Abstract
NF-κB is a ubiquitous transcription factor that regulates various kinds of genes including inflammatory molecules, macrophage infiltration factors, cell adhesion molecules, etc., in various disease processes including cardiac hypertrophy and heart failure (HF). Previously, we have demonstrated that activation of NF-κB was required in myotrophin induced cardiac hypertrophy, in spontaneously hypertensive rats (SHR) and in dilated cardiomyopathy (DCM) human hearts. Moreover, our recent study using the myotrophin overexpressed transgenic mouse (Myo-Tg) model showed that shRNA-mediated knock down of NF-κB significantly attenuated cardiac mass associated with improved cardiac function. Although, it has been shown that NF-κB is substantially involved in cardiovascular remodeling, it is not clear whether the continuous blockade of NF-κB is effective in cardiovascular remodeling. To address this question, we took a genetic approach using IκBα triple mutant mice (3M) bred with Myo-Tg mice (a progressive hypertrophy/HF model). The double transgenic mice (Myo-3M) displayed an attenuated cardiac hypertrophy (9.8 ± 0.62 vs 5.4 ± 0.34, p<0.001) and improved cardiac function associated with significant inhibition of the NF-κB signaling cascade, hypertrophy marker gene expression, inflammatory and macrophage gene expression at 24 weeks of age compared to Myo-Tg mice. NF-κB-targeted gene array profiling displayed several important genes were significantly down regulated in Myo-3M mice compared to Myo-Tg mice. Furthermore, Myo-3M did not show any changes of apoptotic gene expression indicating that complete inhibition of NF-κB activation reduces further pro-inflammatory reactions without affecting susceptibility to apoptosis. Therefore, development of therapeutic strategies targeting NF-κB may provide an effective approach to prevent adverse cardiac pathophysiological consequences.
Keywords: NF-κB, IκBκ dominant negative mice, cardiac hypertrophy, myotrophin, macrophage, NF-κB-gene array
INTRODUCTION
Cardiac hypertrophy is a fundamental process of adaptation to an increased workload due to hemodynamic overload (1, 2). This increase in size can be attributed to a number of diverse factors including physiological, mechanical, humoral and even genetic influences (3). Development of cardiac hypertrophy is initially beneficial since it augments the number of contractile units and reduces ventricular wall stress to normal levels. However, sustained hypertrophy can lead to dilated cardiomyopathy and HF. The hypertrophic growth of cardiomyocytes is triggered by multiple signal transduction pathways and ultimately involves activation of nuclear factors and the regulation of responsive gene expression. Myotrophin, a 12-kDa soluble ankyrin repeat domain protein was identified and characterized from spontaneously hypertensive rat (SHR) hearts and cardiomyopathic human hearts responsible for myocytes growth (4, 5, 6). Myotrophin is related to IκBα, but acts to activate nuclear factor-κB (NF-κB) rather than repress it. We employed in this study, a transgenic mouse model developed by our laboratory, having cardiac specific overexpression of myotrophin (Myo-Tg) in the heart via the α-myosin heavy chain as a promoter (7). The Myo-Tg mice exhibited left ventricular hypertrophy, atrial dilation, myocyte necrosis, multiple focal fibrosis, pleural effusion and compromised cardiac function associated with significant high levels of NF-κB activity (7, 8).
Among several other signal transduction pathways involved in the hypertrophic growth of myocardium, NF-κB signaling cascade has been a major focus of attention as it has recently been shown by many investigator including our laboratories that NF-κB plays a pivotal role in cardiac hypertrophy and HF (9, 10, 11, 12). In un-stimulated cells, NF-κB dimers are retained in the cytoplasm by interaction with IκB inhibitory proteins. Following stimulation, IκB proteins are phosphorylated, ubiquitinated and degraded by 26S proteasomes. Consequently, liberated NF-κB dimers are translocated to the nucleus and regulate the transcription of specific target genes (13, 14). One of the recent conceptual advances in our understanding is that during pathogenesis of cardiac remodeling inflammation occur resulted in activation of pro-inflammatory cytokines/growth factors and the recruitment of macrophages and have direct pathophysiological effects upon cardiac myocytes and non-myocytes, promoting myocardial damage and fibrosis (15, 16). Our previous study showed that NF-κB activation was required in the development of cardiac hypertrophy in SHR (17) and treatment with pyrolidine dithiocarbamate (PDTC, a pharmacological inhibitor of NF-κB) significantly attenuated cardiac mass suggesting NF-κB’s beneficial effect. Moreover, we showed, using explanted human heart (12), that NF-κB-target genes were significantly activated during HF. Since, the effects of NF-κB must be mediated by NF-κB-dependent genes, it would be logical to assess the effect of blockade of NF-κB on its target gene expression and the pro-inflammatory and macrophage infiltration during cardiovascular remodeling.
A genetic approach is the most definitive way to assess the function of any gene due to the specificity of this approach. In fact, direct pharmacological inhibitors of NF-κB do not exist; drugs that do block upstream signaling kinases exist but are not completely selective for NF-κB. Although mice bearing genetic disruptions of all of the rel-family proteins exist, some are lethal (p65), some infertile (RelB), and all of them exhibit defects in inflammatory and immune responses that would likely affect development of cardiac pathophysiology (18, 19, 20, 21). Particularly, since p65 appears to be the major NF-κB subunit activated in hypertrophy and HF, the lethality of homozygous p65 knockout mice precludes their use in studies querying the role of NF-κB in these phenomena. A transgenic mouse expressing a dominant-negative IκBα with triple mutations (3M) of the amino-terminal serine and the tyrosine that mediate NF-κB activation (IκBα S32A, S36A, Y42F) has been shown to exhibit normal cardiac morphology, histopathology and physiology(22). Activation of NF-κB in response to cytokines and TNF-α induced cardiomyopathy is completely absent in these mice (22).
We hypothesize that inhibition of NF-κB activation cascade would be an efficacious therapeutic approach for treatment of cardiac hypertrophy and HF by attenuating the pro-inflammatory and other NF-κB’s target gene expression. In this study, we examined our hypothesis by using double transgenic mice harboring IκBα mutant gene (3M) and Myo-Tg (Myo-3M).
MATERIAL AND METHOD
Generation of myotrophin overexpressed transgenic mice
Generation of transgenic mice was described previously (7). The studies were conducted with the approval of The Cleveland Clinic Foundation’s Institutional Review Board. In all experiments undertaken in this study, age and sex-matched wild type (WT) mice were used for comparison with Myo-Tg mice. We also used WT/3M mice as a comparative control for Myo-3M and Myo-Tg. 3M mice did not show any abnormality and behave as WT. In all experiments, we used either WT/3M breeding pairs as a control except for the study of IκBα protein.
Generation of IκBα dominant negative mice
IκBα dominant negative mice were generated as described previously (22, 23).
Extraction of cytoplasmic, nuclear protein, western blotting and northern blotting
Nuclear and cytoplasmic extracts were made according to the method described by Dignam et al (24) using WT/3M, Myo-Tg and Myo-3M mice hearts of 24-week old. Western blot analysis was performed as described previously (12). Membranes were probed with IκBα, NF-κB p65, pAkt (473) and Akt antibodies (Cell Signaling Technology, Beverly, CA) overnight at 4° C (all at 1:1000 dilution). Histone (for nuclear protein) and Actin (for cytoplasmic protein) as an internal loading control. Total RNA was isolated from the ventricle of WT and Myo-Tg mice according to the protocol of Chomczynsky and Sacchi, 1987 (25).
Electrophoretic mobility shift assay (EMSA), IKKβ activity and histological analysis
EMSA was performed using a double-stranded NF-κB binding site oligonucleotide as a probe, as described previously (11). Left ventricular tissue from age-matched WT/3M and Myo-Tg and Myo-3M were homogenized and IKKβ activity was determined using GST-IκBα as a substrate described previously (12). Sections were then photographed with an Olympus photomicroscope at 20 × magnification as described previously (8). The primary antibodies used in immunohistological analysis included p65 and MCP-1, all at 1: 200 dilution.
RNase protection assay (RPA)
Total RNA was isolated using Trizol reagent (Invitrogen) from WT/3M, Myo-Tg and Myo-3M mice hearts. RPAs were done using the RiboQuant system with mouse multi probe APO-1 (Caspases) and mouse APO-2 (Bcl2 family genes) template set from BD Bioscience. The labeling was done using dUTP according to the manufacturer protocol. The probes (5× 106 cpm) were hybridized with 10 µg of total RNA from each sample at 56°C and resolved on 5% denaturing polyacrylamide gels. Internal house keeping genes (L32 and GAPDH) were analyzed for loading control.
NF-κB target gene array analysis
The NF-κB-target gene array was performed using the TranSignal mouse NF-κB Target Gene Array kit from Panomics, Inc. (Redwood City, CA) as described previously (12).
Determination of Cardiac Function, Data Collection and Data Analysis
Echocardiography and data collection were analyzed as described previously (8).
Statistical Analysis
Results are expressed as mean ± S.E. Differences between groups were tested for statistical significance by paired Student’s t test. Differences were considered significant at p < 0.001. We calculate the inhibitory effect of NF-κB activation cascade and down regulation of gene expression in Myo-3M as a % (down) over Myo-Tg mice. Data were also analyzed by two-way analysis of variance (ANOVA) using GraphPad Prism software (GraphPad Software, Inc., San Diego, USA) for Myo-3M mice. For NF-κB-target gene array analysis, genes are arranged in order by t-statistic, i.e. from largest to smallest standardized difference in mean. We used 0.001 as the critical level (Bonferroni’s correction).
RESULTS
Effect of inhibition of NF-κB on cardiac mass and function in Myo-3M mice
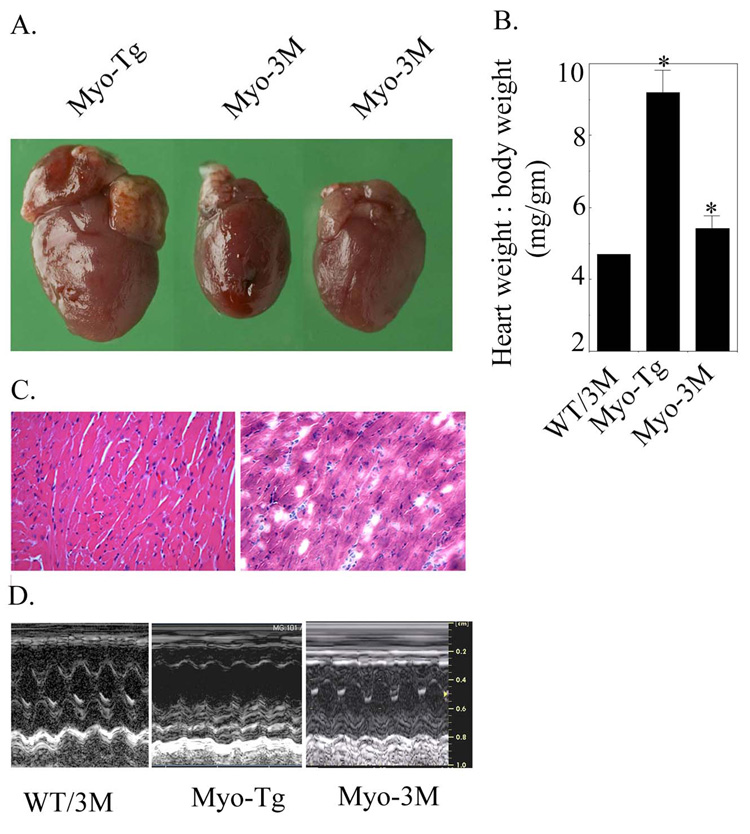
To explore the effect of inhibition of NF-κB on cardiac mass, Myo-Tg mice were crossed with 3M transgenic mice. Double transgenic mice (Myo-3M) were sacrificed at 24 weeks of age and their heart weight to body weight determined as shown in Fig. 1 A and B. Myo-3M mice show a significant attenuation of heart weight to body weight ratio in comparison to Myo-Tg mice (9.8 ± 0.62 vs 5.4 ± 0.34, p<0.001). Furthermore, histological analysis of hearts from both Myo-Tg and Myo-3M showed significant reduction in myocyte cross-section (Fig. 1C). Echocardiographic data from Myo-3M mice showed improvement of cardiac function as compared to Myo-Tg mice. On the contrary, Myo-Tg mice showed impaired cardiac function compared to WT/3M mice. When compared with Myo-Tg, Myo-3M mice showed a trend towards improvement of both ejection fraction (0.57± 5.3% vs 0.77 ± 15.2, p < 0.09) and fractional shortening (24.5 ± 1.16 vs. 42.8 ± 1.9, p < 0.06). The number of animal used are: n=3 for WT-3M, n=5 for Myo-Tg and n=4 for Myo-3M.:
Figure 1. Attenuation of cardiac hypertrophy in Myo-3M mouse.
(A) Typical appearance of heart size in Myo-Tg and Myo-3M mice. (B) Heart weight: body weight ratio in Myo-3M mice. Values represent mean ± SE and p = 0.002 compared with untreated Myo-Tg mice (n=6). (C) H &E staining of Myo-Tg and Myo-3M mice hearts. Sections were visualized and photographed with an Olympus photomicroscope at 20 × magnification. (D) Representative M-mode tracings of the left ventricle obtained in WT/3M, Myo-Tg and Myo-3M mice. Results are presented as the mean SEM and represent four different mice (p < 0.001 compared with the Myo-Tg mice).
Status of NF-κB activation cascade in Myo-3M mice
We analyzed the NF-κB signaling components using WT/3m, Myo-Tg and Myo-3M mice as described below:
a. NF-κB activation
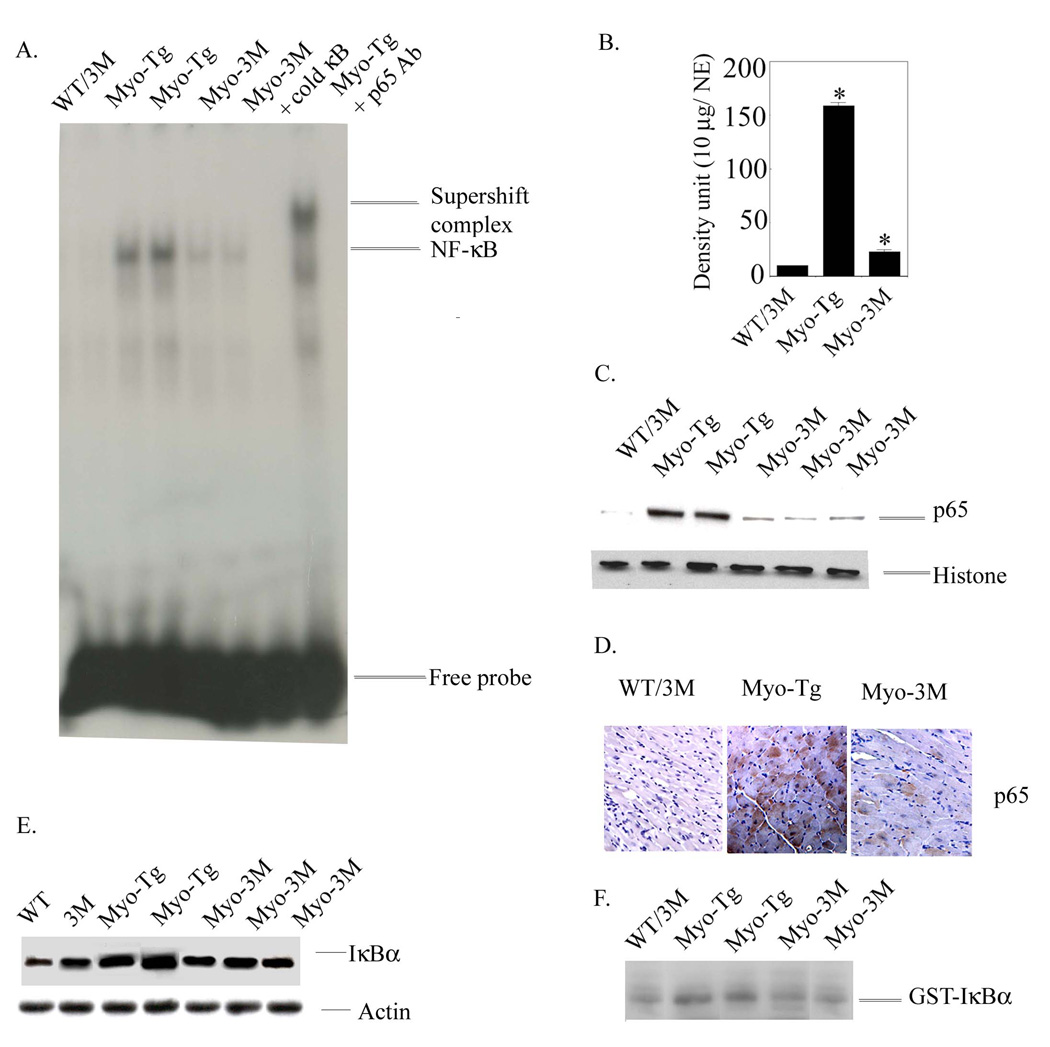
Double transgenic mice (Myo-3M, n=6) were sacrificed at 24 weeks of age. Inhibition of NF-κB activation the hearts from Myo-Tg (i.e. in Myo-3M mice) resulted significant reduction in NF-κB activity (Fig. 2A, 85.93 % reduction relative to untreated Myo-Tg mice, p =0.002, density/10 µg nuclear extract). There was no significant activation of NF-κB detectable in WT or 3M mice. In addition, we determined the translocation of NF-κB-p65 protein into nucleus by western blot analysis. Our data showed a significant inhibition of translocation of active NF-κB-p65 protein into nucleus in Myo-3M mice whereas in Myo-Tg mice there was a robust translocation NF-κB-p65 protein into nucleus (Fig 2 C). Moreover, we assessed the NF-κB-p65 level in the myocardium of both Myo-Tg and Myo-3M mice by immunohistology. The results support inhibition of NF-κB-p65 protein in the myocardium of Myo-3M mice compared to Myo-Tg (Fig 2 D).
Figure 2. NF-κB activation cascades Myo-3M mice hearts.
(A) Nuclear protein was extracted from the hearts of WT/3M, Myo-Tg mice and Myo-3M. Binding reactions were performed with an NF-κB oligonucleotide labeled with 32P-dATP. The complex formation was eliminated with excess unlabeled NF-κB oligonucleotide. The complex formation was confirmed by supershift analysis using p65 antibody. NE: Nuclear extract. (B) Quantification of EMSA using an arbitrary density unit (10 µg/NE). (C) Western blots profile of NF-κB p65 protein in the nucleus. Histone antibody was used as an internal nuclear protein loading control. (D) Expression of p65 active protein in the heart section of both Myo-Tg and Myo-3M mice and were photographed with an Olympus photomicroscope at 20 × magnification. This figure is representative of three different mice in each group (WT/3M and Myo-Tg). (E). Cytoplasmic protein extracts were made from both WT, 3M, Myo-Tg and Myo-3M mouse hearts at 24 weeks of age. Tissue extracts (50 µg) were analyzed for the intracellular level of total IκBα protein content and (F) Actin protein was used as an internal loading control. Results are presented as the mean SEM and represent three different mice in each group (Myo-Tg and Myo-3M (p < 0.001).
b. IκBα total protein level
The appearance of total IκBα cytosolic protein was analyzed by immunoblot analysis using IκBα antibody that does not detect phosphorylated IκBα, as the probe. The data are shown in Fig. 2 E. Myo-Tg mice showed a significant increase in IκBα total protein compared to age-matched WT mice. It is of note that, compared to WT, 3M mice showed 2.5 times more IκBα protein. This is due to it’s overexpression in the heart (Fig 2 E 2nd lane). Compared with Myo-Tg mice, Myo-3M mice showed a significant reduction in IκBα levels (60.8 % reduction in Myo-3M relative to Myo-Tg mice, p< 0.001) (Fig 2 E). Actin protein was used as an internal loading control (Fig 2 E). It is of note that we used separate WT and 3M mice as a comparative control in this particular experiment.
c. IKKβ activity
To explore the involvement of IKKβ, we determined the IKKβ activity in WT/3M, Myo-Tg and Myo-3M mice hearts (Fig.2 F). IKKβ activity was detected in all Myo-Tg mouse hearts and was very low in WT hearts. In Myo-Tg mice, a 3.8-fold increase in IKKβ activity was observed in compare to WT/3M (p < 0.001) at 24 weeks. A significant reduction of IKKβ activity (72 % over Myo-Tg, p < 0.001) was observed in Myo-3M mice compared with Myo-Tg mice.
Determination of hypertrophic marker gene expression in Myo-3M mice
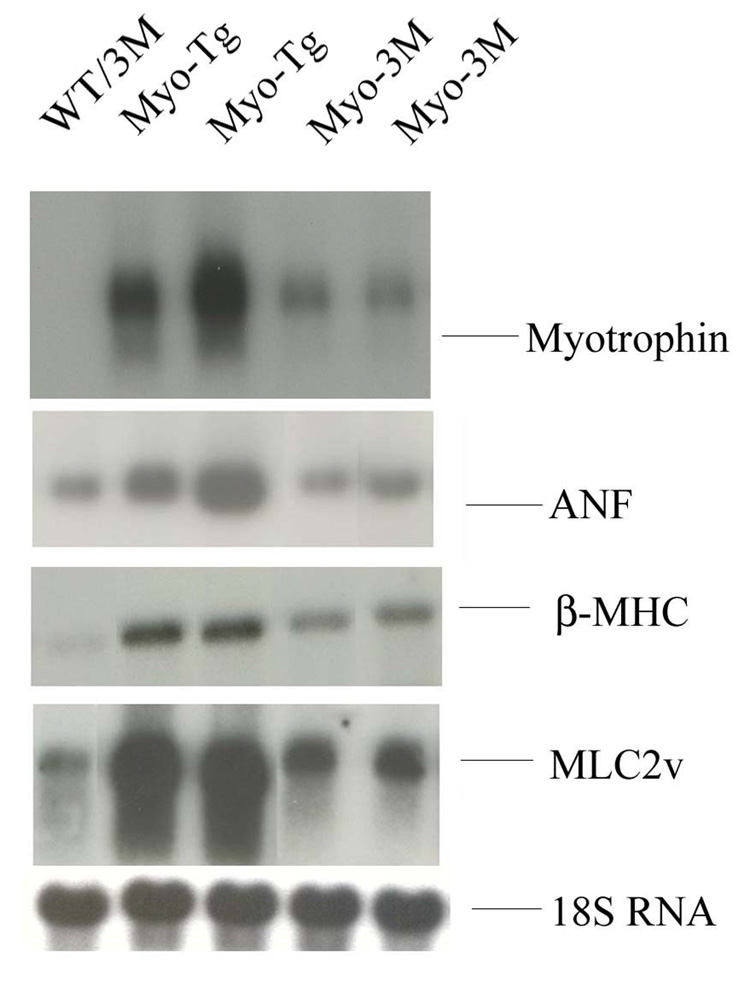
To evaluate the expression of hypertrophy marker genes, ANF, myosin light chain 2 (MLC 2) and β-myosin heavy chain (βMHC) in Myo-3M mice, northern blotting was performed. All three genes were significantly upregulated in Myo-Tg mice (5.75-, 4.8 and 4.1 fold respectively, compared to WT/3M mice, p <0.001). Myo-3M mice showed a significant inhibition of ANF (72.46% over Myo-Tg, p < 0.001) MLC 2 (68.4% over Myo-Tg, p < 0.001) and β-MHC (58.87 over Myo-Tg, p < 0.001) gene expression compared to age-matched Myo-Tg. In all experiments, we did not see any changes in either WT or 3M mice (Fig 3).
Figure 3. Determination of steady state level of ANF, β-MHC and MLC2 (v) gene expressions in 3M mice.
Total RNA was extracted from hearts of 24-week old WT/3M, Myo-Tg and Myo-3M mice. mRNA expression was determined using (A) ANF, (B) β-MHC, (C) MLC2 (v) and (D) 18S rRNA oligonucleotides labeled with 32P-ATP as a probes. Results are presented as the mean SEM and represent three different mice (p < 0.001 compared with the Myo-Tg mice).
Analysis of inflammatory gene expression and macrophage infiltration in Myo-3M mice
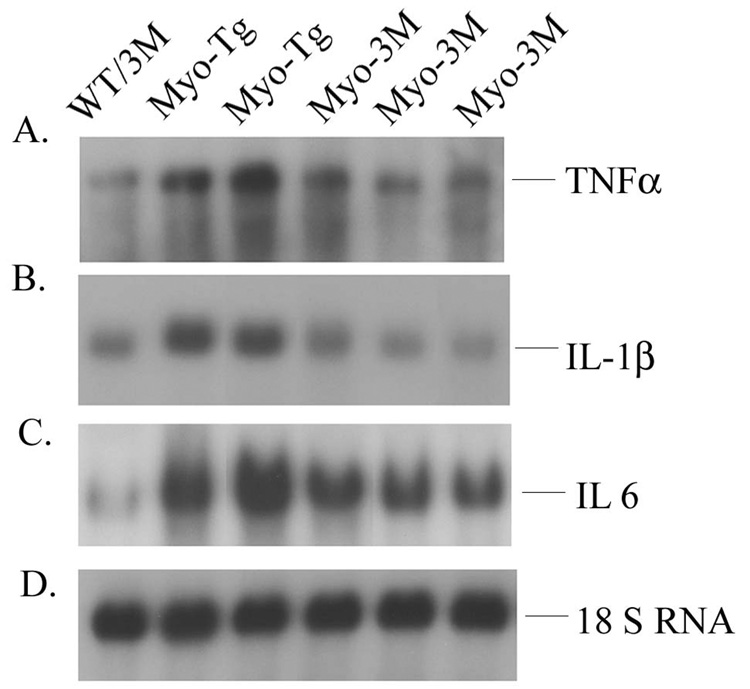
We analyzed inflammatory gene expression in Myo-3M mice by northern blotting. Our data showed significant reduction of the steady state levels of TNFα, IL-6 and IL-1β (59.2, 61.1 and 47.7 % over Myo-Tg, p < 0.001 respectively) in Myo-3M mice compared to Myo-Tg mice. WT/3M mice were used as a control. The results are summarized in Fig. 4.
Figure 4. Determination of steady state level of TNFα, IL-1β and IL-6 in Myo-3M mice hearts.
Total RNA was extracted from hearts of 24-week old WT/3M, Myo-Tg and Myo-3M mice. mRNA expression was determined using (A) TNFα, (B) IL-1β and (C) IL-6 oligonucleotide labeled with 32P-dATP as a probe. (D) 18S rRNA probe was used as a loading control. Results are presented as the mean SEM and represent three different mice (p < 0.001 compared with the Myo-Tg mice).
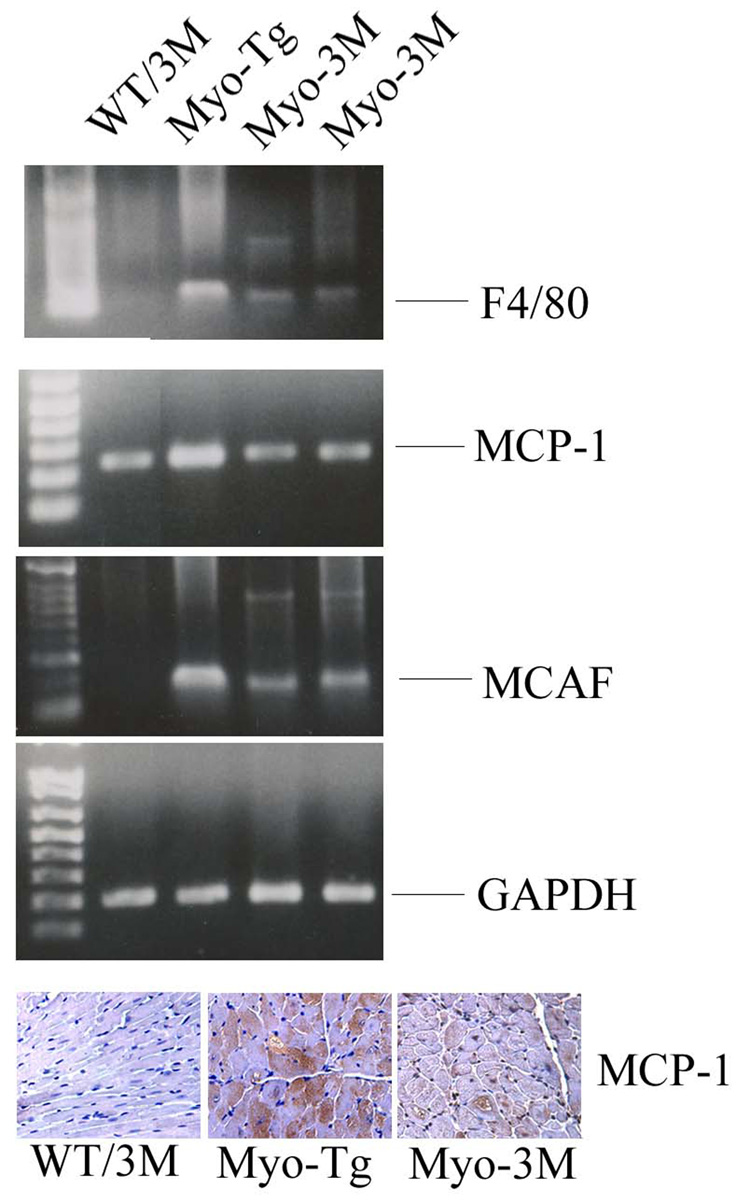
We also determined levels for genetic markers of macrophage infiltration in Myo-3M mice. We included MCP-1, F4/80 and MCAF in this study as they have been reported to play an important role in cardiac diseases. Our data showed that MCP-1, F4/80 and MCAF were significantly reduced (70.5, 62.7 and 67.4% over Myo-Tg mice respectively, p< 0.001) in Myo-3M mice compared with Myo-Tg mice (Fig 5).
Figure 5. Analysis of macrophage infiltration in Myo-3M mice hearts.
Total RNA was extracted from hearts of 24-week old WT/3M, Myo-Tg and Myo-3M mice. Semi-quantitative RT-PCR was performed using (A), F4/80 (B) MCP-1 and (C) MCAF specific primers. Results are presented as the mean SEM and represent three different mice (p < 0.001 compared with the Myo-Tg mice). (D). Immunohistological analysis of MCP-1 in cardiac section of WT/3M, Myo-Tg and Myo-3M at 20X magnification.
Analysis of NF-κB-target gene expression in Myo-3M mice
Previously, we have shown that a wide variety of NF-κB-targeted genes are activated in DCM human hearts as well as Myo-Tg mice (12, 8). To gain further insight into the NF-κB-target gene expression in Myo-3M mice, we used the TranSignal mouse NF-κB Target Gene Array System. The expression of various NF-κB-targeted genes at 24 weeks is summarized in Tables 1a. The genes are arranged in order by t-statistic, i.e. from largest to smallest standardized difference in mean. We used p < 0.001 as the critical level (Bonferroni’s correction). Genes found to be upregulated at 24 weeks of age are shown in Table 1a (41 selected genes, fold value 2.5 and above). The genes included were Alox-12, AHRR, ApoC3, AGER, Bcl2a1a, BGN, BLR-1, Cyclin D1 and D3, CD69, CSF-2 and CSF-3, Fcer2a, F8, HMGN-1, GRO-1, GSTP-1, FB, FasL, Fth, Gly96, HAS-1, IGFBP-2, IFNβ, IFNγ, IRF-2, IL-10, IL-11, IL-6, IL-2, IL-m, IκBα, MadCam-1, myc, NF-κB-1, NF-κB-2, PENK-1, PDGFβ, rel, PTGIS and TNFα. When compared to Myo-3M mice, many genes are found to be down regulated, suggesting a potential role in cardiac hypertrophy. The genes found to be down regulated in Myo-3M mice compared to Myo-Tg are showing in the table 1b (23 selected genes, fold value 2.0 compared to Myo-Tg mice, compare the expression levels in table 1b to those in table 1a). These included Apoc3, BGN, BLr-1, Ccnd-1, CSF-2, CSF-3, GRO-1, GSTP-1, HMGN-1, Gly96, HAS-1, ICAM-1, IL-11, IL-15, IL-1β, IL-6, IRF-2, myc, NF-κB-1, NF-κB-2, IκBα, MadCAM-1, Rel, TNFα and VEGFc. The remainder of the genes on the array showed no significant changes in their expression level compared to Myo-Tg.
Determination of apoptotic gene expression in Myo-3M mice
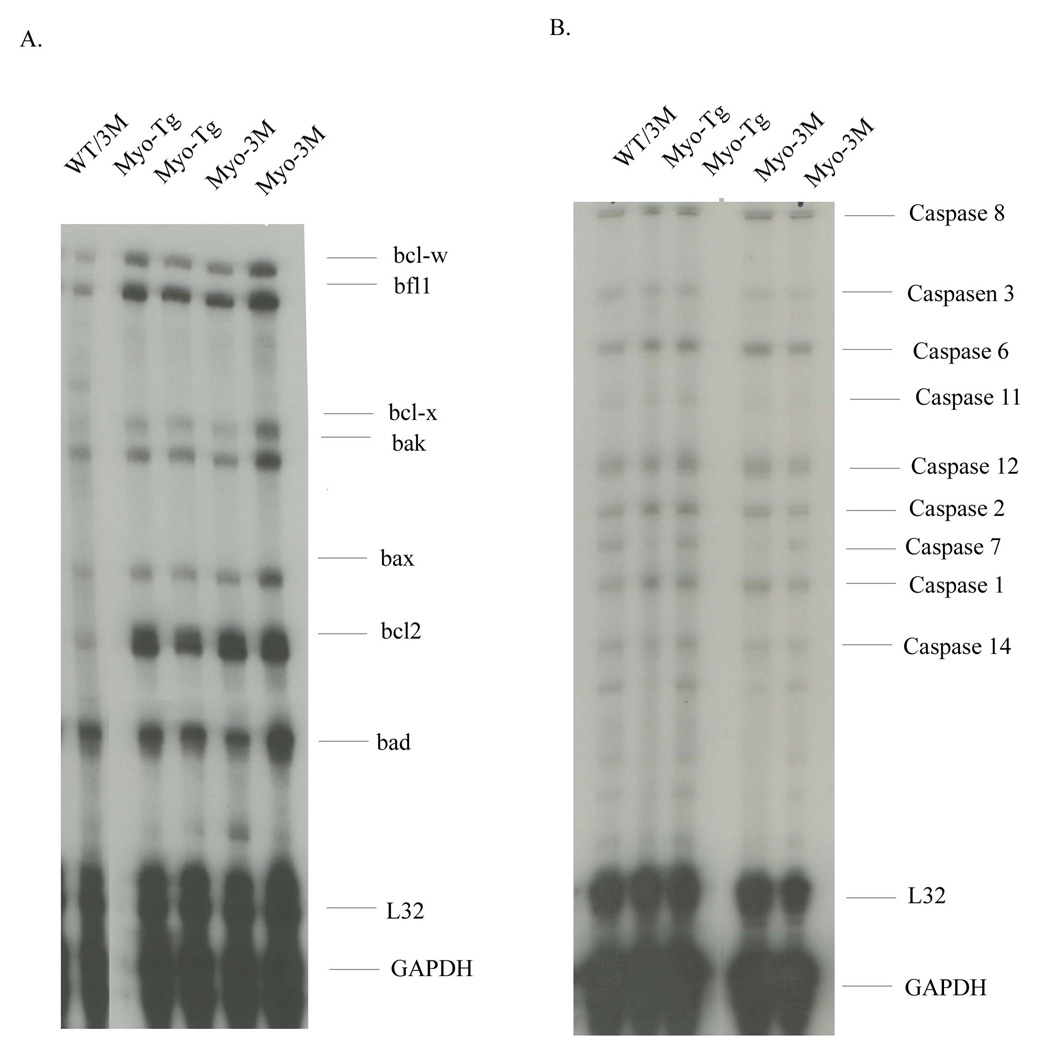
In order to determine the status of apoptotic gene expression profiles we performed RPA analysis using mouse multi-probes APO1 and APO2 kit. This includes caspase family and Bcl2 family genes. The data are presented in Fig. 6 A and B. Several apoptotic genes are induced, as expected, in Myo-Tg at 24 weeks of age compared to WT mice. Significant upregulation of Bcl2 family members was observed in Myo-Tg mice. This includes bcl-w, bfl, bcl-x, bak, bax, bad and bcl2. Among them bcl2 and bcl-w and bfl1 showed maximum upregulation (3.8-, 2.6- and 3.2-fold compared to WT/3M mice, p< 0.001) in Myo-Tg mice. In addition, we determined the caspase family genes, which include caspase 8, 3-, 6-, 11-, 12-, 2-, 7-, 1- and 14. Our data showed an increase level of caspase 8-, 6-, 2- and 1 (1.8-, 2.4-, 2.0- and 2.1 fold compared to WT/3M mice, p < 0.001) in Myo-Tg mice. When analyzed these two sets of apoptotic genes in Myo-3M mice, no significant changes relative to Myo-Tg were observed despite of reduction of cardiac mass.
Figure 6. Status of apoptotic gene expression in Myo-3M mouse hearts.
Total RNA was isolated from hearts of WT/3M, Myo-Tg and Myo-3M mice. RPA was performed using mouse APO-1 and mouse APO-2 kit. Results are presented as the mean SEM and represent three different mice (p < 0.001 compared with the Myo-Tg mice).
Analysis of AKT phosphorylation in Myo-3M mice
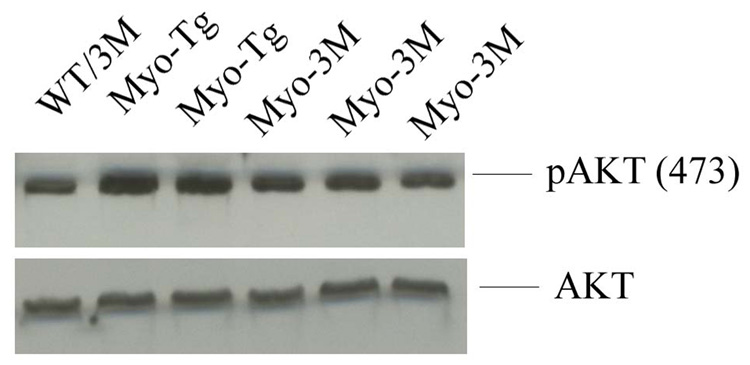
To assess the role of AKT in cardiac hypertrophy, we also examined AKT phosphorylation (at serine 473) in Myo-3M mice compared to Myo-Tg mice. We observed a 2.5 fold increase (p <0.001) in AKT 473 phosphorylation in Myo-Tg relative to Wt/3M (Fig 7). The Myo-3M mice showed a significant decrease in AKT phosphorylation relative to Myo-Tg (P≤ 0.001).
Figure 7. Analysis of Akt phosphorylation in Myo-3M mice hearts.
Total protein extracts were made from both WT/3M, Myo-Tg and Myo-3M mouse hearts. 50 µg of protein extracts were run on 10 % SDS-PAGE and were probed with pAkt (473) and Akt. Results are presented as the mean SEM and represent three different mice (p < 0.001 compared with the Myo-Tg mice).
DISCUSSION
The important observation of this study is that inhibition of NF-κB in Myo-Tg model, using a genetic approach, significantly attenuated cardiac mass and improved cardiac function. These changes are associated with significant reduction in NF-κB activation, NF-κB-dependent target gene mRNA levels, and, importantly, down regulation of inflammatory genes and markers of macrophage infiltration. This is the first report using a genetic approach to dissect out the functional significance of NF-κB in myotrophin-induced cardiac hypertrophy. Our observation that NF-κB underlies much of the pathologic aspects of the hypertrophy in Myo-Tg mice is based on physiological, biochemical and molecular results discussed in detail below.
Although, we achieved significant inhibition of NF-κB activation in Myo-3M mice, we were unable to completely blockade NF-κB activity. This is interesting in light of the fact that we have previously been unable to detect any activation of NF-κB in the 3M transgenic mice after ischemia, I/R, cytokine injection, or in several murine cardiomyopathic models (22, 23) (unpublished observations, WKJ). At this point, it is difficult to explain this residual NF-κB activity in Myo-3M mice. We could speculate that other signal transduction cascades that may activate NF-κB via non-IκBα-dependent mechanisms may be operative during development of cardiac hypertrophy or progression to cardiac failure in the Myo-Tg mice. It is thought that NF-κB is activated in the acute hypertrophic process via different parallel signal transduction pathways regulating various downstream target genes. Another possibility is that this residual NF-κB activation occurs in non-cardiomyocytes. The 3M mice are cardiomyocyte-specific and it has been previously shown that this blocks NF-κB in myocardium after multiple stimuli. This implies that all detectable NF-κB activation occurs in cardiomyocytes. It remains possible however, that, in Myo-Tg mice, NF-κB is activated in non-cardiomyocytes during disease progression; this would not be blocked in the 3M transgenics. We also noted that there is an increase in levels of IκBα in the Myo-Tg mice, that was somewhat reduced in Myo-3M mice. This likely reflects the fact that the endogenous IκBα gene is known to be NF-κB-dependent and is thus upregulated by the NF-κB activation in the Myo-Tg model and repressed in Myo-3M mice. Furthermore, our data showed a significant inhibition of IKKβ levels in Myo-3M vs Myo-Tg mice. Although, the 3M transgenic mice block NF-κB downstream of IKKβ, it is possible that NF-κB regulates the IKK complex either directly, through transcriptional regulation of components, or indirectly through modulation of signaling. Cardiac NF-κB blockade does not lead to cardiac morphological or functional abnormalities (22). This result adds to the growing evidence that NF-κB plays an important role in heart diseases like cardiac hypertrophy and HF (17, 12, 26, 9). Our results demonstrates that blocking of NF-κB activation is functionally coupled to biological signals that lead to attenuation of left ventricular hypertrophy, is entirely consistent with other results (27, 28). It has been demonstrated, using p50 knockout mice challenged with angiotensin II infusion results in dramatic improvement in cardiac hypertrophic response in comparison to WT mice (27). Other studies using p50 knockout mice, it was shown that abrogation of p50 resulted in attenuation of myocardial inflammation and cardiac dysfunction in TNFα transgenic mice (28).
In addition to reduction of ventricular hypertrophy, we observed a significant down regulation of cardiac hypertrophy marker genes, including ANF, β-MHC and MLC-2 in 3M-Myo compared to Myo-Tg mice. These genes are not known to have NF-κB DNA binding sites in their proximal promoters. Reduction of marker gene expression is more likely to be an indirect effect of reduced load on the heart or could be indirectly mediated by the interaction of other transcription factors.
We also show an effect of NF-κB inhibition upon the inflammatory response, indicated by altered expression of pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6. These cytokines are not constitutively expressed in the normal heart, but are upregulated in Myo-Tg mice, in association with pathophysiology. Upregulation and production of these cytokines represent an intrinsic or innate stress response against myocardial injury (29). In this investigation, we found that TNF-α, IL-1β and IL-6 levels decreased noticeably in Myo-3M mice compared with Myo-Tg mice, demonstrating that NF-κB inhibition attenuates gene expression associated with the inflammatory response. One possible mechanism for such a protective effect pertains to the presence of κB-binding domain in their promoter sites (30), directly allowing NF-κB to regulate their expression. During the inflammatory phase, infiltration by inflammatory cells, particularly neutrophils and macrophages, is followed by removal of necrotic tissue and degradation of extracellular matrix components (29, 31). Inhibition of NF-κB activation would thus short-circuit much of this inflammatory program.
In addition to cytokines, our data showed the down regulation of MCP-1, MCAF and F4/F80 genes, markers of tissue inflammation. Recent evidence suggests that macrophage infiltration occurs during the HF process as macrophages produce cytokines and growth factors that influence the process of myocardial remodeling. In addition, macrophages may regulate extracellular matrix metabolism through the synthesis of matrix metalloproteinases and their inhibitors (32). Down regulation of MCP-1, a chemotactic factor in 3M mice is likely due the direct regulation of MCP-1 by NF-κB as the MCP-1 promoter is known to contain NF-κB consensus sites in its promoter region (33). There is evidence in support of a role for anti-MCP-1 therapy in the heart; blockade of MCP-1 reduced LV remodeling after myocardial infarction. This process was mediated by attenuation of macrophage infiltration and interstitial fibrosis (34, 35). This suggests that MCP-1 plays a pivotal role in the recruitment of inflammatory cells that accelerate LV remodeling. MCAF is a chemotactic factor for macrophages and is produced by a variety of tissue and cells, including endothelial cells (36). MCAF enhances intracellular adhesion molecule-1 expression in cultured myocytes, which might also recruit macrophages (37). We found that MCAF, upregulated in Myo-Tg hearts, was significantly reduced by NF-κB blockade. We examined expression of F4/80 because it is the most accurate indicator of the monocytes/macrophage lineage. It is detected in circulating (monocytes) as well as tissue infiltrating stages (macrophage) (38). In our Myo-3M mice, we observed the down regulation of F4/80 suggesting its upregulation plays an injurious role in cardiac remodeling. Thus inhibition of NF-κB potentially reduces myocardial damage, at least in part, by suppressing MCP-1, MCAF and F4/80 in the heart.
The most surprising result of this study was that there was no significant change in expression of apoptotic genes in Myo-Tg vs Myo-3M mice. Apoptosis is an important phenomenon associated with end stage HF (39, 40). It is a highly organized and regulated process wherein cell death follows a genetically program and is executed by caspase-dependent protein cleavage and DNA laddering or fragmentation (41). Ventricular remodeling is associated with increased apoptosis in the myocardium (42). It has been previously shown that the expression of apoptosis inhibitory genes like Bcl2 and Bcl-xl are NF-κB-dependent (43). In our mouse model, NF-κB inhibition attenuated cardiac hypertrophy without any detectable effects on cardiomyocyte apoptosis as indicated by either bcl2 or caspase family genes. This result demonstrates that the effects of NF-κB in myotrophin-driven hypertrophy/HF, does not involve as a primary mechanism, induction of apoptosis. Since the 3M transgenic is driven by the α-MHC gene promoter, this suggests that NF-κB has an important cardiomyocyte-specific role in regulating hypertrophy that is independent of programmed cell death. This result is supported by Bermann et al who showed that NF-κB inhibition by Ad5IκBαΔN in isolated neonatal rat cardiomyocytes resulted in no detectable change of iAP1, bcl-2, or bcl-xL protein levels (44). Alternative interpretations include that there could be compensatory regulation of other transcription factors that affect apoptosis and thus there is no effect of blocking NF-κB upon apoptosis. In any case, inhibition of NF-κB does not appear to sensitize the cells to pro-apoptotic effects. Further studies are needed to elucidate the specific pathophysiological conditions under which the inhibition of NF-κB activation may be beneficial or detrimental.
We further explored the status of NF-κB targeted gene expression in Myo-3M vs Myo-Tg mice using targeted arrays. We observed that several genes were down regulated in Myo-3M relative to Myo-Tg mice, but many more are not changed. The expression of NF-κB target genes depends not only on the activity of NF-κB itself, but also on a number of other factors, including NF-κB’s interaction with other transcription factors. Moreover, the pattern of NF-κB target genes varies under different physiological conditions and therefore, extending this sort of analysis to multiple time points, using conditional transgenesis for blockade and assessing gene expression at the protein level will be necessary. Ultimately, this type of analysis may delineate represent novel cardiac-specific genes that potentially provide a key to unlocking the underlying mechanism of hypertrophy and HF.
Finally, we observed a high level of Akt phosphorylation, indicative of activation, in Myo-3M mice. Akt controls phosphorylation of mTOR, p70S6K and GSK3β, three serine/threonine kinases responsible for increased protein synthesis. Forced expression of constitutively active Akt in the heart of transgenic mice induces increased cardiomyocyte size and concentric hypertrophy (45, 46). Our data showed that inhibition of NF-κB decreases the Akt phosphorylation. This suggests a link between Akt and NF-κB in the cardiac remodeling process. This is in fact, mirror image to our findings in a previous publication, wherein Akt activation was found to be suppressed in TNF1.6 mice with TNF-α-dependent cardiomyopathy (23). The results, taken together, show that, in one model, TNF1.6, NF-κB suppresses Akt, while in the other model, Myo-Tg (herein), NF-κB activates Akt. A good deal of evidence suggests that Akt at low levels is protective, but high levels, chronic activation are pro-disease. Thus NF-κB is implicated as a homeostatic regulator of Akt in the heart but whether this effect is direct or indirect remains to be determined.
In conclusion, our study revealed a global impact of NF-κB inhibition on cardiac mass regression and cardiac dysfunction, suggesting its therapeutic benefit. The literature supports that numerous pathways are involved in the remodeling process. However, NF-κB plays critical roles in hypertrophy, inflammatory cytokine expression and macrophage infiltration, which are clearly all major players in hypertrophy and HF. Therefore, NF-κB inhibition may be considered as a therapeutic means to protect the heart from further damage by modulating multiple critical aspects of the disease process. In addition, inhibition of specific combinations of NF-κB-target genes may offer potential therapeutic opportunities in future. However, a cautionary note is needed as it is unclear at present which components of the NF-κB gene expression network are optimal for therapeutic intervention and this might be different in discrete disease conditions. Thus, additional basic studies of the downstream genes regulated by NF-κB and their effects upon normal physiology and in pathophysiology are needed.
Supplementary Material
ACKNOWLEDGEMENT
This study was supported by the American Heart Association (Ohio Valley Affiliate) through Beginning Grant-in-aid 0565226B to S.G. and the National Institute Health Grant to KJ (HL63034). The author also acknowledges Dr. Subha Sen for providing Myotrophin overexpressing transgenic mouse (Myo-Tg) in this study. The authors acknowledge Ms Linda Vergo (Image Facility and Histology) for her expert technical assistance in immunohistology, the expert secretarial help from Michele Barnard.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
David Young, Department of Molecular Cardiology, NB 50, Lerner Research Institute, Cleveland Clinic, 9500 Euclid Avenue, Cleveland, Ohio-44195.
Zoran B. Popovic, Department of Cardiovascular Medicine, Cleveland Clinic, 9500 Euclid Avenue, Cleveland, Ohio-44195
W. Keith Jones, Department of Pharmacology and Cell Biophysics, University of Cincinnati, OH-.
Sudhiranjan Gupta, Department of Molecular Cardiology, NB 50, Lerner Research Institute, Cleveland Clinic, 9500 Euclid Avenue, Cleveland, Ohio-44195.
Reference List
- 1.Cooper G. Cardiocyte adaptation to chronically altered load. Annu. Rev. Physiol. 1987;49:501–518. doi: 10.1146/annurev.ph.49.030187.002441. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 2.Marian AJ, Roberts R. The molecular genetic basis for hypertrophic cardiomyopathy. J Mol Cell Cardiol. 2001;33:655–670. doi: 10.1006/jmcc.2001.1340. Ref Type: Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 4.Sen S, Kundu G, Mekhail N, Castel J, Misono K, Healy B. Myotrophin: purification of a novel peptide from spontaneously hypertensive rat heart that influences myocardial growth. J. Biol. Chem. 1990;265:16635–16643. Ref Type: Journal. [PubMed] [Google Scholar]
- 5.Sil P, Misono K, Sen S. Myotrophin in human cardiomyopathic heart. Circ. Res. 1993;73:98–108. doi: 10.1161/01.res.73.1.98. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 6.Sil P, Mukherjee DP, Sen S. Quantification of myotrophin from spontaneously hypertensive and normal rat hearts. Circ. Res. 1995;76:1020–1027. doi: 10.1161/01.res.76.6.1020. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 7.Sarkar S, Leaman DW, Gupta S, Sil P, Young D, Morerhead A, Mukherjee D, Ratliff N, Sun Y, Rayborn M, Hollyfield J, Sen S. Cardiac expression of myotrophin triggers myocardial hypertrophy and heart failure in transgenic mice: Changes in gene expression profiles during initiation of hypertrophy and during heart failure measured by DNA microarray analysis. J. Biol. Chem. 2004;279:20422–20434. doi: 10.1074/jbc.M308488200. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 8.Gupta S, Young D, Maitra RK, Gupta A, Popovic ZB, Yong SL, Mahajan A, Wang Q, Sen S. Prevention of cardiac hypertrophy and heart failure by silencing of NF-kappaB. J. Mol. Biol. 2008;375:637–649. doi: 10.1016/j.jmb.2007.10.006. Ref Type: Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ritchie ME. Nuclear factor-kappaB is selectively and markedly activated in humans with unstable angina pectoris. Circulation. 1998;98:1707–1713. doi: 10.1161/01.cir.98.17.1707. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 10.Morgan EN, Boyle EM, Jr, Yun W, Griscavage-Ennis JM, Farr AL, Canty TG, Jr, Pohlman TH, Verrier ED. An essential role for NF-kappaB in the cardioadaptive response to ischemia. Ann. Thorac. Surg. 1999;68:377–382. doi: 10.1016/s0003-4975(99)00646-3. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 11.Gupta S, Purcell NH, Lin A, Sen S. Activation of nuclear factor-kappaB is necessary for myotrophin-induced cardiac hypertrophy. J Cell Biol. 2002;159:1019–1028. doi: 10.1083/jcb.200207149. Ref Type: Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta S, Sen S. Role of the NF-kappaB signaling cascade and NF-kappaB-targeted genes in failing human hearts. J. Mol. Med. 2005;83:993–1004. doi: 10.1007/s00109-005-0691-z. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 13.Baldwin AS., Jr The NF-κB and I-κB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 14.Beg AA, Finco TS, Nantermet PV, Baldwin AS., Jr Tumor necrosis factor and interleukin-1 lead to phosphorylation and loss of I kappa B alpha: a mechanism for NF-kappa B activation. Mol Cell Biol. 1993;13:3301–3310. doi: 10.1128/mcb.13.6.3301. Ref Type: Journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aukrust P, Gullestad L, Ueland T, Damas JK, Yndestad A. Inflammatory and anti-inflammatory cytokines in chronic heart failure: potential therapeutic implications. Ann. Med. 2005;37:74–85. doi: 10.1080/07853890510007232. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 16.Blum A, Miller H. Pathophysiological role of cytokines in congestive heart failure. Annu. Rev. Med. 2001;52:15–27. doi: 10.1146/annurev.med.52.1.15. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 17.Gupta S, Young D, Sen S. Inhibition of NF-kappaB induces regression of cardiac hypertrophy, independent of blood pressure control, in spontaneously hypertensive rats. Am. J. Physiol Heart Circ. Physiol. 2005;289:H20–H29. doi: 10.1152/ajpheart.00082.2005. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 18.Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature. 1995;376:167–170. doi: 10.1038/376167a0. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 19.Burkly L, Hession C, Ogata L, Reilly C, Marconi LA, Olson D, Tizard R, Cate R, Lo D. Expression of relB is required for the development of thymic medulla and dendritic cells. Nature. 1995;373:531–536. doi: 10.1038/373531a0. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 20.Kontgen F, Grumont RJ, Strasser A, Metcalf D, Li R, Tarlinton D, Gerondakis S. Mice lacking the c-rel proto-oncogene exhibit defects in lymphocyte proliferation, humoral immunity, and interleukin-2 expression. Genes Dev. 1995;9:1965–1977. doi: 10.1101/gad.9.16.1965. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 21.Snapper CM, Zelazowski P, Rosas FR, Kehry MR, Tian M, Baltimore D, Sha WC. B cells from p50/NF-kappa B knockout mice have selective defects in proliferation, differentiation, germ-line CH transcription, and Ig class switching. J. Immunol. 1996;156:183–191. Ref Type: Journal. [PubMed] [Google Scholar]
- 22.Brown M, McGuinness M, Wright T, Ren X, Wang Y, Boivin GP, Hahn H, Feldman AM, Jones WK. Cardiac-specific blockade of NF-kappaB in cardiac pathophysiology: differences between acute and chronic stimuli in vivo. Am. J. Physiol Heart Circ. Physiol. 2005;289:H466–H476. doi: 10.1152/ajpheart.00170.2004. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 23.Higuchi Y, Chan TO, Brown MA, Zhang J, DeGeorge BR, Jr, Funakoshi H, Gibson G, McTiernan CF, Kubota T, Jones WK, Feldman AM. Cardioprotection afforded by NF-kappaB ablation is associated with activation of Akt in mice overexpressing TNF-alpha. Am. J. Physiol Heart Circ. Physiol. 2006;290:H590–H598. doi: 10.1152/ajpheart.00379.2005. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 24.Dignam JD, Martin PL, Shastry BS, Roeder RG. Eukaryotic gene transcription with purified components. Methods Enzymol. 1983;101:582–598. doi: 10.1016/0076-6879(83)01039-3. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 25.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 26.Wong SC, Fukuchi M, Melnyk P, Rodger I, Giaid A. Induction of cyclooxygenase-2 and activation of nuclear factor-kappaB in myocardium of patients with congestive heart failure. Circulation. 1998;98:100–103. doi: 10.1161/01.cir.98.2.100. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 27.Kawamura N, Kubota T, Kawano S, Monden Y, Feldman AM, Tsutsui H, Takeshita A, Sunagawa K. Blockade of NF-kappaB improves cardiac function and survival without affecting inflammation in TNF-alpha-induced cardiomyopathy. Cardiovasc. Res. 2005;66:520–529. doi: 10.1016/j.cardiores.2005.02.007. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 28.Kawano S, Kubota T, Monden Y, Tsutsumi T, Inoue T, Kawamura N, Tsutsui H, Sunagawa K. Blockade of NF-{kappa}B improves cardiac function and survival after myocardial infarction. Am. J. Physiol Heart Circ. Physiol. 2006 doi: 10.1152/ajpheart.01175.2005. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 29.Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ. Res. 2004;94:1543–1553. doi: 10.1161/01.RES.0000130526.20854.fa. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 30.Blackwell TS, Christman JW. The role of nuclear factor-kappa B in cytokine gene regulation. Am. J Respir. Cell Mol Biol. 1997;17:3–9. doi: 10.1165/ajrcmb.17.1.f132. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 31.Sun M, Dawood F, Wen WH, Chen M, Dixon I, Kirshenbaum LA, Liu PP. Excessive tumor necrosis factor activation after infarction contributes to susceptibility of myocardial rupture and left ventricular dysfunction. Circulation. 2004;110:3221–3228. doi: 10.1161/01.CIR.0000147233.10318.23. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 32.Lindsey ML. MMP induction and inhibition in myocardial infarction. Heart Fail. Rev. 2004;9:7–19. doi: 10.1023/B:HREV.0000011390.44039.b7. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 33.Ueda A, Ishigatsubo Y, Okubo T, Yoshimura T. Transcriptional regulation of the human monocyte chemoattractant protein-1 gene. Cooperation of two NF-kappaB sites and NF-kappaB/Rel subunit specificity. J. Biol. Chem. 1997;272:31092–31099. doi: 10.1074/jbc.272.49.31092. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 34.Hayashidani S, Tsutsui H, Shiomi T, Ikeuchi M, Matsusaka H, Suematsu N, Wen J, Egashira K, Takeshita A. Anti-monocyte chemoattractant protein-1 gene therapy attenuates left ventricular remodeling and failure after experimental myocardial infarction. Circulation. 2003;108:2134–2140. doi: 10.1161/01.CIR.0000092890.29552.22. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 35.Jones WK, Brown M, Wilhide M, He S, Ren X. NF-kappaB in cardiovascular disease: diverse and specific effects of a "general" transcription factor? Cardiovasc. Toxicol. 2005;5:183–202. doi: 10.1385/ct:5:2:183. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 36.Leonard EJ, Yoshimura T. Human monocyte chemoattractant protein-1 (MCP-1) Immunol. Today. 1990;11:97–101. doi: 10.1016/0167-5699(90)90035-8. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 37.Ban K, Ikeda U, Takahashi M, Kanbe T, Kasahara T, Shimada K. Expression of intercellular adhesion molecule-1 on rat cardiac myocytes by monocyte chemoattractant protein-1. Cardiovasc. Res. 1994;28:1258–1262. doi: 10.1093/cvr/28.8.1258. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 38.Gordon S. Macrophage-restricted molecules: role in differentiation and activation. Immunol. Lett. 1999;65:5–8. doi: 10.1016/s0165-2478(98)00116-3. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 39.Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, Quaini E, Di LC, Beltrami CA, Krajewski S, Reed JC, Anversa P. Apoptosis in the failing human heart. N. Engl. J. Med. 1997;336:1131–1141. doi: 10.1056/NEJM199704173361603. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 40.Narula J, Kharbanda S, Khaw BA. Apoptosis and the heart. Chest. 1997;112:1358–1362. doi: 10.1378/chest.112.5.1358. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 41.Haunstetter A, Izumo S. Apoptosis: basic mechanisms and implications for cardiovascular disease. Circ. Res. 1998;82:1111–1129. doi: 10.1161/01.res.82.11.1111. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 42.Mani K, Kitsis RN. Myocyte apoptosis: programming ventricular remodeling. J. Am. Coll. Cardiol. 2003;41:761–764. doi: 10.1016/s0735-1097(02)02958-3. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 43.Lee R, Collins T. Nuclear factor-kappaB and cell survival: IAPs call for support. Circ. Res. 2001;88:262–264. doi: 10.1161/01.res.88.3.262. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 44.Bergmann MW, Loser P, Dietz R, von HR. Effect of NF-kappa B Inhibition on TNF-alpha-induced apoptosis and downstream pathways in cardiomyocytes. J. Mol. Cell Cardiol. 2001;33:1223–1232. doi: 10.1006/jmcc.2001.1385. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 45.Schiekofer S, Shiojima I, Sato K, Galasso G, Oshima Y, Walsh K. Microarray analysis of Akt1 activation in transgenic mouse hearts reveals transcript expression profiles associated with compensatory hypertrophy and failure. Physiol Genomics. 2006;27:156–170. doi: 10.1152/physiolgenomics.00234.2005. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
- 46.Shiraishi I, Melendez J, Ahn Y, Skavdahl M, Murphy E, Welch S, Schaefer E, Walsh K, Rosenzweig A, Torella D, Nurzynska D, Kajstura J, Leri A, Anversa P, Sussman MA. Nuclear targeting of Akt enhances kinase activity and survival of cardiomyocytes. Circ. Res. 2004;94:884–891. doi: 10.1161/01.RES.0000124394.01180.BE. Ref Type: Journal. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.