Abstract
FcγRIIB is an inhibitory receptor which plays a role in limiting B cell and DC activation. Since FcγRIIB is known to dampen the signaling strength of the BCR, we wished to determine the impact of FcγRIIB on the regulation of BCRs which differ in their affinity for DNA. For these studies, FcγRIIB deficient BALB/c mice were bred with mice expressing the transgene-encoded H chain of the R4A anti-DNA antibody which gives rise to BCRs which express high, low or no affinity for DNA. The deletion of FcγRIIB in R4A BALB/c mice led to an alteration in the B cell repertoire, allowing for the expansion and activation of high affinity DNA-reactive B cells. By 6 to 8 months of age, R4A × FcγRIIB-/- BALB/c mice spontaneously developed anti-DNA antibody titers. These mice also displayed an induction of IFN-inducible genes and an elevation in levels of the B cell survival factor, BAFF. These data demonstrate that FcγRIIB preferentially limits activation of high affinity autoreactive B cells and can influence the activation of DC through an immune complex-mediated mechanism.
Keywords: Antibodies, Autoimmunity, Lupus, B cells, Fc receptor
1. Introduction
B cell autoreactivity can arise at multiple stages of B cell development, and there are regulatory molecules that combat the generation of high affinity autoreactive B cells. In particular, the inhibitory FcγRIIB, which binds IgG with low affinity, seems to play a crucial role in the elimination of autoreactive B cells [1, 2]. FcγRIIB is the sole Fc receptor for IgG on mouse B cells. One the functions of FcγRIIB is to modulate the strength of the BCR [3, 4], and co-ligation of the BCR and FcγRIIB leads to recruitment of the inhibitory phosphatase SHIP and the subsequent attenuation of the BCR signal [5, 6]. FcγRIIB can function also in the absence of BCR signaling [3, 7]. Clinical studies of humans with the autoimmune disease Systemic Lupus Erythematosus (SLE) suggest a role for the inhibitory FcγRIIB in disease. Failure to upregulate FcγRIIB on memory cells and plasma cells has been reported in patients with SLE [8-10]. In addition, several polymorphisms of the human fcgr2b gene have been shown to associate with disease [11, 12], and in particular the Ile232Thr polymorphism leads to an exclusion of FcγRIIB from lipid rafts and impaired activation of SHIP [13, 14]. A deletion polymorphism in the promoter region of the mouse ortholog of fcgr2b is present in the NZB, BXSB, MRL and NOD mouse strains [15, 16], and additional polymorphisms in the putative regulatory regions 3 and 4 of exon 3 associate with a failure to upregulate FcγRIIB on activated B cells and on germinal center B cells and an ensuing hypergammaglobulinemia [17, 18]. Thus, in both human and murine lupus, there is ample evidence to suggest that a dysregulation of FcγRIIB is linked to the disease phenotype.
Much of our understanding of the in vivo role of FcγRIIB in B cells comes from studies of mice with a targeted deletion of the fcgr2 gene generated by Ravetch and colleagues [19]. FcγRIIB-/- mice displayed enhanced humoral and anaphylactic responses [19], and more recently it was shown that plasma cell apoptosis is impaired in the absence of FcγRIIB [7]. These data confirmed early findings that a primary function of FcγRIIB is to limit humoral responses following B cell activation. Studies of FcγRIIB-/- mice revealed a role for FcγRIIB in B cell tolerance also FcγRIIB-/- C57Bl/6 mice develop a spontaneous lupus-like phenotype characterized by the production of anti-DNA antibodies and a fatal immune complex-mediated glomerulonephritis [20]. This phenotype is due to the targeted deletion of the fcgr2 gene since re-introduction of FcγRIIB by retroviral transduction restored tolerance [21]. Interestingly, the disease phenotype was not observed in FcγRIIB-/- BALB/c mice [20].
We wished to further examine the role of FcγRIIB in the regulation of both high affinity and low affinity DNA-reactive B cells. For these studies, BALB/c mice transgenic for the H chain of the R4A anti-DNA mAb were used [22, 23]. R4A BALB/c mice normally maintain tolerance of high affinity DNA-reactive B cells through receptor editing or deletion [24-27], but permit the maturation to immunocompetence of low affinity DNA-reactive B cells [28]. R4A × FcγRIIB-/- BALB/c mice were generated. These mice displayed elevated serum titers of anti-DNA antibodies. We show that FcγRIIB deficiency appeared to modulate not only plasma cell number, but also repertoire selection of naïve DNA-reactive B cells. Expression of the R4A anti-DNA heavy chain in FcγRIIB-/- BALB/c mice led to the survival and activation of high affinity DNA-reactive B cells, and to the generation of pro-inflammatory immune complexes. These data suggest that FcγRIIB deficiency may preferentially sustain high affinity DNA-reactive B cells which produce pro-inflammatory autoantibodies.
2. Materials and methods
2.1. Mice
The conventional R4A transgenic mouse has been previously described [22] and FcγRIIB-/- BALB/c mice were provided by Dr. J. Ravetch (Rockefeller University, New York, NY). FcγRIIB-/- BALB/c mice were mated with R4A BALB/c mice to generate R4A × FcγRIIB-/- BALB/c mice. With the exception of the time course study, which examined mice from 1 to 6 months of age, all mice studied were between the ages of 5 to 10 months. WT BALB/c mice were purchased from Jackson Laboratories. The mice were housed in a specific pathogen-free facility and animal experiments were approved by the Institutional Animal Care and Research Advisory Committee at the Feinstein Institute for Medical Research.
2.2. Anti-DNA antibody ELISA
Anti-DNA antibody measurements were performed as previously described [29]. Briefly, Immulon 2HB 96-well plates (Thermo LabSystems, Franklin, MA) were dry coated with 100 μg/ml of sonicated calf thymus DNA and blocked with 1.0% BSA/PBS. Serum dilutions ranged from 1:100 to 1:500 as described in the text. Specific IgG isotypes were detected using AP-labeled antibodies for specific for IgG2b, IgG2a and IgG1 (Southern Biotech, Birmingham, AL). For anti-DNA antibody quantitation, purified R4A mAb was added at a starting concentration of 50 μg/ml and 2-fold serial dilutions were used to generate a standard curve. Two-fold serial dilutions of serum ranging from 1:100 to 1:800 was used to determine the linear range of reactivity and the concentration of anti-DNA antibody present in the serum samples was calculated.
2.3. Inhibition ELISA
Dilution curves were generated for serum samples from 3 R4A mice and 4 R4A × FcγRIIB-/- mice to determine the linear range of DNA reactivity for each sample. Diluted serum samples were pre-incubated with various concentrations of sonicated DNA (~2.0 kb in length) for 2 hours at 37°C and the remaining DNA reactivity was measured by DNA ELISA. The range of relative affinities of the anti-DNA antibodies present in the sera was calculated as previously described [30].
2.4. Single cell Vκ-Jκ analysis
The splenocytes from 3 R4A or 3 R4A × FcγRIIB-/- mice were pooled and R4A H chain+ B cells (B220+/IgG2b+) were sorted using a MoFlo cell sorter (Dako Cytomation) into 96-well plates as previously described [25]. PCR amplification was carried out in two rounds using a GeneAmp PCR system 9700 PCR machine (Applied Biosystems) with primer sets as previously described [31]. The PCR products were cloned into the TOPO TA cloning vector (Invitrogen Life Technologies) and sequenced. Nucleotide sequences were determined by automated sequencing (Genewiz Inc., NJ). Analysis of the DNA sequences was performed using IgBLAST program (http://www.ncbi.nlm.nih.gov/igblast/).
2.5. Flow cytometry
Splenocytes were isolated and red blood cells were lysed. Pacific Blue-labeled B220, PE-labeled CD138, biotin-labeled anti-IgG2b and PerCP-labeled streptavidin were purchased from Pharmingen. Samples were analyzed with a LSRII cytometer and FlowJo software.
2.6. qPCR
RNA was isolated using the RNAeasy kit from Qiagen and cDNA was synthesized using the iScript cDNA synthesis kit (Bio-Rad). ABI Gene Expression Assays were used and the reactions were performed by using TaqMan primer/probe sets and universal PCR master mix. Polymerase (RNA) II (DNA directed) polypeptide A (polr2a) was used as a housekeeping gene. Relative expression levels were determined using the Pfaffl method [32].
2.7. BAFF and cytokine ELISAs
Serum BAFF was measured by sandwich ELISA based on the method previously described [33]. Costar 96 well plates were coated with the 5A8 BAFF antibody, followed by serum and biotin-labeled 1C9 anti-BAFF antibody (both from Apotech). Two-fold serial dilutions of recombinant BAFF (Apotech) starting at 40 ng/ml were used to generate a standard curve to determine serum BAFF levels. IL-6 and IL-10 levels were measured in the supernatants derived from DC cultures using the anti-mouse cytokine OptEIA set (Pharmingen).
2.8. Serum treatment of DCs
Bone marrow-derived DC cultures were prepared as described [34]. 6-8 week old BALB/c (n = 4) and R4A × FcγRIIB-/- (n = 4) mice were sacrificed and the femurs and tibias were collected. To remove non-DC precursors, cells were incubated with mAb supernatants from the hybridomas TIB120 (anti-I-A), GK1.5 (anti-CD4), TIB146 (anti-B220) and TIB211 (anti-CD8) and rabbit complement for 1hour at 37°C. The cells were resuspended at 1x106/ml in complete media supplemented with 1000 U/ml of recombinant murine GM-CSF (Peprotech, Rockey Hill, NJ) for 6 days. 70-80% of cells CD11c+ displayed an immature DC phenotype as determined by flow cytometry. The activation status was determined by flow cytometry using antibodies specific for CD86 and CD83 (Pharmingen). To stimulate the immature DCs, cells were plated at 1x106 in 1 ml of complete media containing either 2 μg/ml of LPS (Sigma), 10 μl (1.0% final concentration) of R4A sera or 10 μl of R4A × FcγRIIB-/- sera for 16 hours. As a control, serum samples were depleted of IgG by absorption with Protein G sepharose [35].
2.9. Statistical analysis
Two-way ANOVA, Mann-Whitney and Fisher’s exact tests were performed when appropriate as described in the text. A p value of < 0.05 was considered significant.
3. Results
3.1.1. FcγRIIB deficiency leads to the spontaneous activation of DNA-reactive B cells in R4A BALB/c mice
R4A BALB/c mice harbor the transgene for the IgG2b H chain of an anti-DNA antibody [23]. These mice have been shown to maintain B cell tolerance, but to be capable of generating autoantibodies upon exposure to increased levels of estradiol or prolactin [27, 36]. Since the R4A H chain transgene is expressed as an IgG2b molecule, the production of IgG2b anti-DNA antibody from the transgene-expressing (Tg+) B cell population can be distinguished from antibody production from the endogenous B cell population which would be expected to include IgM antibodies as well as other IgG istoypes. A time-course study of serum titers of IgG2b anti-DNA antibodies was performed in aged-matched R4A BALB/c and R4A × FcγRIIB-/- BALB/c mice. As expected, R4A mice displayed no appreciable increase in IgG2b anti-DNA antibody levels with age; however, starting at 4 months of age, R4A × FcγRIIB-/- mice showed a discernible increase in IgG2b anti-DNA antibody levels (Fig. 1A). Titration of serum samples collected from a second cohort of mice confirmed that IgG2b anti-DNA antibody titers were significantly increased in 6 to 8 month-old R4A × FcγRIIB-/- mice (Fig. 1B and 1D). Quantitation ELISAs using the R4A mAb as a standard were performed to measure relative levels of serum anti-DNA antibody. For these studies, sera from 5 to 7 month-old R4A and R4A × FcγRIIB-/- mice were examined. Only 6 out of the 17 R4A mice (~35%) tested displayed measurable levels of IgG2b anti-DNA antibody with an average of 16.6 ± 6.6 μg/ml, whereas 12 out of the 18 R4A × FcγRIIB-/- mice (~67%) displayed significantly higher levels of anti-DNA antibody with an average of 59.7 ± 18.1 μg/ml (Fig. 1C). Furthermore, inhibition ELISAs using soluble DNA showed an apparent affinity of 9 × 10-8 M for serum samples from R4A × FcγRIIB-/- mice and 3 × 10-7 M for serum from R4A mice (data not shown), indicating that high affinity DNA-reactive B cells were spontaneously activated by self antigen in vivo in R4A × FcγRIIB-/- mice.
Figure 1. Spontaneous production of anti-DNA antibody titers in R4A × FcγRIIB-/- BALB/c mice.
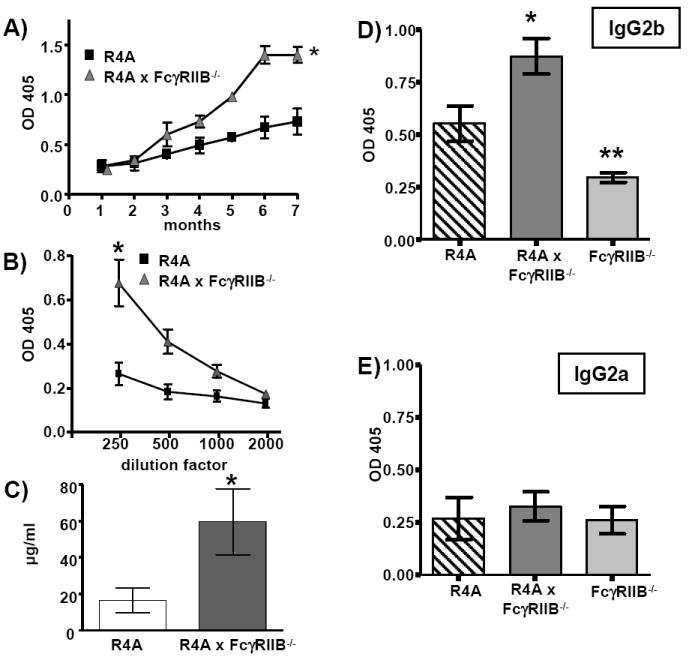
A) Serum was collected once a month beginning at 1 month of age from R4A BALB/c (n = 5) and R4A × FcγRIIB-/- BALB/c (n = 5) mice and IgG2b anti-DNA antibodies were measured by ELISA using a 1:500 dilution of serum. IgG2b anti-DNA antibody levels significantly increased in R4A × FcγRIIB-/- mice with age (*p < 0.0001). B) Serum samples from a second set of 5 to 8 month-old R4A mice (n = 17) and R4A × FcγRIIB-/- mice (n = 18) were tested by ELISA using 2-fold serial dilutions beginning at 1:250. The differences in IgG2b anti-DNA antibody levels were significant (**p < 0.003). C) The R4A mAb was used as a standard to measure levels of anti-DNA antibody in the serum of R4A mice (n = 17) and R4A × FcγRIIB-/- mice (n = 18). The anti-DNA antibody concentrations were significantly higher in R4A × FcγRIIB-/- mice (*p < 0.04). D) IgG2b and E) IgG2a anti-DNA titers were measured using a 1:100 dilution of serum. IgG2a anti-DNA antibody levels were similar for R4A (n = 14), R4A × FcγRIIB-/- (n = 7) and FcγRIIB-/- (n = 9) BALB/c mice. There was a significant increase in IgG2b anti-DNA antibody titers in R4A × FcγRIIB-/- mice (n = 19) versus R4A mice (n = 23) (*p < 0.002) and R4A × FcγRIIB-/- mice versus FcγRIIB-/- mice (n = 7) (**p < 0.001). Statistical significance was determined by two-way ANOVA for experiments in a) and b) and by Mann-Whitney for c), d) and e). Data points are shown as the mean ± SD.
Since IgG2b anti-DNA antibodies are a feature of R4A Tg mice, non-Tg FcγRIIB-/- BALB/c mice did not display these antibodies (Fig. 1D). Moreover, IgG2a anti-DNA antibody titers of R4A × FcγRIIB-/-, R4A and FcγRIIB-/- mice were indistinguishable (Fig. 1E), as well as IgG1 anti-DNA antibody titers (data not shown). Thus, the lack of IgG1, IgG2a or IgG2b anti-DNA antibody titers in control R4A or FcγRIIB-/-mice, and the presence of high affinity IgG2b anti-DNA antibody titers in R4A × FcγRIIB-/- mice indicate that the Tg+ B cell population breached tolerance in R4A × FcγRIIB-/- mice, which was evident by 5 to 8 months of age.
3.1.2. Repertoire selection of B cells in R4A × FcγRIIB-/- BALB/c mice
Since one of the roles of FcγRIIB is to limit B cell autoreactivity, we were interested in ascertaining whether the absence of FcγRIIB led to a shift in the DNA-reactive B cell repertoire in R4A BALB/c mice. Several laboratories including our own have demonstrated that high and low affinity autoreactive B cells are differentially regulated [25, 37, 38]. Our previous analysis of hybridomas has demonstrated that R4A H chain can pair with a spectrum of L chains to generate anti-DNA antibodies with varying affinities for DNA [24, 28, 29]. L chains which generate high affinity DNA-reactive B cells are eliminated by deletion in R4A mice as immature B cells in the bone marrow and at the transitional stage of B cell maturation, while low affinity DNA-reactive B cells survive and are selected into the mature, immunocompetent repertoire [25].
We have established a single cell PCR protocol to identify the Vκ-Jκ usage of Tg+ B cells. Forty-two L chain sequences from R4A and 67 sequences from R4A × FcγRIIB-/- mice were analyzed. In total, 24 different Vκ-Jκ L chains were identified, 8 of which were expressed by the B cells of both R4A and R4A × FcγRIIB-/- mice (Supplemental Table 1). In R4A mice, Vk4/5 L chains predominated, followed Vκ1 and then Vκ21 L chains (Fig. 2), which is a pattern of usage similar to what we have previously observed for R4A mice (CG and JV, unpublished data and [25]). In contrast, Vκ1 L chains predominated in R4A × FcγRIIB-/- mice, followed by Vκ21, Vκ9/10 and Vk23 L chains. Interestingly, this pattern of L usage is the similar to R4A mice in which estradiol treatment was used to break tolerance (CG and JV, unpublished data and [25]). Thus, in the absence of FcγRIIB, there was skewing of the Tg+ B cell repertoire.
Figure 2. Vκ gene usage.
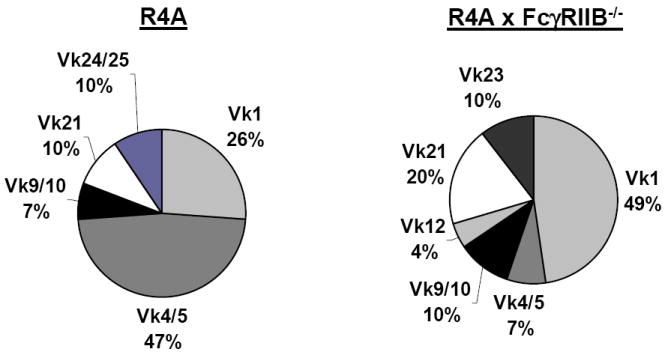
Splenocytes were pooled from three 5 month-old R4A mice or R4A × FcγRIIB-/- BALB/c mice and stained with fluorochrome-labeled B220 and IgG2b to identify Tg+ B cells. Tg+ B cells were individually sorted into the wells of microtiter plates and PCR was performed on genomic DNA to amplify a partial L fragment spanning FR3 and Jκ regions. Sequence analysis was performed and the Vκ gene families were determined (also presented in Supplemental Table I). The data are shown as the percentage of L chains belonging to each Vκ family.
We focused our attention on Tg+ B cells expressing germline-encoded Vκ1A/Jκ1 and Vκ1A/Jκ4 L chains since our previous studies of hybridomas demonstrated that the R4A H chain paired with either of these L chains generate antibodies with high affinity for DNA. Twenty-six of the 67 B cells analyzed from R4A × FcγRIIB-/- mice (~38%) utilized L chains which generate high affinity DNA-reactivity, while only 5 of 42 B cells (~11%) from R4A mice utilized these L chains (Table 1). Thus, in the absence of FcγRIIB there was an increased frequency of high affinity DNA-reactive B cells.
Table 1.
Frequency of high and low affinity DNA-reactive B cells
| High affinitya | Low affinityb | |
|---|---|---|
| R4A (total # =42) | 5 (11.9%) | 3 (7.2%) |
| R4A × FcγRIIB-/- (total # = 67) | 26 (38.8%) | 0 (0%) |
High affinity DNA-reactive B cells utilize Vκ1 (BB1)-Jκ1 and Vκ1-Jκ4 L chains [25]. The frequency of high affinity B cells was significantly increased in R4A × FcγRIIB-/- mice compared to R4A mice (p < 0.003).
Low affinity DNA-reactive B cells utilize the Vκ1-Jκ5 L chain [25]. Statistical significance was determined by Fisher’s exact test.
Interestingly, B cells bearing the Vκ1A/Jκ5 low affinity L chain were not detected in R4A × FcγRIIB-/- mice (Table 1). It is likely that this reflects a failure of these low affinity DNA-reactive B cells to compete for entrance into follicular niches when high affinity DNA-reactive B cells escape tolerance [25]. We have seen this pattern previously in estradiol-treated R4A mice; not only is there an accumulation of high affinity DNA-reactive B cells, but there is a decreased representation of low affinity DNA-reactive B cells suggestive of competition for antigen once tolerance has been breached.
3.1.3. B cell phenotype in R4A × FcγRIIB-/- mice
The B cell phenotype of R4A × FcγRIIB-/- mice was assessed to determine if changes in B cell subsets coincided with the escape of high affinity DNA-reactive B cells and the rise in anti-DNA antibody titers. Analysis of the transitional and the mature follicular and marginal zone B cell subsets in 5 to 9 month-old mice revealed no differences between R4A and R4A × FcγRIIB-/- mice at the time when anti-DNA antibody titers are detectable (data not shown). We expected either an expansion or an increased activation of Tg+ B cells in the spleens of R4A × FcγRIIB-/- mice which would account for the increased anti-DNA antibody titers. There was only a slight increase in the percentage of Tg+ B cells in R4A × FcγRIIB-/- mice as demonstrated by staining for surface IgG2b expression, but this was not significant in either of two cohorts studied (Fig. 3A). Furthermore, levels of the activation markers CD80, CD86 and CD69 were not different between R4A and R4A × FcγRIIB-/- mice (data not shown). Thus, the increase in anti-DNA titers does not appear to be due to an overall expansion or polyclonal activation of the Tg+ B cell population. There was, however, a significant increase in B220lo/CD138+ cells in the spleens of R4A × FcγRIIB-/- mice (Fig. 3B), which is presumably reflective of an increase in plasma cells. Thus, expansion of plasma cells, which is also a feature of FcγRIIB-/- C57Bl/6 mice [39] and is suggestive of faulty regulation of plasma cell development [18] or impaired regulation of plasma cell survival [7], occurred in R4A × FcγRIIB-/- BALB/c mice.
Figure 3. Flow cytometry of B cells in R4A × FcγRIIB-/- BALB/c mice.
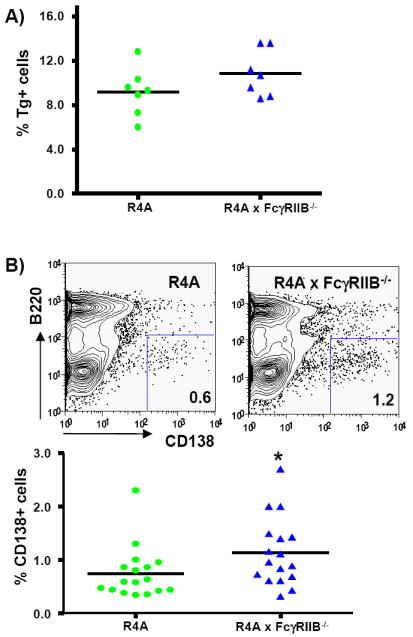
A) Splenocytes from 5 to 10 month-old R4A mice (n = 7) and R4A × FcγRIIB-/- mice (n = 7) mice were analyzed for the percentage of Tg+ B cells using an anti-IgG2b antibody. Analysis of B220+/Tg+ cells revealed no difference by Mann-Whitney in this study, and the analysis of a second set of mice. B) Splenocytes from R4A mice (n = 17) and R4A × FcγRIIB-/- mice (n = 17) were stained with B220 and CD138 to determine the percentage of B220lo/CD138+ plasma cells. Representative contour plots from each mouse are shown. The percentage of B220lo/CD138+ cells were significantly increased in R4A × FcγRIIB-/- mice (*p < 0.04 determined by Mann-Whitney). Data are shown as the mean ± SD.
3.1.4. Induction of IFN-regulated genes and BAFF in R4A × FcγRIIB-/- mice
Based on the increased frequency of high affinity DNA-reactive B cells and the increased titers of high affinity anti-DNA antibody in the serum of R4A × FcγRIIB-/- mice, we reasoned that an important role of FcγRIIB is to limit the generation of autoantibodies that could trigger a pro-inflammatory response. We, therefore, determined IFN-induced gene expression, which is known to occur in patients with lupus [40-43] and in lupus-prone mice [44, 45], and to be a consequence of nucleic acid-containing pro-inflammatory immune complexes [46, 47]. RNA was isolated from splenocytes of R4A and R4A × FcγRIIB-/- mice and qPCR was performed using two IFN-regulated genes, ifi202b [48] and oasl [43], which are extremely sensitive to activation by lupus serum. Both ifi202b and oasl were significantly increased in R4A × FcγRIIB-/- BALB/c mice (Fig.4), showing that an inflammatory response was triggered in these mice.
Figure 4. IFN-inducible gene expression.
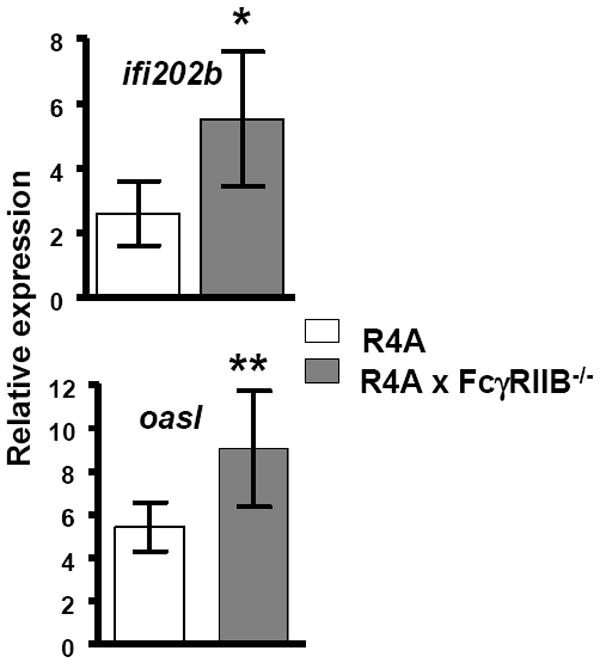
Splenocytes were isolated from 5 to 10 month-old R4A mice or R4A × FcγRIIB-/- mice (n = 6 for each) and qPCR was performed to measure mRNA levels of IFN-regulated genes. Values are expressed as the mean ± SD of the relative expression levels normalized to polr2a. Relative expression of ifi202b and oasl mRNA levels were significantly increased in the splenocytes of R4A × FcγRIIB-/- (*p < 0.005 and **p < 0.02, respectively, determined by Mann-Whitney). Data are shown as the mean ± SD.
Levels of the B cell survival factor, BAFF, were also measured since it has been shown that nucleosome-containing immune complexes can stimulate DCs to make BAFF [49], and that high BAFF levels may permit the survival of autoreactive B cells that would normally be subject to tolerance mechanisms [50, 51]. Analysis of splenic baff mRNA levels revealed a significant increase in R4A × FcγRIIB-/- mice (Fig. 5A). In agreement with the elevation in baff mRNA, serum BAFF levels were also significantly increased in R4A × FcγRIIB-/- mice compared to aged-matched R4A, FcγRIIB-/- and WT BALB/c mice (Fig. 5B).
Figure 5. Induction of BAFF.
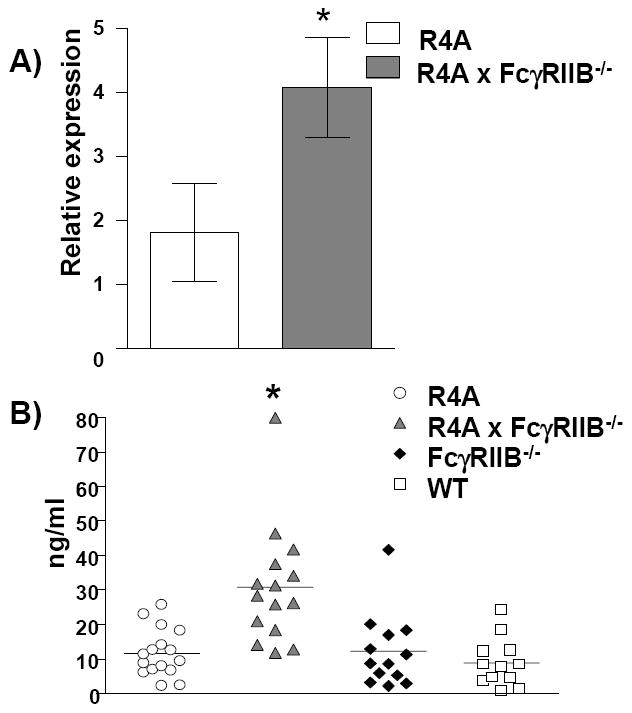
A) Splenocytes were isolated from 5 to 10 month-old R4A mice or R4A × FcγRIIB-/- mice (n = 6 for each) and analyzed for baff mRNA by qPCR. The data were normalized to polr2a which revealed an increase in baff mRNA in R4A × FcγRIIB-/- mice. B) Serum BAFF levels were measured by ELISA for aged-matched WT BALB/c (n = 12), FcγRIIB-/- (n = 13), R4A (n = 16) and R4A × FcγRIIB-/- (n = 15) mice. **BAFF serum levels were significantly increased in R4A × FcγRIIB-/- mice compared to R4A (p < 0.0001), FcγRIIB-/- (p < 0.0008) or WT (p < 0.0001) BALB/c mice. Statistical significance was determined by Mann-Whitney. Data are shown as the mean ± SD.
Based on the increased expression of IFN-inducible genes and of BAFF, we presume that high affinity anti-DNA antibodies generated in R4A × FcγRIIB-/- mice triggered the induction a pro-inflammatory response. This may create a feedback loop in which the increase in BAFF contributed to the enhanced selection of high affinity DNA-reactive B cells [52].
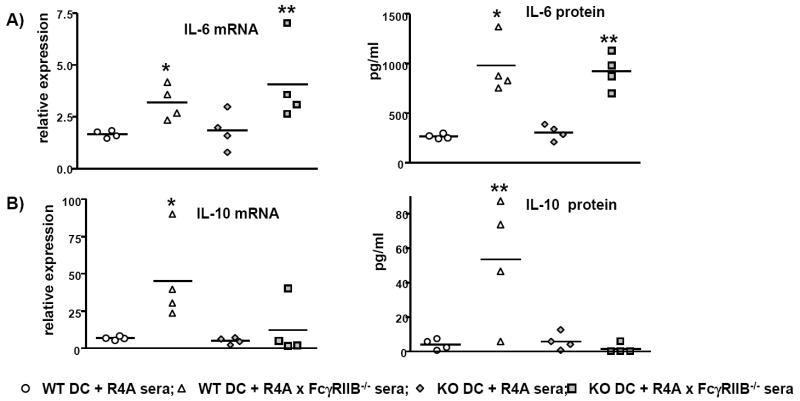
3.1.5. Induction of IL-6 and IL-10 secretion by sera treatment of myeloid dendritic cells
Since we detected evidence for an upregulation of IFN-inducible genes and BAFF, and since the incubation of innate cells with lupus sera has been demonstrated to trigger these and other pro-inflammatory cytokine responses [53, 54], the ability of DNA-reactive sera from R4A × FcγRIIB-/- mice to stimulate cytokine production was assessed. We focused these studies on DCs as this cell type is responsive to FcγR and TLR engagement triggered by nucleic acid-containing immune complexes [49].
Bone marrow-derived DC cultures were generated by treatment of DC precursors with IL-4 and GM-CSF to generate either WT or FcγRIIB-/- DCs. The total number of DCs generated from R4A × FcγRIIB-/- mice was slightly lower compared to WT mice (16.0 × 106 ± 6.0 versus 23.0 × 106 ± 4.7, respectively); however, this difference was not statistically significant. Not surprisingly, FcγRIIB-/- DCs also exhibited a more activated phenotype based on the increased percentage of CD86+ and CD83+ cells (Supplemental Table II).
To determine the pattern of TNFα, IL-6, IL-10 and IL-12 expression induced by the sera of R4A × FcγRIIB-/- mice, equal numbers of WT or FcγRIIB-/- DCs were incubated overnight with 1 μl of pooled sera (1.0% final volume) from 10 R4A or 11 R4A × FcγRIIB-/- mice, and cytokine levels were measured by qPCR or by ELISA. Medium alone or LPS were used as controls to assess basal levels and activation-induced levels of cytokines, respectively. Treatment of the WT or FcγRIIB-/- DCs with medium alone revealed no differences in baseline cytokine mRNA levels, with the exception of IL-12, which was significantly lower in FcγRIIB-/- DCs (Supplemental Table III). Moreover, WT and FcγRIIB-/- DCs responded similarly to LPS with respect to transcription of cytokine genes (Supplemental Table III).
Treatment with R4A × FcγRIIB-/- sera significantly increased IL-6 mRNA and protein levels in WT and FcγRIIB-/- DCs, suggesting that the DNA-reactive sera can induce a pro-inflammatory cytokine response, and that this response was not dependent on DC expression of FcγRIIB (Fig. 6A). The induction of IL-6 was presumably due to the presence of DNA-containing immune complexes since depletion of immunoglobulin from the sera with Protein G abrogated this response (data not shown). Interestingly, treatment with R4A × FcγRIIB-/- sera induced the expression of IL-10 in WT DCs only, indicating that FcγRIIB on innate cells plays a role in immune complex-mediated IL-10 production (Fig. 6B). Again, the ability to induce IL-10 was abrogated by treatment of the sera with Protein G to remove serum immunoglobulin. It is important to note that LPS treatment of DCs from WT and FcγRIIB-/- mice induced IL-10 expression, indicating that the FcγRIIB deficiency does not lead to global defect in the ability of the DC to make IL-10 (Supplemental Table IV).
Figure 6. Induction of IL-6 and IL-10 by serum treatment of DCs.
Bone marrow-derived DCs were generated from WT mice (n = 4) or R4A × FcγRIIB-/- mice (n = 4) and treated overnight with 1.0% pooled sera from 10 R4A mice or from 11 R4A × FcγRIIB-/- mice. Induction of IL-6 and IL-10 was measured by qPCR and ELISA. A) Treatment of either WT or FcγRIIB-/- (KO) DCs with R4A × FcγRIIB-/- sera significantly induced IL-6 mRNA and protein (* and ** p < 0.03). B) Treatment with R4A × FcγRIIB-/- sera induced a significant increase in IL-10 mRNA only in WT DCs (*p < 0.03). IL-10 secretion was increased by treatment with R4A × FcγRIIB-/- sera only in WT DCs, but was not significant when compared to treatment with R4A sera, but showed a trend towards being increased. (**p = 0.057). There was no induction of IL-10 when the FcγRIIB-/- DCs were treated with R4A × FcγRIIB-/- sera. Statistical significance was determined by Mann-Whitney. Data are shown as the mean ± SD.
Treatment of the DC cultures with sera from either R4A or R4A × FcγRIIB-/- mice failed to induce detectable changes in TNFα and IL-12 (Supplemental Table III). As has been previously shown, IL-12 is not normally increased by FcγR engagement, and TNFα production by chromatin-containing immune complexes is not dependent on FcγRIIB [49]. Overall, the salient detectable difference in the DC response to sera with anti-DNA reactivity was an increase in IL-6, which was not dependent of DC FcγRIIB expression, and an increase in IL-10 mRNA seen only in WT DCs. These data suggest that one of the roles of FcγRIIB on DCs may be to help establish compensatory immune inhibition by IL-10 if pro-inflammatory anti-DNA antibody titers transiently rise as a consequence of autoreactive B cell activation.
4. Discussion
In this report we demonstrate that tolerance of high affinity DNA-reactive B cells in transgenic BALB/c mice that express the R4A anti-DNA H chain is abrogated in the absence of FcγRIIB. While several other studies have demonstrated that pristane or mercury induced lupus is enhanced in the absence of FcγRIIB [55, 56], our study is the first to demonstrate that high affinity DNA-reactive B cells are selectively expanded. In agreement with the studies of Ravetch and Bolland [20], we observed that non-transgenic FcγRIIB-/- BALB/c mice did not develop anti-DNA antibody titers. Our findings differ from the previous data of Ravetch and colleagues in which the 56R transgenic mouse model was examined to determine the role of FcγRIIB in the selection and regulation of high affinity DNA-reactive B cells [39]. In those studies, the DNA-reactive B cells of 56R × FcγRIIB-/- BALB/c mice remained tolerant and high affinity reactivity for DNA was extinguished by the hierarchical use of “silencing” editor L chains [39, 57]. In light of our findings, we interpret this to suggest that there are differences between the intrinsic properties of the DNA-reactive BCRs generated in 56R and R4A FcγRIIB deficient BALB/c mice, such as affinity for self antigen or fine specificity and antigenic cross-reactivity. Moreover, BCR-extrinsic factors, such as availability or valency of self antigen may be an important determinant in the ability of FcγRIIB to modulate autoreactive B cells. Thus, we see that different BCRs are differentially affected by the absence of FcγRIIB.
Also of interest, our data demonstrate that the anti-DNA antibody-containing serum of R4A × FcγRIIB-/- BALB/c mice can trigger pro-inflammatory responses such as the increased expression of IFN-regulated genes and of BAFF mRNA and protein. The increase in BAFF is of particular importance since there are recent data to suggest that engagement of the activating FcγR1 receptor on innate cells induces cleavage of membrane BAFF [58]. Since the inhibitory FcγRIIB expressed on innate cells counters the function of the activating FcγRs, it is possible that the unopposed activation of FcγRs by ligand can lead to an elevation in BAFF. However, in the aforementioned study, engagement of FcγR with ligand induced an increase in soluble BAFF without changing the level of baff mRNA, and BAFF cleavage was not affected by engagement of FcγRIIB [58]. Since we observed an increase in baff mRNA as well as BAFF protein in R4A × FcγRIIB-/- mice, our data suggest a distinct role for FcγRIIB-/- in the regulation of BAFF.
Both FcγRIIB-/- and WT DCs made IL-6 in response to sera from R4A × FcγRIIB-/- mice. Moreover, WT DCs made IL-10 in response to sera containing anti-DNA antibodies, while DCs from FcγRIIB-/- mice failed to respond in a similar fashion. When sera were depleted of IgG, there was no serum-induced increased expression of cytokines. These results raise some interesting points. First, several studies demonstrate that the sera of active SLE patients induce innate cells to secrete IL-6 and IL-10 [53, 54, 59]; thus, the DNA-reactive sera of R4A × FcγRIIB-/- BALB/c mice share pro-inflammatory properties with SLE sera. Second, for the DNA-reactive sera of R4A × FcγRIIB-/- BALB/c mice to induce IL-10, the presence of FcγRIIB on DCs was required. The effect of FcγRIIB on the production of IL-10 has not been clearly established in mice, but a study of human PBMCs suggests that blockade of the FcγIIB can inhibit IL-10 production induced by SLE sera [54]. The role of IL-10 in SLE is controversial, with some studies suggesting that it plays a pathogenic role [54, 60-63], perhaps, enhancing B cell hyper-reactivity, while others suggesting that IL-10 is part of a compensatory anti-inflammatory program that counters the production of pro-inflammatory mediators [61, 64, 65].
Our results demonstrate that FcγRIIB deficiency leads to a preferential expansion of B cells bearing BCRs with high affinity for DNA. Given the previous data on this topic, we speculate that fine specificity or even availability and valency of self antigen determine whether the inhibitory effects of FcγRIIB on autoreactive B cells are operative. Importantly, this study emphasizes the importance of studying an array of BCRs with reactivity to a particular self antigen to understand the diverse tolerance checkpoints and mechanisms of tolerance induction that protect against pathogenic autoreactivity. Finally, it is clear that once anti-DNA antibodies are produced, there is downstream dysregulation of many pathways of immune homeostasis.
Supplementary Material
Acknowledgments
This work was supported by grants from NIAMS (BD and CMG). We wish thank Alla Tashmukhamedova for technical assistance and Sylvia Jones for help with the preparation of this manuscript.
Abbreviations used in this paper
- SLE
systemic lupus erythematosus
- κ
kappa
- Tg+
transgene positive
- polr2A
polymerase (RNA) II (DNA directed) polypeptide A
- BAFF
B cell activating factor belonging to the tumor necrosis family
Footnotes
Conflicts of interest There are no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Takai T. Fc receptors and their role in immune regulation and autoimmunity. J Clin Immunol. 2005;25:1–18. doi: 10.1007/s10875-005-0353-8. [DOI] [PubMed] [Google Scholar]
- 2.Tarasenko T, Dean JA, Bolland S. FcgammaRIIB as a modulator of autoimmune disease susceptibility. Autoimmunity. 2007;40:409–17. doi: 10.1080/08916930701464665. [DOI] [PubMed] [Google Scholar]
- 3.Pearse RN, Kawabe T, Bolland S, Guinamard R, Kurosaki T, Ravetch JV. SHIP recruitment attenuates Fc gamma RIIB-induced B cell apoptosis. Immunity. 1999;10:753–60. doi: 10.1016/s1074-7613(00)80074-6. [DOI] [PubMed] [Google Scholar]
- 4.Phillips NE, Parker DC. Fc-dependent inhibition of mouse B cell activation by whole anti-mu antibodies. J Immunol. 1983;130:602–6. [PubMed] [Google Scholar]
- 5.Bolland S, Pearse RN, Kurosaki T, Ravetch JV. SHIP modulates immune receptor responses by regulating membrane association of Btk. Immunity. 1998;8:509–16. doi: 10.1016/s1074-7613(00)80555-5. [DOI] [PubMed] [Google Scholar]
- 6.Aman MJ, Tosello-Trampont AC, Ravichandran K. Fc gamma RIIB1/SHIP-mediated inhibitory signaling in B cells involves lipid rafts. J Biol Chem. 2001;276:46371–8. doi: 10.1074/jbc.M104069200. [DOI] [PubMed] [Google Scholar]
- 7.Xiang Z, Cutler AJ, Brownlie RJ, Fairfax K, Lawlor KE, Severinson E, Walker EU, Manz RA, Tarlinton DM, Smith KG. FcgammaRIIb controls bone marrow plasma cell persistence and apoptosis. Nat Immunol. 2007;8:419–29. doi: 10.1038/ni1440. [DOI] [PubMed] [Google Scholar]
- 8.Mackay M, Stanevsky A, Wang T, Aranow C, Li M, Koenig S, Ravetch JV, Diamond B. Selective dysregulation of the FcgammaIIB receptor on memory B cells in SLE. J Exp Med. 2006;203:2157–64. doi: 10.1084/jem.20051503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su K, Yang H, Li X, Li X, Gibson AW, Cafardi JM, Zhou T, Edberg JC, Kimberly RP. Expression profile of FcgammaRIIb on leukocytes and its dysregulation in systemic lupus erythematosus. J Immunol. 2007;178:3272–80. doi: 10.4049/jimmunol.178.5.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isaak A, Gergely P, Jr, Szekeres Z, Prechl J, Poor G, Erdei A, Gergely J. Physiological up-regulation of inhibitory receptors Fc gamma RII and CR1 on memory B cells is lacking in SLE patients. Int Immunol. 2008;20:185–92. doi: 10.1093/intimm/dxm132. [DOI] [PubMed] [Google Scholar]
- 11.Blank MC, Stefanescu RN, Masuda E, Marti F, King PD, Redecha PB, Wurzburger RJ, Peterson MG, Tanaka S, Pricop L. Decreased transcription of the human FCGR2B gene mediated by the -343 G/C promoter polymorphism and association with systemic lupus erythematosus. Hum Genet. 2005;117:220–7. doi: 10.1007/s00439-005-1302-3. [DOI] [PubMed] [Google Scholar]
- 12.Kyogoku C, Dijstelbloem HM, Tsuchiya N, Hatta Y, Kato H, Yamaguchi A, Fukazawa T, Jansen MD, Hashimoto H, van de Winkel JG, Kallenberg CG, Tokunaga K. Fcgamma receptor gene polymorphisms in Japanese patients with systemic lupus erythematosus: contribution of FCGR2B to genetic susceptibility. Arthritis Rheum. 2002;46:1242–54. doi: 10.1002/art.10257. [DOI] [PubMed] [Google Scholar]
- 13.Floto RA, Clatworthy MR, Heilbronn KR, Rosner DR, MacAry PA, Rankin A, Lehner PJ, Ouwehand WH, Allen JM, Watkins NA, Smith KG. Loss of function of a lupus-associated FcgammaRIIb polymorphism through exclusion from lipid rafts. Nat Med. 2005;11:1056–8. doi: 10.1038/nm1288. [DOI] [PubMed] [Google Scholar]
- 14.Kono H, Kyogoku C, Suzuki T, Tsuchiya N, Honda H, Yamamoto K, Tokunaga K, Honda Z. FcgammaRIIB Ile232Thr transmembrane polymorphism associated with human systemic lupus erythematosus decreases affinity to lipid rafts and attenuates inhibitory effects on B cell receptor signaling. Hum Mol Genet. 2005;14:2881–92. doi: 10.1093/hmg/ddi320. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Y, Hirose S, Abe M, Sanokawa-Akakura R, Ohtsuji M, Mi X, Li N, Xiu Y, Zhang D, Shirai J, Hamano Y, Fujii H, Shirai T. Polymorphisms in IgG Fc receptor IIB regulatory regions associated with autoimmune susceptibility. Immunogenetics. 2000;51:429–35. doi: 10.1007/s002510050641. [DOI] [PubMed] [Google Scholar]
- 16.Pritchard NR, Cutler AJ, Uribe S, Chadban SJ, Morley BJ, Smith KG. Autoimmune-prone mice share a promoter haplotype associated with reduced expression and function of the Fc receptor FcgammaRII. Curr Biol. 2000;10:227–30. doi: 10.1016/s0960-9822(00)00344-4. [DOI] [PubMed] [Google Scholar]
- 17.Rahman ZS, Manser T. Failed up-regulation of the inhibitory IgG Fc receptor Fc gamma RIIB on germinal center B cells in autoimmune-prone mice is not associated with deletion polymorphisms in the promoter region of the Fc gamma RIIB gene. J Immunol. 2005;175:1440–9. doi: 10.4049/jimmunol.175.3.1440. [DOI] [PubMed] [Google Scholar]
- 18.Rahman ZS, Alabyev B, Manser T. FcgammaRIIB regulates autoreactive primary antibody-forming cell, but not germinal center B cell, activity. J Immunol. 2007;178:897–907. doi: 10.4049/jimmunol.178.2.897. [DOI] [PubMed] [Google Scholar]
- 19.Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature. 1996;379:346–9. doi: 10.1038/379346a0. [DOI] [PubMed] [Google Scholar]
- 20.Bolland S, Ravetch JV. Spontaneous autoimmune disease in Fc(gamma)RIIB-deficient mice results from strain-specific epistasis. Immunity. 2000;13:277–85. doi: 10.1016/s1074-7613(00)00027-3. [DOI] [PubMed] [Google Scholar]
- 21.McGaha TL, Sorrentino B, Ravetch JV. Restoration of tolerance in lupus by targeted inhibitory receptor expression. Science. 2005;307:590–3. doi: 10.1126/science.1105160. [DOI] [PubMed] [Google Scholar]
- 22.Offen D, Spatz L, Escowitz H, Factor S, Diamond B. Induction of tolerance to an IgG autoantibody. Proc Natl Acad Sci U S A. 1992;89:8332–6. doi: 10.1073/pnas.89.17.8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shefner R, Kleiner G, Turken A, Papazian L, Diamond B. A novel class of anti-DNA antibodies identified in BALB/c mice. J Exp Med. 1991;173:287–96. doi: 10.1084/jem.173.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spatz L, Saenko V, Iliev A, Jones L, Geskin L, Diamond B. Light chain usage in anti-double-stranded DNA B cell subsets: role in cell fate determination. J Exp Med. 1997;185:1317–26. doi: 10.1084/jem.185.7.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grimaldi CM, Jeganathan V, Diamond B. Hormonal regulation of B cell development: 17 beta-estradiol impairs negative selection of high-affinity DNA-reactive B cells at more than one developmental checkpoint. J Immunol. 2006;176:2703–10. doi: 10.4049/jimmunol.176.5.2703. [DOI] [PubMed] [Google Scholar]
- 26.Venkatesh J, Peeva E, Xu X, Diamond B. Cutting Edge: Hormonal milieu, not antigenic specificity, determines the mature phenotype of autoreactive B cells. J Immunol. 2006;176:3311–4. doi: 10.4049/jimmunol.176.6.3311. [DOI] [PubMed] [Google Scholar]
- 27.Bynoe MS, Grimaldi CM, Diamond B. Estrogen up-regulates Bcl-2 and blocks tolerance induction of naive B cells. Proc Natl Acad Sci U S A. 2000;97:2703–8. doi: 10.1073/pnas.040577497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bynoe MS, Spatz L, Diamond B. Characterization of anti-DNA B cells that escape negative selection. Eur J Immunol. 1999;29:1304–13. doi: 10.1002/(SICI)1521-4141(199904)29:04<1304::AID-IMMU1304>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 29.Iliev A, Spatz L, Ray S, Diamond B. Lack of allelic exclusion permits autoreactive B cells to escape deletion. J Immunol. 1994;153:3551–6. [PubMed] [Google Scholar]
- 30.Nieto A, Gaya A, Jansa M, Moreno C, Vives J. Direct measurement of antibody affinity distribution by hapten-inhibition enzyme immunoassay. Mol Immunol. 1984;21:537–43. doi: 10.1016/0161-5890(84)90070-1. [DOI] [PubMed] [Google Scholar]
- 31.Yamagami T, ten Boekel E, Schaniel C, Andersson J, Rolink A, Melchers F. Four of five RAG-expressing JCkappa-/- small pre-BII cells have no L chain gene rearrangements: detection by high-efficiency single cell PCR. Immunity. 1999;11:309–16. doi: 10.1016/s1074-7613(00)80106-5. [DOI] [PubMed] [Google Scholar]
- 32.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Batten M, Fletcher C, Ng LG, Groom J, Wheway J, Laabi Y, Xin X, Schneider P, Tschopp J, Mackay CR, Mackay F. TNF deficiency fails to protect BAFF transgenic mice against autoimmunity and reveals a predisposition to B cell lymphoma. J Immunol. 2004;172:812–22. doi: 10.4049/jimmunol.172.2.812. [DOI] [PubMed] [Google Scholar]
- 34.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bengtsson AA, Sturfelt G, Gullstrand B, Truedsson L. Induction of apoptosis in monocytes and lymphocytes by serum from patients with systemic lupus erythematosus - an additional mechanism to increased autoantigen load? Clin Exp Immunol. 2004;135:535–43. doi: 10.1111/j.1365-2249.2003.02386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peeva E, Michael D, Cleary J, Rice J, Chen X, Diamond B. Prolactin modulates the naive B cell repertoire. J Clin Invest. 2003;111:275–83. doi: 10.1172/JCI16530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borrero M, Clarke SH. Low-affinity anti-Smith antigen B cells are regulated by anergy as opposed to developmental arrest or differentiation to B-1. J Immunol. 2002;168:13–21. doi: 10.4049/jimmunol.168.1.13. [DOI] [PubMed] [Google Scholar]
- 38.Huang H, Kearney JF, Grusby MJ, Benoist C, Mathis D. Induction of tolerance in arthritogenic B cells with receptors of differing affinity for self-antigen. Proc Natl Acad Sci U S A. 2006;103:3734–9. doi: 10.1073/pnas.0600214103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fukuyama H, Nimmerjahn F, Ravetch JV. The inhibitory Fcgamma receptor modulates autoimmunity by limiting the accumulation of immunoglobulin G+ anti-DNA plasma cells. Nat Immunol. 2005;6:99–106. doi: 10.1038/ni1151. [DOI] [PubMed] [Google Scholar]
- 40.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, Shark KB, Grande WJ, Hughes KM, Kapur V, Gregersen PK, Behrens TW. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A. 2003;100:2610–5. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, Pascual V. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197:711–23. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kirou KA, Lee C, George S, Louca K, Papagiannis IG, Peterson MG, Ly N, Woodward RN, Fry KE, Lau AY, Prentice JG, Wohlgemuth JG, Crow MK. Coordinate overexpression of interferon-alpha-induced genes in systemic lupus erythematosus. Arthritis Rheum. 2004;50:3958–67. doi: 10.1002/art.20798. [DOI] [PubMed] [Google Scholar]
- 43.Feng X, Wu H, Grossman JM, Hanvivadhanakul P, Fitzgerald JD, Park GS, Dong X, Chen W, Kim MH, Weng HH, Furst DE, Gorn A, McMahon M, Taylor M, Brahn E, Hahn BH, Tsao BP. Association of increased interferon-inducible gene expression with disease activity and lupus nephritis in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;54:2951–62. doi: 10.1002/art.22044. [DOI] [PubMed] [Google Scholar]
- 44.Jorgensen TN, Gubbels MR, Kotzin BL. Links between type I interferons and the genetic basis of disease in mouse lupus. Autoimmunity. 2003;36:491–502. doi: 10.1080/08916930310001605864. [DOI] [PubMed] [Google Scholar]
- 45.Xin H, D’Souza S, Jorgensen TN, Vaughan AT, Lengyel P, Kotzin BL, Choubey D. Increased expression of Ifi202, an IFN-activatable gene, in B6.Nba2 lupus susceptible mice inhibits p53-mediated apoptosis. J Immunol. 2006;176:5863–70. doi: 10.4049/jimmunol.176.10.5863. [DOI] [PubMed] [Google Scholar]
- 46.Lovgren T, Eloranta ML, Bave U, Alm GV, Ronnblom L. Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 2004;50:1861–72. doi: 10.1002/art.20254. [DOI] [PubMed] [Google Scholar]
- 47.Vallin H, Perers A, Alm GV, Ronnblom L. Anti-double-stranded DNA antibodies and immunostimulatory plasmid DNA in combination mimic the endogenous IFN-alpha inducer in systemic lupus erythematosus. J Immunol. 1999;163:6306–13. [PubMed] [Google Scholar]
- 48.Rozzo SJ, Allard JD, Choubey D, Vyse TJ, Izui S, Peltz G, Kotzin BL. Evidence for an interferon-inducible gene, Ifi202, in the susceptibility to systemic lupus. Immunity. 2001;15:435–43. doi: 10.1016/s1074-7613(01)00196-0. [DOI] [PubMed] [Google Scholar]
- 49.Boule MW, Broughton C, Mackay F, Akira S, Marshak-Rothstein A, Rifkin IR. Toll-like receptor 9-dependent and -independent dendritic cell activation by chromatin-immunoglobulin G complexes. J Exp Med. 2004;199:1631–40. doi: 10.1084/jem.20031942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lesley R, Xu Y, Kalled SL, Hess DM, Schwab SR, Shu HB, Cyster JG. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity. 2004;20:441–53. doi: 10.1016/s1074-7613(04)00079-2. [DOI] [PubMed] [Google Scholar]
- 51.Thien M, Phan TG, Gardam S, Amesbury M, Basten A, Mackay F, Brink R. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20:785–98. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 52.Kalled SL. The role of BAFF in immune function and implications for autoimmunity. Immunol Rev. 2005;204:43–54. doi: 10.1111/j.0105-2896.2005.00219.x. [DOI] [PubMed] [Google Scholar]
- 53.Mathsson L, Ahlin E, Sjowall C, Skogh T, Ronnelid J. Cytokine induction by circulating immune complexes and signs of in-vivo complement activation in systemic lupus erythematosus are associated with the occurrence of anti-Sjogren’s syndrome A antibodies. Clin Exp Immunol. 2007;147:513–20. doi: 10.1111/j.1365-2249.2006.03313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ronnelid J, Tejde A, Mathsson L, Nilsson-Ekdahl K, Nilsson B. Immune complexes from SLE sera induce IL10 production from normal peripheral blood mononuclear cells by an FcgammaRII dependent mechanism: implications for a possible vicious cycle maintaining B cell hyperactivity in SLE. Ann Rheum Dis. 2003;62:37–42. doi: 10.1136/ard.62.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Desai DD, Harbers SO, Flores M, Colonna L, Downie MP, Bergtold A, Jung S, Clynes R. Fc gamma receptor IIB on dendritic cells enforces peripheral tolerance by inhibiting effector T cell responses. J Immunol. 2007;178:6217–26. doi: 10.4049/jimmunol.178.10.6217. [DOI] [PubMed] [Google Scholar]
- 56.Martinsson K, Carlsson L, Kleinau S, Hultman P. The effect of activating and inhibiting Fc-receptors on murine mercury-induced autoimmunity. J Autoimmun. 2008;31:22–9. doi: 10.1016/j.jaut.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 57.Li H, Jiang Y, Prak EL, Radic M, Weigert M. Editors and editing of anti-DNA receptors. Immunity. 2001;15:947–57. doi: 10.1016/s1074-7613(01)00251-5. [DOI] [PubMed] [Google Scholar]
- 58.Li X, Su K, Ji C, Szalai AJ, Wu J, Zhang Y, Zhou T, Kimberly RP, Edberg JC. Immune opsonins modulate BLyS/BAFF release in a receptor-specific fashion. J Immunol. 2008;181:1012–8. doi: 10.4049/jimmunol.181.2.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chun HY, Chung JW, Kim HA, Yun JM, Jeon JY, Ye YM, Kim SH, Park HS, Suh CH. Cytokine IL-6 and IL-10 as biomarkers in systemic lupus erythematosus. J Clin Immunol. 2007;27:461–6. doi: 10.1007/s10875-007-9104-0. [DOI] [PubMed] [Google Scholar]
- 60.Llorente L, Zou W, Levy Y, Richaud-Patin Y, Wijdenes J, Alcocer-Varela J, Morel-Fourrier B, Brouet JC, Alarcon-Segovia D, Galanaud P, Emilie D. Role of interleukin 10 in the B lymphocyte hyperactivity and autoantibody production of human systemic lupus erythematosus. J Exp Med. 1995;181:839–44. doi: 10.1084/jem.181.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–20. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Llorente L, Richaud-Patin Y, Garcia-Padilla C, Claret E, Jakez-Ocampo J, Cardiel MH, Alcocer-Varela J, Grangeot-Keros L, Alarcon-Segovia D, Wijdenes J, Galanaud P, Emilie D. Clinical and biologic effects of anti-interleukin-10 monoclonal antibody administration in systemic lupus erythematosus. Arthritis Rheum. 2000;43:1790–800. doi: 10.1002/1529-0131(200008)43:8<1790::AID-ANR15>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 63.Ishida H, Muchamuel T, Sakaguchi S, Andrade S, Menon S, Howard M. Continuous administration of anti-interleukin 10 antibodies delays onset of autoimmunity in NZB/W F1 mice. J Exp Med. 1994;179:305–10. doi: 10.1084/jem.179.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ji JD, Tassiulas I, Park-Min KH, Aydin A, Mecklenbrauker I, Tarakhovsky A, Pricop L, Salmon JE, Ivashkiv LB. Inhibition of interleukin 10 signaling after Fc receptor ligation and during rheumatoid arthritis. J Exp Med. 2003;197:1573–83. doi: 10.1084/jem.20021820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Williams L, Bradley L, Smith A, Foxwell B. Signal transducer and activator of transcription 3 is the dominant mediator of the anti-inflammatory effects of IL-10 in human macrophages. J Immunol. 2004;172:567–76. doi: 10.4049/jimmunol.172.1.567. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.