti-rat antisera (diluted 1:200) in TBS-NBS for 60 min. This was followed by incubation of the sections with avidin-biotinylated peroxidase substrate in TBS for 60 min. The staining was then visualized by reacting with 3,3′-diaminobendine containing 0.01% H2O2 for 5 min.
The stained sections were mounted onto gelatin-coated slides and completely dried. The sections were then dehydrated, cleared, and coverslipped. The images were captured digitally using a Leica DMIL microscope (Leica Microsystems, Bannockburn, IL) and optical density readings were quantified in anterior regions of the striatum using Kodak ID image analysis software. To obtain the data point for each animal, the optical density readings from at least three sections were averaged.
2.7. Temperature measurements
The mice (N=69) were randomly assigned to treatment groups, which were the same as those described for the dopamine assays. All of the combinations of drug treatments were given (i.p.) at 2 h intervals, a total of four times. Core body temperature was measured 1 h following each injection (i.p.) of methamphetamine (or saline) with a Thermalert TH-S monitor (Physitemp Instruments Inc., Clifton, NJ). During the temperature measurements, mice were gently held at the base of the tail and a probe (RET-3) inserted approximately 2.5 cm past the rectum into the colon for 8–10 s until a rectal temperature was maintained for 3–4 s.
2.8. Cytotoxicity in NG108-15 cells
NG108-15 cells (American Type Culture Collection, Rockville, MD) were grown on T-75 mm2 culture flasks in Dulbecco’s modified eagle media (DMEM; Sigma Aldrich, St. Louis, MO) which was supplemented with 4500 mg/L glucose, L-glutamine, sodium bicarbonate, pyridoxine HCl, HAT medium supplement (Sigma Aldrich, St. Louis, MO), 10% bovine fetal serum (FBS) containing 1% antibiotic and antimycotic (Sigma Aldrich, St. Louis, MO) in a 5% CO2 incubator at 37° C. The cells were differentiated by reducing the FBS from 10% to 0.5% along with DMSO. Cells from passages 9–12 were used in the experiments. The cells were plated in 96 well plates in growth media for 24 h, after which the growth media was aspirated and replaced with differentiating media containing methamphetamine (0–300 μM) and/or AC927 (0–0.1 μM).
After 24 or 48 h, the morphology of the cells was rated as described by Bowen and coworkers (Vilner et al., 1995). The ratings (N, A, B, C, D) were then converted to a corresponding numerical cell morphology score (indicated in parentheses) to enable quantification of the effects: N (0) = no observable effect on cells, A (1) = significant proportion of cells exhibit loss of fine processes, B (2) = all cells have lost fine processes and some cells remain polygonal with coarse processes, while others become spindle shaped or round, C (3) = most or all cells have become round, D (4) = cell death with presence of cell debris.
2.9. Statistical analyses
For the radioligand binding assays, the data were analyzed using GraphPad Prism (San Diego, CA). Ki values were calculated using the Cheng-Prusoff equation and Kd values that were separately determined. The data from the locomotor measurements, dopamine assays, and immunohistochemical studies were evaluated using analysis of variance. Post-hoc analyses were conducted using either Dunnett’s tests for comparisons to controls or Tukey’s tests for pairwise comparisons. A repeated measures analysis of variance was used to evaluate whether there were significant differences in core body temperature among the treatment groups. Kruskal-Wallis non-parametric statistics, followed by Dunn’s multiple comparisons tests, were used to analyze the cytotoxicity data because the data were not normally distributed. For all of the statistical analyses, P < 0.05 was considered statistically significant.
3. Results
3.1. Radioligand binding
The affinities of AC927 for σ receptors, monoamine transporters, and a select group of other receptors and ion channels are summarized in Table 1. AC927 bound to both σ1 and σ2 subtypes with nanomolar affinities. In contrast, AC927 exhibited micromolar to negligible affinities for the other binding sites tested, thereby demonstrating a high degree of selectivity for σ receptors.
3.2. Locomotor activity
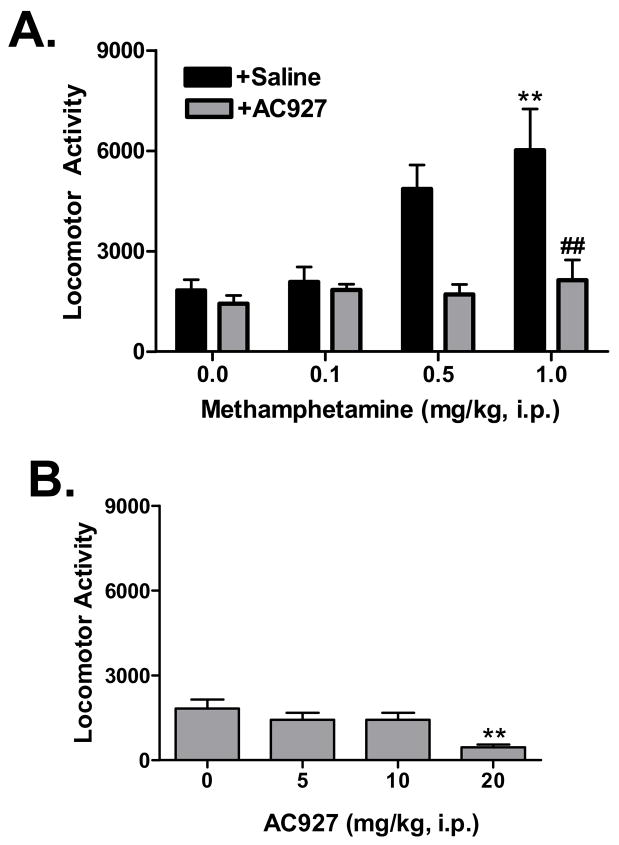
Methamphetamine produced a progressive increase in locomotor activity across the tested dose range (Figure 2A). One-way analysis of variance confirmed that the differences between the methamphetamine doses were statistically significant (F(3,34) =5.93, P < 0.005). Post-hoc analysis using Dunnett’s multiple comparison tests revealed that the 1 mg/kg dose of methamphetamine differed significantly from the saline control (q=3.61, P < 0.01).
Figure 2.
Effects of methamphetamine and AC927 on locomotor activity in mice. (A) Methamphetamine-induced locomotor activity in the absence and presence of AC927 antagonism. Male, Swiss Webster mice were pretreated with either saline (+Saline) or AC927 (+AC927, 10 mg/kg, i.p.), and then challenged 15 min later with a dose of methamphetamine (0–1 mg/kg, i.p.). Methamphetamine produced a dose dependent change in locomotor activity, which differed significantly in the presence of AC927. (B) Dose response curve for AC927 alone. AC927 produced a dose dependent reduction in locomotor activity in male, Swiss Webster mice. Post-hoc tests confirmed that the 20 mg/kg dose caused a significant decrease in activity, while the 10 mg/kg dose of AC927 produced effects that did not differ significantly from saline vehicle injections (0 mg/kg). The 10 mg/kg dose of AC927 was thus used in the antagonism portion of the study shown in panel (A). The data are represented as mean ± S.E.M. **P < 0.01 vs. saline; ##P < 0.01 vs. methamphetamine.
AC927 dose dependently decreased locomotor activity (Figure 2B). One-way analysis of variance showed that there was a significant difference between the AC927 doses (F(2,23)=7.45, P < 0.005). Post-hoc analysis using Dunnett’s multiple comparison tests revealed that the 20 mg/kg dose of AC927 (q=3.80, P < 0.01) differed significantly from the saline control. The 10 mg/kg dose of AC927 produced effects that did not differ significantly from the saline control (q=1.09, n.s.) and was thus chosen as the antagonist dose for further testing in combination with methamphetamine.
When AC927 (10 mg/kg, i.p.) was administered as a pretreatment, it significantly attenuated the locomotor stimulant actions of methamphetamine (Figure 2A). One-way analysis of variance confirmed a significant difference between the treatment groups (F(7,62)=6.55, P < 0.0001). Post-hoc analysis using Tukey-Kramer multiple comparison tests revealed there was a significant difference at the 1 mg/kg dose of methamphetamine in the presence vs. absence of AC927 (q=5.67, P < 0.01). All of the other post-hoc comparisons in the presence vs. absence of AC927 were not statistically significant, although notable trends were evident.
3.3. Dopamine levels
In naïve animals, basal dopamine levels in the striatum were 7.70 ± 0.17 ng/mg tissue, which is consistent with the range obtained using HPLC with electrochemical detection (Gruss and Braun, 2004; Siuciak et al., 2007). In naïve mice, basal dopamine levels in the cerebellum were 0.0037 ± 0.00034 ng/mg tissue. These very low levels of cerebellar dopamine were generally not detectable using traditional HPLC methods, but the values were similar to the few earlier reports in the literature (Gruss and Braun, 2004).
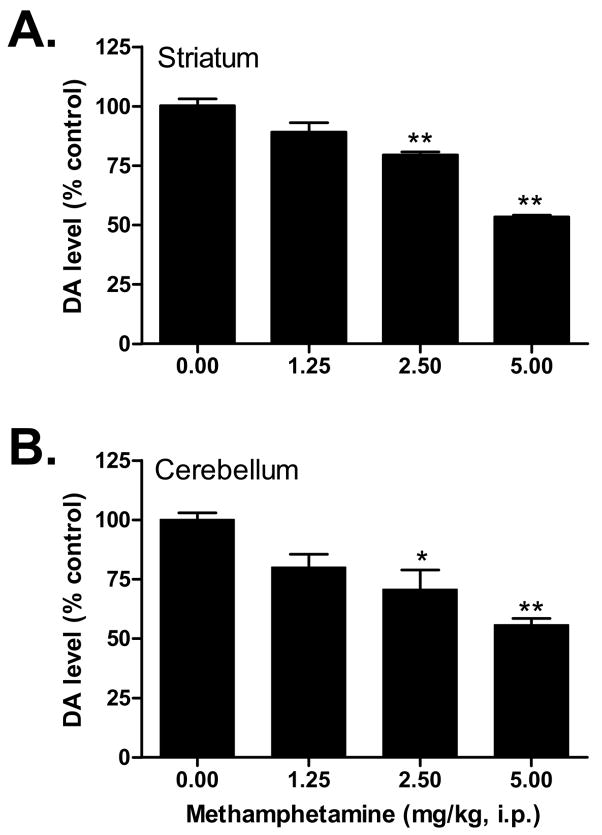
Similar to previous neurotoxicity studies, methamphetamine dose dependently decreased dopamine levels in the striatum and cerebellum (Figure 3). Analysis of variance confirmed significant differences between the methamphetamine doses in both the striatum (F(3,16)=20.22, P < 0.0001) and cerebellum (F(3,16)=5.89, P < 0.01). Post-hoc Dunnett’s tests revealed that the following doses of methamphetamine produced depletions in dopamine that differed significantly from the saline control group: in striatum, 2.5 mg/kg (q=3.57, P < 0.01), 5 mg/kg (q=7.35, P < 0.01) and in cerebellum, 2.5 mg/kg (q=2.96, P < 0.05), 5 mg/kg (q=4.08, P < 0.01).
Figure 3.
Dose response of methamphetamine on dopamine (DA) levels in the striatum (A) and cerebellum (B). Male, Swiss Webster mice were injected (i.p.) with methamphetamine (1.25–5 mg/kg) or saline (0 mg/kg) at 2 h intervals, a total of four times. Striatal and cerebellar dopamine levels were measured one week later and data were reported as mean ± S.E.M. *P < 0.05, **P < 0.01 vs. saline.
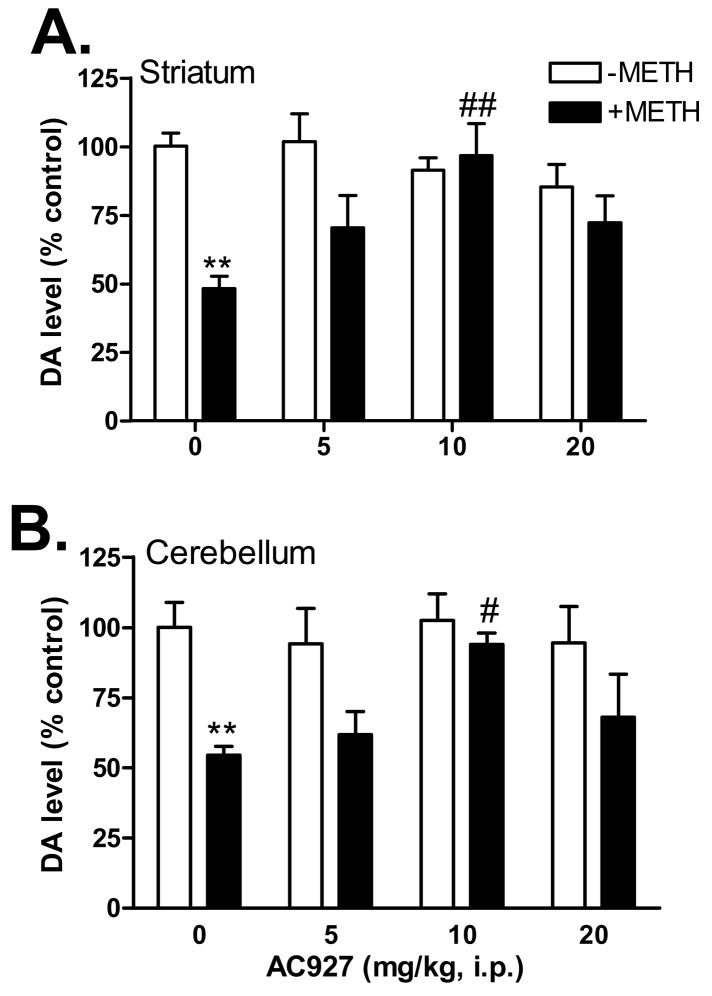
Administration of AC927 in the absence of methamphetamine had no significant effects on dopamine levels in the striatum and cerebellum of the mouse brain (Figure 4). Analysis of variance confirmed that there were no significant differences between the treatment groups in either the striatum (F(3,16)=1.10, n.s.) or the cerebellum (F(3,16)=0.14, n.s.). In contrast, pretreatment of mice with AC927 dose-dependently attenuated the dopamine depletions caused by a challenge dose of methamphetamine (5 mg/kg, i.p.) in both the striatum (F(4,19)=6.26, P < 0.005) and cerebellum (F(4,20)=5.10, P < 0.01). Post-hoc Tukey’s multiple comparison tests confirmed that pretreatment with the 10 mg/kg dose of AC927 significantly attenuated the striatal (q=5.58, P < 0.01) and cerebellar (q=4.55, P < 0.05) dopamine depletions caused by methamphetamine. Moreover, the dopamine levels of mice treated with AC927 (10 mg/kg) + methamphetamine did not differ significantly from the saline control groups.
Figure 4.
Effects of AC927 (0–20 mg/kg, i.p.) on methamphetamine (5 mg/kg, i.p.)-induced dopamine damage in the striatum (A) and cerebellum (B). Male, Swiss Webster mice were pretreated with saline, or AC927 (5, 10, 20 mg/kg, i.p.). The mice were then challenged 15 min later with saline (−METH; 0 mg/kg, i.p.) or methamphetamine (+METH; 5 mg/kg, i.p.). This schedule of treatment was repeated at 2 h intervals, a total of four times. Striatal and cerebellar dopamine levels were measured one week later and data were reported as mean ± S.E.M. **P < 0.01 vs. saline; #P < 0.05, ##P < 0.01 vs. methamphetamine.
3.4. Immunohistochemistry
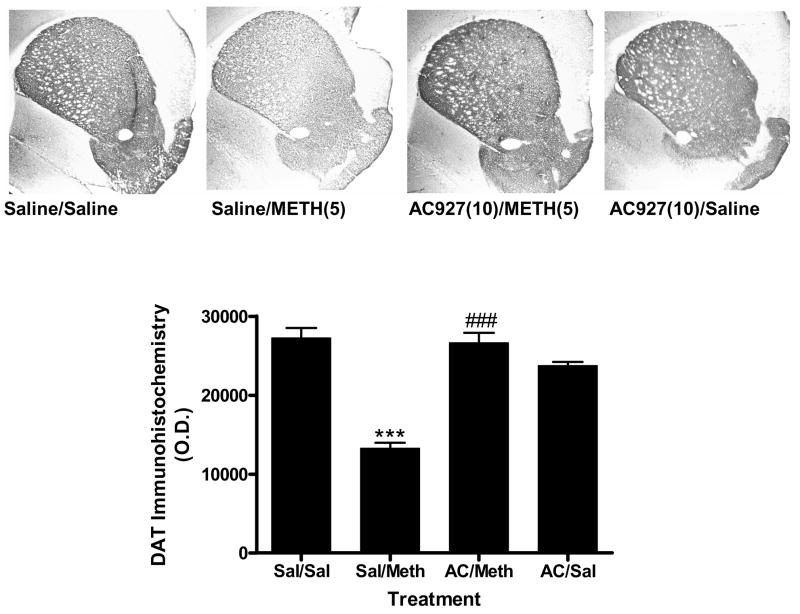
Figure 5 illustrates the effects of methamphetamine and AC927 on DAT immunoreactivity in the mouse striatum. Analysis of variance revealed a significant difference between the treatment groups (F(3,12)=43.76, P < 0.0001). Post-hoc Tukey’s multiple comparison tests confirmed that methamphetamine caused a significant reduction in DAT immunoreactivity (q=14.26, P < 0.001). Pretreatment with AC927 significantly attenuated the methamphetamine-induced neurotoxicity (q=13.63, P < 0.001), whereas treatment with AC927 alone had no significant effects on DAT expression (q=3.56; n.s.).
Figure 5.
Effects of methamphetamine and AC927 on dopamine transporter (DAT) immunoreactivity in the mouse striatum. Male, Swiss Webster mice were pretreated with saline or AC927 (10 mg/kg, i.p.). The mice were then challenged 15 min later with saline or methamphetamine (5 mg/kg, i.p.). This schedule of treatment was repeated at 2 h intervals, a total of four times. One week later, the brains were removed and processed for DAT immunoreactivity. A representative section from each treatment group is shown, along with average optical density readings (mean ± S.E.M.). There was a significant decrease in DAT immunoreactivity in the methamphetamine treatment group (***P < 0.001), which was attenuated by pretreatment with AC927 (###P < 0.001).
3.5. Temperature measurements
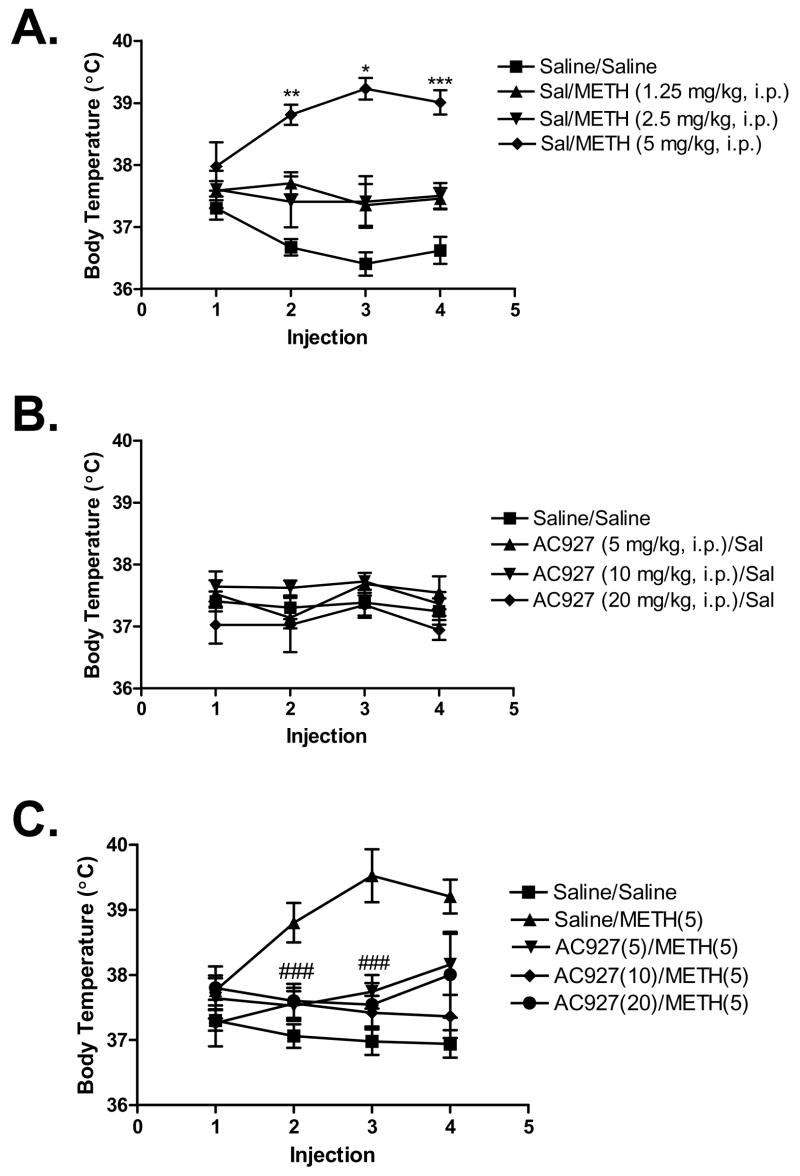
Figure 6 illustrates the effects of methamphetamine and AC927 on core body temperature. Methamphetamine increased body temperature in a dose dependent manner (Figure 6A), with the effects at the higher doses of methamphetamine being statistically significant (after 2nd injection, F(3,20)=4.99, P < 0.01, 3rd injection, F(3,20)=3.23, P < 0.05 and 4th injection, F(3,20)=8.71, P < 0.001). AC927, on the other hand, did not significantly change core body temperature at any of the given doses (Figure 6B). However, pretreatment with AC927 significantly attenuated the ability of methamphetamine to produce its hyperthermic effects (Figure 6C; after 2nd injection, F(4,20)=5.86, P < 0.005 and 3rd injection, F(4,20)=9.12, P < 0.0005).
Figure 6.
Effects of methamphetamine and AC927 on body temperature. (A) Methamphetamine-induced hyperthermia. Male, Swiss Webster mice were injected with methamphetamine (1.25–5 mg/kg, i.p.) or saline at 2 h intervals, a total of four times. Core body temperature was measured 1 h after each injection and data were reported as mean ± S.E.M. (B) Effects of AC927 on body temperature. Male, Swiss Webster mice were injected with AC927 (5–20 mg/kg, i.p.) or saline at 2 h intervals, a total of four times. Core body temperature was measured 1 h after each injection. No significant changes in body temperature were noted after administration of AC927. (C) Effects of AC927 (0–20 mg/kg, i.p.) on methamphetamine (5 mg/kg, i.p.)-induced hyperthermia. Male, Swiss Webster mice were pretreated with saline or AC927 (5, 10, 20 mg/kg, i.p.). The mice were then challenged 15 min later with saline (0 mg/kg, i.p.) or methamphetamine (5 mg/kg, i.p.). This schedule of treatment was repeated at 2 h intervals, a total of four times. Core body temperature was measured 1 h after each injection and data were reported as mean ± S.E.M. *P < 0.05, **P < 0.01, ***P < 0.001 vs. saline; ###P < 0.005 vs. methamphetamine.
3.6. Cytotoxicity in NG-108 cells
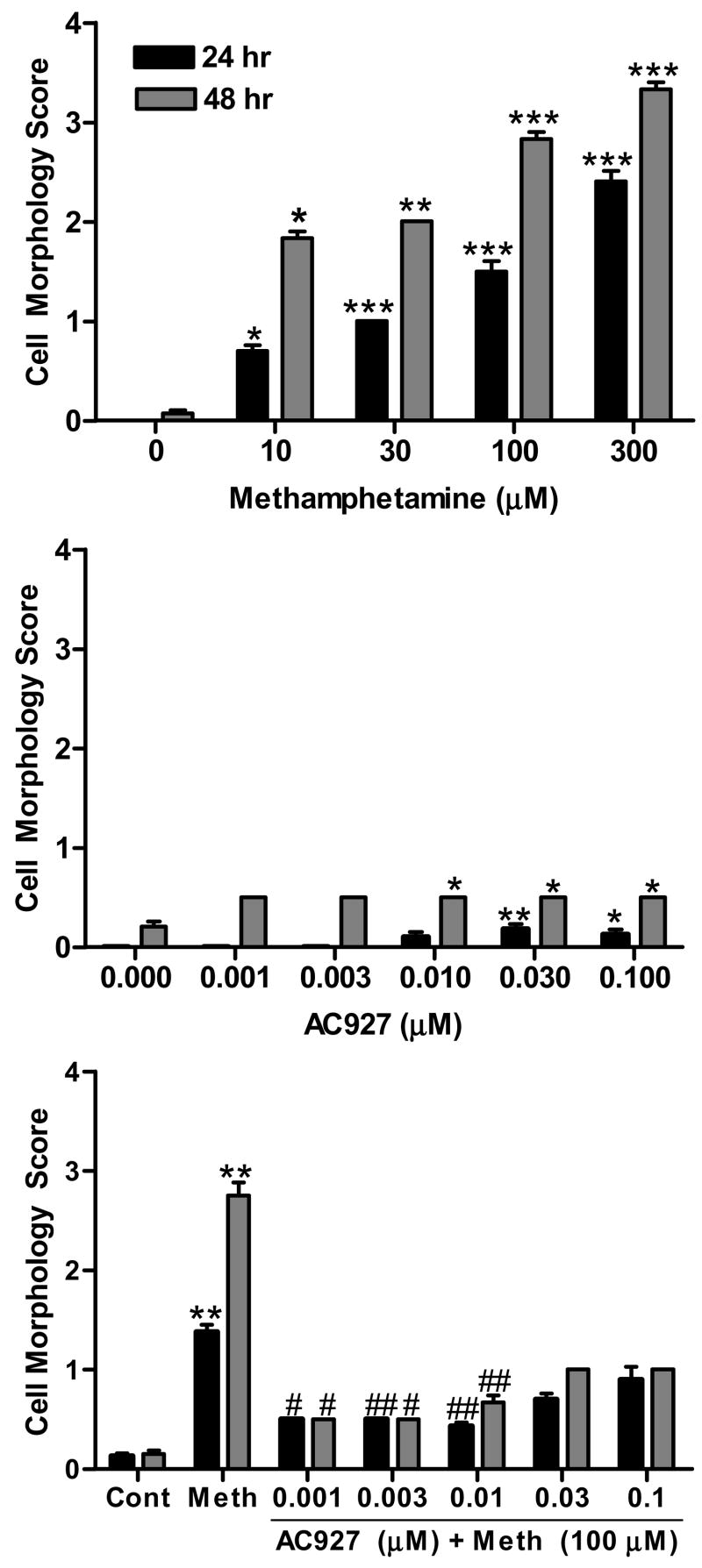
Similar to its effects in vivo, methamphetamine produced a concentration dependent increase in neurotoxicity in vitro (Figure 7A); this effect was statistically significant at both the 24 h (KW = 89.02, P < 0.0001) and 48 h (KW = 56.15, P < 0.0001) time points. Dunn’s multiple comparisons post-hoc tests confirmed that all of the concentrations of methamphetamine were significantly different from the control group: 10 μM (24 h, P < 0.05), 30 μM (24 h, P < 0.001; 48 h, P < 0.05), 100 μM (24 and 48 h, P < 0.001), 300 μM (24 and 48 h, P < 0.001). As shown in Figure 7B, AC927 alone produced small, but statistically significant, changes on cell morphology at 24 h (KW=17.92, P < 0.005) and 48 h (KW=22.01, P < 0.0005). Post-hoc comparisons showed that the following concentrations of AC927 differed significantly from the controls: 0.01 μM (48 h, P < 0.01), 0.03 μM (24 and 48 h, P < 0.01), 0.1 μM (24 h, P < 0.05; 48 h, P < 0.01). Co-incubation of AC927 with methamphetamine significantly attenuated methamphetamine-induced cytotoxicity (Figure 7C), at both the 24 h (KW = 112.23, P < 0.0001) and 48 h (KW = 96.59, P < 0.0001) time points. Dunn’s multiple comparison’s post-hoc tests confirmed that 100 μM of methamphetamine produced significant cytotoxicity relative to the controls (24 and 48 h, P < 0.001). More importantly, co-exposure with the following concentrations of AC927 significantly attenuated the cytotoxicity evoked by methamphetamine: 0.001 μM (24 and 48 h, P < 0.05), 0.003 μM (24 h, P < 0.001; 48 h, P < 0.05), 0.01 μM (24 and 48 h, P < 0.001).
Figure 7.
Effects of methamphetamine and AC927 on NG108-15 cell morphology. (A) Methamphetamine-induced cytotoxicity. NG108-15 cells were incubated with various concentrations of methamphetamine for 24 or 48 h. Cytotoxicity was rated using a standardized scoring system. (B) Effects of AC927 effects on cell morphology. Cells were incubated with various concentrations of AC927 and cell morphology rated after 24 or 48 h of exposure. (C) Effects of AC927 (0.001–0.1 μM) on methamphetamine (100 μM)-induced cytotoxicity. Co-incubation with AC927 significantly attenuated the cytotoxic effects of methamphetamine. The data are represented as mean ± S.E.M. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control; #P < 0.05, ##P < 0.001 vs. methamphetamine.
4. Discussion
These studies demonstrate that AC927 is a highly selective σ receptor ligand with the ability to attenuate a number of methamphetamine-induced effects, including hyperlocomotion, hyperthermia, dopamine damage in the brain, and cytotoxicity in a neuronally-derived cell line. The preferential affinity of AC927 for σ1 and σ2 receptors, compared to 29 other receptors, transporters and ion channels, suggests that its actions are most likely mediated through σ receptors.
The ability of AC927 to attenuate the locomotor stimulant effects of methamphetamine is consistent with earlier reports of the involvement of each of the subtypes, σ1 and σ2, in locomotor behavior. Previous studies have shown that diminution of σ1 receptor function using sequence specific antisense oligonucleotides or σ1-preferring antagonists significantly attenuates locomotor activity under a variety of conditions, including methamphetamine-induced hyperactivity (Nguyen et al., 2005). The known ability of σ2 receptor agonists to produce motor activating effects (Walker et al., 1993) also supports the likelihood that σ2 receptor antagonism contributes to the attenuation of methamphetamine-induced locomotor activity by AC927. When the effects of AC927 on locomotor activity were measured by itself, the compound produced a dose dependent reduction, which was observed at doses that exceeded those that were used in the antagonism studies. This pattern of results suggests that some level of basal locomotor activity is mediated through σ receptors, in addition to the role these receptors play in modifying methamphetamine-induced effects.
The actions of AC927 against the locomotor stimulant effects of methamphetamine were similar to those observed against cocaine, another psychomotor stimulant with significant affinity for σ receptors (Matsumoto et al., 2003). AC927 as well as other σ1 and σ2 preferring antagonists, each have been shown to significantly attenuate the acute locomotor stimulant effects of cocaine (Matsumoto and Mack, 2001; Matsumoto et al., 2002, 2003). In addition, the selective σ receptor antagonist BD1063 has also been shown to attenuate the locomotor stimulant effects of MDMA (3,4-methylenedioxymethamphetamine) through competitive antagonism of σ1 receptors (Brammer et al., 2006). Since MDMA, cocaine and methamphetamine all have measurable affinities for σ receptors (Brammer et al., 2006; Matsumoto et al., 2003; Nguyen et al., 2005), these receptors may represent a common, but relatively unrecognized, target through which psychostimulants mediate some of their actions.
The present experiments also evaluated the effects of AC927 on methamphetamine-induced dopamine damage and hyperthermia in mice. Similar to earlier reports, methamphetamine produced a significant decrease in striatal and cerebellar dopamine in a dose dependent manner (Bowyer et al., 1994; Davidson et al., 2001; Volz et al., 2007). AC927 itself did not have an effect on dopamine levels under the conditions used in the present study. However, when AC927 was used as a pretreatment, it significantly attenuated methamphetamine-induced dopamine depletions. To corroborate that the dopamine depletions reflected neurotoxic damage, dopamine transporter expression was measured in the striatum using immunohistochemical approaches. This well validated marker of methamphetamine-induced neurotoxicity (Volz et al., 2007) confirmed the ability of AC927 to protect against methamphetamine-induced damage.
Temperature is also known to influence methamphetamine-induced dopamine neurotoxicity, with hyperthermia exacerbating the damage (Cadet et al., 2007; Xie et al., 2000) and hypothermia providing neuroprotection (Bowyer et al., 1994; Miller and O’Callaghan, 1994). Pretreatment of mice with AC927 significantly attenuated methamphetamine-induced hyperthermia. However, when administered alone, AC927 had no significant effects on body temperature, suggesting that nonspecific effects on body temperature are unlikely to account for the protective actions of AC927. This interpretation is supported by in vitro data in which experimental conditions controlled for temperature. In NG-108 cells that were maintained at a constant 37° C throughout the experiments, methamphetamine produced the expected concentration- and time-dependent increases in cytotoxicity, which could be attenuated in the presence of AC927. This demonstrates that the cytoprotective effects of AC927 can occur independently of changes in temperature, a finding that is consistent with earlier reports of cytoprotective actions of σ receptor antagonists in tumor cells (Crawford and Bowen, 2002; Vilner and Bowen, 2000).
The precise mechanisms mediating the neuroprotective effects of AC927 have yet to be determined, and are likely to involve both systems- and cellular-based processes. At the systems level, the presence of σ receptors in brain regions that control temperature regulation, such as the hypothalamus (Gundlach et al., 1986) may contribute to the ability of AC927 to attenuate methamphetamine-induced hyperthermia. An earlier study showed that the selective σ receptor agonist, di-o-tolylguanidine (DTG), produces hypothermia in rats (Rawls et al., 2002). The established σ receptor antagonist BD1047 did not affect basal body temperature, but attenuated the DTG-induced changes (Rawls et al., 2002). Together, the results suggest that σ receptors are not critically involved in the tonic maintenance of body temperature, and instead have a modulatory role in thermoregulation.
Several related cellular mechanisms may also have contributed to the observed pattern of results. First, AC927 may inhibit the release of monoamines in neuronal pathways that are involved in the actions of methamphetamine. The ability of methamphetamine to enhance extracellular dopamine is well established (Cadet et al., 2007; Volz et al., 2007), and σ receptor agonists have likewise been shown to facilitate the release of dopamine (Basianetto et al., 1995; Derbez et al., 2002). Under toxic conditions, auto oxidation of dopamine can lead to increases in reactive oxygen species (Cadet et al., 2007; Volz et al., 2007; Yamamoto and Zhu, 1998). AC927 may thus reduce the potential auto oxidation of dopamine by decreasing methamphetamine-induced dopamine release. Such a mechanism would be similar to that reported for BIMU-8, another σ receptor antagonist, which attenuated amphetamine-stimulated dopamine release in vitro (Derbez et al., 2002).
It is also possible that AC927 may modulate oxidative stress responses and inhibit the mobilization of intracellular calcium, thereby preventing apoptosis and possibly nerve ending damage. Increased levels of reactive oxygen species have been shown to follow exposure of tumor cells to a σ2 receptor agonist (Ostenfeld et al., 2005). In contrast, σ receptor antagonists can protect against neural damage in experimental models of stroke and traumatic brain injury where the compromise of energy demands leads to cell injury and death (Church and Andrew, 2005; Schetz et al., 2007). It is therefore possible that reduced oxygen radical formation occurs in the presence of σ receptor antagonists, and consequently, AC927 is able to attenuate methamphetamine-induced dopamine damage and hyperthermia.
In addition, calcium-mediated mechanisms that contribute to apoptosis can be activated by σ2 receptor agonists (Crawford and Bowen, 2002; Vilner and Bowen, 2000). These mechanisms include increases in ceramide levels and concomitant decreases in sphingomyelin (Crawford et al., 2002). AC927 and other σ receptor antagonists block these effects (Bowen, 2007), suggesting that σ2 receptors are involved in the modulation of signaling pathways that regulate cell survival.
The current body of literature suggests that σ2 receptor antagonists can mitigate responses that lead to cell death. However, the influence of the σ1 subtype in these processes appears quite distinct. Accumulating evidence suggests that σ1-mediated neuroprotection is associated with agonist, rather than antagonist actions. Neuroprotective effects of σ1 receptor agonists have been reported in a variety of in vitro and in vivo models (Hayashi and Su, 2007; Katnik et al., 2006; Meunier et al., 2006; Nakazawa et al., 1998; Tchedre and Yorio, 2008; Yang et al., 2007). However, there are also reports of σ1 receptor antagonists providing neuroprotection in vivo (Church and Andrew, 2005; Schetz et al., 2007), suggesting that the influence of these receptors on cell death cascades may vary depending on the pathways and specific mechanisms manipulated, as well as whether the studies are conducted in vitro or in vivo.
The ability of AC927 to mitigate a range of neurotoxic actions of methamphetamine in vivo and in vitro is compelling, but future studies to delineate the cellular mechanisms through which each σ receptor subtype can convey neuroprotective actions are warranted. For example, it may be possible that certain subtypes predominate in mitochondrial vs. endoplasmic rerticulum stress pathways, both of which have been implicated in methamphetamine-induced neurotoxicity (Jayanthi et al., 2004), or at different time points relative to methamphetamine exposure. It will also be important to validate findings both in vitro and in vivo as differences between these two conditions may promote divergent effects through distinct mechanisms.
In summary, the data demonstrate that AC927 exhibits a high degree of selectivity for σ receptors and attenuates the hyperthermic, neurotoxic, and stimulant effects of methamphetamine. Further studies of AC927 and other σ receptor antagonists, particularly those that exhibit specificity for the σ1 and σ2 subtypes, are needed to fully elucidate the mechanisms through which they mitigate the actions of methamphetamine, and other drugs of abuse.
Acknowledgments
5. Role of funding source
Funding for this study was provided by the National Institutes of Health. The NIH had no further role in the design of the study; in the collection, analysis and interpretation of the data; in writing the report; and in deciding to submit the paper for publication.
This work was supported by the National Institute on Drug Abuse (DA11979, DA13978). Andrew Coop is the recipient of an Independent Scientist Award from the National Institute on Drug Abuse (K02 DA19634).
Footnotes
6. Contributors
Rae R. Matsumoto designed the studies, supervised the data collection and analysis, and wrote the paper. Jamaluddin Shaikh performed the in vivo neurotoxicity studies and related data analysis. Lisa L. Wilson performed the locomotor behavioral studies and assisted with the related data analysis. Shreedeepalakshmi Vedam conducted the in vitro cytotoxicity studies and assisted with the data analysis. Andrew Coop synthesized AC927 and obtained some of the radioligand binding data.
7. Conflict of interest
No conflicts of interest exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aydar E, Palmer CP, Klyachko VA, Jackson MB. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron. 2002;34:399–410. doi: 10.1016/s0896-6273(02)00677-3. [DOI] [PubMed] [Google Scholar]
- Barr AM, Paneka WJ, MacEwan GW, Thornton AE, Lang DJ, Honer WG, Lecomte T. The need for speed: an update on methamphetamine addiction. Journal of Psychiatry Neuroscience. 2006;31:301–313. [PMC free article] [PubMed] [Google Scholar]
- Bastianetto S, Roquier L, Perrault G, Sanger DJ. DTG-induced circling behaviour in rats may involve the interaction between σ sites and nigro-striatal dopaminergic pathways. Neuropharmacology. 1995;34:281–287. doi: 10.1016/0028-3908(94)00156-m. [DOI] [PubMed] [Google Scholar]
- Booth RG, Baldessarini RJ. (+)−6,7-Benzomorphan sigma ligands stimulate dopamine synthesis in rat corpus striatum tissue. Brain Research. 1991;557:349–352. doi: 10.1016/0006-8993(91)90159-s. [DOI] [PubMed] [Google Scholar]
- Bowen WD. σ2 Receptors: Regulation of cell growth and implications for cancer diagnosis and therapeutics. In: Matsumoto RR, Bowen WD, Su T-P, editors. Sigma Receptors: Chemistry, Cell Biology and Clinical Implications. Springer; New York: 2007. pp. 215–235. [Google Scholar]
- Bowyer JF, Davis DL, Schmued L, Broening HW, Newport GD, Slikker W, Jr, Holson RR. Further studies of the role of hyperthermia in methamphetamine neurotoxicity. Journal of Pharmacology and Experimental Therapeutics. 1994;268:1571–1580. [PubMed] [Google Scholar]
- Brammer MK, Gilmore DL, Matsumoto RR. Interactions between 3,4-methylenedioxymethamphetamine (MDMA) and σ1 receptors. European Journal of Pharmacology. 2006;553:141–145. doi: 10.1016/j.ejphar.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN, Jayanthi S, Lyles J. Neurotoxicity of substituted amphetamines: molecular and cellular mechanisms. Neurotoxicity Research. 2007;11:183–202. doi: 10.1007/BF03033567. [DOI] [PubMed] [Google Scholar]
- Cappon GD, Pu C, Vorhees CV. Time-course of methamphetamine-induced neurotoxicity in rat caudate-putamen after single-dose treatment. Brain Research. 2000;863:106–111. doi: 10.1016/s0006-8993(00)02107-7. [DOI] [PubMed] [Google Scholar]
- Church AJ, Andrew RD. Spreading depression expands traumatic brain injury in neocortical brain slices. Journal of Neurotrauma. 2005;22:277–290. doi: 10.1089/neu.2005.22.277. [DOI] [PubMed] [Google Scholar]
- Crawford KW, Bowen WD. Sigma-2 receptor agonists activate a novel apoptotic pathway and potentiate antineoplastic drugs in breast tumor cell lines. Cancer Research. 2002;62:313–322. [PubMed] [Google Scholar]
- Crawford KW, Coop A, Bowen WD. σ2 Receptors up regulate changes in sphingolipid levels in breast tumor cells. European Journal of Pharmacology. 2002;443:207–209. doi: 10.1016/s0014-2999(02)01581-9. [DOI] [PubMed] [Google Scholar]
- Davidson C, Gow AJ, Lee TH, Ellinwood EH. Methamphetamine neurotoxicity: necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain Research Reviews. 2001;36:1–22. doi: 10.1016/s0165-0173(01)00054-6. [DOI] [PubMed] [Google Scholar]
- Derbez AE, Mody RM, Werling LL. σ2-Receptor regulation of dopamine transporter via activation of protein kinase C. Journal of Pharmacology and Experimental Therapeutics. 2002;301:306–314. doi: 10.1124/jpet.301.1.306. [DOI] [PubMed] [Google Scholar]
- Gebreselassie D, Bowen WD. σ2 Receptors are specially localized to lipid rafts in rat liver membranes. European Journal of Pharmacology. 2004;493:19–28. doi: 10.1016/j.ejphar.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Gruss M, Braun K. Age- and region-specific imbalances of basal amino acids and monoamine metabolism in limbic regions of female Fmr1 knock-out mice. Neurochemistry International. 2004;45:81–88. doi: 10.1016/j.neuint.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Guitart X, Codony X, Monroy X. Sigma receptors: biology and therapeutic potential. Psychopharmacology. 2004;174:301–319. doi: 10.1007/s00213-004-1920-9. [DOI] [PubMed] [Google Scholar]
- Gundlach AL, Largent BL, Snyder SH. Autoradiographic localization of sigma receptor binding sites in guinea pig and rat central nervous system with (+)3H-3-(3-hydroxyphenyl)-N-(1-propyl)piperidine. Journal of Neuroscience. 1986;6:1757–1770. doi: 10.1523/JNEUROSCI.06-06-01757.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP. σ1 Receptors (σ1 binding sites) form raft-like microdomains and target lipid droplets on the endoplasmic reticulum: roles in endoplasmic reticulum lipid compartmentalization and export. Journal of Pharmacology and Experimental Therapeutics. 2003a;306:718–725. doi: 10.1124/jpet.103.051284. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Intracellular dynamics of σ1 receptors (σ1 binding sites) in NG108-15 cells. Journal of Pharmacology and Experimental Therapeutics. 2003b;306:726–733. doi: 10.1124/jpet.103.051292. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca2+ signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- Hellewell SB, Bruce A, Feinstein G, Orringer J, Williams W, Bowen WD. Rat liver and kidney contain high densities of sigma-1 and sigma-2 receptors. Characterization by ligand binding and photoaffinity labeling. European Journal of Pharmacology, Molecular Pharmacology Section. 1994;268:9–18. doi: 10.1016/0922-4106(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Itzhak Y. Repeated methamphetamine-treatment alters brain σ receptors. European Journal of Pharmacology. 1993;230:243–244. doi: 10.1016/0014-2999(93)90810-5. [DOI] [PubMed] [Google Scholar]
- Jayanthi S, Deng X, Noailles PA, Ladenheim B, Cadet JL. Methamphetamine induces neuronal apoptosis via cross-talks between endoplasmic reticulum and mitochondria-dependent death cascades. FASEB Journal. 2004;18:238–251. doi: 10.1096/fj.03-0295com. [DOI] [PubMed] [Google Scholar]
- Katnik C, Guerrero WR, Pennypacker KR, Herrera Y, Cuevas J. Sigma-1 receptor activation prevents intracellular calcium dysregulation in cortical neurons during in vitro ischemia. Journal of Pharmacology and Experimental Therapeutics. 2006;319:1355–1365. doi: 10.1124/jpet.106.107557. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Mack AL. (±)-SM 21 attenuates the convulsive and locomotor stimulatory effects of cocaine in mice. European Journal of Pharmacology. 2001;417:R1–2. doi: 10.1016/s0014-2999(01)00891-3. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, McCracken KA, Pouw B, Zhang Y, Bowen WD. Involvement of sigma receptors in the behavioral effects of cocaine: evidence from novel ligands and antisense oligodeoxynucleotides. Neuropharmacology. 2002;42:1043–1055. doi: 10.1016/s0028-3908(02)00056-4. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Liu Y, Lerner M, Howard EW, Brackett DJ. Sigma receptors: potential medications development target for anti-cocaine agents. European Journal of Pharmacology. 2003;469:1–12. doi: 10.1016/s0014-2999(03)01723-0. [DOI] [PubMed] [Google Scholar]
- Maeda DY, Williams W, Kim WE, Thatcher LN, Bowen WD, Coop A. N-Arylalkylpiperidines as high affinity sigma-1 and sigma-2 receptor ligands: phenylpropylamines as potential leads for sigma-2 agents. Bioorganic Medicinal Chemistry Letters. 2002;12:497–500. doi: 10.1016/s0960-894x(01)00788-0. [DOI] [PubMed] [Google Scholar]
- Mei J, Pasternak G. Molecular cloning and pharmacological characterization of the rat sigma1 receptor. Biochemical Pharmacology. 2001;62:349–355. doi: 10.1016/s0006-2952(01)00666-9. [DOI] [PubMed] [Google Scholar]
- Meunier J, Ieni J, Maurice T. The anti-amnesic and neuroprotective effects of donepezil against amyloid β25–35 peptide-induced toxicity in mice involve an interaction with the σ1 receptor. British Journal of Pharmacology. 2006;149:998–1012. doi: 10.1038/sj.bjp.0706927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DB, O’Callaghan JP. Environment-, drug- and stress-induced alterations in body temperature affect the neurotoxicity of substituted amphetamines in the C57BL/6J mouse. Journal of Pharmacology and Experimental Therapeutics. 1994;270:752–760. [PubMed] [Google Scholar]
- Nakazawa M, Matsuno K, Mita S. Activation of σ1 receptor subtype leads to neuroprotection in the rat primay neuronal cultures. Neurochemistry International. 1998;32:337–343. doi: 10.1016/s0197-0186(97)00105-8. [DOI] [PubMed] [Google Scholar]
- Nguyen EC, McCracken KA, Pouw B, Matsumoto RR. Involvement of sigma receptors in the actions of methamphetamine: receptor binding and behavioral studies. Neuropharmacology. 2005;49:638–645. doi: 10.1016/j.neuropharm.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Ostenfeld MS, Fehrenbacher N, Hoyer-Hansen M, Thomsen C, Farkas T, Jaattela M. Effective tumor cell death by sigma-2 receptor ligand siramesine involves lysosomal leakage and oxidative stress. Cancer Research. 2005;65:8975–8983. doi: 10.1158/0008-5472.CAN-05-0269. [DOI] [PubMed] [Google Scholar]
- Prasad PD, Li HW, Fei YJ, Ganapathy ME, Fujita T, Plumley LH, Yang-Feng TL, Leibach FH, Ganapathy V. Exon-intron structure, analysis of promoter region, and chromosomal localization of the human type 1 sigma receptor gene. Journal of Neurochemistry. 1998;70:443–451. doi: 10.1046/j.1471-4159.1998.70020443.x. [DOI] [PubMed] [Google Scholar]
- Rawls SM, Baron DA, Geller EB, Adler MW. Sigma sites mediate DTG-evoked hypothermia in rats. Pharmacology Biochemistry Behavior. 2002;73:779–786. doi: 10.1016/s0091-3057(02)00903-6. [DOI] [PubMed] [Google Scholar]
- Schetz JA, Perez E, Liu R, Chen S, Lee I, Simpkins JW. A prototypical sigma-1 receptor antagonist protects against brain ischemia. Brain Research. 2007;181:1–9. doi: 10.1016/j.brainres.2007.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuciak JA, McCarthy SA, Chapin DS, Reed TM, Vorhees CV, Repaske DR. Behavioral and neurochemical characterization of mice deficient in the phosphodiesterase-1B (PDE1B) enzyme. Neuropharmacology. 2007;53:113–124. doi: 10.1016/j.neuropharm.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Stefanski R, Justinova Z, Hayashi T, Takebayashi M, Goldberg SR, Su TP. Sigma1 receptor upregulation alter chronic methamphetamine self-administration in rats: a study with yoked controls. Psychopharmacology. 2004;175:68–75. doi: 10.1007/s00213-004-1779-9. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Miwa Y, Horikomi K. Involvement of σ1 receptors in methamphetamine-induced behavioral sensitization in rats. Neuroscience Letters. 2000;289:21–24. doi: 10.1016/s0304-3940(00)01258-1. [DOI] [PubMed] [Google Scholar]
- Tchedre KT, Yorio T. Sigma-1 receptors protect RGC-5 cells from apoptosis by regulating intracellular calcium, Bax levels, and caspase-3 activation. Investigative Ophthalmology and Visual Science. 2008 doi: 10.1167/iovs.07-1101. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Terleckyj I, Sonsalla PK. The sigma receptor ligand (+/−)BMY 14802 prevents methamphetamine-induced dopaminergic neurotoxicity via interactions at dopamine receptors. Journal of Pharmacology and Experimental Therapeutics. 1994;269:44–50. [PubMed] [Google Scholar]
- Ujike H, Okamura K, Zushi Y, Akiyama K, Otsuki S. Persistent supersensitivity of σ receptors develops during methamphetamine treatment. European Journal of Pharmacology. 1992;211:323–328. doi: 10.1016/0014-2999(92)90388-k. [DOI] [PubMed] [Google Scholar]
- Vilner BJ, Bowen WD. Modulation of cellular calcium by sigma-2 receptors: release from intracellular stores in human SK-N-SH neuroblastoma cells. Journal of Pharmacology and Experimental Therapeutics. 2000;292:900–911. [PubMed] [Google Scholar]
- Vilner BJ, de Costa BR, Bowen WD. Cytotoxic effects of sigma ligands: sigma receptor-mediated alterations in cellular morphology and viability. Journal of Neuroscience. 1995;15:117–134. doi: 10.1523/JNEUROSCI.15-01-00117.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz TJ, Fleckenstein AE, Hanson GR. Methamphetamine-induced alterations in monoamine transport: implications for neurotoxicity, neuroprotection and treatment. Addiction. 2007;102 (Suppl 1):44–48. doi: 10.1111/j.1360-0443.2007.01771.x. [DOI] [PubMed] [Google Scholar]
- Walker JM, Bowen WD, Patrick SL, Williams WE, Mascarella SW, Bai X, Carroll FI. A comparison of (−)-deoxybenzomorphans devoid of opiate activity with their dextrorotatory phenolic counterparts suggests role of σ2 receptors in motor function. European Journal of Pharmacology. 1993;231:61–68. doi: 10.1016/0014-2999(93)90684-a. [DOI] [PubMed] [Google Scholar]
- Walker JM, Bowen WD, Walker FO, Matsumoto RR, de Costa BR, Rice KC. Sigma receptors: Biology and function. Pharmacological Reviews. 1990;42:355–402. [PubMed] [Google Scholar]
- Weiser SD, Patrick SL, Mascarella SW, Downing-Park J, Bai X, Carroll FI, Walker JM, Patrick RL. Stimulation of rat striatal tyrosine hydroxylase activity following intranigral administration of σ receptor ligands. European Journal of Pharmacology. 1995;275:1–7. doi: 10.1016/0014-2999(94)00718-m. [DOI] [PubMed] [Google Scholar]
- Xie T, McCann UD, Kim S, Yuan J, Ricaurte GA. Effect of temperature on dopamine transporter function and intracellular accumulation of methamphetamine: implications for methamphetamine-induced dopaminergic neurotoxicity. Journal of Neuroscience. 2000;20:7838–7845. doi: 10.1523/JNEUROSCI.20-20-07838.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto BK, Zhu W. The effects of methamphetamine on the production of free radicals and oxidative stress. Journal of Pharmacology and Experimental Therapeutics. 1998;287:107–114. [PubMed] [Google Scholar]
- Yang S, Bhardwaj A, Cheng J, Alkayed NJ, Hurn PD, Kirsch JR. Sigma receptor agonists provide neuroprotection in vitro by preserving bcl-2. Anesthesia and Analgesia. 2007;104:1179–1184. doi: 10.1213/01.ane.0000260267.71185.73. [DOI] [PMC free article] [PubMed] [Google Scholar]