Abstract
Current research in the field of anxiety disorders is largely receptor-centric, leaving intracellular pathways largely unexplored. Gαs, the G-protein which stimulates adenylyl cyclase and L-type voltage-gated calcium channels, may be one intracellular molecule regulating anxiety-related behaviors as increased efficacy of Gαs signaling has been noted in patient populations that suffer from anxiety. We report here anxiety-related behaviors in two lines of transgenic mice expressing a constitutively active isoform of Gαs (or Gαs*). The first line expressed Gαs* throughout postnatal forebrain neurons, while the second line of mice conditionally expressed Gαs* selectively in the striatum (Gαs*str mice). In the open field, both lines of mice showed a significant preference for the periphery suggesting that expression of Gαs* in the striatum alone was sufficient to produce an anxiogenic phenotype. In the light/dark box, Gαs*str mice exhibited longer latencies to enter the light and spent significantly less time in the lit compartment. Similarly, Gαs*str mice showed longer latencies to enter the open quadrants and spent less time in the open quadrants of the elevated zero maze. Interestingly, these anxiety-related phenotypes were largely unrelated to developmental effects as mice expressing the Gαs*str transgene during development, but not at testing, were normal on most measures. These observations show that chronic Gαs signaling in the striatum is sufficient to trigger anxiety-related behaviors largely independent of developmental effects and suggest the cAMP pathway or L-type voltage-gated calcium channels may be viable targets for future pharmacological intervention in the treatment of anxiety disorders.
Keywords: GNAS, anxiety, panic disorder, striatum, cAMP, L-VGCC
Introduction
Anxiety disorders are estimated to have a lifetime prevalence rate in the United States of 31% (Kessler et al., 2007). In addition to the psychological symptoms (e.g., irrational fear, worry, restlessness, and sleep disturbances) classically associated with anxiety disorders, patients show an increased risk for a variety of physical illnesses, including heart disease, obesity, and cancer (cf., Balon, 2006). Thus, anxiety disorders pose a threat to both the mental and physical well being of a large percentage of the population, greatly affecting quality of life and producing an economic burden (Hoffman et al., 2008).
Despite the widespread prevalence of anxiety disorders, the biochemical substrates underlying these pathological behavioral responses remain largely unknown. One potential intracellular mechanism contributing to anxiety disorders may be increased signaling through Gαs, the G-protein that stimulates adenylyl cyclase (cf., Neves et al., 2002) and L-type voltage-gated calcium channels (VGCC) (Yatani and Brown, 1989; Lader et al., 1998). Gurguis and colleagues (1999) found increased efficacy of Gαs signaling in neutrophils of drug-free patients with panic disorder as well as in polymorphonuclear leukocytes of patients with panic disorder (Gurguis et al., 1997). In addition, increased levels and/or efficacy of Gαs have repeatedly been measured in peripheral and central measures from patients with bipolar disorder (Young et al., 1991, 1993, 1994; Avissar et al., 1997), a patient population with high rates of comorbid anxiety (cf., Oswald et al., 2007). Together, these data raise the possibility that chronic Gαs signaling may contribute to anxiety-related phenotypes.
Given that disease-related symptoms are likely due to long term changes in neuronal function, we test here the behavioral effect of chronically increasing Gαs signaling. To do so, we evaluated two lines of transgenic mice that express a constitutively active isoform Gαs (Gαs*). In the first line, forebrain-specific expression of Gαs* was driven by the CaMKIIα promoter (see Fig. 1A) (Wand et al., 2001; Kelly et al., 2007, 2008). In the second line, conditional expression of Gαs* was driven with the reversible tetracycline-regulated system (see Fig. 1B), which spatially restricted expression of the transgene to forebrain neurons and enabled expression of the transgene to be suppressed with administration of doxycycline (dox) (see Fig. 1C). By driving expression of our transgene with this reversible system, we were able to determine whether expressing Gαs* during development was sufficient to alter anxiety-related behaviors in adults. By virtue of where in the genome the tetO construct randomly inserted, the new transgenic line showed Gαs* expression enriched in the striatum consistent with increased cAMP levels being found only in this region (referred to hereafter as Gαs*str mice; see Fig. 1). Thus, Gαs*str mice impart enhanced temporal and spatial resolution relative to our initial line. Focusing on anxiety-related behaviors, we tested Gαs* mice in the open field, light/dark box, and elevated zero maze. We show that, across all paradigms, Gαs*str mice exhibited a significant increase in anxiety-related behaviors relative to control littermates and that this phenotype was largely independent of developmental effects.
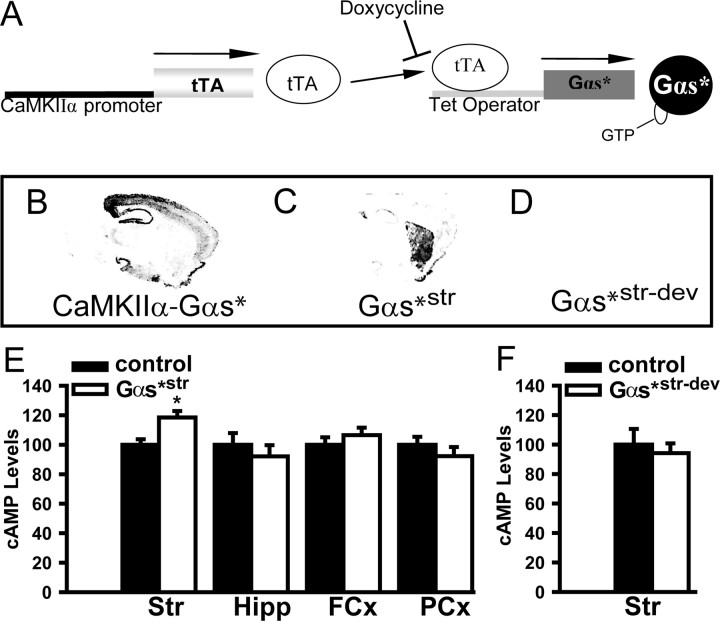
Figure 1.
Gαs*str mice showed striatally enriched transgene expression and cAMP elevation restricted to the striatum. A, The tetracycline-regulated transgenic system used herein employs the CaMKIIα promoter to restrict expression of the transcription factor tTA to postnatal forebrain neurons. The tTA protein then binds the Tet promoter, activating transcription of the ligated transgene. Doxycycline impedes the ability of tTA to bind, thus preventing transcription. As such, the tetracycline system enables expression of a transgene to be suppressed. B, Autoradiographs for CaMKIIα-Gαs* mice showed expression of the transgene throughout postnatal forebrain neurons (nonreversible). C, Autoradiographs for CaMKIIα-tTA/tetO-Gαs* mice showed transgene expression enriched in the striatum (thus, referred to as Gαs*str mice). D, Autoradiographs also showed that transgene expression within Gαs*str mice is reversible with dox, as shown in a Gαs*str-dev mouse (i.e., a mouse that expressed the transgene throughout development but received dox during adulthood). E, As previously measured in CaMKIIα-Gαs* mice (Kelly et al., 2007), Gαs*str mice showed significantly elevated cAMP levels (%CT pmol/mg protein) in the striatum. Gαs*str showed no significant differences in hippocampal or cortical cAMP levels. F, The elevated striatal cAMP levels observed in adult Gαs*str mice were not due to developmental effects, as evidenced by normal cAMP levels in Gαs*str-dev mice. The asterisk (*) versus CT, p = 0.009. FCx, Frontal cortex; Hipp, hippocampus; PCx, parahippocampal cortex; Str, striatum.
Materials and Methods
Subjects
Transgenic mice were bred and group-housed in breeding colonies at the University of Pennsylvania in the hemizygous state on a C57BL/6J background. Transgenic mice express an isoform of the Gαs protein subunit that is constitutively active due to a point mutation (Q227L), as previously described (Wand et al., 2001). This mutation prevents the hydrolysis of bound GTP and, in so doing, results in increased basal adenylyl cyclase activity in the brain (Wand et al., 2001; Kelly et al., 2007). CaMKIIα-Gαs* mice (N12–13 onto C57BL/6J) were bred as previously described (Kelly et al., 2007, 2008). The tetO-Gαs* transgene was microinjected into pronuclei from superovulated B6SJLF1/J mice by the Transgenic and Chimeric Mouse Facility at the University of Pennsylvania. The line reported here (line 1485, referred to as Gαs*str mice) was then backcrossed onto C57BL/6J for 5–6 generations in the hemizygous state before being mated to CaMKIIα-tTA(B) mice (Mayford et al., 1996;N20+ on C57BL/6J). Only mice positive for both the tetO and CaMKIIα driven constructs express the Gαs* transgene. The control group, then, includes wild-type mice (with neither construct), tetO-only mice and CaMKIIα-only mice. For experiments using doxycycline, 200 mg/kg was administered via the chow to both Gαs*str and control littermates either ≥2.5 weeks before test (i.e., Gαs*str-dev mice) or from gestation through testing (i.e., for Gαs*str-dox mice). This dose was chosen based on our previous experience that 40 mg/kg dox was insufficient to reliably suppress transgene expression in our tetracycline regulated Gαswt mice (our unpublished observations). In situ hybridization confirmed that 2.5 weeks of dox was sufficient to suppress transgene expression (see Fig. 1D). Using transgene specific probes, animals are genotyped by Southern blot, but all experiments are conducted blind to genotype. For both lines, transgenic mice 2–5 months old were compared with sex-matched control littermates. All experiments were conducted in accordance with National Institutes of Health guidelines for animal care and use, and all experiments were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Behavior
Locomotor activity.
Locomotor activity was measured for 30 min in a 41 × 41 cm San Diego Instruments Open Field (Stein et al., 2006). The box was equipped with 16 × 16 motion detector beams (every 2.54 cm). Ambulations (breaks of two contiguous beams) and preference for the periphery (percentage of ambulations in outer two beams on all sides) were calculated.
Light/dark box.
Partiality to light versus dark was measured in a modified housing cage (Stein et al., 2006). Animals were introduced to the dark chamber and were allowed to explore the apparatus freely for 10 min. Latency to enter (operationally defined as the time when all four paws first crossed the threshold of the 6 × 6 cm opening) and the total time spent in the lit compartment were recorded.
Elevated zero maze.
Preference for walled versus exposed quadrants was measured in an elevated circular track (Stein et al., 2006). Animals were introduced to a walled quadrant and permitted to explore the elevated zero maze for 10 min. Latency to enter (operationally defined as time when all 4 paws first entered the open) and total time spent in the open quadrants were recorded.
Contextual fear conditioning.
Contextual fear conditioning was conducted as previously described (Kelly et al., 2008), by pairing a training context with a 1.5 mA footshock and measuring the level of freezing exhibited in the same context 24 h later (3 min session).
Prepulse inhibition.
Prepulse inhibition (PPI) was conducted as previously described (Kelly et al., 2007; Kanes et al., 2007), using prepulses 4–16 dB above a 68 dB background and a pulse of 120 dB and measuring the startle response with an accelerometer (San Diego Instruments).
Biochemistry
In situ hybridization and cAMP assays were conducted as previously described (Kelly et al., 2007, 2008). For biochemical experiments, animals were killed by cervical dislocation and brains were immediately harvested, hemisected (one half intact for in situ the other dissected for cAMP assays), and placed on dry ice. “Parahippocampal cortex” was defined as cortical regions overlying the hippocampus. Levels of cAMP were measured from a single hemisphere using a radioimmune competition assay kit (Perkin-Elmer). All animals were killed from the home cage.
Data analyses
Data were analyzed for effect of genotype by t test using Sigmastat (v. 2.03; Systat). In biochemical experiments, balanced groups of animals were raised at different times and killed on different days. Therefore, to control for any confounds due to day of killing, data for each group were normalized and expressed as a percentage of the control (CT) mean, as previously described (Kelly et al., 2007, 2008). Normalized data were then combined and analyzed for effects of genotype. Statistical outliers >2 SDs from the mean were removed from analyses. Significance was determined at p < 0.05. All values reported are means ± SEM.
Results
Gαs*str mice showed increased cAMP levels in the striatum
Autoradiographic in situ hybridization showed transgene expression was more spatially restricted in the Gαs*str mice (Fig. 1C) versus our original CaMKIIα-Gαs* mice (Fig. 1B). Biochemical analyses show that Gαs*str mice show elevated cAMP levels only in striatum (not cortex nor hippocampus) (Fig. 1E) (n = 5–9 per genotype; t(12) = 3.10, p = 0.009), as previously reported in CaMKIIα-Gαs* mice (41% increase; Kelly et al., 2007). Developmental expression of Gαs*str is not sufficient to cause permanent alterations in striatal cAMP levels, because Gαs*str-dev mice show striatal cAMP levels equivalent to those of control littermates (Fig. 1F) (n = 7 per genotype; t(6) = 0.47, p = 0.657).
CaMKIIα-Gαs* and Gαs*str mice exhibited a significant preference for the periphery of an open field
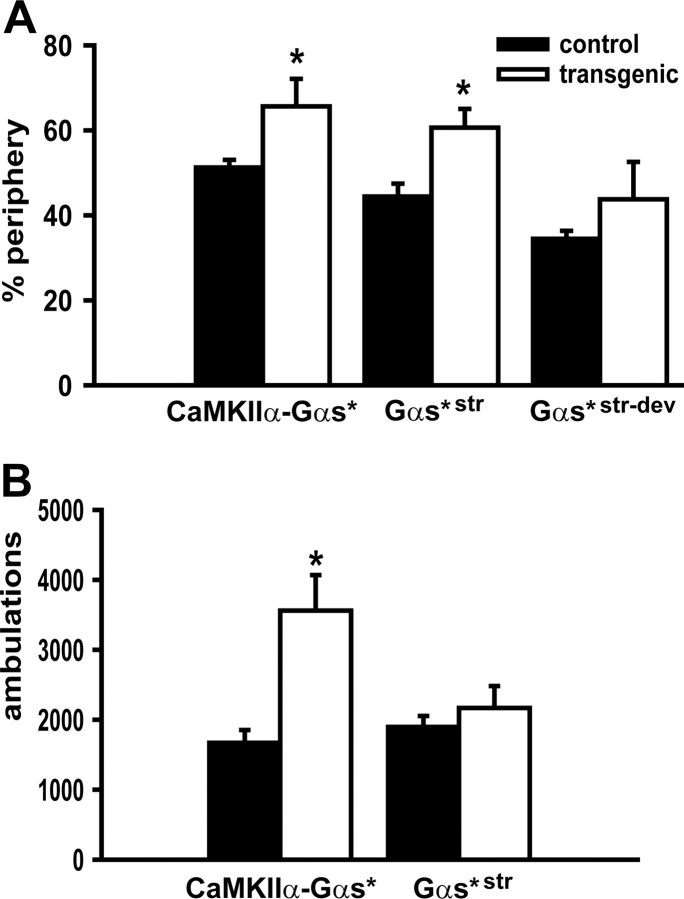
To determine whether expressing Gαs* in forebrain neurons would be sufficient to increase anxiety-related behaviors, we characterized our initial line of CaMKIIα-Gαs* mice in an open field. Relative to wild-type mice (n = 7–8, per genotype), CaMKIIα-Gαs* mice exhibited a higher percentage of ambulations in the periphery of the open field (t(13) = 2.28, p = 0.048) (Fig. 2A). In addition, CaMKIIα-Gαs* mice showed significantly more ambulations overall (t(12) = 3.50, p = 0.004) (Fig. 2B). These results suggest that Gαs* expression is sufficient to cause hyperlocomotion and an increase in anxiety-related behavior.
Figure 2.
Gαs* mice exhibited an increase in anxiety-like behavior in the Open Field. A, Relative to control littermates (CT), CaMKIIα-Gαs* transgenic mice performed a greater percentage of ambulations in the periphery of an Open Field, as did Gαs*str mice. B, In contrast, however, only CaMKIIα-Gαs* mice showed significantly more ambulations in total, suggesting that Gαs* expression in the striatum was not sufficient to produce hyperactivity. The asterisk (*) versus CT, p < 0.05–0.01.
To further isolate where in the brain and when (i.e., development vs adulthood) Gαs* acts to influence locomotor and anxiety-related behavior, we also tested Gαs*str and control littermates in the open field (n = 14–18 per genotype). Consistent with the observations above, Gαs*str mice showed a greater preference for the periphery of the open field (t(30) = 3.19, p = 0.003) (Fig. 2a). In contrast to the CaMKIIα-Gαs* line, however, Gαs*str mice displayed no difference in total ambulations relative to control littermates (Fig. 2B), showing the effect of Gαs* signaling on the anxiety-related phenotype in the open field is dissociable from the effect on hyperactivity.
Gαs*str mice showed anxiety-related phenotypes in a light/dark box and an elevated zero maze
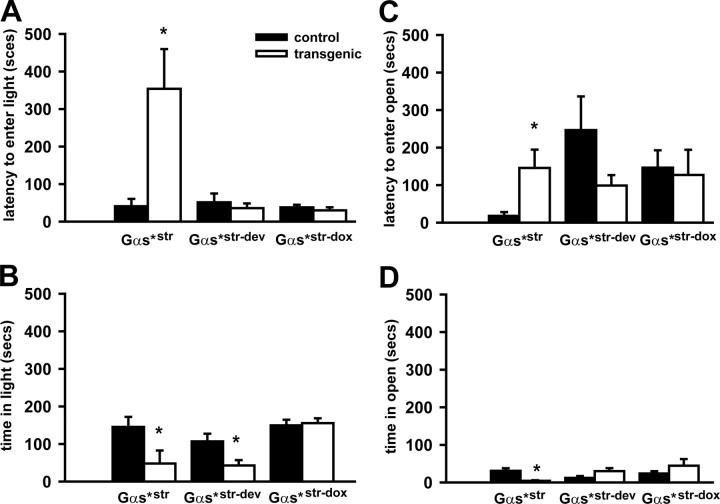
To determine whether the Gαs*-induced anxiety-related phenotype observed above in the open field would extend to additional anxiety-related paradigms, we tested Gαs*str mice and control littermates in the light/dark box and elevated zero maze paradigms. In the light/dark box, Gαs*str mice exhibited a longer latency to enter the lit chamber relative to their control littermates (n = 7–11 per genotype; t(16) = 3.62, p = 0.002) (Fig. 3A). Additionally, Gαs*str mice spent significantly less time in the lit compartment relative to control littermates (t(16) = 2.27, p < 0.037) (Fig. 3B). This pattern of behavior is paralleled in the elevated zero maze, where Gαs*str mice showed a longer latency to enter the open quadrants (n = 7–10, per genotype; t(15) = 3.01, p = 0.009) (Fig. 3C) and spent less total time in the open quadrants relative to control littermates (t(14) = 2.99, p = 0.01) (Fig. 3D). These behaviors confirm the hypothesis that chronically increasing Gαs* signaling is sufficient to increase anxiety-related phenotypes and suggest the effect is localized to the striatum.
Figure 3.
Gαs*str mice showed an increase in anxiety-related behaviors in the light/dark box and elevated zero maze. A, Relative to control littermates (CT), Gαs*str transgenic mice exhibited a longer latency to enter the lit chamber of the light/dark box; however, Gαs*str-dev and Gαs*str-dox mice showed normal behavior. B, Gαs*str mice also spent less time in total in the lit portion of the box. Surprisingly, Gαs*str-dev mice also showed this decrease in time spent in the light. Importantly, however, Gαs*str-dox mice exhibited normal performance. C, Gαs*str mice also exhibited significantly longer latencies to enter the open quadrants of an elevated zero maze and D) and spent less time overall in the open quadrants. Gαs*str-dev mice and Gαs*str-dox mice were normal on all elevated zero maze measures. The asterisk (*) versus CT, p < 0.05–0.01.
Anxiety-related phenotypes observed in Gαs*str mice were largely unrelated to developmental effects
Next we determined whether the anxiety-related phenotypes observed in adult Gαs*str mice were due to developmental effects. To do so, we tested adult Gαs*str mice that experienced transgene expression throughout development, but not at the time of adulthood testing due to doxycycline administration (referred to as Gαs*str-dev mice). Gαs*str-dev mice did not exhibit a significant preference for the periphery of an open field relative to control littermates (Fig. 2A; n = 7 per genotype; t(12) = 1.52, p = 0.154). Similarly, Gαs*str-dev mice showed no significant difference in the latency to enter the lit compartment of the light/dark box (Fig. 3A) (n = 7, per genotype; t(12) = 0.77, p = 0.456). In contrast, however, Gαs*str-dev mice did spend significantly less time in the light in total (Fig. 3B) (t(12) = 2.58, p = 0.024). This effect observed in Gαs*str-dev mice was specifically due to developmental expression of the transgene (as opposed to a confounding effect of transgene insertion) because Gαs*str mice administered dox throughout life (i.e., that never expressed the transgene; Gαs*str-dox) showed no difference with respect to latency to enter the lit chamber (Fig. 3A) (n = 4 per genotype; t(6) = 0.75, p = 0.48) nor total time spent in the light (Fig. 3B) (t(6) = 0.30, p = 0.77). In the elevated zero maze, Gαs*str-dev mice showed no significant difference in the latency to enter the open quadrants (Fig. 3C) (n = 7 per genotype; t(12) = 1.46, p = 0.172) nor in the total time spent in the open quadrants (Fig. 3D) (t(12) = 1.96, p = 0.074).
Discussion
Here, we showed that chronically increasing Gαs signaling in the forebrain triggers an increase in anxiety-related behaviors in the open field, light/dark box, and elevated zero maze. The fact that chronic Gαs signaling increases anxiety-related behaviors largely independent of developmental effects suggests that such anxiety-related pathophysiology is readily reversible in the adult. These observations are particularly relevant to patients with panic disorder, in whom increased efficacy of Gαs signaling has been noted (Gurguis et al., 1997, 1999).
Data presented here suggest that the effect of Gαs* on anxiety-related behaviors is localized to the striatum. This conclusion is based on the fact that Gαs*str mice, which exhibited increased anxiety-related behaviors, showed cAMP changes in striatum but not in hippocampus nor cortex. Further, Gαs*str mice showed intact contextual fear conditioning and PPI (Fig. 4), behaviors influenced by chronic Gαs signaling in the hippocampus and cortex (Kelly et al., 2007, 2008). Although studies here do not rule out completely the possibility that Gαs* is acting elsewhere in the brain, a striatally localized effect of Gαs* on anxiety would be consistent with an extensive literature implicating this brain region as key to the circuitry underlying anxiety-related behaviors and pathologies [e.g., for panic disorder, see Reiman et al. (1989), Yoo et al. (2005); for social anxiety disorder, cf., Mathew and Ho (2006); for bipolar disorder, see Konarski et al. (2008), Killgore et al. (2008)]. Dysfunction within the dorsal striatum, in particular, may disrupt processing of safety signals that would otherwise decrease expression of fearful or anxiety-related behaviors (Rogan et al., 2005).
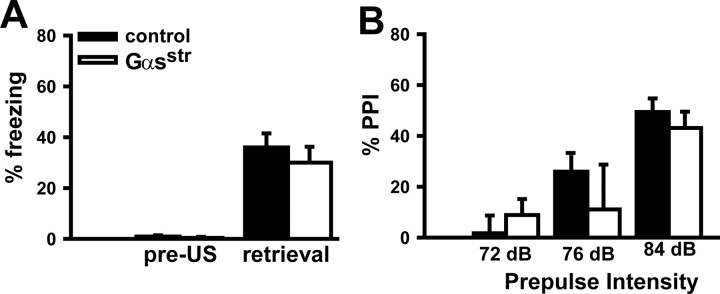
Figure 4.
Gαsstr mice showed normal performance in contextual fear conditioning and prepulse inhibition of acoustic startle. A, Relative to control littermates, Gαs*str mice showed equivalent levels of freezing during training [before administration of the footshock unconditioned stimulus (US)] and 24 h later when tested in the same context (n = 10–13, per genotype; t(21) = 0.72, p = 0.482). B, Relative to control littermates, Gαs*str mice showed normal sensorimotor gating across a range of prepulse intensities. (n = 15–21, per genotype; F(1,68) = 0.38, p = 0.541).
It remains to be determined through which downstream target Gαs* elicits an anxiogenic profile. Chronically increasing cAMP signaling could increase anxiety, by virtue of decreasing GABA functionality (Moss et al., 1992; Shekhar et al., 2003; Kalueff and Nutt, 2007). Although increasing cAMP signaling acutely appears to reduce anxiety-related behaviors in rodents (Silvestre et al., 1999; Barrot et al., 2002; Masood et al., 2008), chronically increasing cAMP signaling has the opposite effect. Genetic deletion of the cAMP-degrading enzyme Phosphodiesterase 4B or overexpression of the striatally enriched cAMP-producing enzyme adenylyl cyclase 5, increases anxiety-related behavior in mice (Kim et al., 2008; Zhang et al., 2008). In addition, genetic deletion of 5HT1A receptors, which couple negatively to adenylyl cylase via Gαi, increases anxiety-related behaviors in mice, and a reduction in 5HT1A receptors has been measured in patients with social anxiety disorder (cf., Lesch et al., 2003). Increased activity of L-type VGCCs could also contribute to Gαs*-induced increases in anxiety as they play a role in the anxiogenic effects associated with amphetamine and nicotine administration (Biala and Budzynska, 2006; Biała and Kruk, 2007; Biala and Kruk, 2008). Further, calcium channel blockers have been proposed as a novel class of anxiolytics, particularly for treatment of panic disorder (Balon and Ramesh, 1996). As such, we hypothesize that Gαs*-induced anxiety is likely due to the chronic nature of the cAMP upregulation and/or increased activation of L-type VGCCs. Our results identify Gαs as an intracellular signaling molecule regulating the anxiety response, suggesting Gαs or it downstream effectors may prove effective therapeutic targets in the treatment of anxiety disorders.
Footnotes
This work was supported by Tourettes Syndrome Association (M.P.K.), National Institute of Mental Health (NIMH) Grant T32 MH019112 [to M.P.K., R. Gur, Principal Investigator (P.I.)], and Merck, Whitehall, and Packard Foundations as well as NIMH Grants R01 MH60244, NIA R01 AG18199, and P50 MH 6404501 (Project 3 to T.A.; R. Gur, Conte Center P.I.). M.P.K. is currently employed by Wyeth Research. We thank Dr. Raquel Gur for helpful discussions and Dr. Jean Richa for efforts in generating our transgenic mice.
References
- Avissar S, Nechamkin Y, Barki-Harrington L, Roitman G, Schreiber G. Differential G protein measures in mononuclear leukocytes of patients with bipolar mood disorder are state dependent. J Affect Disord. 1997;43:85–93. doi: 10.1016/s0165-0327(96)01400-0. [DOI] [PubMed] [Google Scholar]
- Balon R. Mood, anxiety, and physical illness: body and mind, or mind and body. Depress Anxiety. 2006;23:377–387. doi: 10.1002/da.20217. [DOI] [PubMed] [Google Scholar]
- Balon R, Ramesh C. Calcium channel blockers for anxiety disorders. Ann Clin Psychiatry. 1996;8:215–220. doi: 10.3109/10401239609147764. [DOI] [PubMed] [Google Scholar]
- Barrot M, Olivier JD, Perrotti LI, DiLeone RJ, Berton O, Eisch AJ, Impey S, Storm DR, Neve RL, Yin JC, Zachariou V, Nestler EJ. CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci U S A. 2002;99:11435–11440. doi: 10.1073/pnas.172091899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biala G, Budzynska B. Effects of acute and chronic nicotine on elevated plus maze in mice: involvement of calcium channels. Life Sci. 2006;79:81–88. doi: 10.1016/j.lfs.2005.12.043. [DOI] [PubMed] [Google Scholar]
- Biała G, Kruk M. Amphetamine-induced anxiety-related behavior in animal models. Pharmacol Rep. 2007;59:636–644. [PubMed] [Google Scholar]
- Biala G, Kruk M. Calcium channel antagonists suppress cross-tolerance to the anxiogenic effects of d-amphetamine and nicotine in the mouse elevated plus maze test. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:54–61. doi: 10.1016/j.pnpbp.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Gurguis GN, Turkka J, George DT, Linnoila M. Beta-adrenoreceptor coupling to GS protein in alcohol dependence, panic disorder, and patients with both conditions. Neuropsychopharmacology. 1997;16:69–76. doi: 10.1016/S0893-133X(96)00161-3. [DOI] [PubMed] [Google Scholar]
- Gurguis GN, Blakeley JE, Antai-Otong D, Vo SP, Orsulak PJ, Petty F, Rush AJ. Adrenergic receptor function in panic disorder. II. Neutrophil beta 2 receptors: Gs protein coupling, effects of imipramine treatment and relationship to treatment outcome. J Psychiatr Res. 1999;33:309–322. doi: 10.1016/s0022-3956(99)00008-4. [DOI] [PubMed] [Google Scholar]
- Hoffman DL, Dukes EM, Wittchen HU. Human and economic burden of generalized anxiety disorder. Depress Anxiety. 2008;25:72–90. doi: 10.1002/da.20257. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Nutt DJ. Role of GABA in anxiety and depression. Depress Anxiety. 2007;24:495–517. doi: 10.1002/da.20262. [DOI] [PubMed] [Google Scholar]
- Kanes SJ, Tokarczyk J, Siegel SJ, Bilker W, Abel T, Kelly MP. Rolipram: A specific phosphodiesterase 4 inhibitor with potential antipsychotic activity. Neuroscience. 2007;144:239–246. doi: 10.1016/j.neuroscience.2006.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MP, Isiegas C, Cheung YF, Tokarczyk J, Yang X, Esposito MF, Rapoport DA, Fabian SA, Siegel SJ, Wand G, Houslay MD, Kanes SJ, Abel T. Constitutive activation of Galphas within forebrain neurons causes deficits in sensorimotor gating because of PKA-dependent decreases in cAMP. Neuropsychopharmacology. 2007;32:577–588. doi: 10.1038/sj.npp.1301099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MP, Cheung YF, Favilla C, Siegel SJ, Kanes SJ, Houslay MD, Abel T. Constitutive activation of the G-protein subunit Gαs within forebrain neurons causes PKA-dependent alterations in fear conditioning and cortical Arc mRNA expression. Learn Mem. 2008;15:75–83. doi: 10.1101/lm.723708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Angermeyer M, Anthony JC, DE Graaf R, Demyttenaere K, Gasquet I, DE Girolamo G, Gluzman S, Gureje O, Haro JM, Kawakami N, Karam A, Levinson D, Medina Mora ME, Oakley Browne MA, Posada-Villa J, Stein DJ, Adley Tsang CH, Aguilar-Gaxiola S, Alonso J, Lee S, Heeringa S, Pennell BE, Berglund P, Gruber MJ, Petukhova M, Chatterji S, Ustün TB. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization's World Mental Health Survey Initiative. World Psychiatry. 2007;6:168–176. [PMC free article] [PubMed] [Google Scholar]
- Killgore WD, Gruber SA, Yurgelun-Todd DA. Abnormal corticostriatal activity during fear perception in bipolar disorder. Neuroreport. 2008;19:1523–1527. doi: 10.1097/WNR.0b013e328310af58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KS, Lee KW, Baek IS, Lim CM, Krishnan V, Lee JK, Nestler EJ, Han PL. Adenylyl cyclase-5 activity in the nucleus accumbens regulates anxiety-related behavior. J Neurochem. 2008;107:105–115. doi: 10.1111/j.1471-4159.2008.05592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarski JZ, McIntyre RS, Kennedy SH, Rafi-Tari S, Soczynka JK, Ketter TA. Volumetric neuroimaging investigations in mood disorders: bipolar disorder versus major depressive disorder. Bipolar Disord. 2008;10:1–37. doi: 10.1111/j.1399-5618.2008.00435.x. [DOI] [PubMed] [Google Scholar]
- Lader AS, Xiao YF, Ishikawa Y, Cui Y, Vatner DE, Vatner SF, Homcy CJ, Cantiello HF. Cardiac Gsalpha overexpression enhances L-type calcium channels through an adenylyl cyclase independent pathway. Proc Natl Acad Sci U S A. 1998;95:9669–9674. doi: 10.1073/pnas.95.16.9669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, Zeng Y, Reif A, Gutknecht L. Anxiety-related traits in mice with modified genes of the serotonergic pathway. Eur J Pharmacol. 2003;480:185–204. doi: 10.1016/j.ejphar.2003.08.106. [DOI] [PubMed] [Google Scholar]
- Masood A, Nadeem A, Mustafa SJ, O'Donnell JM. Reversal of oxidative stress-induced anxiety by inhibition of phosphodiesterase-2 in mice. J Pharmacol Exp Ther. 2008;326:369–379. doi: 10.1124/jpet.108.137208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew SJ, Ho S. Etiology and neurobiology of social anxiety disorder. J Clin Psychiatry. 2006;67(Suppl 12):9–13. [PubMed] [Google Scholar]
- Mayford M, Bach ME, Huang YY, Wang L, Hawkins RD, Kandel ER. Control of memory formation through regulated expression of a CaMKII transgene. Science. 1996;274:1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- Moss SJ, Smart TG, Blackstone CD, Huganir RL. Functional modulation of GABAA receptors by cAMP-dependent protein phosphorylation. Science. 1992;257:661–665. doi: 10.1126/science.1323140. [DOI] [PubMed] [Google Scholar]
- Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002;296:1636–1639. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- Oswald P, Souery D, Kasper S, Lecrubier Y, Montgomery S, Wyckaert S, Zohar J, Mendlewicz J. Current issues in bipolar disorder: a critical review. Eur Neuropsychopharmacol. 2007;17:687–695. doi: 10.1016/j.euroneuro.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Raichle ME, Robins E, Mintun MA, Fusselman MJ, Fox PT, Price JL, Hackman KA. Neuroanatomical correlates of a lactate-induced anxiety attack. Arch Gen Psychiatry. 1989;46:493–500. doi: 10.1001/archpsyc.1989.01810060013003. [DOI] [PubMed] [Google Scholar]
- Rogan MT, Leon KS, Perez DL, Kandel ER. Distinct neural signatures for safety and danger in the amygdala and striatum of the mouse. Neuron. 2005;46:309–320. doi: 10.1016/j.neuron.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Sajdyk TJ, Gehlert DR, Rainnie DG. The amygdala, panic disorder, and cardiovascular responses. Ann N Y Acad Sci. 2003;985:308–325. doi: 10.1111/j.1749-6632.2003.tb07090.x. [DOI] [PubMed] [Google Scholar]
- Silvestre JS, Fernández AG, Palacios JM. Effects of rolipram on the elevated plus-maze test in rats: a preliminary study. J Psychopharmacol. 1999;13:274–277. doi: 10.1177/026988119901300309. [DOI] [PubMed] [Google Scholar]
- Stein JM, Bergman W, Fang Y, Davison L, Brensinger C, Robinson MB, Hecht NB, Abel T. Behavioral and neurochemical alterations in mice lacking the RNA-binding protein translin. J Neurosci. 2006;26:2184–2196. doi: 10.1523/JNEUROSCI.4437-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wand G, Levine M, Zweifel L, Schwindinger W, Abel T. The cAMP-protein kinase A signal transduction pathway modulates ethanol consumption and sedative effects of ethanol. J Neurosci. 2001;21:5297–5303. doi: 10.1523/JNEUROSCI.21-14-05297.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatani A, Brown AM. Rapid beta-adrenergic modulation of cardiac calcium channel currents by a fast G protein pathway. Science. 1989;245:71–74. doi: 10.1126/science.2544999. [DOI] [PubMed] [Google Scholar]
- Yoo HK, Kim MJ, Kim SJ, Sung YH, Sim ME, Lee YS, Song SY, Kee BS, Lyoo IK. Putaminal gray matter volume decrease in panic disorder: an optimized voxel-based morphometry study. Eur J Neurosci. 2005;22:2089–2094. doi: 10.1111/j.1460-9568.2005.04394.x. [DOI] [PubMed] [Google Scholar]
- Young LT, Li PP, Kish SJ, Siu KP, Warsh JJ. Postmortem cerebral cortex Gs alpha-subunit levels are elevated in bipolar affective disorder. Brain Res. 1991;553:323–326. doi: 10.1016/0006-8993(91)90843-k. [DOI] [PubMed] [Google Scholar]
- Young LT, Li PP, Kish SJ, Siu KP, Kamble A, Hornykiewicz O, Warsh JJ. Cerebral cortex Gs alpha protein levels and forskolin-stimulated cyclic AMP formation are increased in bipolar affective disorder. J Neurochem. 1993;61:890–898. doi: 10.1111/j.1471-4159.1993.tb03600.x. [DOI] [PubMed] [Google Scholar]
- Young LT, Li PP, Kish SJ, Warsh JJ. Cerebral cortex beta-adrenoceptor binding in bipolar affective disorder. J Affect Disord. 1994;30:89–92. doi: 10.1016/0165-0327(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Huang Y, Masood A, Stolinski LR, Li Y, Zhang L, Dlaboga D, Jin SL, Conti M, O'Donnell JM. Anxiogenic-like behavioral phenotype of mice deficient in phosphodiesterase 4B (PDE4B) Neuropsychopharmacology. 2008;33:1611–1623. doi: 10.1038/sj.npp.1301537. [DOI] [PMC free article] [PubMed] [Google Scholar]